Abstract
While antibody mediated hyper-acute vasculitic rejection is rare in liver transplant recipients, acute and chronic rejection have clinical significance. The liver allograft behaves differently to other solid organ transplants as acute rejection generally does not impair graft survival and chronic rejection (CR) is uncommon. The incidence of acute and chronic rejection has declined in current era due to improved immunosuppressive regimens. Acute rejection generally improves with steroid boluses and steroid resistant rejection is uncommon. CR may improve with escalation of immunosuppression or may result in irreversible loss of graft function leading to retransplantation or death. The current review discusses diagnosis and management of acute and chronic liver allograft rejection.
Abbreviations: ACR, acute cellular rejection; APCs, antigen presenting cells; CR, chronic rejection; DDLT, deceased donor liver transplantation; DAIH, de novo autoimmune hepatitis; LDLT, living donor liver transplantation; MHC, major histocompatibility complex
Keywords: liver transplantation, acute cellular rejection, chronic rejection, antibody mediated rejection, steroids
The incidence of acute and chronic rejection has declined with improvement of immunosuppression regimens in liver transplant recipients. Acute cellular rejection (ACR) occurs in 15–25% of liver transplant recipients on Tacrolimus based immunosuppression regimens and generally improves with steroids in majority.1, 2, 3, 4 ACR does not affect long term graft or patient survival in most of cases.1 While acute rejection usually responds well to treatment, chronic rejection (CR) represents a difficult situation and a significant proportion of patients do not respond to increased immunosuppression.5, 6, 7, 8 CR often leads to retransplantation or death.5, 7 The current review discusses management of acute and chronic rejection after liver transplantation.
Immunological Basis and Pathogenesis of Acute Cellular Rejection
The hyperacute antibody mediated (due to preformed antibodies in recipient against donor's major histocompatibility complex, MHC) rejection although described, is quite rare in liver transplantation9 and mainly acute and chronic rejection are of clinical significance. The ACR occurs due recipient T cells that recognize donor alloantigens.10 Transplantation of MHC incompatible tissues causes a T cell dependent cytopathic immune response to donor tissues. Donor MHC molecules are processed after internalized by donor and recipient (antigen presenting cells) APCs. MHC peptide fragments are presented to T cells after intracellular processing. APCs also provide another second signal which may be stimulatory to T cell or may cause anergy if inhibitory in nature, however this anergy may be broken down by viral infections (e.g. CMV). There are several pathways of allorecognition by T cells. The recipient T cells can recognize allogeneic MHC molecules on the surface of donor APCs (direct pathway). The recipient APCs process MHC peptides shed by donor cells and present these to recipient T cells (indirect pathway). The recipient APCs may acquire intact MHC molecules from direct contact with donor APCs and present these to T cells via T cell receptors (semi direct pathway).10, 11, 12 ACR manifests as sudden deterioration of allograft function and biopsy shows infiltration by T cells and other leukocytes with evidence of ductular injury and endothelitis.13
Pathogenesis of Chronic Rejection
In contrast to ACR, pathogenesis of CR is not well characterized. The pathogenesis of CR is multifactorial and includes vascular occlusion, antibodies and cell mediated pathways.5, 14 Pathophysiology of CR is not entirely clear but immune mechanisms are involved as changes of CR does not appear in isografts and sometimes it is extension and result of ACR. CR in solid organ transplantation is characterized by obliterative arteriopathy (caused by arterial inflammation), interstitial inflammation, damage and atrophy of parenchymal cells, interstitial fibrosis and disruption of lymphatics and organ associated lymphoid tissues. CR is characterized by ductopenia in liver allograft as arterial changes affect larger arteries and are difficult to be seen in smaller arteries present in liver biopsy specimen. In CR, various mechanisms lead to ductopenia of liver allograft which include ischemia by obliterative arteriopathy and immune destruction of bile ductular cells.13, 14, 15
Clinical Features and Diagnosis of Acute Cellular Rejection
ACR is generally suspected after elevation of hepatic enzymes (serum aminotransferases, alkaline phosphatase, gamma-glutamyl transpeptidase) and/or bilirubin. However, these liver enzymes or bilirubin abnormalities are not sensitive or specific enough to differentiate ACR from other causes of graft dysfunction.16, 17 A liver biopsy is needed for a definite diagnosis of ACR or CR. Several cytokines have also been studied to diagnose ACR after LT, however it is difficult to differentiate between infections and ACR by use of cytokines.18, 19, 20, 21, 22, 23 The differential diagnosis of ACR and CR include other causes of graft dysfunction like infections (viral, bacterial, fungal), ischemic reperfusion injury, vascular (arterial or venous), biliary strictures, recurrent or de novo diseases after liver transplantation as shown in Table 1. The ACR generally occurs between 5 and 30 days after liver transplantation, although it can occur later also.24 The incidence of ACR and CR has decreased after improvementsin immunosuppression regimens, and in particular after introduction of Tacrolimus. Incidence of ACR varies widely among studies and was reported up to as high as 80% (ACR) with earlier immunosuppression protocols. A systemic review of studies comparing various immunosuppressive regimens from January 2007 to September 2015 showed wide variability of reported ACR incidence in 34 RCTs, it ranged from 5% (Tacrolimus, Mycophenolate, anti IL2 and steroids) to 66% (Antithymocyte globulin + weaning Tacrolimus). However most of studies have shown a incidence of 10–30% in liver transplantation recipients.1, 3 Most episodes of ACR occur within one month post-transplantation.24 The diagnosis of ACR and CR is based on Banff schema.15, 24 ACR changes are divided in to three pathological findings; mixed (predominantly mononuclear activated lymphocytes, neutrophils, and eosinophils) portal inflammation, bile duct inflammation/damage and sub endothelial inflammation of portal veins or terminal hepatic venules.24 Each of these parameters is scored as 1 to 3 and sum is called rejection activity index (Table 2), thus a maximum score of 9 is possible. The various possible rejection grades are as follows: a score of 0–2 is no rejection, 3 borderline (consistent with), 4–5 is mild, 6–7 is moderate and 8–9 as severe ACR. However, higher rejection activity index does not translate into less response to steroids.25 Antibody mediated rejection (AMR) is different and generally occurs in setting of ABO incompatible liver transplantation. AMR behaves clinically like ACR and may overlap with ACR. The proposed features of AMR include donor-specific HLA antibodies in serum, microvascular endothelial cell injury in liver biopsy and linear C4d positivity in liver sinusoids, in the absence of other causes of graft injury.26, 27
Table 1.
