Abstract
Background
Although pleural fluid lactate dehydrogenase (LDH) and adenosine deaminase (ADA) levels are often used to distinguish between tuberculous pleural effusion (TPE) and parapneumonic pleural effusion (PPE), this can be challenging as the LDH level may vary from normal to severely increased in PPE and a significantly elevated ADA is frequently measured in both conditions. In this study, we evaluated use of the pleural fluid LDH/ADA ratio as a new parameter to discriminate TPE from PPE.
Methods
A retrospective study was conducted in patients with pathologically-confirmed TPE (n = 72) and PPE (n = 47) to compare pleural fluid LDH and ADA levels and LDH/ADA ratios between the 2 groups. A receiver operating characteristic (ROC) curve was constructed for identifying TPE.
Results
The median pleural fluid LDH and ADA levels and LDH/ADA ratios in the TPE and PPE groups were: 364.5 U/L vs 4037 U/L (P < .001), 33.5 U/L vs 43.3 U/L (P = .249), and 10.88 vs 66.91 (P < .0001), respectively. An area under the ROC curve of 0.9663 was obtained using the LDH/ADA ratio as the indicator for TPE identification, and the sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were, respectively, 93.62%, 93.06%, 13.48, and 0.068 at a cut-off level of 16.20.
Conclusions
The pleural fluid LDH/ADA ratio, which can be determined from routine biochemical analysis, is highly predictive of TPE at a cut-off level of 16.20. Measurement of this parameter may be helpful for clinicians in distinguishing between TPE and PPE.
Electronic supplementary material
The online version of this article (10.1186/s12890-017-0526-z) contains supplementary material, which is available to authorized users.
Keywords: Pleural fluid, Lactate dehydrogenase, Adenosine deaminase, Tuberculous pleural effusion, Parapneumonic pleural effusion
Background
Pleural effusion, which is a commonly observed clinical manifestation, is associated with more than 50 recognized diseases and disorders. In most parts of world, subtypes of exudative effusions often seen in clinical practice include tuberculous pleural effusion (TPE), parapneumonic effusion (PPE), and malignant pleural effusion (MPE) [1, 2]. It is crucially important to differentiate TPE and PPE, which are curable conditions, from MPE, as misdiagnosis and delayed treatment can result in significant mortality and morbidity [3, 4]. A recent study found that only 31% of patients with TPE have a positive microbiological test result [5]. In contrast, an increased pleural fluid adenosine deaminase (ADA) level is frequently seen in TPE, which usually helps to discriminate it from PPE [6, 7]. Data from a meta-analysis [6] revealed a sensitivity and specificity as high as 92% and 90%, respectively, for the use of ADA in the diagnosis of TPE. However, a similar or even higher ADA level has occasionally been reported in PPE, particularly in patients with empyema [8–10].
Management of pleural effusion is usually initiated after determining its transudative or exudative nature and comparing pleural fluid lactate dehydrogenase (LDH) and serum LDH levels according to Light’s criteria [11]. Clinical practice guidelines endorse the use of pleural fluid LDH and glucose to assist in the classification of patients with complicated parapneumonic effusions (CPPE) [12, 13]. However, an elevated pleural fluid LDH may present in TPE, PPE, and MPE, and the level is likely to range greatly from normal to extremely increased, which limits the use of LDH for identifying PPE in an individual patient due to the low sensitivity [9, 13, 14]. Therefore, it remains a challenge for clinicians to distinguish between patients with TPE and PPE from elevated pleural fluid ADA and LDH levels. As different mechanisms contribute to the elevation of ADA and LDH, it may be helpful to differentiate TPE from PPE by examining the pleural fluid LDH/ADA ratio, which has not been investigated in previous studies.
The aim of the present study was to evaluate the use of the pleural fluid LDH/ADA ratio as a new parameter to discriminate between TPE and PPE.
Methods
Study design and setting
A retrospective, non-randomized study of patients with confirmed diagnoses of TPE and PPE was conducted at a dedicated respiratory center (State Key Laboratory of Respiratory Disease and China Clinical Research Centre of Respiratory Disease, Guangzhou Institute of Respiratory Disease, Guangzhou).
