Abstract
Matrix metalloproteinases (MMPs) are proteinases capable of degrading components of the extracellular matrix and numerous nonmatrix proteins. MMPs along with tissue inhibitors of MMPs, have been implicated in the pathogenesis of liver diseases. Although, the precise mechanism-of-actions of MMPs in various liver related disorders is largely unknown, however, data from diverse experimental models indicate that these proteinases influence cellular activities including proliferation and survival, gene expression, as well as multiple aspects of inflammation. Hence, MMP's are likely key players in the outcomes related to liver disease.
Abbreviations: Col, collagen; ECM, extra cellular matrix; GBD, global burden of disease; HCC, hepato-cellular carcinoma; IRI, ischemia and reperfusion injury; MMP, matrix metalloproteases; NAFLD, non-alcoholic fatty liver disease; NFkB, nuclear factor kappa-B; TNF, tumor necrosis factor; TIMPs, tissue inhibitors of MMPs
Keywords: matrix metalloproteinases, extracellular matrix (ECM), liver fibrosis, cirrhosis, hepatocellular carcinoma
The liver is one of the vital organs of human body, which supports nearly every other organ at some level. Liver is mainly involved in the metabolism process, including metabolism of macromolecules and micro molecules, immune response and blood purification.1, 2 Due to the involvement of liver in metabolic processes, liver is also exposed to many insults which lead to an inflammatory response. Liver or hepatic diseases include a wide range of complex conditions like hepatic inflammation, fatty liver, hepatitis, liver cirrhosis, fibrosis and hepatocellular carcinoma (HCC).3 Among the major liver ailments, cirrhosis is the late stage liver disease, which is caused when healthy liver tissues are replaced by scar tissue (fibrosis),4 further leading to hepatic cancer. Liver is also known for its regenerative potential, which has been observed post hepatectomy and also during recovery, post-ischemic or acute liver injury.5, 6
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases, which are involved in remodeling of extracellular matrix (ECM) under normal physiological and pathologic conditions.7, 8 MMPs are also involved in the process of liver regeneration along with many liver diseases including hepatic inflammation, fatty liver, hepatitis, liver cirrhosis, fibrosis and HCC.9 MMPs are responsible for the turnover of matrix proteins, including collagen, gelatin, elastin and fibronectin, as well as non-matrix substrates like growth factors, chemokines and adhesion molecules.10 Although MMPs are well known for their anti-fibrotic effect due to their ability to degrade ECM proteins, it has been recognized that MMPs can also exert pro-fibrotic effect under various pathological conditions. This review aims to explore the canonical and non-canonical roles of MMPs in liver diseases.
Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases
MMPs are secreted as latent zymogen form and are activated by proteolytic cleavage by various factors.11 Their activities and expressions are regulated at different phases like gene transcription, zymogen activation, enzyme secretion and by their endogenous inhibitors, namely tissue inhibitor of metalloproteinases (TIMPs).12, 13
TIMPs is an ancient family with structural and functional diversity. Any disruption in the activity of MMPs could lead to the development of diseases discussed above. An imbalance in the expression levels of MMP and TIMP has been reported to be the cause of various medical conditions, like tumor invasion, rheumatoid arthritis, atherosclerosis, aneurysms, nephritis, tissue ulcers, fibrosis and endometriosis.14
