Abstract
Background
Long-acting muscarinic antagonists (LAMAs) are recommended for the treatment of chronic obstructive pulmonary disease (COPD). Glycopyrrolate/eFlow® is an investigational drug–device combination of the LAMA glycopyrrolate administered by an eFlow® Closed System (eFlow® CS) nebulizer. The GOLDEN 2 (NCT01706536) and GOLDEN 6 (NCT02038829) Phase II, multicenter studies were conducted to inform dose selection for the GOLDEN Phase III clinical trials. Bronchodilator responses and safety assessments supported dose selection.
Methods
Subjects with moderate-to-severe COPD were randomized into 28-day parallel-group (GOLDEN 2) or 7-day crossover (GOLDEN 6) studies and received placebo, glycopyrrolate (3, 6.25, 12.5, 25, 50 or 100 μg twice daily [BID]) or aclidinium bromide 400 μg BID. The primary endpoint of both studies was change from baseline in trough forced expiratory volume in 1 s (FEV1). Safety assessments included the incidence of treatment-emergent adverse events (TEAEs), treatment-emergent serious adverse events, and discontinuation due to TEAE. Lung function data collected in both studies were pooled.
Results
The combined GOLDEN 2 (n = 282) and GOLDEN 6 (n = 96) studies included 378 subjects. On Days 7 and 28 there were dose-ordered, statistically significant and clinically important lung function improvements in glycopyrrolate treatment groups. Specifically, on Day 7, glycopyrrolate produced >0.100 L placebo-adjusted changes from baseline in trough FEV1 (12.5 μg BID: 0.122 L; 25 μg BID: 0.123 L; 50 μg BID: 0.137 L) and FEV1 AUC0–12 (12.5 μg BID: 0.145 L; 25 μg BID: 0.178 L; 50 μg BID: 0.180 L). The improvements in lung function for the glycopyrrolate 25 and 50 μg BID doses were comparable to those with aclidinium bromide 400 μg BID (FEV1: 0.149 L; FEV1 AUC0−12: 0.172 L). Acceptable safety profiles were observed across all groups in both studies.
Conclusions
The efficacy and safety findings supported selection of glycopyrrolate 25 and 50 μg BID doses for the Phase III GOLDEN studies and provided preliminary evidence for the use of nebulized glycopyrrolate as a maintenance therapy for COPD.
Electronic supplementary material
The online version of this article (10.1186/s12931-017-0681-z) contains supplementary material, which is available to authorized users.
Keywords: COPD, eFlow® CS, GOLDEN, Glycopyrrolate, LAMA, Nebulizer, Phase II
Background
In the United States (US), approximately 12.7 million adults have a diagnosis of chronic obstructive pulmonary disease (COPD), with evidence of impaired lung function in up to 24 million Americans [1]. COPD is the third leading cause of death and is associated with substantial medical and economic disease burdens for patients and healthcare systems [2].
COPD is a heterogeneous disease requiring a spectrum of treatment options to achieve therapeutic goals [3]. Treatment response may depend on the method of delivery, drug preference and tolerability. Use of a metered dose inhaler (MDI), dry powder inhaler (DPI) or a nebulizer is appropriate for self-administered COPD therapy. MDIs and DPIs are widely prescribed, yet up to 70% of COPD patients may not receive an optimal dose due to an inability to inhale rapidly and forcefully, hold their breath after dosing, and/or exhale into the device. Over time, these issues may be associated with suboptimal outcomes [4–6].
A survey of 205 US-based pulmonologists indicated that 63% believed handheld nebulizers may be more effective than MDIs or DPIs in severe COPD, and 70% stated that nebulizers are more effective in the management of acute exacerbations [7]. Given the prevalence of COPD, several million US patients may regularly use nebulizers for COPD; however, treatment compliance can be affected by the need for frequent dosing with short-acting bronchodilators, long delivery times (≥12 min) and limited portability of jet nebulizers. In addition, standard jet nebulizers have poor delivery efficiency that may result in suboptimal drug treatment [8, 9], so there is a need for a new generation of nebulizers that can optimize treatment compliance and deliver long-acting bronchodilators into the lung.
Long-acting muscarinic antagonists (LAMAs) play a central role in the pharmacologic management of COPD [3]. Currently, there is no nebulized LAMA approved for use in COPD. Glycopyrrolate/eFlow® is a drug–device combination of glycopyrrolate administered twice daily (BID) by an investigational, innovative, vibrating membrane nebulizer (eFlow® CS; PARI Pharma GmbH, Starnberg, Germany). The eFlow® CS device delivers an acceptable respirable fraction (72% fine particle fraction) of glycopyrrolate aerosol droplets (3.7 μm mass median aerodynamic diameter, 1.7 geometric standard deviation) into the lung within 3 min with tidal breathing [10], is silent, portable, does not require patient preparation of the drug product, and is amenable to caregiver assistance. The shorter delivery time and portability may address the treatment compliance issues that have been reported with non-portable nebulizers. Nebulized glycopyrrolate delivery occurs over multiple tidal breaths, rather than during a single breath attempt, and may provide a therapeutic alternative for patients who experience difficulty operating handheld devices, exacerbate, and/or are physiologically unable to generate sufficient inspiratory pressure for inhaler use [11–14].
The Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 2 and GOLDEN 6 studies were conducted to inform dose selection for the GOLDEN Phase III clinical trials by characterizing the bronchodilator dose–response relationship and safety profiles of nebulized glycopyrrolate doses administered BID in subjects with moderate-to-severe COPD. Lung function data are presented pooled across both studies and individually.
Methods
Study design and treatment
The GOLDEN 2 study was initiated in October 2012 and completed in April 2013. GOLDEN 6 was initiated in January 2014 and completed in May 2014. The study protocols were approved by an Institutional Review Board and were conducted in accordance with the approved protocols, the International Council for Harmonization Good Clinical Practice guidelines, and the Declaration of Helsinki. All subjects provided written informed consent prior to undergoing any study procedures.
In the GOLDEN 2 study, subjects were randomized to one of five treatment groups following a 1-week placebo run-in period, and stratified by inhaled corticosteroid use and participation (yes/no) in serial spirometry assessments. The subjects received placebo, glycopyrrolate 12.5, 25, 50, or 100 μg BID for 28 days in a double-blind manner (Fig. 1).
Fig. 1.
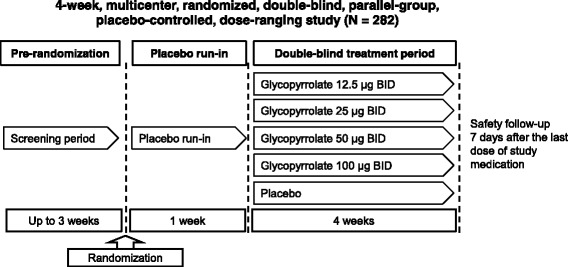
GOLDEN 2 study design. BID, twice daily
GOLDEN 6 used a complete crossover study design, with subjects randomized to a treatment sequence consisting of six 7-day treatment periods followed by a 5- to 7-day washout (Fig. 2). During each treatment period, subjects received placebo, glycopyrrolate 3, 6.25, 12.5, or 50 μg BID, or aclidinium bromide (Tudorza® Pressair®, AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA) 400 μg BID in the morning and evening. All glycopyrrolate and placebo treatments were administered double-blinded by the investigational eFlow® CS nebulizer; dosing of aclidinium bromide was open label.
Fig. 2.
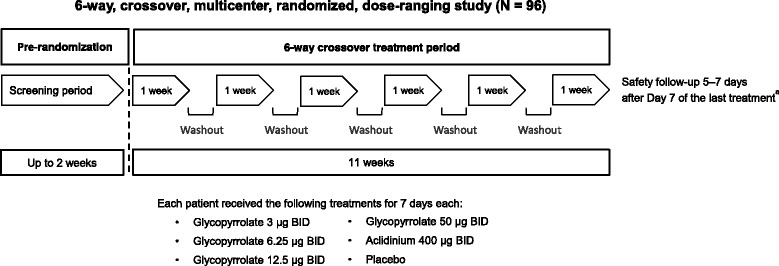
GOLDEN 6 study design. aSafety follow-up was conducted 5–7 days after the Early Termination visit for subjects who discontinued the study prior to completing all scheduled treatment periods. BID, twice daily
Patients
Eligible male and female subjects were 35 to 75 (GOLDEN 2) and 40 to 65 (GOLDEN 6) years old with a minimum 10 pack-year smoking history and a clinical diagnosis of moderate-to-severe COPD (GOLD 2011) [15]. Additional inclusion criteria were a forced expiratory volume in 1 s (FEV1) between 30% and 70% (GOLDEN 2) and 40% and 70% (GOLDEN 6) of the predicted normal, an FEV1/forced vital capacity ratio <0.70 and demonstrated reversibility (FEV1 ≥12% and 0.100 L) following post-bronchodilator (inhalation of ipratropium bromide) spirometry at screening. Subjects with current or history of unstable cardiac and/or respiratory disease (including asthma) or unstable comorbidities were excluded. Other exclusion criteria included systemic steroid therapy, respiratory infection, and a COPD exacerbation requiring hospitalization or need for increased treatments for COPD within 1.5 to 3 months of screening. Subjects using oxygen therapy for >10 h daily were also excluded.
Study objectives and endpoints
The primary objective of both studies was to confirm the efficacy and dose–response relationship of glycopyrrolate BID in subjects with moderate-to-severe COPD, and identify doses for the GOLDEN Phase III clinical trials. Safety and tolerability of glycopyrrolate/eFlow® were secondary objectives.
The GOLDEN 2 primary efficacy endpoint was change from baseline in morning trough FEV1 on Day 28. Secondary endpoints included standardized change from baseline in both FEV1 area under the curve from 0 to 12 h (AUC0–12) and peak FEV1 on Day 28. Other endpoints included change from baseline in FEV1 AUC0–12 and trough FEV1 on Day 7.
The primary and secondary efficacy endpoints for GOLDEN 6 were placebo-adjusted change from baseline in morning trough FEV1 and standardized change from baseline in FEV1 AUC0−12, both on Day 7.