Patients
The available data for a total of 72 patients with TPE and 47 with PPE who were treated at our respiratory center between January 2014 and December 2015 were reviewed retrospectively. The inclusion criteria for patients with TPE were: (1) chronic granulomatous inflammation in pleural tissue; (2) a clinical response to anti-tuberculosis treatment; and (3) no pleural effusion or only a small amount observed in chest ultrasound examinations over the last 12 months. The criteria for inclusion of patients with PPE were: (1) exudative effusions associated with bacterial pneumonia, lung abscesses, or bronchiectasis; (2) absence of Mycobacterium tuberculosis (MTB) in pleural fluid obtained from serial thoracentesis procedures; (3) pathological manifestations of inflammatory pleuritis, pleural fibrosis and plaques, or chronic empyema, without evidence of MTB; and (4) remission and recovery for at least 3 months at follow-up visits after antibiotic treatment. Uncomplicated parapneumonic effusion (UPPE) was defined when patients responded to antibiotic treatment alone; complicated parapneumonic effusion (CPPE) was defined when nonpurulent-appearing effusions required medical interventions such as drainage and other procedures; and empyema was defined when there was frank pus in the pleural space [15].
The demographic and clinical data of the patients were collected for analysis of the pleural fluid LDH and ADA levels and the LDH/ADA ratio.
Statistical analysis
Continuous variables were presented as the median and range, and qualitative variables were presented as the number and percentage. Intergroup differences were analysed statistically using SPSS® 17.0 (SPSS Inc., Chicago, IL, USA). Receiver operating characteristic (ROC) curves were used to identify the optimal cut-off points. The Wilcoxon signed-rank test or Fisher’s exact test were used for comparisons of the test results. Significance for statistical analyses was set at P < .05.
Results
Data for a total of 72 patients with TPE and 47 with PPE were reviewed (Additional file 1: Excel). The data for the TPE patients revealed 4 who had positive MTB cultures (3 in pleural fluid, 2 in sputum), 4 with pleural fluid TB-DNA, and 1 who was positive on acid-fast bacillus (AFB) testing. Among the PPE patients, bacterial pathogens were identified in 10 cases (3 in pleural fluid, 2 in sputum, and 1 in blood 1), including streptococci in 4 patients and staphylococci in 3. In addition, 23 UPPE, 15 CPPE, and 9 empyema cases were respectively identified (according to the definitions specified above).
Clinical and laboratory findings in the patients with TPE and PPE are shown in Table 1. The TPE and PPE groups had median ages of 51.5 years (range,17–82 years) and 59.0 years (range, 26–93 years), respectively (P < .05), and the male sex percentages in the 2 groups were 68.1% and 75%, respectively. Univariate analysis revealed that in comparison with the PPE group, the TPE group had an increased pleural fluid protein level (P < .05), but a significantly lower pleural fluid LDH level (P < .001). In contrast, no significant differences were observed between the 2 groups in blood albumin and blood LDH levels, or in pleural fluid ADA levels (Table 1). However, the pleural fluid LDH/ADA ratio in the TPE group was significantly lower than in the PPE group, and the sensitivity, specificity, positive likelihood ratio (PLR, and negative likelihood ratio (NLR) for identifying TPE were, respectively, 93.62%, 93.06%, 13.48, and 0.068 at a cut-off level of 16.20 (Fig. 1).
Table 1.
Comparison of clinical and laboratory findings between patients with TPE and PPE
| Parameter | TPE (n = 72) | PPE (n = 47) | P Value |
|---|---|---|---|
| Demographic data: | |||
| Age, years | 51.5 (17–82) | 59.0 (26–93) | .014 |
| Male sex | 49 (68.1%) | 33 (75%) | |
| Blood values: | |||
| Albumin, g/dl | 67.25 (55.3–81.6) | 66.1 (52.5–79.5) | .633 |
| LDH, U/L | 81.0 (111–321) | 180.3 (118–433) | .618 |
| Pleural fluid values: | |||
| Protein, g/dl | 50.0 (9.6–65.1) | 47.6 (19.1–65) | .045 |
| LDH, U/L | 364.5 (55–1154) | 4037 (103–48,730) | < .001 |
| ADA, U/L | 33.5 (4.5–75.9) | 43.3 (2.0–344.1) | .249 |
| LDH/ADA ratio | 10.88 (3.65–21.81) | 66.91 (9.04–411.4) | < .0001 |
Continuous variables are presented as the median (range) and qualitative variables as the number and percentage.
ADA, adenosine deaminase; LDH, lactate dehydrogenase; PPE, parapneumonic pleural effusion; TPE, tuberculous pleural effusion
Fig. 1.