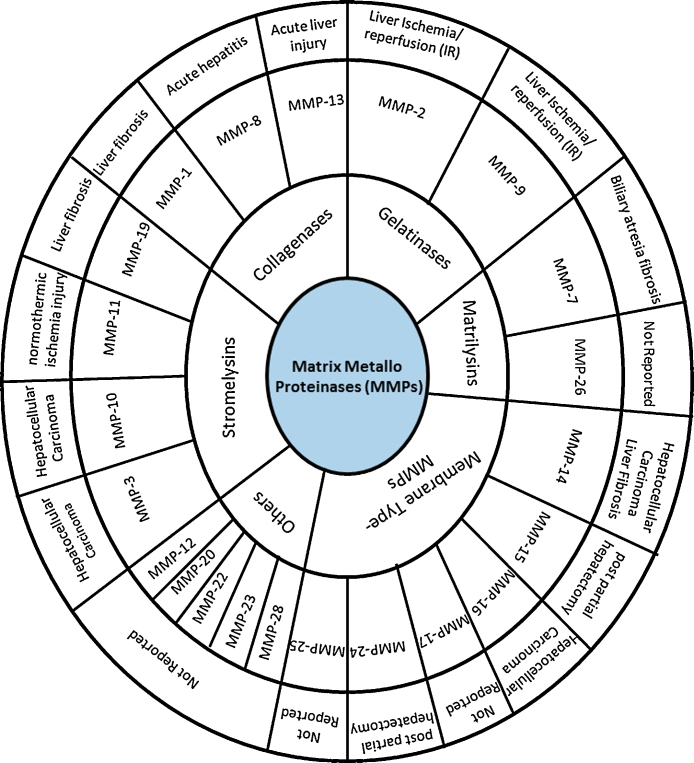
So far, 28 MMPs have been discovered in vertebrates, including 23 in humen15 and have been classified on number basis, MMP1–MMP28.16 MMPs are expressed in a wide variety of cells like connective tissue, including fibroblasts, but also in neutrophils, monocytes, macrophages, and endothelial cells. The biological activity of MMPs is mainly regulated at the level of gene transcription.17 Their essential role in many fundamental physiological events has been observed in tissue remodeling, such as angiogenesis, bone development, wound healing and mammary involution.18, 19 Besides degradation of collagen, MMPs also target several components of ECM, including degradation of basement membrane correlating its role in cancer and angiogenesis.20
ECM is composed of macromolecules required for the purpose of cell adhesion, intercellular interaction, cell migration, proliferation, and survival. ECM maintains the cellular infrastructure by scaffolding the cellular components and is also responsible for regulating the cell behavior. Collagen fibers are an integral part of ECM, which provide the basis for cell adhesion and restrict cell movement. As a consequence of ECM degradation cells start migrating21 which is one of the mechanisms followed in cancer cells invasion and progression. Different MMPs are involved in the process of ECM degradation, like MMP1, which targets collagen and MMP9, which targets gelatin.22, 23 Different MMPs use different substrates for their activity; therefore, substrate specificity has been used as a basis of classification for MMPs which has been summarized in Table 1.
Table 1.
Substrate Based Classification of MMPs and the Associated Liver Pathology.
| Classification of MMPs | Type of MMPs | Substrate | Specific role of MMP in liver diseases |
|---|---|---|---|
| Collagenases | MMP1 | Col I, II, III, VII, VIII, X, gelatin | Over expression in the liver effectively attenuates fibrosis and causes hepatocyte proliferation.23 |
| MMP8 | Col I, II, III, VII, VIII, X, aggrecan, gelatin | Promoted leukocyte infiltration in TNF-induced acute hepatitis.24 | |
| MMP13 | Col I, II, III, IV, IX, X, XIV, gelatin | Acute liver injury and accelerates liver fibrosis.26 | |
| Gelatinases | MMP2 | Gelatin, Col I, II, III, IV, VII, X | Expressed during Liver ischemia/reperfusion (IR) injury.27 |
| MMP9 | Gelatin, Col IV, V | Expressed in leukocytes during Ischemia/Reperfusion Injury.30, 31 | |
| Stromelysins | MMP3 | Col II, IV, IX, X, XI, gelatin | Expressed in hepatocellular carcinoma.25 |
| MMP10 | Col IV, laminin, fibronectin, elastin | Expressed in hepatocellular carcinoma.25 | |
| MMP11 | Col IV, fibronectin, laminin, aggrecan | Expressed in normothermic ischemia injury.29 | |
| MMP19 | # | Promoted TGF-p signaling in development of liver fibrosis.31 | |
| Matrilysins | MMP7 | Fibronectin, laminin, Col IV, gelatin | Expressed in biliary atresia fibrosis.49, 50, 51 |
| MMP26 | # | # | |
| Membrane Type-MMPs | MMP14 | Gelatin, fibronectin | Expressed in highly invasive hepatocellular carcinoma and expressed in liver fibrosis.33 |
| MMP15 | Gelatin, fibronectin | Expression is significantly downregulated post partial hepatectomy. | |
| MMP16 | Gelatin, fibronectin, laminin | Expressed in hepatitis, cirrhosis and hepatocellular carcinoma.32 | |
| MMP17 | Fibrinogen, fibrin | # | |
| MMP24 | # | Expressed post partial hepatectomy.33 | |
| MMP25 | # | # | |
| Others | MMP12 | Elastin, fibronectin, Col IV | # |
| MMP20 | # | # | |
| MMP22 | # | # | |
| MMP23 | # | # | |
| MMP28 | # | # |
#: not reported.