Safety assessments included the incidence of treatment-emergent adverse events (TEAEs), serious TEAEs, discontinuations due to TEAEs, changes in clinical laboratory assessments, vital signs and electrocardiograms (ECG, including QT interval), and physical examination findings. Vital signs, clinical laboratory assessments, and ECG were collected at Days 1, 7 and 28 for GOLDEN 2, and at Days 1 and 7 for GOLDEN 6.
Statistical analyses
For GOLDEN 2, 45 subjects per treatment arm were required to provide approximately 80% power to detect a treatment difference of 0.12 L in the primary efficacy endpoint (mean change in trough FEV1) between each glycopyrrolate dose group and placebo (at a significance level of 0.05, assuming a standard deviation [SD] of 0.200 L and using a two-sided test). For GOLDEN 6, 66 subjects were required to provide approximately 90% power to detect a treatment difference of 0.10 L in the primary efficacy endpoint (mean change in trough FEV1) between each glycopyrrolate dose group and placebo (at a significance level of 0.0125, assuming an SD of 0.176 L and using a two-sided test). Additional subjects (total n = 78) were required in GOLDEN 6 for the trial to have sufficient power for the key secondary endpoint, FEV1 AUC0–12. Accounting for potential dropouts, the planned enrollment for GOLDEN 2 was 275 subjects and 96 subjects for GOLDEN 6.
All subjects who were randomized and received at least one dose of study drug were included in the statistical analyses of baseline characteristics, efficacy, and safety. Missing data were treated as missing at random. Sensitivity analyses were performed to assess the impact of missing data.
Each study was analyzed separately. Subsequently, the data were pooled to increase the sample size in the overlapping glycopyrrolate dose groups (12.5 and 50 μg BID) and fully characterize the dose–response profile. Pooling of these Phase II data was justified based on the overlap of doses, BID dose regimen, blinding of doses, nebulizer device, time points, similarity of study populations, and primary endpoint (trough FEV1).
Trough FEV1 was calculated as the mean of two FEV1 values obtained between 23 and 24 h after the morning dose of study drug on Day 7 (GOLDEN 6) or Day 28 (GOLDEN 2). Change in trough FEV1 was calculated as trough FEV1 minus baseline FEV1 (the mean of two FEV1 values obtained at 45 and 15 min prior to the morning dose on Day 1).
Least squares (LS) means and 95% confidence intervals (CIs) for the pooled study data were derived from an analysis of covariance (ANCOVA) model with change from baseline in trough FEV1 or FEV1 AUC0–12 as the response variable, a factor for treatment group, and with baseline FEV1 as a covariate.
Safety parameters were analyzed descriptively for the safety population. All adverse events (AEs) were classified using the Medical Dictionary for Regulatory Activities Version 15.1.
All statistical procedures were performed using SAS® Version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics and baseline characteristics
A total of 282 subjects were randomized in GOLDEN 2, and 96 were randomized in GOLDEN 6. In the pooled population of 378 subjects, 27 (7%) discontinued; the most frequent reason was AEs (14 [5%] in GOLDEN 2 and 5 [5.2%] in GOLDEN 6).
Pooled patient demographics and baseline characteristics are presented in Table 1 (data for the individual studies are available in Additional file 1: Tables S1 and S2). Mean age was 59 years (range: 40–75 years). Most subjects were white (90%) and 52% were female. The proportion of subjects with severe COPD ranged from 37.2% to 51.9%. The majority of subjects (60.3%) were current smokers and the mean duration of smoking history ranged from 50.1 to 54.0 pack-years.
Table 1.
Patient demographics and baseline characteristics (pooled population)
| Parameter | Placebo | Glycopyrrolate | Aclidinium | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 149) | 3 μg BID (n = 91) |
6.25 μg BID (n = 92) |
12.5 μg BID (n = 145) |
25 μg BID (n = 54) |
50 μg BID (n = 149) |
100 μg BID (n = 59) |
400 μg BID (n = 94) |
(N = 378) | |
| Mean (SD) age, years | 57.7 (7.65) | 54.5 (5.90) | 54.4 (5.90) | 56.9 (7.37) | 59.6 (8.98) | 56.4 (7.49) | 59.4 (7.65) | 54.6 (5.92) | 59.0 (8.05) |
| Age <65 years, n (%) | 120 (80.5) | 89 (97.8) | 90 (97.8) | 124 (85.5) | 40 (74.1) | 126 (84.6) | 42 (71.2) | 92 (97.9) | 278 (73.5) |
| Gender, n (%) | |||||||||
| Female | 80 (53.7) | 49 (53.8) | 49 (53.3) | 76 (52.4) | 30 (55.6) | 73 (49.0) | 34 (57.6) | 50 (53.2) | 197 (52.1) |
| Male | 69 (46.3) | 42 (46.2) | 43 (46.7) | 69 (47.6) | 24 (44.4) | 76 (51.0) | 25 (42.4) | 44 (46.8) | 181 (47.9) |
| Race, n (%) | |||||||||
| White | 131 (87.9) | 81 (89.0) | 83 (90.2) | 132 (91.0) | 51 (94.4) | 135 (90.6) | 49 (83.1) | 84 (89.4) | 339 (89.7) |
| Black/African American | 17 (11.4) | 10 (11.0) | 9 (9.8) | 13 (9.0) | 3 (5.6) | 13 (8.7) | 10 (16.9) | 10 (10.6) | 37 (9.8) |
| American Indian/Alaskan Native | 1 (0.7) | 0 | 0 | 0 | 0 | 1 (0.7) | 0 | 0 | 2 (0.5) |
| Post-bronchodilator FEV1, n (%) | |||||||||
| <50% predicted | 60 (40.3) | 34 (37.4) | 34 (37.0) | 59 (40.7) | 28 (51.9) | 62 (41.6) | 25 (42.4) | 35 (37.2) | NA |
| ≥50% predicted | 89 (59.7) | 57 (62.6) | 58 (63.0) | 86 (59.3) | 26 (48.1) | 87 (58.4) | 34 (57.6) | 59 (62.8) | NA |
BID twice daily, FEV 1 forced expiratory volume in 1 s, NA not available, SD standard deviation
Treatment compliance during the double-blind treatment period was 95.8–97.2% across treatment groups in GOLDEN 2, and 98.9–99.3% in GOLDEN 6.