Differential Diagnosis of Acute and Chronic Rejection.
| Category | Etiology | Time after liver transplantation |
|---|---|---|
| Surgical issues | Hepatic artery thrombosis Biliary issues |
More common in early period Any time |
| Infections | Cytomegalovirus, atypical viral infections | Higher chances in early months |
| Rejection | Acute cellular rejection Chronic rejection (CR) Antibody mediated Plasma cell rich rejection |
Any time after liver transplantation, majority of ACR occur early (initial 3 months) CR occur late (months to years) Evolving literature on antibody mediated rejection Plasma cell rich rejection occurs after months to years |
| Recurrence of primary disease | Hepatitis B, hepatitis C, non-alcoholic steatohepatitis, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis | Generally >1 year after liver transplantation, viral can manifest any time |
Table 2.
| Category | Criteria | Score |
|---|---|---|
| Portal inflammation | Mostly lymphocytic inflammation involving, but not noticeably expanding, a minority of the triads | 1 |
| Expansion of most of all of the triads, by a mixed infiltrate containing lymphocytes with occasional blasts, neutrophils and eosinophils. If eosinophils are conspicuous and accompanied by edema and microvascular endothelial cell hypertrophy is prominent, acute antibody mediated rejection should be considereda | 2 | |
| Marked expansion of most or all of the triads by a mixed infiltrate containing numerous blasts and eosinophils with inflammatory spillover into the periportal parenchyma | 3 | |
| Bile duct inflammation damage | A minority of the ducts are cuffed and infiltrated by inflammatory cells andshow only mild reactive changes such as increased nuclear: cytoplasmic ratio of the epithelial cells | 1 |
| Most or all of the ducts infiltrated by inflammatory cells. More than an occasional ductshows degenerative changes such as nuclear pleomorphism, disordered polarity and cytoplasmic vacuolization of the epithelium | 2 | |
| As above for 2, with most or all of the ducts showing degenerative changes or foacal luminal disruption | 3 | |
| Venous endothelial inflammation | Subendothelial lymphocytic infiltration involving some, but not a majority of the portal and/or hepatic venules | 1 |
| Subendothelial infiltration involving most or all of the portal and/or hepatic venules with or without confluent hepatocyte necrosis/dropout involving a minority of perivenular regions | 2 | |
| As above for 2, with moderate or severe perivenular inflammation that extends into the perivenular parenchyma and is associated with perivenular hepatocyte necrosis involving a majority of perivenular regions | 3 |
Added later in 2015 modification.
Clinical Features and Diagnosis of Chronic Rejection
Although CR is chronic by definition and occurs months to years after liver transplantation, no time limit is intended in definition and it may occur even after few months and may lead to graft failure in first year of liver transplantation.14, 15, 28 CR generally presents with progressive cholestatic graft dysfunction and may results graft loss.14 CR occurs in 3–17% liver transplant recipients. The incidence of CR is lower in Tacrolimus based studies as compared to earlier Cyclosporin based studies.14, 15 CR is characterized by obliterative arteriopathy and ductopenia, the diagnostic criteria of CR are given in Table 3.
Table 3.
Early Versus Late CR (from Ref. 15).
