Abstract
This study examined the relationship between hepatic and myocardial iron concentration, assessed by T2*-MRI in 66 patients (3–82 years) with transfusion-dependent anemias and thalassemia intermedia, to determine whether hepatic iron levels alone suffice for chelation adjustments. We found a poor correlation between hepatic and myocardial iron (r = 0.10, P = 0.43) and identified a subgroup (14%) with increased myocardial iron without a matched degree of hepatic hemosiderosis. Left ventricular ejection fraction was insensitive for detecting elevated myocardial iron. These findings were present in both adult and pediatric patients. We recommend therapeutic monitoring of iron burden by evaluation of both liver and myocardial iron with T2*-MRI.
Introduction
Transfusion therapy has greatly improved the outlook in patients with chronic anemias such as thalassemia major, sickle cell disease, and bone marrow failure syndromes; however, the resultant iron load damages tissues including the heart, liver, and endocrine organs. Iron chelation treatment diminishes the risk of such organ damage but has its own toxicities, associated complications, and expense. Close monitoring of organ iron concentration and function as well as chelator dosing is thus important for optimal patient care. It has long been accepted that hepatic iron concentration (HIC) is the major indicator of overall iron burden and the principal index by which to manage therapy [1]. HIC monitored by serial liver biopsies was presumed to reflect iron concentration in other organs such as the heart as well.
Recently, accurate MRI techniques for monitoring iron concentration have been developed and provide a means to noninvasively assess iron load in various organs. Specifically, MRI measurements of the tissue relaxation parameter T2*, have been shown to correlate well with biopsy-derived iron concentrations in the liver [2,3] and the heart [4–7], and myocardial T2* values <20 ms (indicating elevated iron) were found to be associated with left ventricular (LV) dysfunction [2,8].
The purpose of this study was to analyze the relationship between liver and heart iron concentration as assessed by T2*-MRI in a cohort of chronically transfused adults and children and patients with thalassemia intermedia and determine whether HIC alone is sufficient for clinical management.
Results
A total of 66 patients had both liver and myocardial iron load assessment by T2*-MRI at Children’s Hospital Boston through March, 2007. Their gender, age, and diagnosis are shown in Table I. The T2*-MRI-derived HIC ranged from 0.95 to 41.8 mg/g dry weight with a median of 9.1 mg/g dry weight (Table I). HIC did not significantly correlate with age or gender. Forty-six patients had a normal myocardial T2* value ≥20 ms and 20 had a T2* value <20 ms indicating increased iron concentration. Myocardial T2* did not significantly correlate with age or gender.
TABLE I.
Characteristics of the Study Cohort
| Number of patients | 66 |
| Gender, n (%) | |
| Male | 40 (61) |
| Female | 26 (39) |
| Age | |
| >21 years, n (%) | 31 (47) |
| ≤21 years, n (%) | 35 (53) |
| Mean ± SD (years) | 23.1 ± 13.5 |
| Median (range) (years) | 19 (3–82) |
| Diagnosis, n (%) | |
| Thalassemia major | 38 (58) |
| Thalassemia intermedia | 7 (11) |
| α-Thalassemia | 1 (1.5) |
| Sickle cell disease | 8 (12) |
| Diamond-Blackfan anemia | 2 (3) |
| Leukemia | 4 (6) |
| Other | 6 (9) |
| Myocardial T2*, n (%) | |
| ≥20 ms | 46 (70) |
| <20 ms | 20 (30) |
| LV ejection fraction, n (%) | |
| ≥56% | 57 (88) |
| <56% | 8 (12) |
| MRI-derived HIC (mg/g dry weight) | |
| Median (range) | 9.1 (0.95–41.8) |
HIC, hepatic iron concentration; LV, left ventricular; SD, standard deviation.