Recent studies have added new dimensions to the role of MMPs, which have now shown to have a role in regulating extracellular tissue signaling networks as well as in maintaining the homeostasis of several tissues. They have also shown to contribute to many physiological processes like bone remodeling, angiogenesis, immunity and wound healing.23
Diverse Roles of Matrix Metalloproteinases in Liver Disease
The liver is the central vital organ involved in many important biological functions like the metabolism of macromolecules including carbohydrates, proteins, and lipids. MMPs play a key role in regulating the immune response via responding to various pathological conditions including alcoholic and non-alcoholic fatty liver disease (NAFLD), steatosis, cirrhosis, and infection. In addition to its role in regulating metabolic homeostasis, liver is also known to have regenerative potential as observed post-hepatectomy, hepatic injury or infection.
ECM degradation and its remodeling have been reported to be a major factor for several diseases including liver cirrhosis and hepatic cancer. MMPs are widely involved as master regulators in many diseases, cellular invasion, and attachment, as well as immune responses and regeneration.36 Primarily, all MMPs are involved in ECM degradation in a canonical manner. However, studies performed by different groups provide evidence that MMPs are also involved in many other biological processes in a non-canonical fashion, for instance, activation of human hepatic stellate cells (HSCs).24
The non-canonical role of different MMPs has been described in the next section, which implicates MMPs versatile characteristic nature.
Collagenases
Matrix metalloproteinase-1 (MMP-1) also known as interstitial collagenase or fibroblast collagenase has a role in the regression of liver fibrosis in rodents. However, in humans MMP-1 is known to enhance tissue fibrosis in non-alcoholic steatohepatitis (NASH), which suggests that MMP-1 might contribute to the repair and regeneration of liver. Matrix metalloproteinase-8 (MMP-8), also known as collagenase-2, is considered to be a hallmark for liver cirrhosis in alcoholics. MMP-8 activity and concentrations, along with MMP-2 and MMP-9 have been reported to be high in patients with Liver cirrhosis (stage C).25 Studies performed on MMP8 knockout mice suggest that the MMP8 deficient mice were unaffected against TNF-induced acute hepatitis.26 Matrix metalloproteinase-13 (MMP-13) also known as collagenase-3, is expressed in acute liver injury. It has also shown to have an anti-fibrotic effect due to persistent fibrosis observed in Mmp13−/− double knockout mice. It also plays a role in supporting hepatocyte proliferation and repair as reported by Endo and co-workers.37
Gelatinases
Matrix metalloproteinase-2 (MMP-2), also known as gelatinase A, is involved in ECM remodeling.27 MMP2 is secreted as a proenzyme and activated by membrane type-MMPs (MT-MMP), such as MT1-MMP. In liver fibrosis, MMP-2 is highly expressed in myofibroblasts and believed to have a profibrogenic role.28 Matrix metalloproteinase-9 (MMP-9), also known as Gelatinase-B is expressed by leukocytes in liver ischemia and reperfusion injury (IRI). MMP-9 is a multi-faceted metalloproteinase having a role in impairing liver regeneration, post liver IRI.29 The activity of MMP-2, a member of gelatinase family of MMPs, is inversely proportional to the MMP-9 activity. Lack of MMP-2 also leads to spontaneous leukocyte infiltration in naïve livers, and amplified MMP-9-dependent transmigration of leukocytes in vitro and after hepatic IRI.30
Stromelysins
Matrix metalloproteinase-3 (MMP-3), also termed as stromelysin-1, is known for its degrading activity against collagens, proteoglycans, fibronectin, laminin, and elastin, hence regulating matrix remodeling. MMP-3 plays a vital role in activating MMP-1, MMP-7, and MMP-9 explaining its involvement in connective tissue remodeling.