| Parameter | Early CR (at least 2 findings should be present) | Late CR (at least 2 findings should be present) |
|---|---|---|
| Small bile ducts (<60 μm) | Senescence-related changes involving a majority of ducts; bile duct loss | Degenerative changes in remaining bile ducts, loss in ≥50% of portal tracts |
| Terminal hepatic venules and zone 3 hepatocytes |
Perivenular mononuclear inflammation Lytic zone 3 necrosis and inflammation Mild perivenular fibrosis |
Focal obliteration Variable inflammation Moderate to severe (bridging) fibrosis |
| Portal tract hepatic arterioles | Occasional loss involving <25% of portal tracts | Loss involving >25% of portal tracts |
| Large perihilar hepatic artery branches | Intimal inflammation, focal foam cell deposition without lumenal compromise | Lumenal narrowing by subintimal foam cells Fibrointimal proliferation |
| Large perihilar bile ducts | Inflammation damage and focal foam cell deposition | Mural fibrosis |
| Other | So-called “transition” hepatitis with spotty necrosis of hepatocytes | Sinusoidal foam cell accumulation; marked cholestasis |
Risk Factors for Acute Cellular Rejection and Chronic Rejection
As ACR and CR are immune related, predisposing factors are largely common. These include autoimmune etiology of underlying liver disease before liver transplantation (primary biliary sclerosis, primary sclerosing cholangitis and autoimmune hepatitis), cytomegalovirus infection, low levels or noncompliance to immunosuppression, positive lymphocyte cross-match, higher lower recipient age, donor-recipient ethnic origin, male donor into female recipient, higher donor age, higher cold ischemia time and living versus deceased donor liver translantation.4, 14, 29, 39 A review of 18 studies (1437 transplant recipients) showed that ACR developed in 24–80% (mean 49%) of recipients among various studies.31 It is presumed that as donors in living donor liver transplantation are generally genetically related, ACR may have less incidence in living donor liver transplantation (LDLT) as compared to deceased donor liver transplantation (DDLT), however it is not an universal finding.3, 32, 33 Liu et al. showed 16/50 (32%) ACR in LDLT patients versus 36/49 (73%) ACR in deceased-donor recipients and this difference was attributed to sibling related donors as ACR rates were not different in non-sibling related living donors and deceased donors.32 Shaked et al. analyzed A2ALL data of 380 LDLT versus 213 DDLT and could not find less ACR in LDLT group.33 In addition to above mentioned factors, CR is more common in patients with higher number and severity of acute rejection episodes, retransplantation for CR, male donor into female recipient, higher donor age, higher cold ischemia time and genetically unrelated donors when compared to genetically related donors in LDLT.7, 13, 28, 34, 35
Late Onset Acute Cellular Rejection
While different studies have used different definitions late onset ACR generally occurs 3–6 months after transplantation.36, 37, 38, 39, 40 The late onset acute cellular rejection (LAR) generally occurs at the time of cessation of initial higher immunosuppression. In a systemic review of late ACR including 9 studies, the incidence of late ACR ranged from 7% to 40% however, only one study reported >25% incidence. Late acute rejection causes graft loss, decreased patient survival, CR and worse prognosis.40 Thurairajah et al. analyzed 970 adult liver transplants; LAR developed in 11% of recipients and it was significantly more common in seronegative hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis. The younger recipient age, primary biliary cirrhosis, and previous graft loss were independent predictors in logistic regression analysis. Low mean trough Tacrolimus levels preceded LAR. LAR was associated with poor survival and poor response to treatment, it also lead to development of ductopenic rejection and half of these patients with CR died.36 Umera et al. analyzed data of 1604 adult transplants from 1985 to 2003. A total of 19.0% developed LAR, which was more common in autoimmune etiology before liver transplant. The LAR was more common in females and younger recipients. Both patient and graft survival was lower in LAR group and PTLD was associated with LAR.37
Ramji et al. showed that LAR occurred in 23% of recipients and 5% were steroid-resistant. Patients with viral etiologies had significantly less LAR (hazard ratio, 0.52). The patients with LAR had higher incidence of CR.38 The LAR was more common with Cyclosporine as compared with Tacrolimus group and MMF decreases risk of LAR.41 One of the differential diagnosis of LAR is plasma cell rich rejection (de novo autoimmune hepatitis or DAIH). The recurrence of autoimmune hepatitis after liver transplantation is well known; autoimmune hepatitis like disease may in patients without autoimmune hepatitis before liver transplantation (called plasma cell rich rejection or DAIH). The diagnostic criteria of DAIH are described recently.15 The diagnostic criteria include 2 mandatory criteria and one desirable, but not absolutely required criteria. The mandatory criteria include presence of portal and/or perivenular plasma cell rich (>30%) infiltrates with periportal/interface and/or perivenular necroinflammatory activity and original disease other than autoimmune hepatitis. The desirable feature is presence of lymphocytic cholangitis.15 The patents with plasma cell rich rejection have significant RAI score due to perivenular and portal inflammation.15 Representative histopathological images of ACR, CR and plasma cell rich rejection are shown in Figure 1, Figure 2, Figure 3.
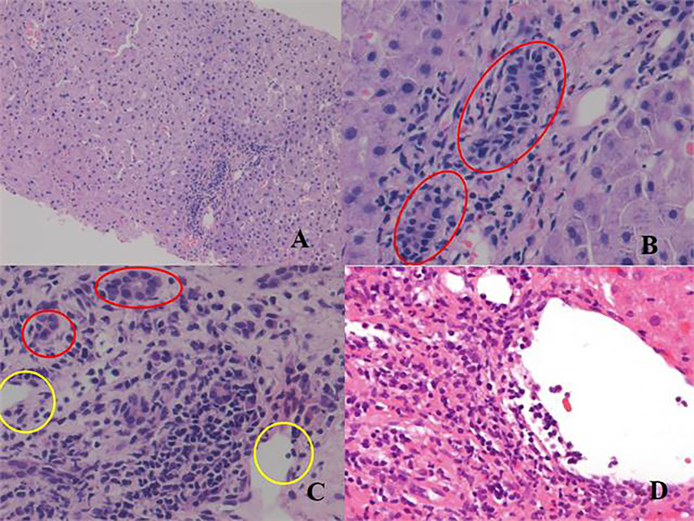
Figure 1.
