Abstract
We describe monozygotic female twins discordant for hemophilia A, born to a carrier mother and normal father. Affected twin A presented at age 1 year with excessive bruising and factor VIII procoagulant activity (FVIII:C) of less than 1% of normal. Twin B is an asymptomatic carrier with FVIII:C level of 42%. Peripheral blood DNA was tested for X-chromosome inactivation (methylation) patterns of the X-linked human androgen receptor gene, comparing the twins’ patterns to parental. Twin A showed nonrandom inactivation skewed toward the paternal X, whereas twin B showed random X-inactivation. This is the first reported case of discordance for hemophilia A between female monozygotic twins.
Introduction
Hemophilia A (factor VIII deficiency) results from mutations in the factor VIII gene, located on distal chromosome Xq28. Affected males with severe hemophilia A have recurrent soft tissue and joint bleeding. Heterozygous female carriers of factor VIII deficiency are usually asymptomatic with normal or near-normal FVIII:C levels, although the normal, stochastic process of X-inactivation leads to a range of factor VIII gene expression in heterozygotes. Females with severe factor VIII deficiency have been reported. The mechanisms for X-linked disease in females include monosomy X (45 X, Turner syndrome) [1], homozygous factor VIII mutations (e.g., consanguinity) [2], compound heterozygous factor VIII mutations (e.g., from an affected father and carrier mother, or from de novo mutation(s)) [3–5], and skewed X-inactivation in a heterozygous female carrier [6–9].
Extreme discordance for expression of disease in monozygotic (MZ) female twin pairs in several other X-linked diseases has been reported previously [10–14]. Here, we describe a pair of MZ twin girls carrying the same novel factor VIII gene mutation, who are discordant for severe hemophilia A. Valleix et al. reported a pair of female twins with concordant skewing of X-inactivation toward the paternally derived (normal factor VIII gene) chromosome, so that both twins exhibited the clinical manifestations of hemophilia [8]. In our study, severe hemophilia A in the affected twin is the result of complete (nonrandom) inactivation of the paternally derived, normal X-chromosome, whereas the unaffected twin has normal random X-inactivation.
Case Report
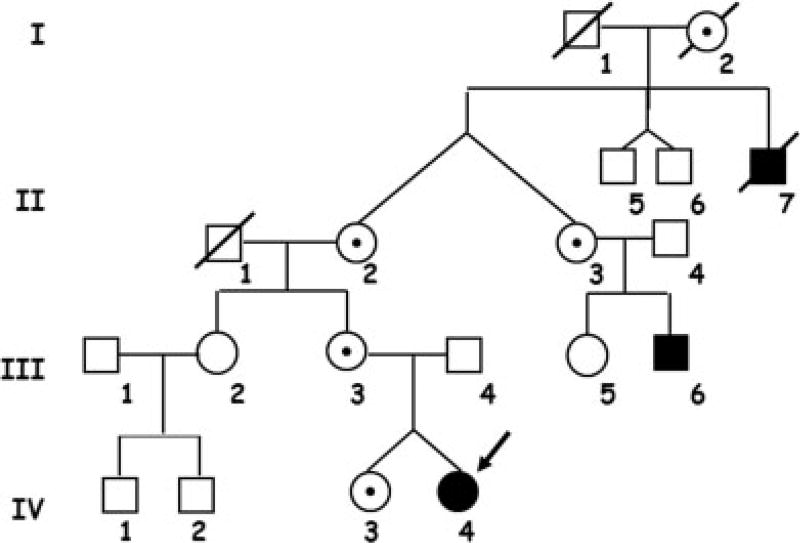
The pedigree of the family is shown in Fig. 1. The proband (Twin A) and her sister (Twin B) were born by vaginal delivery to nonconsanguineous, healthy parents after an uneventful pregnancy at 37 weeks gestation. Placentation status was not determined. Twin A developed excessive bruising and prolonged bleeding from trivial insults as an infant, whereas twin B had no such history. Twin A had FVIII:C level of less than 1% of pooled plasma values (normal FVIII:C 50–150%), with no evidence of an inhibitor, whereas her sister’s FVIII:C was 42%. The peripheral blood karyotype was 46 XX. Family history was significant for severe hemophilia A in a maternal cousin and great uncle. Von Willebrand antigen and ristocetin cofactor activity levels were normal in both sisters. Twin A was diagnosed with severe hemophilia A, whereas twin B had no bleeding manifestations and FVIII:C levels consistent with heterozygous (carrier) status.
