Abstract
Background and aims
With the rising prevalence of obesity and metabolic syndrome, nonalcoholic fatty liver disease (NAFLD) has become the most common liver disorder in both developed and developing nations. Several studies on NAFLD have described waist circumference, a surrogate marker of visceral fat accumulation and waist height ratio as a better screening tool for NAFLD and metabolic syndrome than body mass index (BMI). We conducted this study to assess simple abdominal obesity indices as a predictor of NAFLD and determine the appropriate cut-off levels with reference to NAFLD.
Methods
1000 subjects with NAFLD detected ultrasonographically and 360 controls attending a Gastroenterology Clinic at Cuttack, Odisha were included in the study and subjected to detailed anthropometric measurements. The abdominal anthropometric cut offs were determined using ROC analysis. Statistical analysis was performed by using SPSS software version 16.
Results
All receiver operating curve (ROC) curves of waist circumference, waist-height ratio and BMI were significantly above the diagonal line. There were no significant differences in the area under the curve values among these abdominal obesity indices in each gender. The appropriate cut-off point of waist circumference in screening for NAFLD was 89 cm for men and 84 cm for women and the optimal cut-off point of waist-height ratio was 0.53 for men and 0.57 for women and the cut-off point of waist to hip ratio was 0.94 for men and 0.87 for women with very good sensitivity and specificity.
Conclusions
The simple anthropometric parameters, such as BMI, waist circumference, waist-hip ratio and waist-height ratio are useful for predicting NAFLD in Indian adults. The anthropometry cut offs would be very useful in setting target points of life style modification and weight reduction. Besides, our study also clearly demonstrated that a simple assessment of BMI is as efficacious as other anthropometry parameters in predicting NAFLD.
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; ROC, receptor operator curves
Keywords: Anthropometry, BMI, Nonalcoholic fatty liver disease, Waist circumference, Waist-height ratio
Non-alcoholic steatohepatitis (NASH) was first coined by Ludwig et al. at Mayo clinic in 1980.1 Ever since the inception of the term, there has been a lot of research in the field of fatty liver in the past two decades. nonalcoholic fatty liver disease (NAFLD) is slowly becoming a universal phenomenon. The prevalence of NAFLD is a staggering 25% in India,2 in contrast to the popular belief that it is disease of the West. The alarming rise in the prevalence can be attributed to adoption of sedentary lifestyle, dietary transition and the obesity epidemic.3 NAFLD is a cause for concern, since it is not a benign static disease, but can progress to steatohepatitis, fibrosis, cirrhosis and rarely hepatocellular carcinoma.4, 5
Central obesity rather than overall obesity has a strong pathogenetic link to NAFLD. The abdominal fat is considered the important determinant of metabolic risk, since the pro-inflammatory adipokines secreted by visceral fat are related to increased blood pressure, dyslipidemia and insulin resistance.6, 7 In obese patients, high levels of pro-inflammatory adipokines (leptins) and reduced levels of anti-inflammatory adipokines (adiponectin) are associated with metabolic disorders, such as, insulin resistance, cardiovascular disease, dyslipidemia and fatty liver.8, 9 Waist circumference, waist-to height ratio and waist-to-hip ratio are the surrogate markers of abdominal obesity. Several studies have shown that metabolic syndrome can be predicted using these anthropometric indices.10 Since NAFLD is believed to be the hepatic manifestation of metabolic syndrome, it is possible that NAFLD patients may be identified at the outpatient department using these simple anthropometric obesity markers. The other aspect to consider is that despite so much research on fatty liver, very limited therapeutic options are available. Weight loss and lifestyle modification still assume the cornerstone of management.11 Thus if the appropriate cut offs of anthropometric obesity markers to predict the presence of fatty liver can be determined in Asian Indians, therapeutic goals for obesity reduction can be set too. The metabolic and anthropometric profile of Indians is quite different from the Western population.12, 13 Many studies have determined the cut offs of abdominal obesity indices for metabolic syndrome, however there is limited research on the anthropometric profile of NAFLD patients in the Indian context.
Aims and Objectives of the Study
The aim of this study was to identify whether simple anthropometric indices like body mass index (BMI), waist circumference, waist-to-height ratio and waist-to-hip ratio can predict the presence of NAFLD in both genders and to identify which obesity marker is the best. The other objective was to determine appropriate cut off points of these anthropometric indices which can help in predicting NAFLD.