MMP3 expression regulates the induction of liver cirrhosis in rats.31 It has been suggested that MMP3 is also involved in the hepatocyte growth factor-induced invasion of human HCC cells.32
Membrane-Type Matrix Metalloproteinases
Matrix metalloproteinase-14 (MMP-14) also known as Membrane Type-1 MMP is associated with leukocyte recruitment in liver IR.33 MMP-14 is also noticed to be expressed in highly invasive HCC. Matrix metalloproteinase-15 (MMP-15) also known as Membrane Type-2 MMP is downregulated after partial hepatectomy. Matrix metalloproteinase-16 (MMP-16) also known as Membrane Type-3 MMP is mainly expressed in hepatitis as well as in cirrhosis and HCC. MMP25—matrix metalloproteinase-25 (MMP-25) is a membrane-type MMP (MT-MMP) attached to the plasma membrane via a glycosylphosphatidyl inositol bond. MMP-25 has also been found to be involved in tumor invasion and metastasis through activation of MMP.35, 36 As a response to inflammation, MMP-25 attenuates alpha-1 proteinase inhibitor, a major tissue protectant against proteolytic enzymes released by activated neutrophils, facilitating the trans-endothelial migration of neutrophils to inflammatory sites.37, 38, 39
Matrilysin
MMP7—matrix metalloproteinase-7 (MMP-7), also known as matrilysin, is primarily associated with tissue remodeling during biliary atresia associated liver fibrosis.40, 41 MMP7 expression has been observed to be upregulated in many human primary cancers like breast, lung, prostate and ovarian cancer. Upregulation of MMP7 has also been observed in benign and malignant colorectal tumors.41, 42, 43
Metalloelastase
MMP12—matrix metalloproteinase-12 (MMP-12,) also known as metalloelastase, is primarily expressed in normothermic ischemia injury. MMP12 is involved in the degradation of elastin in hepatic fibrosis and liver injury.44, 45, 46 Ratio of MMP-12 to its inhibitor (TIMP-1) also regulates elastin turnover. MMP12 also regulates inflammation and IL-13 induced hepatic fibrosis.47, 48
Other Matrix Metalloproteinases
Matrix metalloproteinase-19 (MMP-19), also known as MMP-RASI, is considered to be pro-fibrotic in the initial phase of post-injury and anti-fibrotic in the late phase. MMP-19 has been reported to promote TGF-β signaling in the development of liver fibrosis. However, Mmp19−/− mice were shown to have reduced liver fibrosis as compared to the controls.49
Studies performed on zebrafish suggest that Matrix metalloproteinase-23 (MMP-23) has a role in the development of liver and hepatocyte proliferation via activating TNF pathway.50, 51
Matrix metalloproteinase-28 (MMP-28) activity increases with alcohol-mediated hepatocyte damage and up-regulates further with inflammation progression and liver diseases.52, 53, 54, 55 An increase in MMP-9 activity has been noticed in the alcohol induced liver damage, due to the production of oxidative stress and inflammatory response.56, 57 Alcohol also leads to increased nuclear translocation of nuclear factor kappa-B (NFkB) and increases degradation of NFkB in hepatocytes.58, 59 AP-1 mediated signaling network also gets activated under chronic alcoholic conditions which as a consequence lead to the regulation of MMPs at the transcriptional level .60
Figure 1.