Figure 1.
Pedigree. Severe factor VIII deficiency was known to be present in the proband (arrow), IV-4, the maternal great uncle II-7, and cousin III-6. Intermediary females, I-2 and II-3, are therefore obligate carriers.
Results
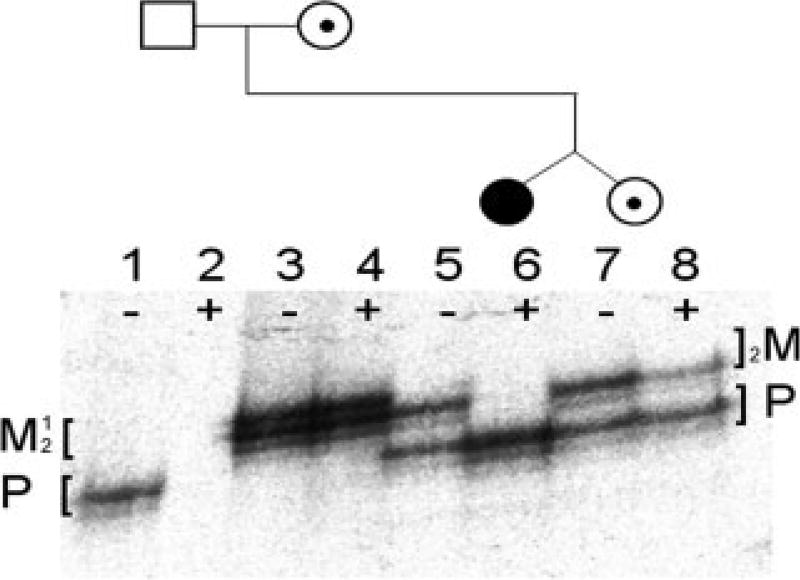
Monozygosity of the twins was established with 99% probability by the testing of microsatellite markers. Karyotype analysis revealed both twins to be normal, 46 XX, eliminating the diagnosis of Turner Syndrome or other large chromosomal abnormality. Direct mutational analysis of the factor VIII gene revealed a single base substitution C5027T in exon 14 of the coding sequence. This mutation, previously unreported in the hemophilia mutation database, leads to a nonsense mutation, Q1659X, producing a truncated protein molecule. Linkage analysis showed that this factor VIII haplotype in the twins, their mother, and maternal grandmother was identical. The results of X-chromosome methylation analysis (Fig. 2) demonstrate complete nonrandom X-inactivation of the normal (paternally derived) X-chromosome in twin A. Because her maternal (unmethylated), active X-chromosome carries the familial factor VIII mutation, she has no normal factor VIII protein expressed, and consequently has severe hemophilia. In contrast, twin B has a normal, expected pattern of methylation and has random X-inactivation and her FVIII:C level is consistent with carrier status.
Figure 2.
X-inactivation patterns for a pair of identical twin females discordant for hemophilia A and their parents using HUMARA assay. Amplification was performed before (−) and after (+) HhaI digestion. Mother and twins are heterozygous for CAG repeats and both alleles are present before digestion represented by two major PCR products on the gel (maternal alleles, lane 3, M1 and M2; twin A and B alleles, lanes 5 and 7, M2 and P). Mother and twin B show random X-inactivation with both alleles active and therefore equally amplified after digestion (lane 4, M1 and M2, and lane 8, M2 and P), whereas twin A shows nonrandom inactivation with only the lower, paternally inherited allele represented (lane 6, P only). Father’s single X-chromosome (hemizygous) is always active (lane 1, P) and disappears after digestion (lane 2). Fainter stutter bands are products of slippage by DNA polymerase. The selective amplification of one allele in the affected twin shows that it is always methylated (i.e., inactive) and resistant to HhaI digestion.