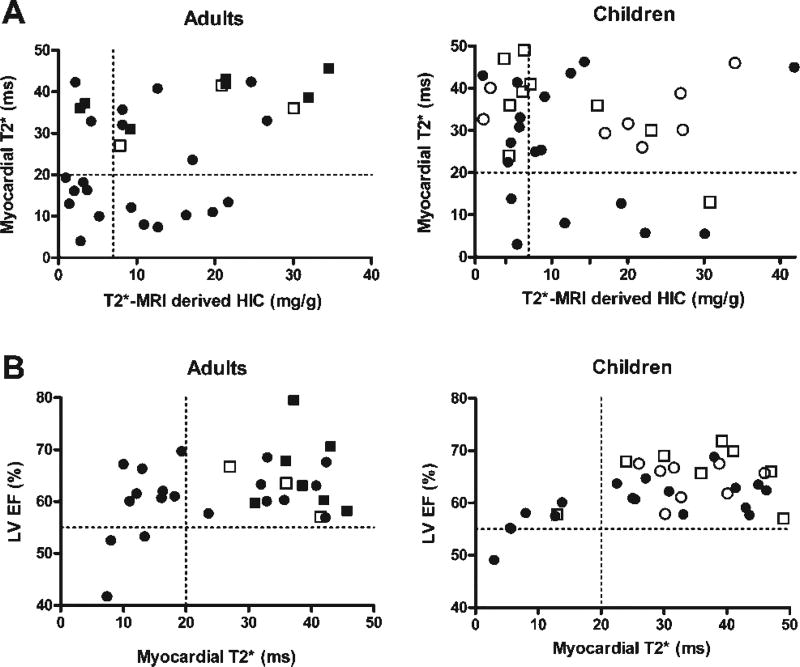
There was no significant correlation between myocardial T2* (or its reciprocal, R2*) and HIC in the cohort as a whole (r = 0.10, P = 0.43) or in the adult and pediatric subgroups: adult, age ≥21 years, n = 31; r = 0.22, P = 0.24 and pediatric, age <21 years, n = 35; r = −0.02, P = 0.90 (Fig. 1A). Of the 20 patients with elevated myocardial iron, 2 were age 9–10 years, 3 age 15–17 years, 2 age 18–21 years, and 13 age ≥21 years. We identified a subgroup of nine subjects, 14% of the total cohort [2/35 children (age, 10 and 17 years) and 7/31 adults)], with increased myocardial iron (T2* < 20 ms) but HIC within target range (<7 mg/g dry weight). All of these patients had thalassemia major and two of them had an LV EF <56%.
Figure 1.
(A) The relationship between myocardial T2* (ms) and T2*-MRI derived hepatic iron concentration (HIC) in adults and children. (B) The relationship between MRI-derived left ventricular ejection fraction (LV EF) and myocardial T2*. The dotted lines represent the limits of normal values for HIC (+7 mg/g dry weight), myocardial T2* (≥20 ms), and LV EF (>56%). ●: Thalassemia major (age, 6–48 years); ○: sickle cell disease (age, 9–19 years); ■: thalassemia intermedia (age, 23–82 years); □: other (age, 3–27 years).
Low myocardial T2* (i.e., increased iron load) correlated with low LV EF (<56%) (r = 0.36, P = 0.004). This relationship was present in both the adult and pediatric subgroups (Fig. 1B). All eight patients with a decreased LV EF had evidence of elevated myocardial iron concentration (T2* < 20 ms). Eighteen percent of patients who had LV EF measured (4/34 children and 8/31 adults) had a normal LV EF (≥56%) but evidence of elevated myocardial iron (T2* < 20 ms).
There were 38 patients with thalassemia major and eight patients with transfusion-dependent sickle cell disease. The sickle cell disease patients (age, 9–18 years) had been transfused a median of 5 years (range, 2–11 years) and one patient was treated with exchange transfusion. The HIC tended to be higher in the sickle cell group (median HIC 21.0 vs. 8.2 mg/g dry weight, P = 0.09). However, none of the patients with sickle cell disease had evidence of elevated myocardial iron (T2* < 20 ms), whereas 19 patients (50%) with thalassemia major had elevated myocardial iron (T2* < 20 ms) (P = 0.01).