Efficacy
In the pooled data, nebulized glycopyrrolate showed significant improvements in change from baseline in trough FEV1 and FEV1 AUC0−12 on Day 7 (Tables 2 and 3). Individual study data for the glycopyrrolate doses of 12.5 and 50 μg BID are reported below and for the remaining treatment groups in Additional file 1: Tables S3–S7.
Table 2.
Change from baseline in trough FEV1 on Day 7 (pooled population)
| Parameter | Placebo | Glycopyrrolate | Aclidinium | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 149) | 3 μg BID (n = 91) |
6.25 μg BID (n = 92) |
12.5 μg BID (n = 145) |
25 μg BID (n = 54) |
50 μg BID (n = 149) |
100 μg BID (n = 59) |
400 μg BID (n = 94) |
|
| Baseline FEV1 | ||||||||
| n | 149 | 91 | 92 | 144 | 54 | 149 | 59 | 94 |
| Mean (SD), L | 1.296 (0.429) | 1.363 (0.429) | 1.380 (0.440) | 1.302 (0.421) | 1.205 (0.425) | 1.321 (0.433) | 1.202 (0.463) | 1.395 (0.464) |
| FEV1 on Day 7 | ||||||||
| n | 139 | 86 | 88 | 137 | 51 | 139 | 57 | 86 |
| Mean (SD), L | 1.304 (0.419) | 1.375 (0.422) | 1.454 (0.482) | 1.396 (0.435) | 1.313 (0.434) | 1.436 (0.440) | 1.359 (0.491) | 1.508 (0.434) |
| Change from baseline in FEV1 on Day 7 | ||||||||
| Mean (SD), L | −0.024 (0.214) | −0.012 (0.186) | 0.063 (0.200) | 0.100 (0.193) | 0.108 (0.202) | 0.113 (0.214) | 0.154 (0.170) | 0.120 (0.187) |
| LS mean (SE), L | −0.024 (0.017) | −0.007 (0.021) | 0.068 (0.021) | 0.097 (0.017) | 0.099 (0.028) | 0.113 (0.017) | 0.145 (0.026) | 0.125 (0.021) |
| 95% CI | −0.057, 0.009 | −0.049, 0.034 | 0.026, 0.109 | 0.064, 0.131 | 0.045, 0.153 | 0.081, 0.146 | 0.094, 0.196 | 0.083, 0.167 |
| Placebo-adjusted change from baseline in FEV1 on Day 7 | ||||||||
| LS mean (SE), L | – | 0.017 (0.027) | 0.092 (0.027) | 0.122 (0.024) | 0.123 (0.032) | 0.137 (0.024) | 0.169 (0.031) | 0.149 (0.027) |
| 95% CI | – | −0.036, 0.070 | 0.039, 0.144 | 0.075, 0.168 | 0.060, 0.186 | 0.091, 0.184 | 0.108, 0.230 | 0.096, 0.202 |
BID twice daily, CI confidence interval, FEV 1 forced expiratory volume in 1 s, LS least squares, SD standard deviation, SE standard error
Table 3.