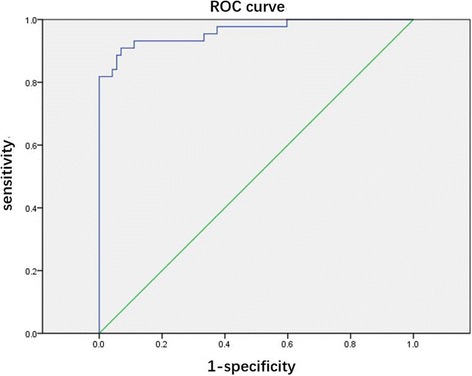
Receiver operating characteristic (ROC) curve for the LDH/ADA ratio in differentiating between TPE and PPE. An area-under-the-curve (AUC) value of 0.9663 was obtained using the pleural fluid LDH/ADA ratio as the indicator for TPE identification, and the sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were, respectively, 93.62%, 93.06%, 13.48, and 0.068 at a cut-off level of 16.20
Subgroup analysis showed no significant differences in pleural fluid LDH levels between patients with TPE and UPPE, or in pleural ADA levels between patients with TPE and CPPE. However, there were significant differences in pleural fluid LDH/ADA ratios between patients with TPE and each of the PPE subgroups (UPPE, CPPE, or empyema) [P < .0001 for each subgroup].
Discussion
Currently, a definitive diagnosis of TPE is made on the basis of the following criteria: (1) a positive AFB smear or positive cultures for MTB in pleural fluid and pleural tissue; (2) chronic granulomatous inflammation in pleural tissue; and (3) a clinical response to anti-tuberculosis treatment [3, 5]. However, in most studies, an ADA level ≥ 40 U/L in a lymphocytic exudate obtained via thoracentesis has been the most widely accepted indicator for a diagnosis of TPE [16]. Although PPE can be confirmed by a pleural exudate in patients with bacterial pneumonia, lung abscesses or bronchiectasis, it is still difficult to make a differential diagnosis due to the variety of PPE subcategories (from UPPE to empyema) [10], as well as the absence of disease-specificity in pleural effusion biomarkers [17, 18]. Making a definitive diagnosis of UPPE is always challenging as such patients may or may not present with symptoms and signs of pneumonia. Therefore, histological examinations via pleural biopsy need to be employed for a definitive diagnosis of pleural effusions. In the present study, ultrasound-guided cutting-needle biopsy was used in combination with a standard pleural biopsy to diagnose pleural effusions in all patients [19].
Use of the ADA level in pleural fluid has demonstrated high sensitivity and specificity for the differential diagnosis of TPE [6, 20]. However, conflicting data were obtained by Zaricet et al. [21] who reported a poor specificity as low as 70.4% for the ADA level in diagnosing TPE, despite an acceptable sensitivity of 89.2%. Furthermore, although a higher ADA level in pleural fluid is considered to be associated with a greater chance of TPE, most patients with UPPE and CPPE have a pleural ADA level of around 40 U/L or below [10, 22], and an extremely high ADA level should raise a suspicion of empyema or lymphoma [10]. Although a similar pleural fluid ADA level was evident in patients with TPE, PPE and CPPE in the present study, a significantly lower pleural fluid ADA level was seen in patients with UPPE in comparison with those with TPE (P < .0001), and a significantly higher pleural fluid ADA level was seen in patients with empyema (P < .0001).
Pleural fluid LDH is a frequently used biomarker to differentiate CPPE from UPPE, and a very high and isolated pleural fluid LDH level might be of specific diagnostic significance, especially for empyema [22]. We found that the pleural fluid LDH level was significantly lower in patients with TPE than in those with PPE (P < .001), and an even greater difference was evident when patients with TPE were compared with the CPPE and empyema subgroups (P < .0001). However, there was no significant significance in the pleural fluid LDH level between patients with TPE and the UPPE subgroup (P = .291). Consequently, the results of our study are consistent with previous research [23] suggesting that use of ADA and LDH levels in pleural fluid for discriminating between TPE and PPE in clinical practice can be challenging.
