Summary
Background
Platinum-based chemotherapy doublets are a standard of care for women with ovarian cancer recurring 6 months after completion of initial therapy. In this study, we aimed to explore the roles of secondary surgical cytoreduction and bevacizumab in this population, and report the results of the bevacizumab component here.
Methods
The multicentre, open-label, randomised phase 3 GOG-0213 trial was done in 67 predominantly academic centres in the USA (65 centres), Japan (one centre), and South Korea (one centre). Eligible patients were adult women (aged ≥18 years) with recurrent measurable or evaluable epithelial ovarian, primary peritoneal, or fallopian tube cancer, and a clinical complete response to primary platinum-based chemotherapy, who had been disease-free for at least 6 months following last infused cycle of platinum. Patients were randomly assigned (1:1) to standard chemotherapy (six 3-weekly cycles of paclitaxel [175 mg/m2 of body surface area] and carboplatin [area under the curve 5]) or the same chemotherapy regimen plus bevacizumab (15 mg/kg of bodyweight) every 3 weeks and continued as maintenance every 3 weeks until disease progression or unacceptable toxicity. Individuals who participated in both the bevacizumab objective and surgical objective (which is ongoing) were randomly assigned (1:1:1:1) to receive either of these two chemotherapy regimens with or without prior secondary cytoreductive surgery. Randomisation for the bevacizumab objective was stratified by treatment-free interval and participation in the surgical objective. The primary endpoint was overall survival, analysed by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00565851.
Findings
Between Dec 10, 2007, and Aug 26, 2011, 674 women were enrolled and randomly assigned to standard chemotherapy (n=337) or chemotherapy plus bevacizumab (n=377). Median follow-up at the end of the trial on Nov 5, 2014, was 49·6 months in each treatment group (IQR 41·5–62·2 for chemotherapy plus bevacizumab; IQR 40·8–59·3 for chemotherapy), at which point 415 patients had died (214 in the chemotherapy group and 201 in the chemotherapy plus bevacizumab group). Based on pretreatment stratification data, median overall survival in the chemotherapy plus bevacizumab group was 42·2 months (95% CI 37·7–46·2) versus 37·3 months (32·6–39·7) in the chemotherapy group (hazard ratio [HR] 0·829; 95% CI 0·683–1·005; p=0·056). We identified incorrect treatment-free interval stratification data for 45 (7%) patients (equally balanced between treatment groups); a sensitivity analysis of overall survival based on the audited treatment-free interval stratification data gave an adjusted HR of 0·823 (95% CI 0·680–0·996; p=0·0447). In the safety population (all patients who initiated treatment), 317 (96%) of 325 patients in the chemotherapy plus bevacizumab group had at least one grade 3 or worse adverse event compared with 282 (86%) of 332 in the chemotherapy group; the most frequently reported of these in the chemotherapy plus bevacizumab group compared with the chemotherapy group were hypertension (39 [12%] vs two [1%]), fatigue (27 [8%] vs eight [2%]), and proteinuria (27 [8%] vs none). Two (1%) treatment-related deaths occurred in the chemotherapy group (infection [n=1] and myelodysplastic syndrome [n=1]) compared with nine (3%) in the chemotherapy plus bevacizumab group (infection [n=1], febrile neutropenia [n=1], myelodysplastic syndrome [n=1], secondary malignancy [n=1]; deaths not classified with CTCAE terms: disease progression [n=3], sudden death [n=1], and not specified [n=1]).
Interpretation
The addition of bevacizumab to standard chemotherapy, followed by maintenance therapy until progression, improved the median overall survival in patients with platinum-sensitive recurrent ovarian cancer. Although the intention-to-treat analysis for overall survival was not significant, our sensitivity analysis based on corrected treatment-free interval stratification indicates that this strategy might be an important addition to the therapeutic armamentarium in these patients.
Funding
National Cancer Institute and Genentech.
Introduction
Recurrent ovarian cancer remains among the most clinically challenging events in the natural history of the disease owing to its relentless trajectory to eventual drug resistance. Whether an intrinsic feature or an acquired event under the selective pressure of serial chemotherapy regimens, the resistant phenotype is mainly responsible for the disease's poor long-term survivorship.1 Outcomes for individual patients vary greatly on the basis of various factors, such as primary postoperative disease volume, primary response to platinum-based combination chemotherapy, genotype (eg, BRCA mutational status), and general performance status. Because precise molecular events driving tumour biology at any given point are unknown or, if suspected, might not be targetable, decisions for treatment of recurrent disease are frequently left to the evaluation of these clinicopathological factors.2
One of the most important phenotypic characteristics driving both prognostic and predictive features of recurrent ovarian tumours is the retention of platinum sensitivity. Although this characteristic is not universally predictable, it can be estimated, in part, by the genotype and by the duration of disease-free survival following completion of initial platinum-based therapy.3 In this setting, patients can achieve noteworthy clinical responses to platinum-based retreatment. Unfortunately, despite what can be substantial survivorship after first progression, resistance eventually develops, which leads to a clinical conundrum in the search for effective therapy. This clinical scenario, aligned with a limited cache of effective agents in this setting, highlights the unmet medical need for the development of new treatment approaches.
Acquired resistance to chemotherapy has driven investigation into alternative strategies with biological relevance for ovarian cancer. One of these mechanisms is angiogenesis. Among the many factors governing this process, VEGF has emerged as both a biologically and clinically relevant target.4–7 Eight randomised phase 3 trials8–15 have studied anti-angiogenesis therapy in newly diagnosed advanced epithelial ovarian cancer, in maintenance therapy following primary treatment, in platinum-sensitive recurrent disease, and in platinum-resistant recurrent disease. Each of these trials met their primary endpoint, indicating that angiogenesis inhibition is an important therapeutic strategy in this disease. Two of these trials8,10 targeted VEGF-dependent angiogenesis exclusively in patients with platinum-sensitive recurrent ovarian cancer: one trial8 used the multitargeted tyrosine kinase inhibitor, cediranib, in combination with paclitaxel and carboplatin, and the other10 used bevacizumab in combination with gemcitabine and carboplatin. Although a significant increase in progression-free survival was reported, neither trial was powered to address the effect of angiogenesis targeting in combination with chemotherapy on overall survival. Additionally, secondary surgical cytoreduction has often been advocated for patients with potentially chemosensitive disease (ie, the potential to respond to platinum-based chemotherapy). Again, neither trial was designed to address this important intervention in the context of chemotherapy.
The present trial was designed to assess two interventions—anti-VEGF targeting (with bevacizumab) and secondary cytoreduction—and their effect on overall survival in women with recurrent platinum-sensitive ovarian, primary peritoneal, or fallopian tube cancer. Here, we report the effect of anti-angiogenesis therapy on survival in women who had remained in clinical remission for at least 6 months following primary therapy. The data for the effect of secondary cytoreduction on overall survival are not yet mature and will be reported separately.
Methods
Study design and participants
The multicentre, open-label, randomised phase 3 Gynecologic Oncology Group (GOG)-0213 trial was done in 67 predominantly academic centres in the USA (65 centres), Japan (one centre), and South Korea (one centre; appendix p 1), and was designed to assess two primary objectives: the role of bevacizumab added to standard chemotherapy with paclitaxel and carboplatin followed by bevacizumab maintenance therapy (bevacizumab objective) and the role of secondary cytoreduction before initiation of chemotherapy (surgical objective). Eligible patients were initially assessed for surgical candidacy, and those meeting surgical eligibility criteria could participate in the surgical objective; this approach was predicated on the belief that recurrent disease could be completely resected before initiating chemotherapy. Those not meeting surgical eligibility directly entered chemotherapy randomisation.
Eligible patients were women aged 18 years and older with recurrent clinically evident (measurable according to Response Evaluation Criteria in Solid Tumors [RECIST] version 1.0 criteria [appendix p 95–98] or evaluable) epithelial ovarian, primary peritoneal, or fallopian tube cancer. Patients were required to have had a clinical complete response to at least three cycles of primary platinum-based chemotherapy as determined by negative clinical examination and a normal CA125 level, as well as be disease-free for at least 6 months following last infused cycle of platinum. If performed as an assessment of response, abdominopelvic CT or MRI had to be negative for disease, and if assessment was made by second-look surgery, all surgical specimens had to be pathologically negative. Patients were required to have a GOG performance status of 0–2; adequate bone marrow, renal, and hepatic function; and a platelet count of at least 100 000/μL (appendix pp 56–57).