Acute cellular rejection: (A) low power view showing portal inflammation with normal parenchyma, (B) high power view of ‘A’ showing eosiniphils (red colored cells) and ductulitis (circles), (C) ductulitis (red circles) and endothelitis (yellow circles), many plasma cells are visible between 2 yellow circles, (D) Endothelitis.
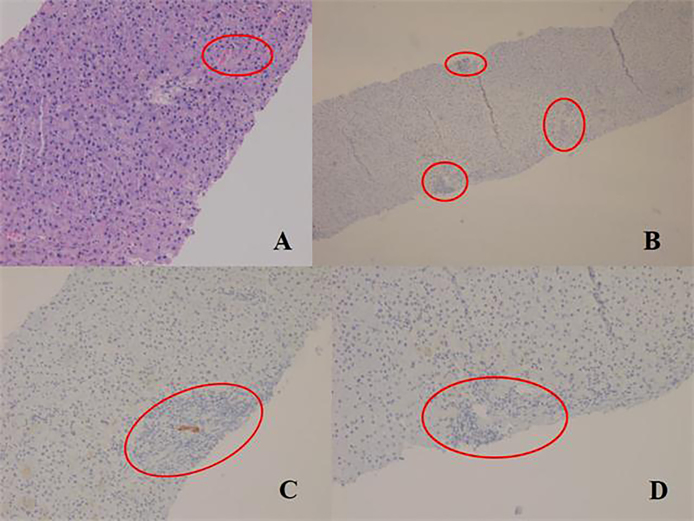
Figure 2.
Chronic rejection: (A) low power view shows atretic portal tracts (red circles) and an area of foamy degeneration, (B) immunohistochemistry (CK 19) image showing CK 19 staining in only 1 (upper) out of 3 portal tracts (marked by red circles), (C) focal CK19 staining (degenerating duct), (D) no CK19 staining is visible in this portal tract (within red circle).
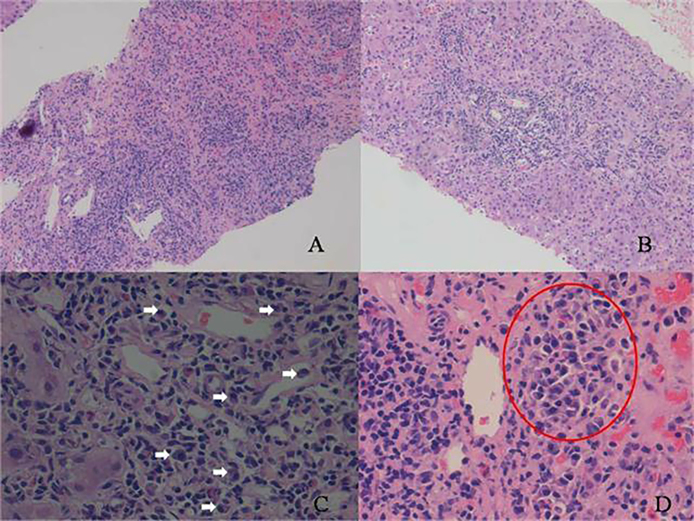
Figure 3.
Plasma cell rich rejection: (A and B) interface hepatitis, (C) many plasma cells (white arrows), (D) a cluster of plasma cells (within red circle).
Treatment of Acute Cellular Rejection
Most of acute rejection episodes improve with steroid boluses or with escalation of immunosuppression, and generally it does not have adverse impact on graft or patient survival in long term.42, 43 It is important to differentiate ACR from post transplantation hepatitis C recurrence as sometimes it is very difficult on histology.44 In HCV steroid boluses tend to cause severe histological recurrence, higher progression of fibrosis and lower survival in treated patients and it was difficult to treat in Peginterferon era due to poor response.45, 46 However, hepatitis C treatment is completely revolutionized with availability of direct acting antivirals in current era and HCV recurrence is less of a concern in the current era.47 Sometimes, ACR may occur in absence of biochemical derangement and improves on its own without escalation of immunosuppression.48 In a review of 15 studies including 1566 patients with protocol biopsies in early post-transplant period, 67% had histological evidence of ACR. Thirty two percent of these patients had no associated biochemical dysfunction, only 14% of these developed biochemical graft dysfunction requiring adjuvant immunosuppression in follow up. Steroid-resistant rejection and CR had aprevalence of 4% in untreated histological ACR and no biochemical graft dysfunction. The authors concluded that withholding adjuvant immunosuppression from patients with histological ACR and no biochemical graft dysfunction seems to be safe, as long as graft function is carefully monitored. The rationale for performing protocol biopsies in the absence of biochemical graft dysfunction is questionable.48 High dose corticosteroids are standard of care for ACR and doses vary at different centers. Usually 500–1000 mg pulse of methylprednisolone is given for 1–3 days followed by taper.42 Volpin et al49 compared three 1 gm pulses of methylprednisolone with a lower tapering dose of steroids in a controlled clinical trial. Patients with grade II or III ACR were randomized to methylprednisolone 1000 mg for first day followed by 200 mg on second day and then tapered by 40 mg every day for five days and 20 mg baseline dose at 7th day (group A, n = 18). The group B consisted of 20 patients who received methylprednisolone 1000 mg/day intravenously for three days followed by baseline dose of 20 mg on 4th day. The response was significantly more in group A (83% versus 50%, P = 0.03) with advantage of significantly less infections (55% versus 90%, P = 0.01).