In view of the limitations of using pleural fluid ADA and LDH levels alone as biomarkers for differentiating between TPE and PPE, we combined the 2 parameters in an attempt to develop a predictor of TPE with acceptable specificity and sensitivity. Our findings revealed a significantly lower pleural fluid LDH/ADA ratio in the TPE group compared with the PPE group (P < .0001) and this difference was also evident in comparison with the 3 PPE subgroups (UPPE, CPPE, and empyema) [P < .0001]. A pleural fluid LDH/ADA ratio < 16.20 was found to provide a sensitivity of 93.62%, a specificity of 93.06%, a PLR of 13.48, and a NLR of 0.068 for TPE identification, yielding an area-under-the-curve (AUC) of 0.9663. Therefore, it was concluded that a pleural fluid LDH/ADA ratio lower than 16.20 is highly predictive of TPE, and that 16.20 can be used as the cut-off value to discriminate between TPE and PPE. This finding could be helpful in early clinical decision-making for the management of these patients, as it could lead to a better prognosis and avoidance of potential adverse consequences.
ADA, an enzyme secreted by mononuclear cells, lymphocytes, neutrophils and red blood cells (RBCs) [10, 24], is categorized as ADA-1 and ADA-2. However, only total ADA is routinely measured in our clinical practice. ADA-2, which is mainly expressed in and released from mononuclear cells and macrophages, correlates with intracellular infection such as TPE, and high levels of ADA-1 are always present in empyema [25, 26]. LDH, as a ubiquitous cytoplasmatic enzyme in virtually all major organ systems, usually increases in a nonspecific manner in response to cell damage or cell death [27]. Consequently, an elevated pleural fluid LDH level in exudative pleural effusions (such as TPE and PPE), is indicative of lung or pleural tissue damage and endothelial injury [27]. Most patients with TPE show chronic granulomatous inflammation in pleural tissue, and infiltration of mononuclear cells and macrophages. However, in those with PPE, pleural tissues always demonstrate acute inflammation and infiltration of neutrophil cells, with a large number of pus cells. In the present study, the pleural fluid LDH/ADA ratio was significantly lower in patients with TPE compared with those with PPE (P < .0001) which may be due to differences in the pathological nature of the 2 conditions, since TPE is a chronic granulomatous inflammation characterized by infiltration of mononuclear cells and marcrophages while PPE is an acute inflammatory condition depending on its pathological course. A similar extent of pleural tissue damage in patients with TPE and UPPE was reflected in the absence of a significant difference in pleural fluid LDH levels between these groups in our study (P = .291), but the level of mononuclear cells and macrophages was higher in UPPE than in TPE. Among the PPE subgroups, the severity of pleural cell damage was least in UPPE and greatest in empyema, leading to increased LDH levels and corresponding increases in the LDH/ADA ratio in the 3 subgroups (Table 2). These results indicate that the LDH/ADA ratio may be a useful indicator of pleural inflammatory responses.
Table 2.
Comparison of pleural fluid LDH, ADA, and LDH/ADA ratio values between patients with TPE, UPPE, CPPE, and empyema
| Parameter | TPE [A] (n = 72) | UPPE [B] (n = 23) | CPPE [C] (n = 15) | Empyema [D] (n = 9) | P Values | ||
|---|---|---|---|---|---|---|---|
| A vs B | A vs C | A vs D | |||||
| Pleural LDH (U/L) | 364.5 (55–1154) | 316.8 (103–852) | 2275 (1012–4446) | 16,479 (1919–48,730) | .291 | < .0001 | < .0001 |
| Pleural ADA (U/L) | 33.5 (4.5–75.9) | 10.55 (2.0–26.1) | 37.9 (14.3–68.6) | 137.9 (31.5–344.1) | < .0001 | .377 | < .0001 |
| Pleural LDH/ADA ratio | 10.88 (3.65–21.81) | 41.33 (9.04–162.4) | 67.3 (41.96–260.9) | 87.50 (15.11–411.4) | < .0001 | < .0001 | < .0001 |
Values are medians (range)
ADA, adenosine deaminase; CPPE, complicated parapneumonic effusions; LDH, lactate dehydrogenase; PPE, parapneumonic pleural effusion; TPE, tuberculous pleural effusion UPPE; uncomplicated parapneumonic effusion
The main limitation of our study was that only blood and pleural fluid levels of LDH and ADA were analyzed, which was a consequence of the retrospective nature of the study. However, the pleural fluid LDH/ADA ratio has been shown to be superior to LDH or ADA alone as a parameter for differentiation between TPE and PPE. Another limitation was that the patients with TPE and PPE included in our study were not representative of those with pleural effusions caused by other conditions such as connective tissue diseases [28] or MPE [29], who may also have a high pleural fluid ADA or LDH levels. In addition, the sample size was small, and prospective studies are required to verify the study’s results.