Key exclusion criteria were concurrent immunotherapy or radiotherapy, or previous radiotherapy to any portion of the abdominal cavity or pelvis; more than one previous chemotherapy regimen (although maintenance therapy was allowed if administered after a complete clinical or pathological response, but must have been discontinued ≥6 months before documentation of recurrent disease); previous chemotherapy for any abdominal or pelvic tumour; uncontrolled infection; clinically significant cardiovascular disease; active bleeding or conditions associated with a high risk of bleeding; or a history of CNS disease. The full exclusion criteria are listed in the appendix (pp 57–60). Patients who had received maintenance biological therapy (eg, bevacizumab) or hormonal therapy were eligible provided their first recurrence was documented at least 6 months from primary cytotoxic chemotherapy completion and at least 4 weeks since their last treatment.
We made two major amendments to the eligibility criteria during the trial: the first (Aug 4, 2008) removed the exclusion of prior anti-VEGF therapy; the second (June 22, 2009) removed the exclusion of previous hormone agents. Both changes were approved by the National Cancer Institute.
Patients could only be enrolled onto the trial after Aug 28, 2011, if they could take part in the surgical objective (not reported here) because the numbers of patients needed to be enrolled for the bevacizumab objective had been accrued: therefore, they had to meet surgical eligibility criteria and consent to their surgical treatment being determined by randomisation. Diffuse carcinomatosis, extra-abdomino pelvic disease, and ascites were general exclusion criteria for the surgical objective; however, no other additional specific exclusion criteria were prespecified. Patients undergoing elective secondary surgical cytoreduction prior to the study were not eligible for either objective.
Written informed consent was obtained from each patient following research ethics board approval of the study at each participating centre or by a central institutional review board before initiation of study procedures. The contributing institutions, their principal investigators, site accrual, original protocol, the amended protocol at the time of closure of the bevacizumab objective, original statistical analysis plan, final statistical analysis plan, and all protocol amendments are available in the appendix.
Randomisation and masking
Individuals who participated only in the bevacizumab objective were randomly assigned (1:1) to receive paclitaxel and carboplatin chemotherapy or paclitaxel and carboplatin chemotherapy with bevacizumab followed by bevacizumab maintenance therapy until disease progression or unacceptable toxicity, whichever occurred first. Individuals who participated in both the bevacizumab and surgical objectives were randomly assigned (1:1:1:1) to receive either of these two drug regimens with or without prior secondary cytoreductive surgery. Study treatments were allocated sequentially from lists composed of random permuted blocks of random sizes of the study treatments. The list of treatments was prepared by the GOG Statistical and Data Center (Buffalo, NY, USA) and remained concealed during conduct of the study. For the bevacizumab objective the treatment allocation procedure was stratified by previous treatment-free interval (6–12 months vs >12 months from last platinum infusion) and by participation in the surgical objective (yes vs no). An automated electronic web-based procedure was used to enrol patients and randomly assign them to treatments. Each individual's treatment assignment remained concealed until after she was successfully enrolled, and this report includes an account of all individuals who enrolled for the bevacizumab objective. This trial was open label: patients and study physicians were aware of treatment assignment.
Procedures
The chemotherapy administered in this part of the trial was paclitaxel (175 mg/m2 of body surface area) administered intravenously over 3 h, followed by carboplatin (area under the curve 5) over 1 h, with standard antiemetic and hypersensitivity medications. In patients who developed dose-limiting peripheral neuropathy or hypersensitivity, paclitaxel was replaced with docetaxel (75 mg/m2), which was administered intravenously over 1 h. In the experimental group, bevacizumab (15 mg/kg bodyweight) was administered intravenously initially over 90 min (if tolerated, this time was reduced to 60 min, and could be further reduced to a minimum of 30 min) sequenced between paclitaxel and carboplatin on the day of infusion. In the maintenance phase, bevacizumab (15 mg/kg) was administered intravenously over 60 min every 3 weeks until disease progression or unacceptably toxicity. Chemotherapy in both groups was planned for six 3-weekly cycles, but two additional cycles were allowed for a documented response (either partial or complete). Bevacizumab was started on cycle 2 if surgery was done to avoid wound healing complications.
During chemotherapy (both groups) and during bevacizumab maintenance therapy, laboratory parameters, adverse events, and tolerance were assessed with each infusion. Administration of myeloid growth factor was allowed only to manage febrile neutropenia or grade 4 neutropenia (absolute neutrophil count <500 cells per m3) persisting for 7 days or longer or as subsequent prophylaxis. One dose level reduction each was permitted for paclitaxel and carboplatin. The bevacizumab dose was modified only in patients whose weight changed by more than 10%, but could be delayed or discontinued depending on the occurrence, duration, and severity of uncontrolled hypertension (systolic blood pressure >150 mm Hg or diastolic blood pressure >90 mm Hg), proteinuria (urine protein–creatinine ratio >3·5), wound or bowel wall disruption (of any grade, during cycle 2 or later), reversible posterior leukoencephalopathy syndrome, arterial thrombosis (grade 3 at any time or grade 2 during cycle 2 or later), and venous thrombosis, coagulopathy, intestinal obstruction, or hypersensitivity of grade 3 or worse severity (appendix pp 80–86).
Disease was assessed in all patients by abdominopelvic CT or MRI within 28 days of first treatment, after cycles 3 and 6 (and 8, if administered) of study treatment, every 3 months for 2 years and then every 6 months thereafter. Interpretation was investigator-assessed. Patients randomly allocated to secondary cytoreductive surgery underwent repeat radiographic assessment 14 or fewer days before chemotherapy administration. Physical examinations were done and serum CA125 levels were measured at the beginning of each cycle of chemotherapy; in the maintenance phase, these procedures were done every 6 weeks.
We considered progression-free survival to have ended at the time of cancer progression as shown on radiography, according to the RECIST version 1.0 criteria (appendix pp 95–98); an increase in the CA125 level according to Gynecologic Cancer InterGroup (GCIG) criteria;16,17 global deterioration of health; or death from any cause. If progression was defined solely on the basis of increased CA125 level, an imaging study similar to that used at baseline was to be completed within 2 weeks of meeting GCIG criteria. If disease was noted on imaging, the date of progression would be the date of the scan; if not, the date of progression would be the date meeting GCIG criteria.
A FastFact checklist of all eligibility criteria was used to ensure patient eligibility (appendix pp 102–104). Quality of life and patient-reported outcome surveys among all cohorts were assessed by the trial outcome index (TOI) of the Function Assessment of Cancer Therapy-Ovary (FACT-O TOI),18 the FACT-O subscales Treatment Side Effects (TSE)-Bevacizumab (TSE-B) and TSE-Surgery (TSE-S; results of subscales not reported here), and the physical functioning subscale of the RAND 36-item short form survey (SF-36). These assessments took place at six possible timepoints: before surgery (for those patients randomly allocated to cytoreductive surgery for the surgical objective), before initiation of chemotherapy, before cycle 3 (6 weeks after starting chemotherapy), before cycle 6 (15 weeks after starting chemotherapy), 6 months after starting chemotherapy, and 12 months after starting chemotherapy.
Adverse events, graded with the use of National Cancer Institute Common Terminology Criteria for adverse events (version 3), were reported until 30 days after the last study treatment had been administered, and were summarised for patients who received at least one cycle of treatment.19
Outcomes
The primary endpoint for both the bevacizumab objective and the surgical objective was overall survival, measured from randomisation to death from any cause. Secondary endpoints were investigator-assessed progression-free survival, defined as the time from randomisation to disease progression or death due to any cause; the incidence of carboplatin and paclitaxel hypersensitivity; and the effect of treatment on patient-reported outcomes and quality of life. As additional exploratory outcomes, we investigated several translational science outcomes— namely, molecular omics and biochemical profiles associated with the duration of overall survival, progression-free survival, and adverse effects, as well as determinants of sensitivity or resistance to carboplatin and paclitaxel with or without bevacizumab (which will be reported at a later date). A further exploratory outcome was to bank DNA from whole blood to evaluate the association between single nucleotide polymorphisms and clinical outcomes.