Methods
This was a prospective, single-centre study of cases and controls attending a Gastroenterology clinic at Cuttack, Orissa from October 2012 to October 2013. The study was approved by the Kalinga Gastroenterology Foundation (KGF) Ethical Committee. Only those subjects who provided informed consent for the study were included. One thousand consecutive NAFLD patients and three hundred sixty controls were analysed in this study.
Patients
These were consecutive NAFLD patients (n = 1000). The diagnosis of NAFLD was made on the basis of ultrasonography and histological confirmation whenever possible. Subjects were considered as cases if they presented with fatty liver defined according to the standard criteria accepted by the American Gastroenterology Association: An increase in hepatic echogenicity taking renal echogenicity as a reference, the presence of enhancement and lack of differentiation in periportal intensity and the vascular wall due to great hyper-echogenicity of the parenchyma.14
Controls
Consecutive patients who had a normal abdominal ultrasonography, which was performed for various causes, such as abdominal pain and dyspepsia, served as controls.
Exclusion Criteria
Patients and controls consuming alcohol > 20 g/day, having other known liver diseases (hepatitis viruses A to E, autoimmune disease and Wilson's disease) and those on medications known to induce fatty liver or insulin sensitization, such as estrogens, amiodarone, methotrexate, tamoxifen, glitazones and metformin were excluded.
The anthropometric assessment included measurements of weight, height, and waist circumference (WC) and hip circumference (HC). BMI was calculated as weight (kg)/stature (m2). The WC and HC were measured at the level midway between the lowest rib and the iliac crest and at the level of the great trochanter, respectively. The waist/height ratio and waist/hip ratio were calculated. The measurements of fasting glucose, triglycerides, cholesterol and high-density lipoprotein (HDL) cholesterol and liver function tests were performed by standard laboratory methods.
Statistical Analysis
Sample size of the study was calculated using results of a previous study from our centre.3 Taking power of study as 90% and P value = 0.001, we calculated the sample size to be 109 (81 cases and 27 controls) by using Real statistic add-in of excel software (2013 version). As we had a large database of NAFLD subjects (1182 subjects) we utilized the available data for maximum power of the study.
Normally distributed continuous variables were expressed as mean ± standard deviation (SD). Student's t-test for unpaired data were used to compare groups when variables are normally distributed. Chi square test was used to compare differences in categorical variables. All analyses were done by using an SPSS software version 16. P value of less than 0.05 was taken as significant. We calculated standard indices of validity including sensitivity, specificity, and area under the Receiver Operating Curve (ROC AUC) for BMI, waist, waist hip ratio and waist height ratio. From the ROC co-ordinates cut off levels for BMI, waist, waist hip ratio and waist height ratio were calculated with maximum sensitivity and specificity.
Results
A total of 1182 NAFLD patients and 409 controls were screened for the study. 182 of cases and 49 of controls were excluded as per the exclusion criteria (Figure 1) After exclusion, 1000 NAFLD patients and 360 controls were enrolled in the study. In the NAFLD and control groups, the male-to-female ratio was 3:1 and 2.27:1, respectively (Table 1). The NAFLD patients were slightly older with the mean age being 42.67 ± 11.27 years, while it was 39.13 ± 13.27 years in non-NAFLD patients. Table 1 shows the anthropometric indices such as BMI, waist circumference, waist/height ratio and waist hip ratio were dramatically greater in the NAFLD group as compared to the control group. Similarly as shown in Table 2, fasting blood glucose and serum triglycerides levels were significantly higher in the NAFLD group as compared to the nonNAFLD group.
Figure 1.
Study consort diagram.
Table 1.
Baseline Clinical and Anthropometric Parameters of NAFLD Patients and Controls.
| Parameters | NAFLD (n = 1000) | Control (n = 360) | P value |
|---|---|---|---|
| Age | 42.67 ± 11.27 | 39.13 ± 13.27 | <0.001 |
| Males | 750 | 250 | <0.001 |
| BMI | 26.92 ± 4.12 | 21.42 ± 3.43 | <0.001 |
| Waist circumference | 95.1 ± 8.7 | 79.1 ± 11.4 | <0.001 |
| Waist hip ratio | 0.97 ± 0.09 | 0.89 ± 0.08 | <0.001 |
| Waist height ratio | 0.59 ± 0.06 | 0.49 ± 0.07 | <0.001 |
Table 2.