Role of MMPs in different liver relate diseases.
Conclusion
MMPs are not only restricted to ECM degradation but are also found to possess a non-canonical role in multiple biological activities including diseases like angiogenesis, cirrhosis, wound repair and cancer. For example, MMP1 and MMP7 roles have been investigated during injury, and found to support re-epithelialization, facilitating wound repair by breaking down different ECM components. MMP1 has also been found to have a role in regulating smooth muscle cells de-differentiation through protease-activated receptor-1 (PAR-1).61 Similarly, MMP8 has been reported to play role in the activation of HSCs in a non-canonical fashion.62Recently, MMP13 have been reported to work as an agonist for PAR1. Studies performed in the area of cardiovascular development showed that inhibition of MMP13 could avoid harmful cardiac effects.
On the other hand, MMPs like MMP2, 9 and MT1-MMP have been found to regulate bone development and cellular homeostasis. The imbalance between MMPs expression and their endogenous inhibitors could be master regulators in some of the diseases and therefore MMPs expression should be properly balanced.
Conflicts of Interest
The authors have none to declare.
Acknowledgements
The authors are thankful to the Council of Scientific and Industrial Research (CSIR) funded grant (37(1664)/15/EMR-II) to MSB. The authors also gratefully acknowledge the Indian Institute of Technology Indore for providing facilities and other support.
References
- 1.Bogdanos D.P., Gao B., Gershwin M.E. Liver immunology. Compr Physiol. 2013;3(2):567–598. doi: 10.1002/cphy.c120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Protzer U., Maini M.K., Knolle P.A. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 3.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;5:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee P., Jana S., Chakraborty S., Swarnakar S. Inflammation and MMPs in alcohol-induced liver diseases and protective action of antioxidants. Indian J Biochem Biophys. 2013;5:377–386. [PubMed] [Google Scholar]
- 5.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 6.Diehl A.M., Chute J. Underlying potential: cellular and molecular determinants of adult liver repair. J Clin Invest. 2013;123:1858–1860. doi: 10.1172/JCI69966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein T., Bischoff R. Physiology and pathophysiology of matrix metalloproteinases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duarte S., Baber J., Fujii T., Coito A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy G., Docherty A.J. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992;7(2):120–125. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- 11.Brew K., Dinakarpandian D., Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1–2):267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 12.Brew K., Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amălinei C., Căruntu I.D., Bălan R.A. Biology of metalloproteinases. Roman J Morphol Embryol. 2007;48(4):323–334. [PubMed] [Google Scholar]
- 14.Rodríguez D., Morrison C.J., Overall C.M. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Puente X.S., Sanchez L.M., Overall C.M., Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4(7):544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 16.Nagase H., Woessner J.F. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–41999. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 17.Gill S.E., Parks W.C. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40(6–7):1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manicone A.M., McGuire J.K. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19(1):34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabłońska-Trypuć A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31(suppl 1):177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 20.Folgueras A.R., Pendás A.M., Sánchez L.M., López-Otín C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48(5–6):411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 21.Opdenakker G., Van den Steen P.E., Dubois B. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukocyte Biol. 2001;69(6):851–859. [PubMed] [Google Scholar]