Treatment of Steroid Resistant Acute Cellular Rejection
While majority of ACR episodes improve with steroid therapy or repeat steroid therapy (infrequently needed); steroid resistant ACR may happen in approximately 10% of ACRs.50, 51 Various treatments used for steroid resistant rejection include conversion to Tacrolimus, Sirolimus, Mycophenolate, anti thymocyte globulin, anti-CD3 monoclonal antibody (OKT3) and anti interleukin 2 agents.51, 52, 53, 54, 55, 56, 57, 58, 59
Chronic Rejection
CR is a major cause of graft dysfunction/loss in long term in various solid organ transplantrecipients.4 The outcomes of liver transplantation have significantly improved as compared to earlier days due to improvement in immunosuppression regimens. While CR was common in Cyclosporine era, it has become uncommon after Tacrolimus-based immunosuppression.7 CR occurs more commonly in other solid organ transplantation as compared to liver transplantation. Incidence of CR in liver transplants is reported as between 3% and 17% as compared to 25–60% in heart transplants, 20–40% in pancreatic and kidney transplantation, 30–70% in pancreatic transplantation and 28–45% in lung transplantation.4 The incidence of CR has declined in Tacrolimus era as compared to Cyclosporine era, and Tacrolimus has been shown to reverse CR in some of patients on Cyclosporine-based immunosuppression.5 A meta-analysis comparing Cyclosporine and Tacrolimus showed that Tacrolimus based therapy was associated with less CR and better long-term outcomes.60 The later studies with Tacrolimus based immunosuppression showed a 2–9% incidence of CR in liver transplantation as shown in Table 4.7, 8, 28, 34, 61, 62, 63 In the largest series published in the Tacrolimus era, CR was present in only 3.1% (32/1048).7
Table 4.
Incidence of Chronic Rejection in Various Studies and Response to Treatment.
| Author | Year | n | CR incidence | Comments, response |
|---|---|---|---|---|
| Freese60 | 1991 | 110 | 10 (9.1%), | All 10 patients were children, 3 improved |
| Blakolmer28 | 1999 | 916 | 23 (2.5%) | 10 recovered, patients with bile duct loss in more than 50% of the portal tracts (P < 0.01), severe (bridging) perivenular fibrosis (P = 0.05), and the presence of foam cell clusters (P = 0.06) were more likely to require retransplantation |
| Jain7 | 2001 | 1048 | 32 (3%) | Mean FU of 6 years, 40% responded |
| Yilmaz8 | 2006 | 132 | 3 (2%) | 2 children responded, one re-transplant |
| Ma61 | 2010 | 516 | 12 (2.3%) | Early CR was reversible |
| Ali34 | 2017 | 308 | 29 (9.4%) | All LDLT, CR 7% in genetically related donor versus 14.7% in the unrelated group, P = 0.03 |
| Kumar62 | 2016 | 1200 | 23 (1.9%) | LDLT population, 13 responded to increased immunosuppression (Everolimus was added in majority) |
Treatment of Chronic Rejection
Liver graft differs from other solid organ transplants in that CR may be reversible in some patients.5, 6, 7, 8, 28, 61, 62, 63 Nonresponder often need retransplantation and has poor outcome in absence of retransplantation.3, 7, 28, 63 Treatment of CR is either escalation of immunosuppression or retransplantation in absence of response. As pathogenesis of CR is complex and multifactorial, it is not always reversible with escalation of immunosuppression. Some studies have tried mTOR inhibitors (Sirolimus or Everolimus) as an additional agent to immunosuppressive regimen, these agents provide additional site of action in the immunity cascade63 and thus provide additional immunosuppression without increasing some of CNI toxicities. Addition of mTOR inhibitors has been shown to reverse CR in approximately half of patients.64, 65, 66 Nishita et al. used mTOR inhibitors in 16 patients and found response in 8 patients,65 in another study from Neff, 61% (13/21) responded to Sirolimus. The authors also did a biopsy after 6 months of Sirolimus treatment and found significant increase in bile duct artery ratio.66 Various studies have shown following factors as predictors of non-recovery among patients with CR; donor age, total serum bilirubin, more extensive bile duct loss, small arterial loss, presence of foam cell clusters and higher total bilirubin and aspartate aminotransferase values.14, 28 In our experience published as an abstract, a total of 23/1200 patients (20 male and 3 female) were diagnosed to have CR (1.9%) at a mean interval of 24.5 ± 19.5 months post liver transplantation. A history of biopsy proven ACR was present in 9 patients. Donor specific antibodies were not tested. The graft dysfunction improved in 11/17 (64.7%) patients after rescue with mTOR inhibitors (Sirolimus in 4, Everolimus in 13) and in 2/6 (33%) with escalation of baseline immunosuppression with CNI.62
Conclusion
ACR and CR present as graft dysfunction and a liver biopsy is needed for definitive diagnosis. While early ACR does not affect graft survival, late ACR is associated with inferior graft survival as compared to early ACR and may evolve into CR. CR responds to increased immunosuppression in approximately half of cases. Non-responding CR is associated with high mortality in absence of retransplantation.
Conflicts of Interest
The authors have none to declare.
Acknowledgements
Mr. Yogesh Saini (research coordinator).