Discussion
Hemophilia in genetic females is rare, but can occur by Turner syndrome, by de novo mutation in one parent if the other is affected, by de novo mutations in both parents or in offspring of a hemophilic father and carrier mother. Hemophilia A in twin sisters has previously been reported [8]. In that family, both twins demonstrated relatively skewed inactivation of the paternal X-chromosome which carried a normal factor VIII gene. To our knowledge, the family in our report represents the most discrepant FVIII:C levels demonstrated in female identical twins heterozygous for factor VIII deficiency, as well as complete inactivation of the unaffected X in one female twin, and random inactivation and carrier FVIII:C levels in the other.
The phenomenon of unequal lyonization in one member of a pair of MZ twins has been observed for several X-linked diseases, including fragile X syndrome [10], Duchenne muscular dystrophy [11], red-green color blindness [12], Lesch-Nyhan disease [13], Hunter syndrome [14], and others. Monochorionic-MZ twinning occurs just after X-inactivation, and unequal splitting of the inner cell mass may result in only one affected twin with absolute inactivation of one X-chromosome [15,16], because the fetal tissues are derived from one or very few cells, while the second twin has typical (random) inactivation (fetus derived from more of the initial inner cell mass), as observed in our patient and her sister. The presence of X-linked disease in only one female twin with skewed X-inactivation supports the hypothesis that X-inactivation occurs in embryonic cells at the early blastocyst stage, before the twinning event. In the study by Valleix et al., the female twins were monochorionic and diamniotic and displayed concordant nonrandom X-inactivation [8]. Although the placentation of our patients was not determined or not reported, it is likely that they were monochorionic and monoamniotic, the more common situation for identical twins whose twinning event occurs just after the blastocyst stage.
We call particular attention to the unique clinical status of twin A based on what we know of her biology. First, she carries one completely normal factor VIII gene. It is possible, though not proven, that this normal gene would render her less likely to develop inhibitory antibodies against exogenous normal factor VIII, because even subdetectable amounts of antigen can be immunologically protective, and low level gene expression from inactive X-chromosomes can sometimes be detected with sensitive methods. Second, our studies confirm that twin B is genetically identical to her affected sister and therefore represents an ideal potential donor of cells or tissue (for example, endothelial progenitors or split liver transplant) that would be curative for Twin A’s severe hemophilia, with the highly favorable identical-twin donor status.
Methods
The study “Genetic Basis of Blood Diseases” was approved by the Committee on Clinical Investigation at Children’s Hospital, Boston, and consent was obtained from the participants or their parents. Monozygosity testing was performed by a commercial laboratory. DNA was isolated from cheek swabs from both twins and amplified via PCR. Microsatellite markers from at least seven of the nine following polymorphic loci (D351358, vWA, FGA, D8S1179, D21S11, D18S51, D5S818, D13S317, D7S820) were analyzed. Factor VIII mutational analysis was performed using genomic DNA purified from peripheral blood of both twins, their mother, and the maternal grandmother. Factor VIII inversion mutation analysis was performed on DNA obtained from twin A by intron 22 Southern blot. Factor VIII linkage analysis by polymorphic markers at the factor VIII locus using Southern blot analyses of informative factor VIII-linked markers (ST14/TaqI and DX13/BglII) and PCR amplification of intragenic markers (F8A and Bcl I) was performed. X-inactivation analysis was performed as previously described [17,18]. Genomic DNA samples from both twins and their parents were digested with the methylation-sensitive restriction enzyme HhaI. The highly polymorphic CAG-repeat sequences in the first exon of the human androgen receptor A (HUMARA) gene were amplified via radioactive PCR. This region contains adjacent HhaI and HpaI sites, at which methylation correlates with X-chromosome inactivation. The PCR products, both before and after enzyme digestions, were separated by gel electrophoresis.
Acknowledgments
The authors thank the patient and her family for participating in the study.
Contract grant sponsor: NHLBI; Contract grant number: K24 HL004184; Contract grant sponsor: John Butler Mulliken Foundation.
Footnotes
Conflict of Interest: Nothing to report.