Statistical analysis
The statistical analysis plan is available in the appendix (pp 105–114). Patients were stratified a priori by treatment-free interval, defined as the time from completion of primary platinum-based chemotherapy (6–12 months vs >12 months) as reported from the site on the faxed FastFact checklist, and by participation in surgical randomisation (yes vs no). These variables, as well as previous bevacizumab exposure in first-line therapy, were pre-planned analytical subgroups. We assumed no interaction between the two randomised treatments (chemotherapy and surgery). We limited the type I error to 2·5% (one tail) for each of the two study objectives. Previous phase 2 studies by the GOG indicated that the median survival time for platinum-sensitive patients treated with a platinum–taxane chemotherapy regimen who did not undergo debulking surgery was 22 months from the diagnosis of recurrence. The target enrolment for this study was 660 patients; we based this target on the bevacizumab objective. The primary analysis of the bevacizumab objective was to be done when at least 214 deaths were reported among patients randomly assigned to chemotherapy alone. The design provides 81% power for a true hazard ratio (HR) of 0·75. The primary analysis of the surgical objective is planned to be done when at least 250 deaths have occurred in those patients randomly assigned to secondary cytoreductive surgery.
A pre-planned interim analysis of the bevacizumab objective included an assessment of treatment efficacy and futility when at least 110 deaths had occurred in patients randomly assigned to standard chemotherapy alone. We calculated critical values of the interim analysis according to Lan and DeMets proposal,20 which provides O'Brien and Fleming-like type I error and type II error spending.
The primary analysis of overall survival was done by intention to treat. Safety analyses included only those patients who had started study therapy. We calculated progression-free survival and overall survival from the date of randomisation. The primary analysis used a log-rank test stratified by treatment-free interval (declared at enrolment) and participation in the surgical objective to compare overall survival or progression-free survival between the treatment groups.21 If patients remained free of progression at their last follow-up visit, data for duration of progression-free survival were censored at the time of last contact. We used a proportional hazards model to estimate treatment HRs with their corresponding confidence intervals.22 We used SAS version 9.4 for all analyses.
The primary tool to assess the effect of therapy on patient-reported outcomes was the FACT-O TOI.18 In this instrument, a six-point difference in score between groups is considered clinically meaningful. We assessed treatment differences in patient-reported outcomes between the groups and during therapy with the linear mixed model, adjusting for patient's pretreatment score, assignment of cytoreductive surgery, and age at enrolment. We first tested interactions between assessment timepoints and treatment assignments for the constant differential effects of treatments over time.23 If the interaction effect was significant, we estimated the treatment differences for each assessment timepoint. Otherwise, we estimated the overall treatment effect by a weighted average of estimates from each timepoint. We compared assessment compliance across assigned groups using a generalised estimating equation. We examined differences between the groups in the severity of adverse events with Fisher's exact test.
This study is registered at ClinicalTrials.gov, number NCT00565851.
Role of the funding source
The NRG Oncology–Gynecologic Oncology Group and GOG designed and conducted the trial. The data were collected, held, and analysed by the NRG Oncology– Gynecologic Oncology Group and GOG, with reviews by the data and safety monitoring committee. The National Cancer Institute distributed bevacizumab to the GOG under a cooperative research and development agreement. Genentech provided supplemental support to the GOG. Representatives from the funders reviewed the protocol and the manuscript, but the authors determined the final content. The corresponding author (RLC) vouches for the integrity of the data and analyses reported and for the fidelity of the trial to the protocol. RLC and MFB had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication.
Results
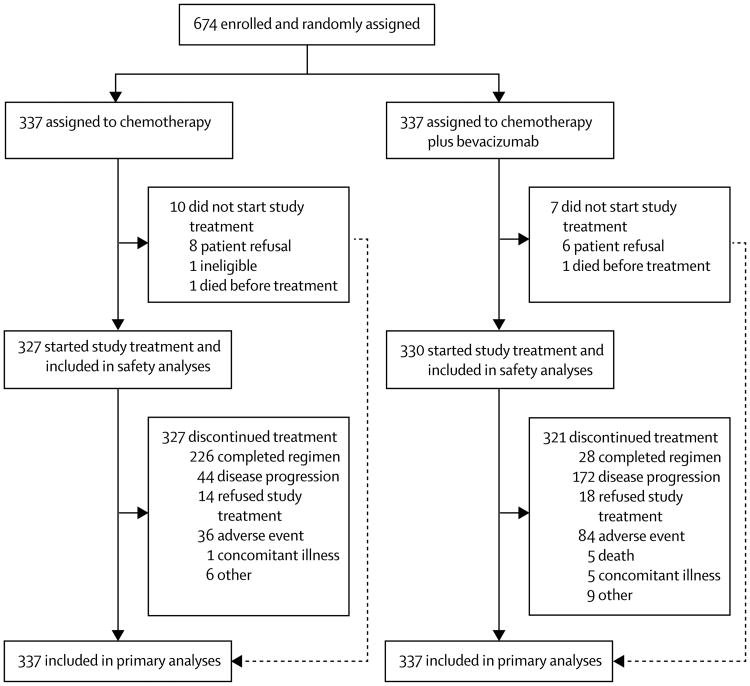
Between Dec 10, 2007, and Aug 26, 2011, 674 patients were enrolled (28 from South Korea, 49 from Japan, and 597 from the USA) and randomly allocated to standard carboplatin and paclitaxel chemotherapy (n=337) or standard chemotherapy plus bevacizumab (n=337), and all included in the primary analysis (figure 1). Ten (3%) of 337 patients in the chemotherapy group and seven (2%) of 337 in the chemotherapy plus bevacizumab group did not initiate their assigned study therapy, and were therefore not included in the safety analyses (figure 1). The data were considered mature for analysis of the bevacizumab objective when 214 deaths had occurred in the chemotherapy group (Nov 5, 2014). At time of writing, the surgical objective is continuing to accrue its selected population; data will be mature when at least 250 deaths have occurred in the surgical cytoreduction group and the findings for this objective will be reported separately at a later date.
Figure 1. Trial profile.
80% (536) of the 674 patients were white (table 1), and most patients were enrolled in the USA. Most patients were stage III or IV at initial presentation, and most tumours were high grade and of serous histology. Notable characteristics of first-line therapy before enrolment were intraperitoneal chemotherapy (n=121 [18%]), maintenance therapy following initial chemo therapy (n=89 [13%]), and prior bevacizumab (n=67 [10%]), which were balanced between the treatment cohorts (table 1). With regard to the stratification variables, 107 (16%) patients were considered surgical candidates and underwent randomisation for secondary cytoreduction; this cohort was equally distributed between the chemotherapy and chemotherapy plus bevacizumab groups (table 1). 232 (69%) patients in each group reported treatment-free interval to be at least 12 months on the FastFact checklist registration form (table 1). However, a review of treatment-free interval from the audited electronic case-report forms identified a discrepancy for the binomial stratification variable in 45 (7%) patients, which was balanced between the two groups (25 [7%] in the chemotherapy group and 20 [6%] in the chemotherapy plus bevacizumab group). This discrepancy was a miscalculation based on time from last treatment to recurrence—most notably, among patients receiving maintenance therapy, in which the reported treatment-free interval was referenced from the last cycle of any therapy instead of from the last cycle of platinum-based primary chemotherapy. Once corrected, the proportion of patients with a treatment-free interval of at least 12 months increased to 75% (254 of 337) in the chemotherapy group and 73% (246 of 337) in the chemotherapy plus bevacizumab group. We subsequently use the term platinum-free interval in this report to reflect the planned stratification variable.