- 22.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behaviour. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iimuro Y., Nishio T., Morimoto T. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124(2):445–458. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- 24.Van Lint P., Wielockx B., Puimege L., Noel A., Lopez-Otin C., Libert C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J Immunol. 2005;175:7642–7649. doi: 10.4049/jimmunol.175.11.7642. [DOI] [PubMed] [Google Scholar]
- 25.Bodey B., Bodey B., Jr., Siegel S.E., Kaiser H.E. Immunocyto-chemical detection of MMP-3 and -10 expressions in hepatocellular carcinomas. Anticancer Res. 2000;20:4585–4590. [PubMed] [Google Scholar]
- 26.Corbel M., Boichot E., Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz J Med Biol Res. 2000;33(7):749–754. doi: 10.1590/s0100-879x2000000700004. [DOI] [PubMed] [Google Scholar]
- 27.Chambers A.F., Matrisian L.M. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89(17):1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 28.Fingleton B. MMPs as therapeutic targets – still a viable option? Semin Cell Dev Biol. 2008;19:61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cursio R., Mari B., Louis K. Rat liver injury after normothermic ischemia is prevented by a phosphinic matrix metalloproteinase inhibitor. FASEB J. 2002;16:93–95. doi: 10.1096/fj.01-0279fje. [DOI] [PubMed] [Google Scholar]
- 30.Hamada T., Fondevila C., Busuttil R.W., Coito A.J. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47:186–198. doi: 10.1002/hep.21922. [DOI] [PubMed] [Google Scholar]
- 31.Nart D., Yaman B., Yilmaz F., Zeytunlu M., Karasu Z., Kilic M. Expression of matrix metalloproteinase-9 in predicting prognosis of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2010;16:621–630. doi: 10.1002/lt.22028. [DOI] [PubMed] [Google Scholar]
- 32.Arai I., Nagano H., Kondo M. Overexpression of MT3-MMP in hepatocellular carcinoma correlates with capsular invasion. Hepatogastroenterology. 2007;54:167–171. [PubMed] [Google Scholar]
- 33.Mohammed F.F., Pennington C.J., Kassiri Z. MetalloproteinaseinhibitorTIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41:857–867. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- 35.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 36.Prystupa A., Szpetnar M., Boguszewska-Czubara A., Grzybowski A., Sak J., Zaluska W. Activity of MMP1 and MMP13 and amino acid metabolism in patients with alcoholic liver cirrhosis. Med Sci Monitor. 2015;21:1008–1014. doi: 10.12659/MSM.892312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endo H., Niioka M., V X. Matrix metalloproteinase-13 promotes recovery from experimental liver cirrhosis in rats. Pathobiology. 2011;78(5):239–252. doi: 10.1159/000328841. [DOI] [PubMed] [Google Scholar]
- 38.Onozuka I., Kakinuma S., Kamiya A. Cholestatic liver fibrosis and toxin-induced fibrosis are exacerbated in matrix metalloproteinase-2 deficient mice. Biochem Biophys Res Commun. 2011;406(1):134–140. doi: 10.1016/j.bbrc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Radbill B.D., Gupta R., Ramirez M.C. Loss of matrix metalloproteinase-2 amplifies murine toxin-induced liver fibrosis by upregulating collagen I expression. Dig Dis Sci. 2011;56(2):406–416. doi: 10.1007/s10620-010-1296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore C., Shen X.-D, Gao F., Busuttil R.W., Coito A.J. Fibronectin-41 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am J Pathol. 2007;170(2) doi: 10.2353/ajpath.2007.060456. Investigative Pathology D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duarte S., Hamada T., Kuriyama N., Busuttil R.W., Coito A.J. TIMP-1 deficiency leads to lethal partial hepatic ischemia and reperfusion injury. Hepatology. 2012;56:1074–1085. doi: 10.1002/hep.25710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato H., Duarte S., Liu D., Busuttil R.W., Coito A.J. Matrix metalloproteinase-2 (MMP-2) gene deletion enhances MMP-9 activity, impairs PARP-1 degradation, and exacerbates hepatic ischemia and reperfusion injury in mice. PLoS ONE. 2015;10(9):e0137642. doi: 10.1371/journal.pone.0137642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herbst H., Heinrichs O., Schuppan D., Milani S., Stein H. Temporal and spatial patterns of transin/stromelysin RNA expression following toxic injury in rat liver. Virchows Archiv B Cell Pathol Incl Mol Pathol. 1991;60:295–300. doi: 10.1007/BF02899560. [DOI] [PubMed] [Google Scholar]