Standardized change from baseline in FEV1 AUC0–12 on Day 7 (pooled population)
| Parameter | Placebo | Glycopyrrolate | Aclidinium | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 149) | 3 μg BID (n = 91) |
6.25 μg BID (n = 92) |
12.5 μg BID (n = 145) |
25 μg BID (n = 54) |
50 μg BID (n = 149) |
100 μg BID (n = 59) |
400 μg BID (n = 94) |
|
| Baseline FEV1 | ||||||||
| n | 149 | 91 | 92 | 144 | 54 | 149 | 59 | 94 |
| Mean (SD), L | 1.296 (0.429) | 1.363 (0.429) | 1.380 (0.440) | 1.302 (0.421) | 1.205 (0.425) | 1.321 (0.433) | 1.202 (0.463) | 1.395 (0.464) |
| Standardized change from baseline in FEV1 AUC0–12 on Day 7 | ||||||||
| n | 146 | 91 | 92 | 143 | 53 | 146 | 59 | 90 |
| Mean (SD), L | −0.016 (0.187) | 0.034 (0.178) | 0.068 (0.181) | 0.130 (0.174) | 0.167 (0.200) | 0.163 (0.201) | 0.210 (0.143) | 0.153 (0.224) |
| LS mean (SE), L | −0.016 (0.016) | 0.036 (0.020) | 0.071 (0.020) | 0.129 (0.016) | 0.162 (0.026) | 0.163 (0.016) | 0.205 (0.025) | 0.156 (0.020) |
| 95% CI | −0.047, 0.014 | −0.003, 0.074 | 0.032, 0.109 | 0.098, 0.160 | 0.112, 0.213 | 0.133, 0.194 | 0.157, 0.253 | 0.117, 0.195 |
| Placebo-adjusted change from baseline in FEV1 AUC0–12 on Day 7 | ||||||||
| LS mean (SE), L | – | 0.052 (0.025) | 0.087 (0.025) | 0.145 (0.022) | 0.178 (0.030) | 0.180 (0.022) | 0.221 (0.029) | 0.172 (0.025) |
| 95% CI | – | 0.003, 0.101 | 0.038, 0.136 | 0.102, 0.188 | 0.120, 0.237 | 0.137, 0.223 | 0.164, 0.278 | 0.123, 0.221 |
AUC area under the curve, BID twice daily, CI confidence interval, FEV 1 forced expiratory volume in 1 s, LS least squares, SD standard deviation, SE standard error
Change from baseline in trough FEV1
Glycopyrrolate 12.5 and 50 μg BID showed significant improvement in LS mean placebo-adjusted change from baseline in trough FEV1 on Day 7 and Day 28 in GOLDEN 2, and on Day 7 in GOLDEN 6 (GOLDEN 2, Day 7: 0.118 and 0.149 L; GOLDEN 2, Day 28: 0.117 and 0.146 L; GOLDEN 6, Day 7: 0.109 and 0.138 L, respectively) (Additional file 1: Tables S3 and S4). Change from baseline in FEV1 over 24 h on Day 28 (GOLDEN 2) and Day 7 (GOLDEN 6), for all doses, is presented in Additional file 1: Figs. S1 and S2.
For the pooled data, the change from baseline in trough FEV1 on Day 7 was significantly greater for all doses of nebulized glycopyrrolate (except the glycopyrrolate 3 μg BID) than for placebo (Table 2, Fig. 3). The placebo-adjusted LS mean change from baseline on Day 7 showed dose-related increases in trough FEV1 of 0.122, 0.123, 0.137, and 0.169 L for the glycopyrrolate 12.5, 25, 50, and 100 μg BID doses respectively. Placebo-adjusted changes from baseline in trough FEV1 of subjects who received glycopyrrolate 50 μg BID showed similar increases to those of subjects who received aclidinium bromide (0.137 and 0.149 L, respectively).
Fig. 3.
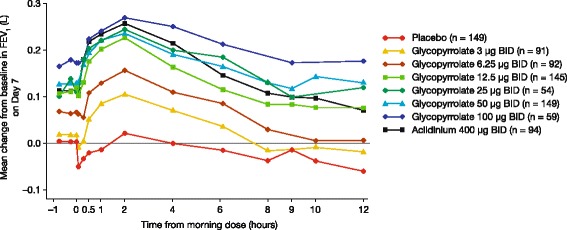
Mean change from baseline in FEV1 over time on Day 7 (pooled population). BID, twice daily. FEV1, forced expiratory volume in 1 second
Change from baseline in FEV1 AUC0–12
In GOLDEN 2, on Day 7 (Fig. 4a) and Day 28, both glycopyrrolate 12.5 and 50 μg BID doses produced significant increases in LS mean placebo-adjusted change from baseline in FEV1 AUC0–12 (Day 7: 0.143 and 0.153 L; Day 28: 0.136 and 0.105 L, respectively; Additional file 1: Table S5).
Fig. 4.
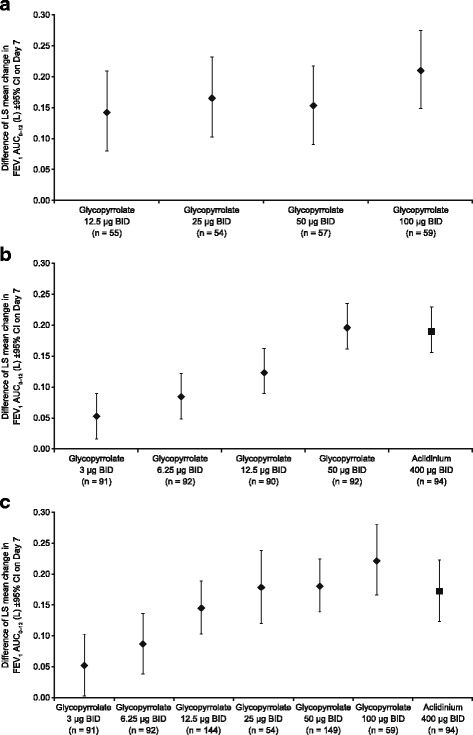
Placebo-adjusted change from baseline in FEV1 AUC0-12 on Day 7 (a: GOLDEN 2; b: GOLDEN 6; c: pooled population). AUC, area under the curve. BID, twice daily. CI, confidence interval. FEV1, forced expiratory volume in 1 second. LS, least squares
Increases in LS mean placebo-adjusted change from baseline in FEV1 AUC0–12 on Day 7 (Fig. 4b) in GOLDEN 6 were observed for both glycopyrrolate 12.5 μg BID (0.126 L) and 50 μg BID (0.196 L), with the improvement for glycopyrrolate 50 μg BID being comparable to that with aclidinium 400 μg BID (0.190 L) (Additional file 1: Table S6).