Table 1. Baseline characteristics.
| Standard chemotherapy (n=337) | Standard chemotherapy plus bevacizumab (n=337) | |
|---|---|---|
| Age (years) | ||
|
| ||
| Median (IQR) | 60·6 (53·6-67·7) | 59·5 (53·6-66·4) |
| Range | 23–85 | 26–84 |
|
| ||
| Race | ||
|
| ||
| American Indian | 1 (<1%) | 4 (1%) |
| Asian | 44 (13%) | 48 (14%) |
| African American | 15 (4%) | 15 (4%) |
| Native Hawaiian/Pacific islander | 0 | 1 (<1%) |
| White | 271 (80%) | 266 (79%) |
| Missing/unknown | 6 (2%) | 3 (1%) |
|
| ||
| Stage | ||
|
| ||
| I | 17 (5%) | 22 (7%) |
| II | 36 (11%) | 18 (5%) |
| III | 246 (73%) | 261 (77%) |
| IV | 38 (11%) | 36 (11%) |
|
| ||
| Histology | ||
|
| ||
| Serous* | 272 (81%) | 273 (81%) |
| Endometrioid | 23 (7%) | 20 (6%) |
| Clear cell | 14 (4%) | 10 (3%) |
| Mucinous | 2 (1%) | 2 (1%) |
| Other† | 26 (8%) | 32 (9%) |
|
| ||
| Grade of differentiation | ||
|
| ||
| Well | 17 (5%) | 20 (6%) |
| Moderate | 44 (13%) | 53 (16%) |
| High | 253 (75%) | 249 (74%) |
| Not available | 22 (7%) | 15 (4%) |
|
| ||
| Cytoreductive surgery‡ | ||
|
| ||
| Randomised, no surgery | 27 (8%) | 27 (8%) |
| Randomised, surgery | 27 (8%) | 26 (8%) |
| Not randomised | 283 (84%) | 284 (84%) |
|
| ||
| Previous treatment-free interval§ | ||
|
| ||
| 6–12 months | 105 (31%) | 105 (31%) |
| >12 months | 232 (69%) | 232 (69%) |
|
| ||
| Previous platinum-free interval ¶ | ||
|
| ||
| 6–12 months | 84 (25%) | 91 (27%) |
| >12 months | 253 (75%) | 246 (73) |
|
| ||
| Measurable disease | ||
|
| ||
| No | 50 (15%) | 63 (19%) |
| Yes | 287 (85%) | 274 (81%) |
|
| ||
| Previous therapies | ||
|
| ||
| Systemic chemotherapy | ||
| Yes | 337 (100%) | 337 (100%) |
| No | 0 | 0 |
| Intraperitoneal chemotherapy | ||
| Yes | 66 (20%) | 55 (16%) |
| No | 271 (80%) | 282 (84%) |
| Hormonal therapy | ||
| Yes | 10 (3%) | 11 (3%) |
| No | 327 (97%) | 326 (97%) |
| Anti-VEGF or bevacizumab | ||
| Yes | 33 (10%) | 34 (10%) |
| No | 303 (90%) | 303 (90%) |
| Not specified | 1 (<1%) | 0 |
|
| ||
| First-line maintenance therapies | ||
|
| ||
| Taxane | ||
| Yes | 27 (8%) | 20 (6%) |
| No | 310 (92%) | 317 (94%) |
| Bevacizumab | ||
| Yes | 22 (7%) | 14 (4%) |
| No | 315 (93%) | 323 (96%) |
| Other | ||
| Yes | 2 (1%) | 4 (1%) |
| No | 335 (99%) | 333 (99%) |
Data are n (%) unless stated otherwise.
Low-grade serous cancer was not excluded but was not specifically defined in this trial.
Other includes adenocarcinoma, not otherwise specified.
Stratification variable.
Treatment-free interval reported here is data submitted immediately before randomisation and not data recorded in the electronic case-report forms.
Variable as reported on the electronic case-report forms.
Compliance with chemotherapy was good, with 277 (85%) of 327 patients in the chemotherapy group and 279 (85%) of 330 patients in the chemotherapy plus bevacizumab group receiving six cycles of all chemotherapy drug components. Five patients in the chemotherapy plus bevacizumab group only received chemotherapy alone. The mean number of bevacizumab cycles administered was 19·36 (SD 11·86), the median was 16 cycles (IQR 8–24), and the range was 1–111 (see appendix pp 30–34 for details of number of cycles, dosages, and dose intensity for chemotherapy and bevacizumab administered through out the trial). Overall, 101 (31%) of 327 assessable patients in the chemotherapy group and 93 (28%) of 330 in the chemotherapy plus bevacizumab group missed one or more planned doses of carboplatin or paclitaxel (with a delay >3 weeks counting as a missed dose), and 100 (31%) patients in the chemotherapy group and 88 (27%) in the chemotherapy plus bevacizumab group substantially delayed (by >1 week) one or more planned doses. In the chemotherapy plus bevacizumab group, 157 (48%) of 330 patients missed or substantially delayed at least one planned dose of bevacizumab.
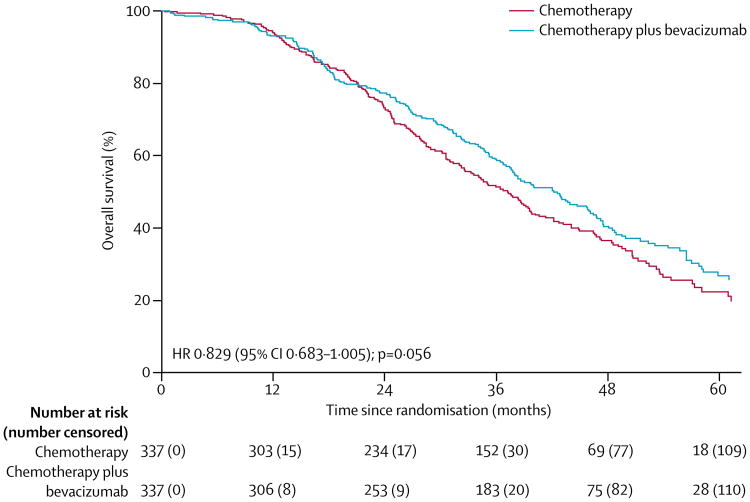
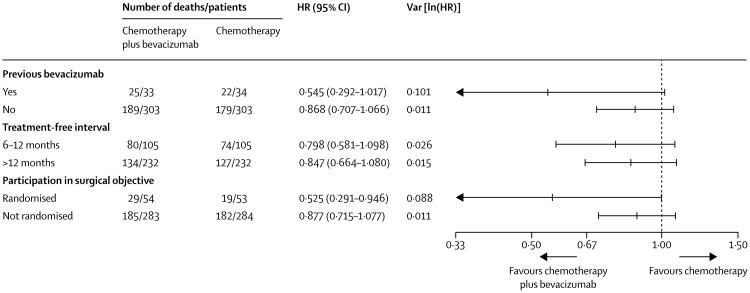
At time of database lock, 415 deaths had occurred (214 in the chemotherapy group and 201 in the bevacizumab group). Median follow-up was 49·6 months in each treatment group (IQR 41·5–62·2 for chemotherapy plus bevacizumab; 40·8–59·3 for chemotherapy alone). Median overall survival was 42·2 months (95% CI 37·7–46·2) in the chemotherapy plus bevacizumab group and 37·3 months (32·6–39·7) in the chemotherapy group (HR 0·829 [95% CI 0·683–1·005], p=0·056; figure 2). Because we had not anticipated the error in the calculated platinum-free interval, we did a post-hoc sensitivity analysis using the actual platinum-free interval calculated from the electronic case-report forms. This exercise adjusted the HR for death slightly to 0·823 (95% CI 0·680-0·996; p=0·0447). The estimated effect on overall survival in the prespecified subgroups was consistent with the primary analysis (figure 3).
Figure 2. Primary analysis of overall survival, adjusted for stratification factors.
Median overall survival is based on the originally submitted treatment-free interval data.
Figure 3. Pre-planned stratified subgroup analysis of overall survival.
Previous bevacizumab was stratified by both treatment-free interval and participation in the surgical objective. Treatment-free interval and participation in the surgical objective were stratified for each other in this analysis. The inclusive lines for each datapoint represent 95% CIs. HR=hazard ratio.