References
- 1.Rodríguez-Perálvarez M., Rico-Juri J.M., Tsochatzis E., Burra P., De la Mata M., Lerut J. Biopsy-proven acute cellular rejection as an efficacy endpoint of randomized trials in liver transplantation: a systematic review and critical appraisal. Transpl Int. 2016;29:961–973. doi: 10.1111/tri.12737. [DOI] [PubMed] [Google Scholar]
- 2.Gruttadauria S., Vasta F., Mandalà L. Basiliximab in a triple-drug regimen with tacrolimus and steroids in liver transplantation. Transpl Proc. 2005;37:2611. doi: 10.1016/j.transproceed.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 3.Maluf D.G., Stravitz R.T., Cotterell A.H. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transpl. 2005;5:149. doi: 10.1111/j.1600-6143.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner R.H., Demetris A.J., Belle S.H. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28:638–645. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 5.Demetris A.J., Murase N., Lee R.G. Chronic rejection. A general overview of histopathology and pathophysiology with emphasis on liver, heart and intestinal allografts. Ann Transpl. 1997;2:27–44. [PMC free article] [PubMed] [Google Scholar]
- 6.Sher L.S., Cosenza C.A., Michel J. Efficacy of tacrolimus as rescue therapy for chronic rejection in orthotopic liver transplantation: a report of the US Multicenter Liver Study Group. Transplantation. 1997;64:258–263. doi: 10.1097/00007890-199707270-00014. [DOI] [PubMed] [Google Scholar]
- 7.Jain A., Demetris A.J., Kashyap R. Does tacrolimus offer virtual freedom from chronic rejection after primary liver transplantation? Risk and prognostic factors in 1,048 liver transplantations with a mean follow-up of 6 years. Liver Transpl. 2001;7:623–630. doi: 10.1053/jlts.2001.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz F., Aydin U., Nart D. The incidence and management of acute and chronic rejection after living donor liver transplantation. Transpl Proc. 2006;38:1435–1437. doi: 10.1016/j.transproceed.2006.02.108. [DOI] [PubMed] [Google Scholar]
- 9.Della-Guardia B., Almeida M.D., Meira-Filho S.P. Antibody-mediated rejection: hyperacute rejection reality in liver transplantation? A case report. Transpl Proc. 2008;40:870–871. doi: 10.1016/j.transproceed.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 10.Afzali B., Lechler R.I., Hernandez-Fuentes M.P. Allorecognition and the alloresponse: clinical implications. Tissue Antigens. 2007;69:545–556. doi: 10.1111/j.1399-0039.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Fueyo A., Strom T.B. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140:51–64. doi: 10.1053/j.gastro.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afzali B., Lombardi G., Lechler R.I. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transpl. 2008;13:438–444. doi: 10.1097/MOT.0b013e328309ee31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neil D.A., Hübscher S.G. Current views on rejection pathology in liver transplantation. Transpl Int. 2010;23:971–983. doi: 10.1111/j.1432-2277.2010.01143.x. [DOI] [PubMed] [Google Scholar]
- 14.Demetris A., Adams D., Bellamy C. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An international panel. Hepatology. 2000;31:792–799. doi: 10.1002/hep.510310337. [DOI] [PubMed] [Google Scholar]
- 15.Demetris A.J., Bellamy C., H€ubscher S.G. 2016 comprehensive update of the Banff Working Group on Liver Allograft Pathology: introduction of antibody-mediated rejection. Am J Transpl. 2016;16:2816–2835. doi: 10.1111/ajt.13909. [DOI] [PubMed] [Google Scholar]
- 16.Abraham S.C., Furth E.E. Receiver operating characteristic analysis of serum chemical parameters as tests of liver transplant rejection and correlation with histology. Transplantation. 1995;59:740–746. doi: 10.1097/00007890-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 17.Henley K.S., Lucey M.R., Appelman H.D. Biochemical and histopathological correlation in liver transplant: the first 180 days. Hepatology. 1992;16:688–693. doi: 10.1002/hep.1840160312. [DOI] [PubMed] [Google Scholar]
- 18.Slapak G.I., Saxena R., Portmann B. Graft and systemic disease in long-term survivors of liver transplantation. Hepatology. 1997;25:195–202. doi: 10.1002/hep.510250136. [DOI] [PubMed] [Google Scholar]
- 19.Prieto M., Berenguer M., Rayón J.M. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999;29:250–256. doi: 10.1002/hep.510290122. [DOI] [PubMed] [Google Scholar]
- 20.Akoglu B., Kriener S., Martens S. Interleukin-2 in CD8+ T cells correlates with Banff score during organ rejection in liver transplant recipients. Clin Exp Med. 2009;9:259–262. doi: 10.1007/s10238-009-0042-4. [DOI] [PubMed] [Google Scholar]
- 21.Conti F., Calmus Y., Rouer E. Increased expression of interleukin-4 during liver allograft rejection. J Hepatol. 1999;30:935–943. doi: 10.1016/s0168-8278(99)80150-0. [DOI] [PubMed] [Google Scholar]