In the pooled data, on Day 7, a dose-related improvement was apparent for glycopyrrolate, in terms of the placebo-adjusted standardized change from baseline in FEV1 AUC0–12 (Table 3; Fig. 4c). Compared with the placebo-adjusted change in FEV1 AUC0–12 versus baseline with aclidinium 400 μg BID (0.172 L), there was less improvement with glycopyrrolate 12.5 μg BID (0.145 L), and more improvement with glycopyrrolate 25 μg BID (0.178 L) and glycopyrrolate 50 μg BID (0.180 L).
Change from baseline in peak FEV1 (GOLDEN 2 only)
At Day 28, in GOLDEN 2, improvements were seen in placebo-adjusted change from baseline in peak FEV1 for all glycopyrrolate doses including 12.5 and 50 μg BID (0.168 and 0.165 L, respectively) (Additional file 1: Table S7).
Characterization of the dose–response relationship for glycopyrrolate/eFlow®
Improvements in placebo-adjusted trough FEV1 with nebulized glycopyrrolate were clinically meaningful within the dose range of 12.5 to 100 μg BID. Although the group mean changes with glycopyrrolate 12.5 μg BID were >0.100 L, the lower bound of the 90% CI was <0.100 L. In addition, a statistical comparison between the 12.5 μg and 50 μg groups in GOLDEN 6 showed that the 50 μg BID dose was statistically superior, as measured by change from baseline in FEV1 AUC0–12. Exploratory model-fitting indicated that a sigmoidal model was the best fit to the trough FEV1 data, and showed that glycopyrrolate doses of 3 to 50 μg were situated on the monotonically increasing dose–response curve.
Safety
Treatment-emergent adverse events
Overall, TEAEs were reported for 87 subjects in GOLDEN 2, and for 62 subjects in GOLDEN 6. In GOLDEN 2, the total incidence of TEAEs was comparable between the placebo group (26%) and the glycopyrrolate groups (12.5 μg BID, 35%; 25 μg BID, 33%; 50 μg BID, 32%; 100 μg BID, 29%; Table 4). Similar TEAE incidences were observed in the treatment groups in GOLDEN 6 (glycopyrrolate 3 μg BID, 24%; 6.25 μg BID, 25%; 12.5 μg BID, 27%; 50 μg BID, 15%; aclidinium 400 μg BID, 26%), with an incidence of 12% in the placebo group. The most frequent TEAEs seen with glycopyrrolate were COPD exacerbation (1.7–7.4%; placebo 1.8%) and headache (0–5.1%; placebo 1.8%) in GOLDEN 2, and hypertension (1.1–4.4%; placebo 0%) and cough (3.3–6.7%; placebo 2.2%) in GOLDEN 6.
Table 4.
Summary of treatment-emergent adverse events
| GOLDEN 2 | GOLDEN 6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Glycopyrrolate | Placebo | Glycopyrrolate | Aclidinium | |||||||
| (n = 57) | 12.5 μg BID (n = 55) |
25 μg BID (n = 54) |
50 μg BID (n = 57) |
100 μg BID (n = 59) |
(n = 92) | 3 μg BID (n = 91) |
6.25 μg BID (n = 92) |
12.5 μg BID (n = 90) |
50 μg BID (n = 92) |
400 μg BID (n = 94) |
|
| Any TEAE, n (%) | 15 (26.3) | 19 (34.5) | 18 (33.3) | 18 (31.6) | 17 (28.8) | 11 (12.0) | 22 (24.2) | 23 (25.0) | 24 (26.7) | 14 (15.2) | 24 (25.5) |
| Potentially related TEAEa, n (%) | 0 | 5 (9.1) | 2 (3.7) | 2 (3.5) | 4 (6.8) | 4 (4.3) | 5 (5.5) | 5 (5.4) | 9 (10.0) | 7 (7.6) | 11 (11.7) |
| Serious TEAE, n (%) | 2 (3.5) | 2 (3.6) | 2 (3.7) | 1 (1.8) | 3 (5.1) | 1 (1.1) | 0 | 2 (2.2) | 1 (1.1) | 0 | 3 (3.2) |
| Discontinuations due to TEAE, n (%) | 2 (3.5) | 3 (5.5) | 4 (7.4) | 3 (5.3) | 1 (1.7) | 1 (1.1) | 0 | 0 | 1 (1.1) | 0 | 2 (2.1) |
BID twice daily, TEAE treatment-emergent adverse event
aConsidered by the Investigator to have a definite, probable, or possible relationship to study drug
Neither study showed evidence of a dose-related relationship in terms of the incidence of any specific TEAE. In subjects treated with aclidinium 400 μg BID, the most common TEAEs were dysgeusia (8.5%), and bronchitis, cough, and nausea (all 2.1%).