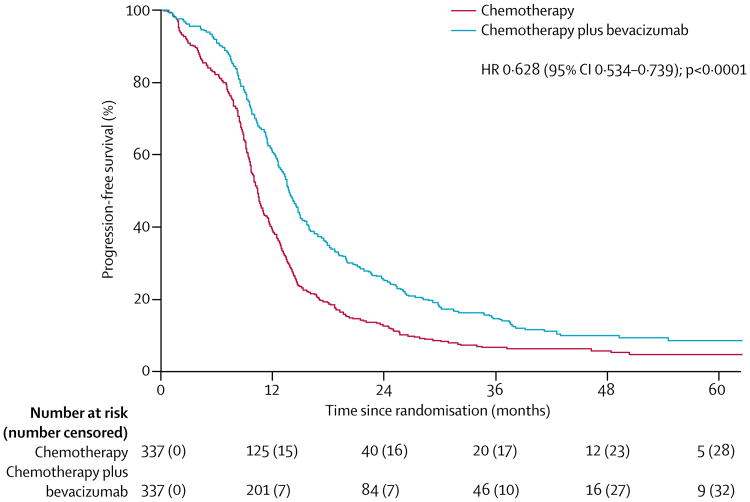
Median progression-free survival was significantly longer with the addition of bevacizumab versus chemotherapy alone (10·4 months [95% CI 9·7–11·0] with chemotherapy alone vs 13·8 months [13·0–14·7] with chemotherapy plus bevacizumab; adjusted HR for surgery and treatment-free interval for progression or death 0·628 [95% CI 0·534–0·739], p<0·0001; figure 4). These results were consistent across the subgroups of interest (participation in the surgical objective, platinum-free interval, and previous bevacizumab treatment; appendix p 35), and after adjustment for actual platinum-free interval (appendix p 35).
Figure 4. Progression-free survival, adjusted for stratification factors.
An exploratory post-hoc analysis of investigator-assessed objective response (complete or partial response according to RECIST version 1.1) was done for the 509 (76%) patients with measurable disease in whom serial imaging was available to assess response. A significantly higher proportion of these patients achieved objective response in the chemotherapy plus bevacizumab group (196 [78%] of 249 patients) compared with the chemotherapy group (152 [59%] of 260 patients; p<0·0001), including a higher number of patients who achieved a complete response (79 [32%] of 249 patients vs 46 [18%] of 260 patients).
Overall, the adverse event profiles for treated patients in both groups were consistent with the known safety profile of the agents under study (table 2, table 3). As noted previously, five patients in the chemotherapy plus bevacizumab group did not receive bevacizumab. Reported here are the adverse events related to the intention-to-treat allocation; adverse events related to actual administered therapy are reported in the appendix (pp 36–38). In the chemotherapy alone group, the worst grade of adverse event to be experienced by individual patients was grade 1–2 in 45 (14%) of 327 patients, grade 3 in 108 (27%), grade 4 in 172 (5%), and grade 5 in two (1%). In the chemotherapy plus bevacizumab group, the worst grade of adverse event to be experienced by individual patients was grade 1–2 in 13 (4%) of 330 patients, grade 3 in 93 (28%), grade 4 in 216 (65%), and grade 5 in nine (3%). More patients in the chemotherapy plus bevacizumab group had at least one adverse event of grade 3 or worse (317 [96%] of 330 patients) than in the chemotherapy group (282 [86%] of 327 patients; table 2). The most frequently reported treatment-related adverse events of grade 3 or worse in the chemotherapy plus bevacizumab group were hypertension (grade 3 in 39 [12%] patients in the chemotherapy plus bevacizumab group vs two [1%] patients in the chemotherapy group), fatigue (27 [8%] patients vs eight [2%] patients), and proteinuria (27 [8%] patients vs none (table 3; appendix pp 3–27).
Table 2. Adverse events by maximum grade and CTCAE (version 3) category.
| Standard chemotherapy (n=327) | Standard chemotherapy plus bevacizumab (n=330) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
| Auditory/ear | 23 (7%) | 0 | 0 | 0 | 29 (9%) | 3 (1%) | 0 | 0 |
| Allergy/immunology | 68 (21%) | 25 (8%) | 1 (<1%) | 0 | 100 (30%) | 31 (9%) | 1 (<1%) | 0 |
| Coagulation | 3 (1%) | 0 | 0 | 0 | 6 (2%) | 6 (2%) | 0 | 0 |
| Constitutional symptoms | 266 (81%) | 9 (3%) | 0 | 0 | 258 (78%) | 29 (9%) | 0 | 0 |
| Cardiac | 28 (9%) | 5 (2%) | 0 | 0 | 126 (38%) | 41 (12%) | 5 (2%) | 0 |
| Dermatology/skin | 277 (85%) | 1 (<1%) | 0 | 0 | 286 (87%) | 8 (2%) | 1 (<1%) | 0 |
| Death (progressive disease or unknown cause) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (2%) |
| Endocrine | 33 (10%) | 0 | 0 | 0 | 47 (14%) | 1 (<1%) | 0 | 0 |
| Gastrointestinal | 207 (91%) | 18 (6%) | 1 (<1%) | 0 | 259 (78%) | 36 (11%) | 2 (1%) | 0 |
| Renal/genitourinary | 39 (12%) | 3 (1%) | 0 | 0 | 58 (18%) | 5 (2%) | 0 | 0 |
| Haemorrhage/bleeding | 24 (7%) | 3 (1%) | 0 | 0 | 134 (41%) | 5 (2%) | 1 (<1%) | 0 |
| Blood/bone marrow | 63 (19%) | 88 (27%) | 170 (52%) | 1 (<1%) | 41 (12%) | 76 (23%) | 208 (63%) | 1 (<1%) |
| Infection | 70 (21%) | 19 (6%) | 0 | 0 | 83 (25%) | 37 (11%) | 4 (1%) | 2 (1%) |
| Lymphatics | 42 (13%) | 0 | 0 | 0 | 44 (13%) | 0 | 0 | 0 |
| Secondary malignancy | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 1 (<1%) |
| Musculoskeletal/soft tissue | 34 (10%) | 1 (<1%) | 0 | 0 | 70 (21%) | 6 (2%) | 0 | 0 |
| Metabolic/laboratory | 102 (31%) | 29 (9%) | 3 (1%) | 0 | 143 (43%) | 60 (18%) | 7 (2%) | 0 |
| Neurology | 255 (78%) | 15 (5%) | 2 (1%) | 0 | 235 (71%) | 25 (8%) | 4 (1%) | 0 |
| Ocular/visual | 48 (15%) | 1 (<1%) | 0 | 0 | 72 (22%) | 3 (1%) | 0 | 0 |
| Pulmonary/upper respiratory | 124 (38%) | 6 (2%) | 0 | 0 | 173 (52%) | 13 (4%) | 1 (<1%) | 0 |
| Pain | 225 (69%) | 16 (5%) | 0 | 0 | 222 (67%) | 48 (15%) | 2 (1%) | 0 |
| Vascular | 2 (1%) | 2 (1%) | 2 (1%) | 0 | 10 (3%) | 10 (3%) | 4 (1%) | 0 |
All adverse events are listed regardless of cause. A detailed breakdown by category is presented in the appendix (pp 3–27). CTCAE=Common Terminology Criteria for Adverse Events.
Table 3. Selected adverse events related to bevacizumab treatment.
| Standard chemotherapy (n=327) | Standard chemotherapy plus bevacizumab (n=330) | |
|---|---|---|
| Any adverse event of special interest | 75 (23%) | 237 (72%) |
| Grade 3–5 | 25 (8%) | 98 (30%) |
| Serious | 19 (6%) | 49 (15%) |
| Bleeding (CNS) | 2 (1%) | 1 (<1%) |
| Bleeding (non-CNS)* | 36 (11%) | 137 (42%) |
| Congestive heart failure | 0 | 1 (<1%) |
| Fistula or abscess (non-gastrointestinal), grade 3–4 | 0 | 0 |
| Gastrointestinal perforations | 1 (<1%) | 6 (2%) |
| Hypertension | 10 (3%) | 135 (41%) |
| Grade 3† | 2 (1%) | 39 (12%) |
| Neutropenia and associated complications (eg, infections) | 26 (8%) | 40 (12%) |
| Grade 3–4 | 14 (4%) | 23 (7%) |
| Posterior reversible encephalopathy syndrome | 0 | 0 |
| Proteinuria | 3 (1%) | 56 (17%) |
| Grade 3–4 | 0 | 27 (8%) |
| Secondary primary malignancies | 1 (<1%) | 0 |
| Thromboembolic event (arterial) | 6 (2%) | 22 (7%) |
| Thromboembolic event (venous) | 0 | 0 |
| Wound healing complication | 2 (1%) | 10 (3%) |
Adverse events shown are for all grades unless otherwise stated.
The most frequent events of non-CNS bleeding (all grades) were epistaxis (6 [2%] in the chemotherapy group vs 109 [33%] in the chemotherapy plus bevacizumab group), contusion (10 [3%] vs 13 [4%]), and rectal haemorrhage (6 [2%] vs 20 [6%]). The patient narrative for the one case of intracranial haemorrhage is presented in the appendix (p 38).