Discussion
In patients with transfusion-dependent anemia and thalassemia intermedia, we demonstrated a poor correlation between hepatic and myocardial iron, and identified a population with significant cardiac iron deposition (T2* < 20 ms) without a matched increased HIC (<7 mg/g dry weight). The sensitivity of HIC to predict abnormal myocardial iron loading was 55%. These observations are consistent with reports from other groups who used similar MRI techniques [2,8] and extend the findings to the pediatric group. Both hepatic and myocardial T2* correlated poorly with age and gender. The weak association between hepatic and myocardial iron content likely reflects differences in organ and cellular iron transport kinetics.
We also found that elevated myocardial iron concentration as evidenced by lower T2* was associated with LV systolic dysfunction. In fact, all patients in the cohort with LV dysfunction had an abnormal T2* (<20 ms), a pattern reported by other groups as well [2,8]. The observation, along with the validation studies showing a close relationship between T2* and myocardial iron concentration [4–7], imply that patients with an abnormal myocardial T2* and normal systolic function are at higher risk for cardiac events than those with a normal myocardial T2*.
The poor correlation between liver and myocardial iron suggests that relying on HIC alone risks missing myocardial iron loading and a window of opportunity to potentially prevent devastating cardiac events [9]. Specifically, 14% of our patients would be considered low-risk based on their HIC of <7 mg/g dry weight, yet had elevated myocardial iron by T2* measurement. Our data show that systolic function also has poor sensitivity (40%) for detecting elevated myocardial iron as 18% of patients had elevated myocardial iron with a normal ejection fraction and only 8 of the 20 with low T2* had abnormal LV EF.
We also found that our patients with transfusion-dependent sickle cell disease tended to have a higher HIC than those with thalassemia major but none had increased myocardial iron (T2* < 20 ms), whereas 50% of those with thalassemia major had abnormal myocardial iron values. Among the thalassemia intermedia cohort, five of seven had an elevated HIC and all had normal myocardial iron indices. Small patient numbers, particularly of the sickle cell patients, limit the broad application of this observation; however, other investigators have also found a low prevalence of myocardial iron loading in patients with sickle cell disease [8,10,11]. Possible reasons for this observation include differences in iron absorption and metabolism, the duration or intensity of transfusion, and the effective use of transferrin-bound iron in bone marrow.
Limitations of this study include its retrospective cross-sectional study design, performance at a single center, and relatively small study population. Nevertheless, the data provide a solid basis for prospective longitudinal studies to verify the power of myocardial T2* measurements to predict cardiac events and to better define iron transport kinetics.
In conclusion, as measured by T2*-MRI, HIC correlates poorly with myocardial iron concentration, and there is an important subgroup with significant cardiac iron deposition but HIC within the accepted therapeutic range. We therefore recommend that both liver and myocardial T2*-MRI measurements along with LV ejection fraction be used in concert for chelation adjustments in patients with transfusion-dependent anemias and thalassemia intermedia.
Methods
A database search identified all patients with transfusion-dependent anemia and thalassemia intermedia who had both liver and heart iron load assessment by T2*-MRI at Children’s Hospital Boston through March, 2007. In this cross-sectional study, only the most recent MRI data are included below for patients with more than one MRI study. Permission to conduct this retrospective database review and analysis was granted by the Committee on Clinical Investigation, Children’s Hospital Boston.