Discontinuations due to TEAEs are presented in Table 4.
Serious treatment-emergent adverse events and deaths
The overall incidence of serious TEAEs reported for glycopyrrolate/eFlow® was comparable to that for placebo and aclidinium (GOLDEN 2: placebo, 3.5%; glycopyrrolate 12.5 μg BID, 3.6%; 25 μg BID, 3.7%; 50 μg BID, 1.8%; 100 μg BID, 5.1%. GOLDEN 6: placebo, 1.1%; glycopyrrolate 3 μg BID, 0%; 6.25 μg BID, 2.2%; 12.5 μg BID, 1.1%; 50 μg BID, 0%; aclidinium 400 μg BID, 3.2%; Table 4). The only serious TEAE to occur in more than one subject receiving glycopyrrolate was COPD exacerbation, which occurred in two subjects in GOLDEN 2 (one in the 25 μg BID and one in the 100 μg BID dose groups) and two subjects in GOLDEN 6 (one in the 6.25 μg BID and one in the 12.5 μg BID dose groups).
Two subjects died, one in GOLDEN 2 (a female subject who had severe cardiac arrest prior to receiving double-blind medication) and one in GOLDEN 6 (a male with presumed poly-drug [i.e. opiate] toxicity 2 days after the last dose of glycopyrrolate 6.25 μg BID, which was not considered to be treatment related).
Vital signs, clinical laboratory, and electrocardiogram parameters
In both studies, no clinically meaningful findings or trends were noted in vital signs, clinical laboratory assessments, or ECG (including QTc-F) parameters between treatment groups (data not shown).
Discussion
This analysis of pooled data from two Phase II randomized, placebo- and/or active-controlled studies was conducted to characterize the dose–response relationship for nebulized glycopyrrolate, and inform dose selection for the Phase III studies. In subjects with moderate-to-severe COPD, treatment with glycopyrrolate/eFlow® was associated with significant improvements in pulmonary function versus placebo, and was generally well tolerated.
Analysis of the pooled population suggests a sigmoidal dose–response relationship. Glycopyrrolate 3 μg BID was deemed the ‘no effect’ dose, with doses up to 50 μg BID situated on the monotonically increasing curve, indicating increased response with increasing dose. The glycopyrrolate 6.25 μg BID and 12.5 μg BID doses had statistically significant but clinically suboptimal effects. At doses of 25 and 50 μg BID, clinically meaningful improvements in the change from baseline in trough FEV1 and change from baseline in FEV1 AUC0–12 were observed, compared with placebo, with greater improvements seen with 50 μg BID than with 25 μg BID. These improvements with glycopyrrolate/eFlow® were either comparable or superior to those observed with aclidinium 400 μg BID. Based on these data, glycopyrrolate doses of 25 μg BID and 50 μg BID were selected for further evaluation. Pooled and individual study data supported the dose selection.
Although previous studies with glycopyrrolate/eFlow® administered once daily (QD) showed statistically significant increases in trough FEV1 [16, 17], a substantial incremental improvement was seen with BID dosing. Glycopyrrolate 50 μg QD administered for 7 days improved the trough FEV1 by 0.068 L, while the pooled improvement in trough FEV1 with glycopyrrolate 50 μg BID for 7 days was 0.137 L in these studies.
Conclusion
In the Phase II GOLDEN 2 and 6 studies, the statistical and clinical improvements in lung function, relative to placebo on Days 7 and 28, and acceptable safety profile support the selection of the glycopyrrolate 25 and 50 μg BID doses for the GOLDEN Phase III program. The combined study results provide preliminary evidence for the use of nebulized glycopyrrolate BID as a maintenance therapy for the treatment of COPD. Nebulized glycopyrrolate may provide patients and physicians with an additional treatment option for moderate-to-severe COPD, and provide a therapeutic alternative for patients who experience physical and/or physiological difficulty using handheld inhalers.
Acknowledgments
Medical writing support was provided by Frances Weir of FireKite, an Ashfield company, part of UDG Healthcare plc, and was funded by Sunovion Pharmaceuticals, Inc.
Funding
This study was funded by Sunovion Pharmaceuticals, Inc.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to proprietary ownership by Sunovion Pharmaceuticals Inc., but are available from the corresponding author on reasonable request.