No grade 4 hypertension events occurred.
The proportion of patients with a serious adverse event was higher in the chemotherapy plus bevacizumab group (92 [28%] of 330 patients) than in the chemotherapy group (37 [11%] of 327). The only serious adverse event that occurred in more than 5% of patients in either group was febrile neutropenia, affecting 17 (5%) patients in the chemotherapy plus bevacizumab group and seven (2%) in the chemotherapy group. The most common toxicities of grade 3 or more for chemotherapy plus bevacizumab versus chemotherapy alone were abdominal pain (40 [12%] vs 0), nausea (30 [9%] vs 10 [3%]), small bowel obstruction (20 [6%] vs 10[3%]), hypertension (20 [6%] vs 0), proteinuria (20 [6%] vs 0), and dyspnoea (20 [6%] vs 0). One patient (described in detail in appendix pp 36–38) was diagnosed with a grade 4 intracranial haemorrhage after six cycles of carboplatin and paclitaxel and eight cycles of bevacizumab. This serious adverse event was attributed to bevacizumab.
Estimation of the incidence of drug-related infusion reactions (attributed hypersensitivity) was a stated secondary objective in this trial. Overall, no grade 4 or 5 hypersensitivity events occurred. However, all-grade hyper sensitivity reactions occurred in 171 (26%) participants overall (82 [25%] patients in the chemotherapy group and 89 [27%] patients in the chemotherapy plus bevacizumab group), with grade 3 reactions occurring in 56 (9%) patients overall (26 [9%] patients in the chemotherapy group and 30 [9%] in the chemotherapy plus bevacizumab group).
Study investigators attributed treatment-related deaths with a low and similar frequency among the treatment groups: two (1%) patients in the chemotherapy group versus nine (3%) patients in the chemotherapy plus bevacizumab group. These events were recorded as neutropenic infection (n=1) and myelodysplastic syndrome (n=1) in the chemotherapy group, and neutropenic infection (n=1), febrile neutropenia (n=1), myelodysplastic syndrome (n=1), and secondary malignancy possibly related to treatment (n=1). Additionally, five deaths were attributed to treatment in the chemotherapy plus bevacizumab group where no Common Terminology Criteria for Adverse Events term was used. These deaths were described as “Death—disease progression” (n=3), “Sudden death” (n=1) and “Death, not otherwise specified” (n=1). More patients in the chemotherapy plus bevacizumab group (84 [25%]) than in the chemotherapy group (36 [11%]) were withdrawn from any study drug owing to an adverse event. Most of the adverse events leading to study treatment discontinuation occurred in less than 1% of patients in either treatment group. However, discontinuation of therapy due to paclitaxel-attributed adverse events (ie, neutropenia, neuropathy, and arthralgia or fatigue) was observed in three (1%) patients in the chemotherapy group and in eight (2%) patients in the chemotherapy plus bevacizumab group. Details of adverse events leading to treatment discontinuation and protocol violations in the safety population are given in the appendix (pp 36–38).
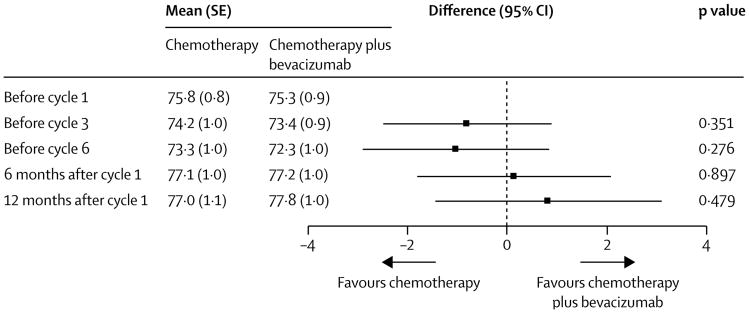
In the intention-to-treat population, FACT-O questionnaire compliance was similar: compliance was 93% in both treatment groups at baseline (313 of 337 patients in the chemotherapy plus bevacizumab group and 311 of 316 patients in the chemotherapy group [one patient died before completing chemotherapy]), and at subsequent timepoints compliance differed between groups by approximately 1–5% (before cycle 3: 89% [297 of 333] chemotherapy plus bevacizumab vs 88% [295 of 335] chemotherapy; before cycle 6: 86% [285 of 332] vs 81% [269 of 334]; at 6 months after cycle 1: 83% [273 of 328] vs 80% [263 of 328]). The difference in compliance between the groups was more pronounced 12 months after start of systemic therapy, when 257 (83%) of 309 patients in the chemotherapy plus bevacizumab group were compliant compared with 229 (74%) of 311 patients in the chemotherapy group. After adjustment for baseline score, age at enrolment, time since enrolment, participation in the surgical objective, and treatment-by-time interaction, the overall estimated difference in mean FACT-O TOI score (accounting for all datapoints) between the chemotherapy plus bevacizumab group and the chemotherapy group was –0·37 (95% CI –1·80 to 1·06; p=0·62; figure 5). Treatment in both groups was associated with a decrease in total FACT-O-TOI scores during therapy, but differences in scores between the groups were neither significant nor considered to be clinically meaningful at any timepoint. By 6 months after the first cycle, FACT-O TOI scores returned to above baseline scores in both groups.
Figure 5. Patient-reported outcomes with FACT-O TOI scores.
Means at baseline are raw means. Means at follow-ups are least-squared means estimated from the fitted linear mixed model. Treatment differences are estimated from the fitted linear mixed model. FACT-O TOI=Function Assessment of Cancer Therapy-Ovary trial outcome index.
Further treatment after disease progression on this trial was not a prespecified outcome but was commonly reported (appendix pp 28–29). 291 (86%) of 337 patients in the control group and 275 (82%) of 337 in the experimental group received subsequent therapy, including 165 (49%) patients in the chemotherapy group and 148 (44%) patients in the chemotherapy plus bevacizumab group who received three or more lines of additional treatment. More patients received bevacizumab in the chemotherapy group (57 [17%] of 337 patients) than in the chemotherapy plus bevacizumab group (27 [8%] of 337 patients). Conversely, more patients received subsequent platinum-based therapy either as a single agent or in combination in the chemotherapy plus bevacizumab group (104 [31%] patients) than in the chemotherapy group (77 [23%]). The proportion of patients undergoing additional surgery, radiotherapy, endocrine or hormonal therapy, or other biologicals was low and similar between the treatment groups (table 1).
Discussion
In our study of bevacizumab added to paclitaxel and carboplatin in women with recurrent, platinum-sensitive ovarian cancer, the upper limit in the adjusted, two-sided overall survival analysis breaches 1·0 (1·005), suggesting no statistically significant difference in overall survival between the treatment groups. However, our sensitivity analysis accounting for misclassification of the stated platinum-free interval places the upper limit of the 95% CI at 0·996, bolstering the observed treatment effect of bevacizumab. Thus, we believe that the improvement in median overall survival of about 5 months in the bevacizumab group is clinically meaningful for both patients and clinicians. Notably, the morphologies of the overall survival curves closely approximate each other for about 22 months before the event (death) rate increases in the chemotherapy alone group. The specific reasons for this pattern are not known, but might be related to the relatively few deaths that occurred during the first year of treatment exposure, the drug–drug interaction of paclitaxel and bevacizumab (as noted in the AURELIA trial9,24), and the effect of bevacizumab maintenance therapy in this setting. Furthermore, we also show that investigator-assessed progression-free survival and objective response (which was analysed post hoc) were significantly improved with bevacizumab administered during chemotherapy and as maintenance therapy.
Although cross-trial comparisons are an acknowledged hazard, they do provide context for consideration of these data. The median overall survival for this trial's control group is longer than that of any previous report, and might represent the (balanced) effect of inclusion of patients amenable to secondary cytoreduction, the inclusion of postoperative patients with no measurable disease, and a relatively high quotient of patients with a platinum-free interval longer than 12 months. Existing standards of more complete primary cytoreduction and improved adjuvant therapy are increasing the duration of primary progression-free survival in patients who ultimately develop recurrent disease. Many patients completing primary chemotherapy for advanced disease will have a platinum-free interval of at least 6 months and, indeed, more than 75% of patients entering primary-therapy clinical trials will have a platinum-free interval longer than 6 months.1,11–13,25 In these women, platinum-based therapy and secondary cytoreduction are important considerations.