References
- 1.Chuansumrit A, Sasanakul W, Goodeve A, et al. Inversion of intron 22 of the factor VIII gene in a girl with severe hemophilia A and Turner’s syndrome. Thromb Haemost. 1999;82:1379. [PubMed] [Google Scholar]
- 2.Al-Mondhiry HA. Inherited bleeding syndromes in Iraq. Thromb Haemost. 1977;37:549–555. [PubMed] [Google Scholar]
- 3.Cai XH, Wang XF, Dai J, et al. Female hemophilia A heterozygous for a de novo frameshift and a novel missense mutation of factor VIII. J Thromb Haemost. 2006;4:1969–1974. doi: 10.1111/j.1538-7836.2006.02105.x. [DOI] [PubMed] [Google Scholar]
- 4.Windsor S, Lyng A, Taylor SA, et al. Severe haemophilia A in a female resulting from two de novo factor VIII mutations. Br J Haematol. 1995;90:906–909. doi: 10.1111/j.1365-2141.1995.tb05213.x. [DOI] [PubMed] [Google Scholar]
- 5.Seeler RA, Vnencak-Jones CL, Bassett LM, et al. Severe haemophilia A in a female: A compound heterozygote with nonrandom X-inactivation. Haemophilia. 1999;5:445–449. doi: 10.1046/j.1365-2516.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 6.David D, Morais S, Ventura C, et al. Female haemophiliac homozygous for the factor VIII intron 22 inversion mutation, with transcriptional inactivation of one of the factor VIII alleles. Haemophilia. 2003;9:125–130. doi: 10.1046/j.1365-2516.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 7.Favier R, Lavergne J-M, Costa J-M, et al. Unbalanced X-chromosome inactivation with a novel FVIII gene mutation resulting in severe hemophilia A in a female. Blood. 2000;96:4373–4375. [PubMed] [Google Scholar]
- 8.Valleix S, Vinciguerra C, Lavergne JM, et al. Skewed X-chromosome inactivation in monochorionic diamniotic twin sisters results in severe and mild hemophilia A. Blood. 2002;100:3034–3036. doi: 10.1182/blood-2002-01-0277. [DOI] [PubMed] [Google Scholar]
- 9.Renault NK, Dyack S, Dobson MJ, et al. Heritable skewed X-chromosome inactivation leads to haemophilia A expression in heterozygous females. Eur J Hum Genet. 2007;15:628–637. doi: 10.1038/sj.ejhg.5201799. [DOI] [PubMed] [Google Scholar]
- 10.Willemsen R, Olmer R, De Diego Otero Y, et al. Twin sisters, monozygotic with the fragile X mutation, but with a different phenotype. J Med Genet. 2000;37:603–604. doi: 10.1136/jmg.37.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards CS, Watkins SC, Hoffman EP, et al. Skewed X inactivation in a female MZ twin results in Duchenne muscular dystrophy. Am J Hum Genet. 1990;46:672–681. [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen AL, Philip J, Raskind WH, et al. Different patterns of X inactivation in MZ twins discordant for red-green color-vision deficiency. Am J Hum Genet. 1992;51:291–298. [PMC free article] [PubMed] [Google Scholar]
- 13.De Gregorio L, Jinnah HA, Harris JC, et al. Lesch-Nyhan disease in a female with a clinically normal monozygotic twin. Mol Genet Metab. 2005;85:70–77. doi: 10.1016/j.ymgme.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Winchester B, Young E, Geddes S, et al. Female twin with Hunter disease due to nonrandom inactivation of the X-chromosome: A consequence of twinning. Am J Med Genet. 1992;44:834–838. doi: 10.1002/ajmg.1320440625. [DOI] [PubMed] [Google Scholar]
- 15.Chitnis S, Derom C, Vlietinck R, et al. X chromosome-inactivation patterns confirm the late timing of monoamniotic-MZ twinning. Am J Hum Genet. 1999;65:570–571. doi: 10.1086/302502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteiro J, Derom C, Vlietinck R, et al. Commitment to X inactivation precedes the twinning event in monochorionic MZ twins. Am J Hum Genet. 1998;63:339–346. doi: 10.1086/301978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boye E, Yu Y, Paranya G, et al. Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest. 2001;107:745–752. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen RC, Zoghbi HY, Moseley AB, et al. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]