- 22.Millán O., Rafael-Valdivia L., Torrademé E. Intracellular IFN-γ and IL-2 expression monitoring as surrogate markers of the risk of acute rejection and personal drug response in de novo liver transplant recipients. Cytokine. 2013;61:556–564. doi: 10.1016/j.cyto.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Bathgate A.J., Lee P., Hayes P.C., Simpson K.J. Pretransplantation tumor necrosis factor-alpha production predicts acute rejection after liver transplantation. Liver Transpl. 2000;6:721–727. doi: 10.1053/jlts.2000.18472. [DOI] [PubMed] [Google Scholar]
- 24.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 25.Höroldt B.S., Burattin M., Gunson B.K. Does the Banff rejection activity index predict outcome in patients with early acute rejection following liver transplantation? Liver Transpl. 2006;12:1144. doi: 10.1002/lt.20779. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski T., Rubinas T., Nickeleit V. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011;17:357. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary J.G., Kaneku H., Demetris A.J. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transpl. 2014;20:218. doi: 10.1002/lt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blakolmer K., Seaberg E.C., Batts K. Analysis of the reversibility of chronic liver allograft rejection implications for a staging schema. Am J Surg Pathol. 1999;23:1328–1339. doi: 10.1097/00000478-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Candinas D., Gunson B.K., Nightingale P., Hubscher S., McMaster P., Neuberger J.M. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet. 1995;346:1117–1121. doi: 10.1016/s0140-6736(95)91797-7. [DOI] [PubMed] [Google Scholar]
- 31.Fisher L.R., Henley K.S., Lucey M.R. Acute cellular rejection after liver transplantation: variability, morbidity, and mortality. Liver Transpl Surg. 1995;1:10–15. doi: 10.1002/lt.500010104. [DOI] [PubMed] [Google Scholar]
- 32.Liu L.U., Bodian C.A., Gondolesi G.E. Marked differences in acute cellular rejection rates between living-donor and deceased-donor liver transplant recipients. Transplantation. 2005;80:1072. doi: 10.1097/01.tp.0000176483.52769.5a. [DOI] [PubMed] [Google Scholar]
- 33.Shaked A., Ghobrial R.M., Merion R.M. A2ALL study group. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transpl. 2009;9:301–308. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali M.A., Elshobari M.M., Salah T. Impact of donor-recipient genetic relationship on outcome of living donor liver transplantation. Liver Transpl. 2017;23:43–49. doi: 10.1002/lt.24599. [DOI] [PubMed] [Google Scholar]
- 35.Choudhary N.S., Saigal S., Saraf N. Outcome of living donor liver transplantation between genetically related and unrelated donors: experience of 1206 adult living donor liver transplantations from a single centre. Transplantation. 2017;101(5S2):1–416. P-282. [Google Scholar]
- 36.Thurairajah P.H., Carbone M., Bridgestock H. Late acute liver allograft rejection: a study of its natural history and graft survival in the current era. Transplantation. 2013;95:955–959. doi: 10.1097/TP.0b013e3182845f6c. [DOI] [PubMed] [Google Scholar]
- 37.Uemura T., Ikegami T., Sanchez E.Q. Late acute rejection after liver transplantation impacts patient survival. Clin Transpl. 2008;22:316–323. doi: 10.1111/j.1399-0012.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramji A., Yoshida E.M., Bain V.G., Kneteman N.M., Scudamore C.H., Ma M.M. Late acute rejection after liver transplantation the Western Canada experience. Liver Transpl. 2002;8(10):945–951. doi: 10.1053/jlts.2002.34969. [DOI] [PubMed] [Google Scholar]
- 39.Akamatsu N., Sugawara Y., Tamura S., Keneko J., Matsui Y., Hasegawa K. Late-onset acute rejection after living donor liver transplantation. World J Gastroenterol. 2006;12:6674–6677. doi: 10.3748/wjg.v12.i41.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nacif L.S., Pinheiro R.S., Pécora R.A. Late acute rejection in liver transplant: a systematic review. Arq Bras Cir Dig. 2015;28:212–215. doi: 10.1590/S0102-67202015000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiesner R.H., Steffen B.J., David K.M., Chu A.H., Gordon R.D., Lake J.R. Mycophenolate mofetil use is associated with decreased risk of late acute rejection in adult liver transplant recipients. Am J Transpl. 2006;6:1609–1616. doi: 10.1111/j.1600-6143.2006.01382.x. [DOI] [PubMed] [Google Scholar]
- 42.Goddard S., Adams D.H. Methylprednisolone therapy for acute rejection: too much of a good thing? Liver Transpl. 2002;8:535. doi: 10.1053/jlts.2002.33486. [DOI] [PubMed] [Google Scholar]
- 43.Seiler C.A., Renner E.L., Czerniak A. Early acute cellular rejection: no effect on late hepatic allograft function in man. Transpl Int. 1999;12:195. doi: 10.1007/s001470050210. [DOI] [PubMed] [Google Scholar]
- 44.Burton J.R., Jr., Rosen H.R. Acute rejection in HCV-infected liver transplant recipients: the great conundrum. Liver Transpl. 2006;12:S38. doi: 10.1002/lt.20944. [DOI] [PubMed] [Google Scholar]
- 45.Berenguer M. Hot topic in hepatitis C virus research: the type of immunosuppression does not matter. Liver Transpl. 2011;17(suppl 3):S24–S28. doi: 10.1002/lt.22347. [DOI] [PubMed] [Google Scholar]