Baseline Glycemic and Lipid Profile of NAFLD Patients and Controls.
| Parameters | NAFLD (n = 1000) | Control (n = 360) | P value |
|---|---|---|---|
| FPG | 104.1 ± 30.6 | 91.4 ± 10.8 | <0.001 |
| TG | 193.6 ± 97.7 | 131.9 ± 53.1 | <0.001 |
| Chol | 194.0 ± 44.6 | 180.0 ± 72.7 | 0.533 |
| HDL | 43.7 ± 12.4 | 44.9 ± 7.8 | 0.220 |
| LDL | 112.7 ± 35.0 | 102.1 ± 36.9 | 0.272 |
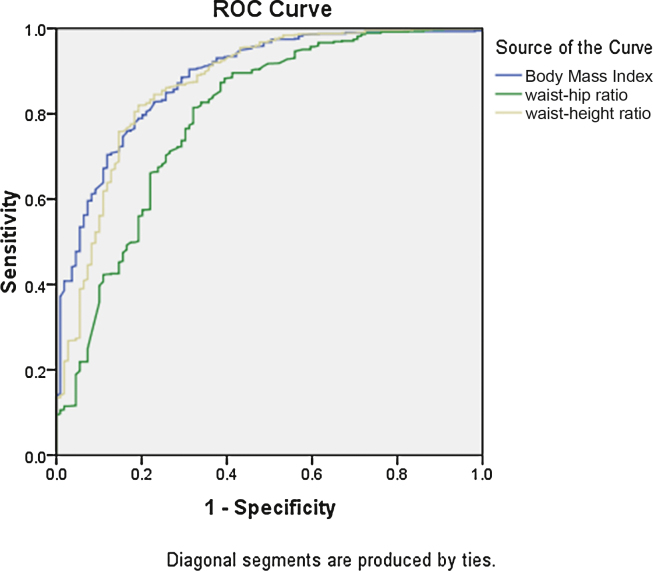
Figure 1, Figure 2 depict the receptor operator curves (ROC) curves of BMI, waist circumference, waist-to-height ratio and waist-to-hip ratio in males and females respectively, which were used to identify subjects with NAFLD. All four curves in men and women were significantly above the diagonal line (all P-value < 0.001). Table 3 presents the area under curve (AUC) values to predict the male subjects with NAFLD while Table 4 shows the AUC values to predict female subjects with NAFLD. The AUC values in males were 0.89 for BMI, 0.87 for waist circumference, 0.87 for the waist-to-height ratio, and 0.79 for waist to hip ratio. In women, the AUC values were 0.87 for BMI, 0.88 for waist circumference, 0.88 for the waist-to-height ratio, 0.73 for waist-to-hip ratio. There were no significant differences in the AUC values among these abdominal obesity indices when compared with each other in both genders, suggesting that waist circumference and waist-to-height ratio were not inferior to the other more complicated abdominal obesity indices for detecting NAFLD.
Figure 2.
ROC for male patients.
Table 3.
Area Under ROC With Sensitivity and Specificity of Cut-offs for Diagnosis of NAFLD by Anthropometry Indices for Males.
| Anthropometric parameters | AUROC | Cutoff values | Sensitivity | Specificity |
|---|---|---|---|---|
| BMI | 0.884 | 23.6 kg/m2 | 79.7% | 79.8% |
| Waist circumference | 0.874 | 88.5 cm | 77.9% | 81.9% |
| Waist/height ratio | 0.870 | 0.53 | 82% | 80% |
| Waist/hip ratio | 0.793 | 0.94 | 71.2% | 73.4% |
Table 4.
Area Under ROC With Sensitivity and Specificity of Cut-offs for Diagnosis of NAFLD by Anthropometry Indices for Females.