- 44.Duarte S., Shen X.-D., Fondevila C., Busuttil R.W., Coito A.J. Fibronectin-α4β1 interactions in hepatic cold ischemia and reperfusion injury: regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway. Am J Transpl. 2012;12(10):2689–2699. doi: 10.1111/j.1600-6143.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H., Lafdil F., Wang L., Yin S., Feng D., Gao B. Tissue inhibitor of metalloproteinase 1 (TIMP-1) deficiency exacerbates carbon tetrachloride-induced liver injury and fibrosis in mice: involvement of hepatocyte STAT3 in TIMP-1 production. Cell Biosci. 2011;1(1):1–14. doi: 10.1186/2045-3701-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Q., Weber C.R., Sohail A. MMP25 (MT6-MMP) is highly expressed in human colon cancer, promotes tumor growth, and exhibits unique biochemical properties. J Biol Chem. 2007;282:21998–22010. doi: 10.1074/jbc.M701737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monvoisin A., Bisson C., Si-Tayeb K., Balabaud C., Desmoulière A., Rosenbaum J. Involvement of matrix metalloproteinase type-3 in hepatocyte growth factor-induced invasion of human hepatocellular carcinoma cells. Cancer Cell Biol. 2001;97(2):157–162. doi: 10.1002/ijc.1595. [DOI] [PubMed] [Google Scholar]
- 48.Soria-Valles C., Gutiérrez-Fernández A., Osorio F.G. MMP-25 metalloprotease regulates innate immune response through NF-κB signaling. J Immunol. 2016;197(1):296–302. doi: 10.4049/jimmunol.1600094. [DOI] [PubMed] [Google Scholar]
- 49.Huang C.C., Chuang J.H., Chou M.H. Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod Pathol. 2005;18:941–950. doi: 10.1038/modpathol.3800374. [DOI] [PubMed] [Google Scholar]
- 50.Kerola A., Lampela H., Lohi J. Increased MMP-7 expression in biliary epithelium and serum underpins native liver fibrosis after successful portoenterostomy in biliary atresia. J Pathol Clin Res. 2016;2(3):187–198. doi: 10.1002/cjp2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng Z.S., Shu W.P., Cohen A.M., Guillem J.G. Matrix metalloproteinase-7 expression in colorectal cancer liver metastases: evidence for involvement of MMP-7 activation in human cancer metastases. Clin Cancer Res. 2002;8:144–148. [PubMed] [Google Scholar]
- 52.Pellicoro A., Aucott R.L., Ramachandran P. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
- 53.Madala S.K., Pesce J.T., Ramalingam T.R. Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis. J Immunol. 2010;184(7):3955–3963. doi: 10.4049/jimmunol.0903008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jirouskova M., Zbodakova O., Gregor M. Hepatoprotective effect of MMP-19 deficiency in a mouse model of chronic liver fibrosis. PLoS ONE. 2012;7(10):e46271. doi: 10.1371/journal.pone.0046271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi F., Song J., Yang H. Mmp23b promotes liver development and hepatocyte proliferation through the TNF pathway in zebrafish. Hepatology. 2010;52(6):2158–2166. doi: 10.1002/hep.23945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodgers U.R., Kevorkian L., Surridge A.K. Expression and function of matrix metalloproteinase (MMP)-28. Matrix Biol. 2009;28(5–3):263–272. doi: 10.1016/j.matbio.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu R., Huang H., Zhang Z., Wang F.S. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11:224–231. doi: 10.1038/cmi.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luedde T., Schwabe R.F. NF-kB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(2):108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y.P. Matrix Metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21(suppl 3):S88–S91. doi: 10.1111/j.1440-1746.2006.04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Austin K.M., Covic L., Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121(3):431–439. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baig M.S., Yaqoob U., Cao S., Saqib U., Shah V.H. Non-canonical role of matrix metalloprotease (MMP) in activation and migration of hepatic stellate cells (HSCs) Life Sci. 2016;155:155–160. doi: 10.1016/j.lfs.2016.04.031. [DOI] [PubMed] [Google Scholar]