- 46.Saigal S., Choudhary N.S., Saraf N. Genotype 3 and higher low-density lipoprotein levels are predictors of good response to treatment of recurrent hepatitis C following living donor liver transplantation. Indian J Gastroenterol. 2015;34:305–309. doi: 10.1007/s12664-015-0578-z. [DOI] [PubMed] [Google Scholar]
- 47.Charlton M., Everson G.T., Flamm S.L. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Bartlett A.S., Ramadas R., Furness S., Gane E., McCall J.L. The natural history of acute histologic rejection without biochemical graft dysfunction in orthotopic liver transplantation: a systematic review. Liver Transpl. 2002;8:1147. doi: 10.1053/jlts.2002.36240. [DOI] [PubMed] [Google Scholar]
- 49.Volpin R., Angeli P., Galioto A. Comparison between two high-dose methylprednisolone schedules inthe treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl. 2002;8:527. doi: 10.1053/jlts.2002.33456. [DOI] [PubMed] [Google Scholar]
- 50.Adams D.H., Neuberger J.M. Patterns of graft rejection following liver transplantation. J Hepatol. 1990;10:113. doi: 10.1016/0168-8278(90)90081-2. [DOI] [PubMed] [Google Scholar]
- 51.Aydogan C., Sevmis S., Aktas S., Karakayali H., Demirhan B., Haberal M. Steroid-resistant acute rejections after liver transplant. Exp Clin Transpl. 2010;8:172–177. [PubMed] [Google Scholar]
- 52.Lee J.G., Lee J., Lee J.J. Efficacy of rabbit anti-thymocyte globulin for steroid-resistant acute rejection after liver transplantation. Medicine (Baltimore) 2016;95:e3711. doi: 10.1097/MD.0000000000003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandes M.L., Lee Y.M., Sutedja D. Treatment of steroid-resistant acute liver transplant rejection with basiliximab. Transpl Proc. 2005;37:2179. doi: 10.1016/j.transproceed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 54.Ramirez C.B., Doria C., di Francesco F., Iaria M., Kang Y., Marino I.R. Anti-IL2 induction in liver transplantation with 93% rejection free patient and graft survival at 18 months. J Surg Res. 2007;138:198. doi: 10.1016/j.jss.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 55.Koch M., Niemeyer G., Patel I. Pharmacokinetics, pharmacodynamics, and immunodynamics of daclizumab in a two-dose regimen in liver transplantation. Transplantation. 2002;73:1640. doi: 10.1097/00007890-200205270-00020. [DOI] [PubMed] [Google Scholar]
- 56.Jara P., Robledo M.J., Frauca E. Tacrolimus for steroid-resistant liver rejection in children. Transpl Int. 1998;11(suppl 1):S275–S277. doi: 10.1007/s001470050478. [DOI] [PubMed] [Google Scholar]
- 57.Demirağ A., Kalayci M., Ekci B., Gülçelik T., Gökçe O. Sirolimus for rescue of steroid and anti-thymocyte globulin-resistant recurrent acute rejection after liver transplantation: report of one case. Transpl Proc. 2009;41:435–436. doi: 10.1016/j.transproceed.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 58.Akamatsu N., Sugawara Y., Tamura S. Efficacy of mycofenolate mofetil for steroid resistant acute rejection after living donor liver transplantation. World J Gastroenterol. 2006;12:4870. doi: 10.3748/wjg.v12.i30.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu X.J., Chen Y.H., Ma Y., Zhu X.F., He X.S. Strategies in clinical diagnosis and treatment of steroid-resistant acute rejection after orthotopic liver transplantation. Zhonghua Gan Zang Bing Za Zhi. 2016;24:297–301. doi: 10.3760/cma.j.issn.1007-3418.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 60.Freese D.K., Snover D.C., Sharp H.L., Gross C.R., Savick S.K., Payne W.D. Chronic rejection after liver transplantation: a study of clinical, histopathological and immunological features. Hepatology. 1991;13:882–891. [PubMed] [Google Scholar]
- 61.Ma Y., He X.S., Hu R.D. Clinical and pathological analysis of chronic rejection following orthotopic liver transplantation. Zhonghua Wai Ke Za Zhi. 2010;15(48):288–292. [PubMed] [Google Scholar]
- 62.Kumar N., Saraf N., Rai R. Revisiting chronic rejection following LDLT in the tacrolimus era: a single centre experience abstract O-29, ILTS 2016. Transplantation. 2016;100(5S):S89. [Google Scholar]
- 63.McAlister V.C., Haddad E., Renouf E., Malthaner R.A., Kjaer M.S., Gluud L.L. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transpl. 2006;6:1578–1585. doi: 10.1111/j.1600-6143.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 64.Choudhary N.S., Saigal S., Shukla R., Kotecha H., Saraf N., Soin A.S. Current status of immunosuppression in liver transplantation. J Clin Exp Hepatol. 2013;3:150–158. doi: 10.1016/j.jceh.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishida S., Pinna A., Verzaro R. Sirolimus (rapamycin)-based rescue treatment following chronic rejection after liver transplantation. Transpl Proc. 2001;33:1495. doi: 10.1016/s0041-1345(00)02566-5. [DOI] [PubMed] [Google Scholar]
- 66.Neff G.W., Montalbano M., Slapak-Green G. A retrospective review of sirolimus (Rapamune) therapy in orthotopic liver transplant recipients diagnosed with chronic rejection. Liver Transpl. 2003;9:477–483. doi: 10.1053/jlts.2003.50119. [DOI] [PubMed] [Google Scholar]
Further reading
- 30.Christina S., Annunziato R.A., Schiano T.D. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20:1168–1177. doi: 10.1002/lt.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]