| Anthropometric parameters | AUROC | Cut-off values | Sensitivity | Specificity |
|---|---|---|---|---|
| BMI | 0.871 | 24.4 kg/m2 | 82% | 76.6% |
| Waist circumference | 0.878 | 84.5 cm | 84.8% | 80.2% |
| Waist/height ratio | 0.877 | 0.57 | 81.6% | 80.2% |
| Waist/hip ratio | 0.727 | 0.89 | 71.2% | 71.2% |
Table 3 lists the optimal cutoff points, sensitivity and specificity for the four abdominal obesity indices (BMI, waist circumference, waist-to-height ratio, waist-to-hip ratio) in detecting male individuals with NAFLD while Table 4 shows the optimal cutoff points, sensitivity and specificity for the abdominal indices in detecting female subjects with NAFLD. In men, the optimal cutoff point of waist circumference in screening for NAFLD was 89 cm, which had 78% sensitivity and 82% specificity. In women, the optimal cutoff value of waist circumference was 84.5 cm, with 85% sensitivity and 80% specificity. The appropriate cutoff values of waist-to-height ratio were 0.53 for men and 0.57 for women, which also had very high sensitivity and specificity values (82%, 80% and 82%, 80% respectively). The optimal cutoff points of BMI for detecting NAFLD were 23.6 kg/m2 for males (sensitivity 79.7% and specificity 79.8%) and 24.4 kg/m2 for females (sensitivity 82% and specificity 76.6%). The optimal cutoff points of waist-to-hip ratio for the prediction of NAFLD were 0.94 for men (sensitivity 71%, specificity 73%) and 0.89 for women (sensitivity 71% and specificity 71%). Thus waist-to-hip ratio has slightly lower sensitivity and specificity as compared to the other indices.
Discussion
The present study showed a higher proportion of males in the NAFLD group, which was similar to the earlier studies from India.3, 4 NAFLD patients had a mean age of 42.15 years, which matches the age of the cohort in the NAFLD series of Madan et al.12 and Duseja et al.13 The metabolic factors such as fasting blood sugar levels and serum triglyceride levels were significantly higher in the NAFLD group as compared to the control population. This result is expected as NAFLD is considered to be the hepatic manifestation of metabolic syndrome.15
The primary objective of the study was to see if anthropometric markers of central obesity can identify patients with NAFLD in both genders. The rationale is that visceral fat is central to the pathogenesis to metabolic syndrome and NAFLD. Adipose tissue is an endocrine organ and a potent source of hormones, peptides and adipokines involved in food intake regulation, glucose and lipid metabolism and inflammation.6, 7 Furthermore, visceral fat has been identified to be harmful, because it is associated with abnormal adipokines production and the activation of several pro-inflammatory signalling pathways. This low-grade inflammation is characterized by high levels of TNF-α, IL-6, leptin, C reactive protein, resistin, and low levels of adiponectin. In this way, abdominal obesity is central to the pathogenesis of metabolic syndrome, insulin resistance and steatohepatitis and is associated with increased cardiovascular mortality.16
There are various studies which show that abdominal obesity indices can predict the presence of metabolic syndrome in patients.17 As a result, BMI and waist circumference have been incorporated in ATP III criteria for diagnosis of metabolic syndrome.18 However, studies on use of anthropometric indices for diagnosing NAFLD are lacking. In this study, it was seen that simple anthropometric indices such as waist circumference, BMI and waist-to-height ratio are very useful in identifying patients with fatty liver in both genders with good sensitivity and specificity. Yoo et al. from Korea have shown that the simple anthropometric parameters, such as waist circumference and waist-to-height ratio, are as useful as CT scan for predicting NAFLD in Korean adults.19 Zheng et al. in their study on Chinese adults have shown that anthropometric indices, especially waist-to-hip ratio, are quite useful in predicting NAFLD.20
Fatty liver is a health concern that cannot be ignored due to two reasons. The first is that it is not a stable, benign disease but indeed a progressive disease which can lead to steato-hepatitis, fibrosis and even cirrhosis and hepato-cellular carcinoma.4, 5 The second reason for identifying and treating fatty liver early is that it has significant association with metabolic syndrome and adverse cardio-vascular events.21 Therefore, the identification of anthropometric predictors of fatty liver is crucial in clinical practice, since it can be used as an easy and fast method in identifying the disease and improve early detection and management.
There was an attempt to identify the best anthropometric parameter in predicting NAFLD. Waist circumference is a well-known surrogate marker of abdominal fat accumulation and is associated with cardiometabolic disease risk.22 Waist circumference, however, has a limitation; in that, it may underestimate the relative amount of abdominal fat in short subjects and may overestimate it in tall subjects.23 Some reports have indicated that the waist-to-height ratio corresponds better to metabolic risk than waist circumference does.24 Others studies have identified waist-to-hip ratio as the better marker of centripetal obesity and a significant risk factor for the development of metabolic syndrome and increased cardiovascular mortality.20, 25 However, in our study, BMI, waist circumference and waist-to-height ratio were not inferior to each other when used for NAFLD screening, with AUROC more than 0.8. Besides, the AUROC of waist-to-hip ratio was less when compared to other indices in both genders.