Iron in the myocardium and liver was quantified by measuring R2* (1/T2*), a MR relaxation parameter which has been shown to vary proportionally with tissue iron concentration [2,3,5]. This technique has high reproducibility and inter-MRI scanner agreement [2,12–14]. MRI measurements were performed using a 1.5-T clinical MRI scanner (initially a General Electric TwinSpeed, General Electric Medical Systems, Waukesha, WI; more recently a Philips Achieva, Philips Medical System, Best, The Netherlands) and a torso surface coil. Liver R2* was measured from a single axial mid-hepatic slice using a multiecho gradient echo (8–16 echoes) sequence with a minimum echo time (TE) of 1.1 ms and a repetition time of 50 ms, obtained in a single breath-hold [9,13]. Myocardial T2* was assessed from a single midpapillary ventricular short-axis slice using a cardiac-gated, segmented, multiecho gradient echo sequence obtained in a single breath-hold, similar to the technique described by Westwood et al. [13]. Eight echoes with a minimum echo time of 2.0 ms, an echo spacing of 2.2 ms, and a repetition time of 19.1 ms were obtained.
R2* values were calculated using custom-written software developed in MATLAB (The MathWorks®, Natick, MA) by Dr. John C. Wood and adapted by Dr. Yansong Zhao. A region of interest was manually defined encompassing the ventricular septum or liver. The signal decay of each pixel within the region of interest was fit to a monoexponential decay with a constant offset (S = S0e−TE·R2* + C) and the mean value was used as the R2* for that region. To make the liver R2* values more clinically meaningful, they were converted to iron concentrations (mg/g liver dry weight) according to the biopsy-based calibration equation reported by Wood et al. ([Fe] = 0.0254·R2* + 0.202) [3]. A similar calibration for myocardial R2* values is not firmly established. Measurements of ventricular ejection fraction (EF) were made using standard cardiac MRI techniques.
Relationships between variables were calculated with Spearman’s correlation coefficient, Fisher’s exact test, and the two-sample t-test or Wilcoxon test. Data were analyzed using SAS software (SAS, Cary, NC). P-values are two-sided and considered significant when P ≤ 0.05.
Acknowledgments
Contract grant sponsors: Higgins Family Noninvasive Cardiac Imaging Research Fund and the National Institutes of Health: K12HL08716401 and K24 HL004184-06A2.
Footnotes
Conflict of Interest: Nothing to report.
References
- 1.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- 2.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 3.Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westwood MA, Sheppard MN, Awogbade M, et al. Myocardial biopsy and T2* magnetic resonance in heart failure due to thalassaemia. Br J Haematol. 2005;128:2. doi: 10.1111/j.1365-2141.2004.05234.x. [DOI] [PubMed] [Google Scholar]
- 5.Wood JC, Otto-Duessel M, Aguilar M, et al. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation. 2005;112:535–543. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghugre NR, Enriquez CM, Gonzalez I, et al. MRI detects myocardial iron in the human heart. Magn Reson Med. 2006;56:681–686. doi: 10.1002/mrm.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavrogeni SI, Markussis V, Kaklamanis L, et al. A comparison of magnetic resonance imaging and cardiac biopsy in the evaluation of heart iron overload in patients with β-thalassemia major. Eur J Haematol. 2005;75:241–247. doi: 10.1111/j.1600-0609.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 8.Wood JC, Tyszka JM, Carson S, et al. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: A prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 10.Westwood MA, Shah F, Anderson LJ, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn Reson Imaging. 2007;26:564–568. doi: 10.1002/jmri.21018. [DOI] [PubMed] [Google Scholar]
- 11.Vichinsky E, Butensky E, Fung E, et al. Comparison of organ dysfunction in transfused patients with SCD or β-thalassemia. Am J Hematol. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- 12.Westwood MA, Firmin DN, Gildo M, et al. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging. 2005;21:531–538. doi: 10.1007/s10554-005-0651-2. [DOI] [PubMed] [Google Scholar]
- 13.Westwood M, Anderson LJ, Firmin DN, et al. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18:33–39. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 14.Westwood MA, Anderson LJ, Firmin DN, et al. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging. 2003;18:616–620. doi: 10.1002/jmri.10396. [DOI] [PubMed] [Google Scholar]