In general, surgery is considered in patients with limited sites of recurrence for which complete cytoreduction is anticipated and in patients with strong potential to respond to chemotherapy.25–27 However, the inclusion of patients undergoing secondary cytoreduction in a recurrent platinum-sensitive chemotherapy trial is not unique to our study. For example, in the OCEANS trial,10 an open-label, randomised, phase 3 trial of gemcitabine and carboplatin with or without bevacizumab in patients with a platinum-free interval of more than 6 months, surgical patients (11% of the total randomised population) were required to have measurable postoperative disease before randomisation. In this cohort, the HR for progression-free survival for the bevacizumab, gemcitabine, and carboplatin group was nearly the same as that of the non-surgical cohort (HR 0·50, 95% CI 0·24–1·01 for the surgical cohort vs 0·49, 0·39–0·62 for the non-surgical cohort). In the CALYPSO trial,28,29 a randomised, phase 3 trial of paclitaxel and carboplatin versus pegylated liposomal doxorubicin and carboplatin, patients under going secondary debulking before randomisation (19% of the study population) were eligible if they had measurable or evaluable postoperative residual disease. However, secondary surgery in that trial had a significant effect on both progression-free survival and overall survival, but no differences in these endpoints were observed between the treatment groups.28,29 In our trial, patients amenable for surgery were randomly allocated to secondary cytoreduction (or not) before randomisation to the chemotherapy treatment groups of the bevacizumab objective. Thus, by contrast to OCEANS10 and CALYPSO,28,29 a sizeable proportion of patients entering chemotherapy randomisation after surgery in our study would have done so with minimal or no residual disease. Because recurrent disease distribution dictates the candidacy of surgical intervention, addressing this endpoint specifically requires a separate randomisation sequence, with its own statistical considerations to bear validity. The proportion of patients who had entered the surgical objective at the point of maturity of the bevacizumab objective was just 16%, reflecting both the need for a separate randomisation sequence and investigator partiality to the potential merits of surgery in recurrent platinum-sensitive disease. The effect of surgery on overall survival is a separate primary endpoint, but has not witnessed enough events for analysis at this time and will be reported separately when the data are mature. Furthermore, because objective response, progression-free survival, and overall survival are temporally related and affected by platinum-free interval, the duration of platinum-free interval in this study should be reviewed when analysing trial results and inferring the population under study. In this study, the proportion of patients with a platinum-free interval of at least 12 months was 69% (74% audited), which is higher than in previous trials such as OCEANS (58%),10 CALYPSO (65%),28 and ICON6 (67%).8 This increased proportion reflects the prognostically favourable cohort and also exemplifies the rationale for the chosen stratification variables—platinum-free interval and participation in the surgical objective. Random imbalance of either or both could have had a substantial effect on the interpretability of the results. We did not stratify by BRCA mutation status because its independent effect on response to an anti-angiogenesis agent was unknown.
Previous bevacizumab administration has been purported to have an adverse effect,30 no effect,31 or a beneficial effect32 with subsequent exposure among patients with ovarian cancer. In this trial, just 67 (10%) patients had previous bevacizumab in the first-line setting. However, our inclusion of patients with previous bevacizumab did not seem to affect the overall directionality of the bevacizumab-related treatment effect. Furthermore, a substantial proportion of both cohorts went on to receive bevacizumab as post-progression therapy, although the ratio was 2:1 between groups. A prospective, randomised, phase 3 trial will specifically address the effect of bevacizumab post-progression in platinum-sensitive ovarian cancer (ClinicalTrials.gov, number NCT01802749).
Fortunately, we observed no new safety signals in this trial. As has been reported in other phase 3 trials9–12 of bevacizumab in patients with ovarian cancer, grade 3–4 hypertension, proteinuria, and venous thromboembolism were more common in the chemotherapy plus bevacizumab group than with chemotherapy alone. However, severe gastrointestinal perforation, fistula, or abscess were infrequent and not significantly increased with the addition of bevacizumab.
Drug-related hypersensitivity was common, affecting around 26% of all participants. Bevacizumab infusion did not seem to affect the incidence of all-grade or grade 3 hypersensitivity. Nevertheless, this incidence is substantially higher than that observed in the two other non-taxane-based phase 3 trials, CALYPSO28 (15·9% in the pegylated liposomal doxorubicin group) and the AGO trial33 of carboplatin versus carboplatin plus gemcitabine (5·7% in the carboplatin plus gemcitabine group), but similar to that reported in the paclitaxel and carboplatin group of CALYPSO (32·9%).28 A more mature toxicity assessment is forthcoming with the conclusion of the surgical objective of our trial.
An important context in which to consider these results is the effect that treatment has on patient-reported outcomes. Previous trials have documented that ascites and carcinomatosis can contribute to baseline symptomatology and represent a potential alterable situation with combination chemotherapy and bevacizumab.9 Although we were unable to specifically evaluate these conditions in our trial, we noted that treatment in both groups was associated with a decrease in total scores while on therapy, but without difference between the groups. By 6 months after the first cycle, FACT-O TOI scores returned above baseline in both groups. These results are considered robust given the high rate of form completion compliance (≥80%) in both groups from study entry to 12 months following treatment initiation.
Some important considerations might have limited the interpretations drawn from this trial. First, a prespecified stratification variable was compromised by inaccurate data regarding the treatment-free interval. The effect of this discrepancy has been discussed; however, as noted, stratification procedures are conducted at the time of randomisation to ensure balance of known prognostic factors. Fortunately, the groups did not seem to be adversely affected by this small cohort of patients with misclassified platinum-free interval (which were balanced between the treatment groups). Second, the two-step randomisation sequence considers the two interventions, chemotherapy and surgery, as independent factors. However, surgery might serve as a treatment-effect modiffer, because patients with recurrent disease amenable to surgical resection are likely to have longer platinum-free intervals and possibly more likely to respond to chemotherapy or bevacizumab. The multivariate Cox regression model clearly demonstrates the differential prognostic effect this cohort serves. The independent effect of this observation should be minimised because it was a stratification variable and equally considered within the two groups. Third, the cohort of patients entering the surgical objective might not represent the general population at large because of investigator preference or interpretation of existing, non-randomised, and retro spective reports of secondary cytoreduction. The generalisation of our future trial findings might be limited by this case selection. Finally, the trial population was mostly enrolled in the USA, which might underestimate the toxicity or inaccurately represent the therapeutic efficacy of therapy in our Asian participants. Such factors have been reported with other anti-angiogenesis inhibitors.15
In summary, we show in this phase 3 trial that bevacizumab added to paclitaxel and carboplatin might favourably affect overall survival in women with platinum-sensitive recurrent ovarian cancer. Additionally, it significantly improves progression-free survival and objective response. We did not observe any new safety signals nor toxicity that differentially increased treatment discontinuation. We believe the data provide important guidance for treatment in this population.
Supplementary Material
Research in context.
Evidence before this study
Prior to the design of this study, we searched PubMed and clinical trial registries for ovarian cancer clinical trials that assessed the efficacy of platinum-combination chemotherapy in women with platinum-sensitive recurrent disease between Jan 1, 1996, and Dec 31, 2005. We used search terms “recurrent ovarian cancer”, “platinum-sensitive”, “secondary cytoreduction”, “platinum”, “platinum-combination therapy”, “platinum and paclitaxel”, “bevacizumab”, bevacizumab combination therapy”, “adverse effects”, “toxicity”, “survival”, “surgical morbidity”, “surgical candidacy”, “anti-angiogeneis agents”, and “biomarkers for anti-angiogenesis”. Identified reports were further evaluated for patient enrolment characteristics and administered therapy, such as specific chemotherapy, surgery, or both. Studies and clinical reports of interest included those reported in English and those that demonstrated efficacy or safety characteristics of a treatment population receiving platinum combinations, surgery, or both. As our trial progressed, reports appearing in the public domain from publication or from conference proceedings, particularly those related to adverse events and efficacy from bevacizumab combinations across tumour types, were considered at the time of protocol amendments for adjustments in adverse event management. At the time of protocol activation, ICON4—a trial of paclitaxel-platinum versus platinum—was the only phase 3 trial in platinum-sensitive patients with recurrent ovarian cancer to have been published. However, the proportion of patients who were taxane-naive entering that trial was substantial. Because platinum-paclitaxel is a global standard for primary therapy, our study was undertaken to evaluate the effect of retreatment in this cohort with a more homogenous population receiving primary therapy. Further, at the time of study activation, no randomised clinical trials of secondary cytoreduction in platinum-sensitive patients with recurrent ovarian cancer had been identified, highlighting the need for this randomisation in GOG-0213. Because more than 75% of patients with advanced-stage ovarian cancer will present with recurrent disease more than 6 months from completion of primary chemotherapy, platinum-based therapy and secondary cytoreductive surgery are important considerations. Bevacizumab has been investigated in this setting, showing a significant improvement in progression-free survival, but none of these clinical trials were powered for overall survival.