Conclusions
This study has provided evidence that the pleural fluid LDH/ADA ratio is a useful indicator to distinguish TPE from PPE. The LDH/ADA ratio may also reflect the nature of pleural inflammation and the response to inflammation. Consequently, it may be useful for the early clinical management of patients with pleural effusion.
Acknowledgements
The authors would like to thank Weizhan Luo for his assistance in statistical analysis of the results. Editorial assistance with the manuscript was provided by Content Ed Net, Shanghai Co. Ltd.
Funding
This work was supported by the Foundation of Guangzhou Health Development Planning Commision (Award Number:20131A011139).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- ADA
adenosine deaminase
- AFB
acid-fast bacillus
- CPPE
complicated parapneumonic effusions
- LDH
lactate dehydrogenase
- MPE
malignant pleural effusion
- MTB
Mycobacterium tuberculosis
- NLR
negative likelihood ratio
- PLR
positive likelihood ratio
- PPE
parapneumonic pleural effusion
- TB
tuberculosis
- TPE
tuberculous pleural effusion
- UPPE
uncomplicated parapneumonic effusion
Additional file
Excel: Pleural fluid results of LDHADA of the TPE and PPE patients. (XLS 26 kb)
Author contributions
YZ takes responsibility for the integrity of the data and the accuracy of the data analysis. JW, JH, and YZ were involved in the study concept and design. JL, XX, and P.S. were involved in the acquisition of data, and JW, JL, and JH were involved in the statistical analysis and interpretation of the data. JW, JL, and YZ drafted the manuscript. All authors critically reviewed/revised the manuscript and approved the final version.
Ethics approval and consent to participate
The study design and protocol were approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. A written informed consent was obtained from each participant.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12890-017-0526-z) contains supplementary material, which is available to authorized users.
Contributor Information
Jinlin Wang, Email: drjlwang@126.com.
Jun Liu, Email: 407180260@qq.com.
Xiaohong Xie, Email: gyxxh@126.com.
Panxiao Shen, Email: 2289219440@qq.com.
Jianxing He, Email: 3194598804@qq.com.
Yunxiang Zeng, Email: yunxiangzeng@126.com.
References
- 1.Light RW. Clinical practice Pleural effusion. N Engl J Med. 2002;346(25):1971–1977. doi: 10.1056/NEJMcp010731. [DOI] [PubMed] [Google Scholar]
- 2.Sahn SA, Heffner JE. Pleural fluid analysis. In: Light RW, Lee YC, editors. Textbook of Pleural Diseases. 2. London: Hodder Arnold; 2008. pp. 209–226. [Google Scholar]
- 3.Lin MT, Wang JY, Yu CJ, Lee LN, Yang PC. Mycobacterium tuberculosis and polymorphonuclear pleural effusion: incidence and clinical pointers. Respir Med. 2009;103(6):820–826. doi: 10.1016/j.rmed.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Kim CH, Lee SY, Lee YD, et al. Atypical pleural fluid profiles in tuberculous pleural effusion: sequential changes compared with parapneumonic and malignant pleural effusions. Intern Med. 2016;55(13):1713–1719. doi: 10.2169/internalmedicine.55.5803. [DOI] [PubMed] [Google Scholar]
- 5.Bielsa S, Palma R, Pardina M, Esquerda A, Light RW, Porcel JM. Comparison of polymorphonuclear- and lymphocyte-rich tuberculous pleural effusions. Int J Tuberc Lung Dis. 2013;17(1):85–89. doi: 10.5588/ijtld.12.0236. [DOI] [PubMed] [Google Scholar]
- 6.Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102(5):744–754. doi: 10.1016/j.rmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Krenke R, Korczynski P. Use of pleural fluid levels of adenosine deaminase and interferon gamma in the diagnosis of tuberculous pleuritis. Curr Opin Pulm Med. 2010;16(4):367–375. doi: 10.1097/MCP.0b013e32833a7154. [DOI] [PubMed] [Google Scholar]
- 8.van Keimpema AR, Slaats EH, Wagenaar JP. Adenosine deaminase activity, not diagnostic for tuberculous pleurisy. Eur J Respir Dis. 1987;71(1):15–18. [PubMed] [Google Scholar]