The other objective of the study was to determine appropriate cut off of the abdominal obesity index which can reasonably predict the presence of fatty liver in both genders. Although many studies have identified these cut off in the context of metabolic syndrome, there are very limited studies on NAFLD especially from the Asian subcontinent. The metabolic profile and abdominal obesity profile of Asians, especially Indians is quite different from the western population.12, 13 Even the cut offs of anthropometric indices for Asian patients are different from the Western population in the context of metabolic syndrome.26
The cut offs deduced from our study for waist circumference to identify NAFLD were 89 cm in males and 85 cm in females. These figures are similar to the cut offs of the Asian Pacific criteria to diagnose metabolic syndrome (90 cm for males and 80 cm for females) which were determined for cardiovascular disease, but were much lower than that mentioned in the ATP III guidelines18 which have taken a cut off of 102 cm in males and 88 cm in females. This suggests that the measurements for assessment of central obesity for Asians are quite different from that for Western population (Figure 3).
Figure 3.
ROC for female patients.
The appropriate cut off values of waist-to-height ratio were 0.53 for men and 0.57 for women which were slightly more than the values obtained by the study done on Korean adults.19 Thus ethnicity plays an important role while determining the appropriate cut off levels for screening of fatty liver and metabolic syndrome.
Further, our study clearly establishes that a single simple assessment of BMI, a time tested parameter, which is calculated routinely in all out patient departments is as efficacious as any other anthropometry parameters including waist circumference and waist-to-height ratio. The earlier hype over central obesity to the exclusion of BMI was probably the result of assessing obesity by wrongly using western cut off of BMI (25 kg/m2). This BMI cut off resulted in exclusion of individuals with BMI between 23 and 25 kg/m2 who were inappropriately considered to have normal weight by western standards. Hence, a simple lowering of BMI cut off to 23 kg/m2 included all the individuals (19.5%) in our study into the higher BMI category (Table 5).
Table 5.
Distribution and Frequency of NAFLD Patients According to BMI.
| BMI | Total NAFLD patients (n = 1000) | Percentage |
|---|---|---|
| <23 kg/m2 | 136 | 13.6% |
| 23–25 kg/m2 | 195 | 19.5% |
| >25 kg/m2 | 669 | 66.9% |
A limitation of the study is the use of ultrasonography to diagnose liver steatosis. Even if ultrasonography is reasonably accurate as compared with nuclear magnetic resonance spectroscopy and liver biopsy, it cannot identify fatty infiltration of the liver below a threshold of 30%.27 However, although liver biopsy is the gold standard to diagnose fatty liver, it is neither feasible nor ethically justified in a condition with low risk of progression and no definite treatment. Besides, ideally the controls should have been age and gender matched. We did attempt for an age and sex matched control population during the enrolment. However, although there was a very small difference between the groups with regard to age (42.67 ± 11.27 versus 39.13 ± 13.27) and sex (75% versus 69.4%), the P values turned out to be significant due to the inordinately large number of NAFLD subjects.
Conclusion
In conclusion, our study demonstrated that simple anthropometric indices such as BMI, waist circumference and waist-to-height ratio are useful markers for detecting NAFLD in both genders. The appropriate cut off points for waist circumference for the screening of NAFLD were 89 cm for men and 85 cm for women in Indians. The cut off values of waist-to-height ratio were 0.53 for men and 0.57 for women. These anthropometric cut offs will be very useful in screening for NAFLD and setting target points for life style modification and weight reduction interventions in these patients, and the general population too.
Further, our study also establishes that a simple assessment of BMI, which is calculated routinely in all out patient departments is as efficacious as other anthropometry parameters including waist circumference and waist height ratio.
Conflicts of Interest
The authors have none to declare.