Added value of this study
This trial provides a mature analysis of overall survival following bevacizumab use in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Progression-free survival, objective response, and toxicity were additionally assessed. This results of this trial confirm those of previous studies with bevacizumab plus gemcitabine and carboplatin, particularly in regard to progression-free survival, response, and toxicity, but with more optimistic results of overall survival after a sensitivity analysis adjusted for incorrect platinum-free interval stratification data. The difference in median overall survival (5 months) is clinically meaningful and is bolstered by a significant increase in both progression-free survival and objective and complete responses in the bevacizumab group. Importantly, toxicity was not associated with diminution of patient-reported outcomes.
Implications of all the available evidence
The totality of data from this trial and other studies serves as evidence for the merit of bevacizumab in combination with chemotherapy for platinum-sensitive recurrent ovarian cancer, which should be considered in general clinical practice. The better-than-expected outcomes in both groups compared with other trials in this clinical domain might relate to inclusion of prognostically more favourable patients, duration of bevacizumab exposure, or the interaction of bevacizumab with taxane-based therapy—a hypothesis also raised in a study of bevacizumab in platinum-resistant recurrent disease.
Acknowledgments
This work was fully supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1U10 CA180822), and NRG Operations (U10CA180868). Roche/Genentech supported the NCI-CRADA enabling this trial. RLC is supported by the Ann Rife Cox Chair in Gynecology and Judy Reis/ Albert Pisani Ovarian Cancer Research Fund.
RLC has received grant funding from Genentech/Roche and serves on the scientific steering committee as the principal investigator for the trial. MFB has received grant funding from Genentech/Roche. TJH serves on an advisory board for Genentech/Roche. KF has received grant funding and speakers' fees from Chugai/Roche, and serves on their advisory board. KST has received grant funding and is on the speaker's bureau for Genentech/Roche.
Footnotes
Declaration of interests: All other authors declare no competing interests.
Contributors: RLC served as principal investigator, wrote the protocol, collected and reviewed the data, audited the data, analysed the data, wrote the report, had access to all data elements, and interpreted the results. MFB served as principal statistical lead, wrote the protocol, analysed and interpreted the data, and contributed to editing the report. TJH, PS, DKA, and NMS served as co-principal investigators, reviewed patient data and data queries, performed data analysis and interpretation, and edited the report. JLW, B-GK, KF, KST, DMO'M, SAD, SCR, PD, and RSM contributed patient materials, reviewed patient data and data queries, performed data analysis and interpretation, and edited the report. KB-E and JKC served as co-principal investigators for patient-reported outcomes, contributed patient materials, reviewed patient data and data queries, performed data analysis and interpretation, and edited the report. HH did quality-of-life data analysis, and edited the report. RA reviewed the pathology slides and the pathology data for enrolment into the study.
References
- 1.Coleman RL, Monk BJ, Sood AK, et al. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nature Rev Clin Oncol. 2013;10:211–24. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 3.Monk BJ, Coleman RL. Changing the paradigm in the treatment of platinum-sensitive recurrent ovarian cancer: from platinum doublets to nonplatinum doublets and adding antiangiogenesis compounds. Int J Gynecol Cancer. 2009;19(suppl 2):S63–67. doi: 10.1111/IGC.0b013e3181c104fa. [DOI] [PubMed] [Google Scholar]
- 4.Schlaeppi JM, Eppenberger U, Martiny-Baron G, et al. Chemiluminescence immunoassay for vascular endothelial growth factor (vascular permeability factor) in tumor-tissue homogenates. Clin Chem. 1996;42:1777–84. [PubMed] [Google Scholar]
- 5.Ueda M, Hung YC, Terai Y, et al. Vascular endothelial growth factor-C expression and invasive phenotype in ovarian carcinomas. Clin Cancer Res. 11:3225–32. doi: 10.1158/1078-0432.CCR-04-1148. [DOI] [PubMed] [Google Scholar]
- 6.Jackson AL, Eisenhauer EL, Herzog TJ. Emerging therapies: angiogenesis inhibitors for ovarian cancer. Expert Opin Emerg Drugs. 2015;20:331–46. doi: 10.1517/14728214.2015.1036739. [DOI] [PubMed] [Google Scholar]
- 7.Graybill W, Sood AK, Monk BJ, et al. State of the science: emerging therapeutic strategies for targeting angiogenesis in ovarian cancer. Gynecol Oncol. 2015;138:223–26. doi: 10.1016/j.ygyno.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Ledermann JA, Embleton AC, Raja F, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387:1066–74. doi: 10.1016/S0140-6736(15)01167-8. [DOI] [PubMed] [Google Scholar]
- 9.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–08. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 10.Aghajanian C, Blank SV, Gof BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perren TJ, Swart AM, Pfsterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 12.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 13.du Bois A, Kristensen G, Ray-Coquard I, et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17:78–89. doi: 10.1016/S1470-2045(15)00366-6. [DOI] [PubMed] [Google Scholar]
- 14.Monk BJ, Poveda A, Vergote I, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:799–808. doi: 10.1016/S1470-2045(14)70244-X. [DOI] [PubMed] [Google Scholar]
- 15.du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32:3374–82. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Rustin GJ, Nelstrop AE, Bentzen SM, et al. Use of tumour markers in monitoring the course of ovarian cancer. Ann Oncol. 1999;10(suppl 1):21–27. doi: 10.1023/a:1008351216605. [DOI] [PubMed] [Google Scholar]
- 18.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19:1809–17. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute of the National Institutes of Health. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) Bethesda, MD: Cancer Therapy Evaluation Program; 2006. [Google Scholar]
- 20.Lan KK, DeMets DL. Changing frequency of interim analysis in sequential monitoring. Biometrics. 1989;45:1017–20. [PubMed] [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chem Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life tables. J Royal Statist Soc. 1972;34:187–220. [Google Scholar]
- 23.Wenzel LB, Huang HQ, Armstrong DK, et al. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:437–43. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 24.Poveda AM, Selle F, Hilpert F, et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol. 2015;33:3836–38. doi: 10.1200/JCO.2015.63.1408. [DOI] [PubMed] [Google Scholar]
- 25.du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–44. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 26.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Bristow RE, Lagasse LD, Karlan BY. Secondary surgical cytoreduction for advanced epithelial ovarian cancer. Patient selection and review of the literature Cancer. 1996;78:2049–62. [PubMed] [Google Scholar]
- 28.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28:3323–29. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 29.Lee CK, Lord S, Grunewald T, et al. Impact of secondary cytoreductive surgery on survival in patients with platinum sensitive recurrent ovarian cancer: analysis of the CALYPSO trial. Gynecol Oncol. 2015;136:18–24. doi: 10.1016/j.ygyno.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Petrillo M, Amadio G, Salutari V, et al. Impact of bevacizumab containing first line chemotherapy on recurrent disease in epithelial ovarian cancer: a case-control study. Gynecol Oncol. 2016;142:231–36. doi: 10.1016/j.ygyno.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Monk BJ, Sill MW, Walker JL, et al. Randomized phase II evaluation of bevacizumab versus bevacizumab plus fosbretabulin in recurrent ovarian, tubal, or peritoneal carcinoma: an NRG Oncology/ Gynecologic Oncology Group Study. J Clin Oncol. 2016;34:2279–86. doi: 10.1200/JCO.2015.65.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Backes FJ, Richardson DL, McCann GA, et al. Should bevacizumab be continued after progression on bevacizumab in recurrent ovarian cancer? Int J Gynecol Cancer. 2013;23:833–38. doi: 10.1097/IGC.0b013e318290ea69. [DOI] [PubMed] [Google Scholar]
- 33.Pfsterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.