- 9.Porcel J, Vives M, Esquerda A, Ruiz A. Usefulness of the British Thoracic Society and the American College of Chest Physicians guidelines in predicting pleural drainage of non-purulent parapneumonic effusions. Respir Med. 2006;100(5):933–937. doi: 10.1016/j.rmed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Porcel JM, Esquerda A, Bielsa S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med. 2010;21(5):419–423. doi: 10.1016/j.ejim.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 12.Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest. 2000;118(4):1158–1171. doi: 10.1378/chest.118.4.1158. [DOI] [PubMed] [Google Scholar]
- 13.Davies CW, Gleeson FV, Davies RJ. Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the management of pleural infection. Thorax. 2003;(58 Suppl):2:ii18-ii28. [DOI] [PMC free article] [PubMed]
- 14.Lee J, Yoo SS, Lee SY, Cha SI, Park JY, Kim CH. Pleural fluid adenosine deaminase/serum C-reactive protein ratio for the differentiation of tuberculous and parapneumonic effusions with neutrophilic predominance and high adenosine deaminase levels. Infection. 2016; Aug 3 [Epub ahead of print]. [DOI] [PubMed]
- 15.Light RW. Pleural diseases. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 152–153. [Google Scholar]
- 16.Jeon D. Tuberculous pleurisy: an update. Tuberc Respir Dis. (Seoul) 2014;76(4):153–159. doi: 10.4046/trd.2014.76.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero-Candeira S, Hernández L, Romero-Brufao S, Orts D, Fernandez C, Martin C. Is it meaningful to use biochemical parameters to discriminate between transudative and exudative pleural effusions? Chest. 2002;122(5):1524–1529. doi: 10.1378/chest.122.5.1524. [DOI] [PubMed] [Google Scholar]
- 18.Porcel JM, Peña JM. Vicente de Vera C, Esquerda A, Vives M, Light RW. Bayesian analysis using continuous likelihood ratios for identifying pleural exudates. Respir Med. 2006;100(11):1960–1965. doi: 10.1016/j.rmed.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Zhou X, Xie X, Tang Q, Shen P, Zeng Y. Combined ultrasound-guided cutting-needle biopsy and standard pleural biopsy for diagnosis of malignant pleural effusions. BMC Pulm Med. 2016;16(1):155. doi: 10.1186/s12890-016-0318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greco S, Girardi E, Masciangelo R, Capoccetta GB, Saltini C. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis. 2003;7(8):777–786. [PubMed] [Google Scholar]
- 21.Zarić B, Kuruc V, Milovancev A, et al. Differential diagnosis of tuberculous and malignant pleural effusions: what is the role of adenosine deaminase? Lung. 2008;186(4):233–240. doi: 10.1007/s00408-008-9085-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Lee SY, Lim JK, et al. Radiologic and laboratory differences in patients with tuberculous and parapneumonic pleural effusions showing non-lymphocytic predominance and high adenosine deaminase levels. Infection. 2015;43(1):65–71. doi: 10.1007/s15010-014-0697-y. [DOI] [PubMed] [Google Scholar]
- 23.Davies HE , Davies RJ , Davies CW; BTS Pleural Disease Guideline Group. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2: ii41-ii53. [DOI] [PubMed]
- 24.Boonyagars L, Kiertiburanakul S. Use of adenosine deaminase for the diagnosis of tuberculosis: a review. J Infect Dis Antimicrob Agents. 2010;27(2):111–118. [Google Scholar]
- 25.Valdes L, San Jose E, Alvarez D, Valle JM. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: diagnostic role, and relevance to the origin of increased ADA in tuberculous pleurisy. Eur Respir J. 1996;9(4):747–751. doi: 10.1183/09031936.96.09040747. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Rodriguez E, Jiménez CD. The use of adenosine deaminase isoenzymes in the diagnosis of tuberculous pleuritis. Curr Opin Pulm Med. 2010;6(4):259–266. doi: 10.1097/00063198-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9(8):1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 28.Porcel JM. Tuberculous pleural effusion. Lung. 2009;187(5):263–270. doi: 10.1007/s00408-009-9165-3. [DOI] [PubMed] [Google Scholar]
- 29.Verma A, Phua CK, Sim WY, et al. Pleural LDH as a prognostic marker in adenocarcinoma lung with malignant pleural effusion. Medicine (Baltimore). 2016;95(26):e3996. doi: 10.1097/MD.0000000000003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