References
- 1.Ludwig J., Viggiano T.R., Mcgill D.B., Oh B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Singh S.P., Nayak S., Swain M. Prevalence of nonalcoholic fatty liver disease in coastal eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–79. [PubMed] [Google Scholar]
- 3.Singh S.P., Singh A., Misra D. Risk factors associated with non-alcoholic fatty liver disease in Indians: a case–control study. J Clin Exp Hepatol. 2015;5:295–302. doi: 10.1016/j.jceh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 5.Bugianesi E., Leone N., Vanni E. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 6.Piya M.K., McTernan P.G., Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013;216:T1–T15. doi: 10.1530/JOE-12-0498. [DOI] [PubMed] [Google Scholar]
- 7.Schuster J., Vogel P., Eckhardt C., Morelo S.D. Applicability of the visceral adiposity index (VAI) in predicting components of metabolic syndrome in young adults. Nutr Hosp. 2014;30:806–812. doi: 10.3305/nh.2014.30.4.7644. [DOI] [PubMed] [Google Scholar]
- 8.Damaso A.R., de Piano A., Campos R.M. Multidisciplinary approach to the treatment of obese adolescents: effects on cardiovascular risk factors, inflammatory profile, and neuroendocrine regulation of energy balance. Int J Endocrinol. 2013;27:2013. doi: 10.1155/2013/541032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Piano A., de Mello M.T., Sanches P.de L. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur J Gastroenterol Hepatol. 2012;24:1313–1324. doi: 10.1097/MEG.0b013e32835793ac. [DOI] [PubMed] [Google Scholar]
- 10.Bosy-Westphal A., Geisler C., Onur S. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes. 2006;30:475–483. doi: 10.1038/sj.ijo.0803144. [DOI] [PubMed] [Google Scholar]
- 11.Tilg H., Moschen A. Weight loss: cornerstone in the treatment of non-alcoholic fatty liver disease. Minerva gastroenterologica e dietologica. 2010;56:159–167. [PubMed] [Google Scholar]
- 12.Madan K., Batra Y., Gupta S.D. Non-alcoholic fatty liver disease may not be a severe disease at presentation among Asian Indians. World J Gastroenterol. 2006;12:3400–3405. doi: 10.3748/wjg.v12.i21.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duseja A., Das A., Das R. The clinicopathological profile of Indian patients with nonalcoholic fatty liver disease (NAFLD) is different from that in the West. Dig Dis Sci. 2007;52:2368–2374. doi: 10.1007/s10620-006-9136-y. [DOI] [PubMed] [Google Scholar]
- 14.Gore R.M. Diffuse liver disease. In: Gore R.M., Levine M.S., Laufer I., editors. Textbook of Gastrointestinal Radiology. WB Saunders; Philadelphia: 1994. pp. 1968–2017. [Google Scholar]
- 15.Boppidi H., Daram S.R. Nonalcoholic fatty liver disease: hepatic manifestation of obesity and the metabolic syndrome. Postgrad Med. 2008;120:E01–E7. doi: 10.3810/pgm.2008.07.1800. [DOI] [PubMed] [Google Scholar]
- 16.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014 doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Després J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 18.Grundy S., Becker D., Clark L.T. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Circulation-Hagertown. 2002;106 3143-3421A. [PubMed] [Google Scholar]
- 19.Yoo H.J., Park M.S., Lee C.H. Cutoff points of abdominal obesity indices in screening for non-alcoholic fatty liver disease in Asians. Liver Int. 2010;30:1189–1196. doi: 10.1111/j.1478-3231.2010.02300.x. [DOI] [PubMed] [Google Scholar]
- 20.Zheng R.D., Chen Z.R., Chen J.N., Lu Y.H., Chen J. Role of body mass index, waist-to-height and waist-to-hip ratio in prediction of nonalcoholic fatty liver disease. Gastroenterol Res Pract. 2012 doi: 10.1155/2012/362147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Targher G., Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Klein S., Allison D.B., Heymsfield S.B. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity. 2007;15:1061–1067. doi: 10.1038/oby.2007.632. [DOI] [PubMed] [Google Scholar]
- 23.Schneider H.J., Friedrich N., Klotsche J. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010;95:1777–1785. doi: 10.1210/jc.2009-1584. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh S.D., Yoshinaga H., Muto T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. Int J Obes. 2003;27:610–616. doi: 10.1038/sj.ijo.0802259. [DOI] [PubMed] [Google Scholar]
- 25.Czernichow S., Kengne A.P., Huxley R.R. Comparison of waist-to-hip ratio and other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: a prospective cohort study from ADVANCE. Eur J Cardiovasc Prev Rehab. 2011;18:312–319. doi: 10.1097/HJR.0b013e32833c1aa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra A., Vikram N.K., Gupta R., Pandey R.M., Wasir J.S., Gupta V.P. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int J Obes. 2006;30:106–111. doi: 10.1038/sj.ijo.0803111. [DOI] [PubMed] [Google Scholar]
- 27.Saadeh S., Younossi Z.M., Remer E.M. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]