Abbreviations
- AE
Adverse event
- ANCOVA
Analysis of covariance
- AUC
Area under the curve
- BID
Twice daily
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- DPI
Dry powder inhaler
- ECG
Electrocardiogram
- FEV1
Forced expiratory volume in one second
- GOLDEN
Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer
- LAMA
Long-acting muscarinic antagonist
- LS
Least squares
- MDI
Metered dose inhaler
- QD
Once daily
- QTc-F
Corrected QT (Fridericia’s formula)
- SD
Standard deviation
- SE
Standard error
- TEAE
Treatment-emergent adverse event
- US
United States
Additional file
Online Supplementary Data. Table S1. Patient demographics and baseline characteristics, GOLDEN 2 (ITT population). Table S2. Patient demographics and baseline characteristics, GOLDEN 6 (safety population). Table S3. Change from baseline in trough FEV1 on Day 7 and Day 28, GOLDEN 2 (ITT population). Table S4. Change from baseline in trough FEV1 on Day 7, GOLDEN 6 (efficacy population). Table S5. Standardized change from baseline in FEV1AUC0–12 on Day 7 and Day 28, GOLDEN 2 (ITT population). Table S6. Standardized change from baseline in FEV1 AUC0–12 on Day 7, GOLDEN 6 (efficacy population). Table S7. Change from baseline in peak FEV1 on Day 28, GOLDEN 2 (ITT population). Figure S1. Least squares mean change from baseline in FEV1 over time on Day 28 (GOLDEN 2 Substudya ITT population). Figure S2. Mean change from baseline in FEV1 over time on Day 7 (GOLDEN 6 efficacy population). (DOCX 269 kb)
Authors’ contributions
JFD was a study investigator, AW was involved in designing the studies, RT and TG helped coordinate the studies and analysed the output. All authors contributed substantially to the writing of the manuscript and approved the submitted version.
Ethics approval and consent to participate
Ethics approval was provided by:
GOLDEN 2
Central IRB: Shulman Associates IRB, 4445 Lake Forest Drive, Suite 300, Cincinnati, OH 45242 (Chair: Susan Nelson MSN, RN, CNS).
Local IRB: Dr. Nicola Hanania, Baylor College of Medicine Office of Research, One Baylor Plaza, Suite 600D, Houston, TX 77030 (Chair: Vernon R Sutton, MD, BS).
GOLDEN 6
Central IRB: Quorum Review IRB, 1501 Fourth Ave, Suite 800, Seattle, WA 98101 (Chair: Steven Rosenfeld MD, MBA).
Consent for publication
Not applicable
Competing interests
JFD has served as a consultant to Sunovion, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Novartis, Theravance, and has served on Drug Safety Monitoring Boards for AstraZeneca, Novartis, Gilead, INSMED and Grifolds. RT and TG are employees of Sunovion. AW was an employee of Sunovion at the time of the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12931-017-0681-z) contains supplementary material, which is available to authorized users.
References
- 1.American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. 2013. http://www.lung.org/assets/documents/research/copd-trend-report.pdf. Accessed 20 Feb 2017.
- 2.Centers for Disease Control and Prevention Chronic obstructive pulmonary disease among adults — United States, 2011. Morb Mortal Wkly Rep. 2012;61:938–943. [PubMed] [Google Scholar]
- 3.Global Strategy for the Diagnosis. Management and Prevention of COPD . Global initiative for chronic obstructive lung disease (GOLD) 2017. [Google Scholar]
- 4.Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, allergy, and immunology. Chest. 2005;127:335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 5.Rau JL. Practical problems with aerosol therapy in COPD. Respir Care. 2006;51:158–172. [PubMed] [Google Scholar]
- 6.Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. doi: 10.2147/CIA.S52999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braman SS, Carlin BW, Dhand R, Hanania NA, Mahler DA, Ohar JA, et al. Results of a pulmonologist survey regarding attitudes and practices with inhalation devices for COPD. Am J Respir Crit Care Med. 2016;193:A7816. doi: 10.4187/respcare.05717. [DOI] [PubMed] [Google Scholar]
- 8.Bauer A, McGlynn P, Bovet LL, Mims PL, Curry LA, Hanrahan JP. The influence of breathing pattern during nebulization on the delivery of arformoterol using a breath simulator. Respir Care. 2009;54:1488–1492. [PubMed] [Google Scholar]
- 9.Tiffin N, Zeman K, Bennett W. Efficacy and variability of aerosol delivery from portable DC-powered compressor/nebulizer systems. Can J Respir Ther. 2011;10:9–14. [Google Scholar]
- 10.Pham S, Ferguson GT, Kerwin E, Goodin T, Wheeler A, Bauer A. In-vitro characterisation of the eFlow® closed system (eFlow® CS) nebulizer with glycopyrrolate inhalation solution (SUN-101). Am J Resp Crit Care Med. 2017;195:A5472. [DOI] [PMC free article] [PubMed]
- 11.Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9:58–72. doi: 10.3109/15412555.2011.630047. [DOI] [PubMed] [Google Scholar]
- 12.Fink JB, Colice GL, Hodder R. Inhaler devices for patients with COPD. COPD. 2013;10:523–535. doi: 10.3109/15412555.2012.761960. [DOI] [PubMed] [Google Scholar]
- 13.Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377:1032–1045. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- 14.Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re-evaluation? Age Ageing. 2007;36:213–218. doi: 10.1093/ageing/afl174. [DOI] [PubMed] [Google Scholar]
- 15.Global Strategy for the Diagnosis. Management and Prevention of COPD . Global initiative for chronic obstructive lung disease (GOLD) 2011. [Google Scholar]
- 16.Kerwin E, Wheeler A, Schaefer K, Claus R, Tutuncu A, Hanania N. A randomized, double-blind, placebo-controlled study of SUN-101 (glycopyrrolate inhalation solution) in subjects with moderate to severe COPD. Chest. 2014;146((4) Suppl 2):68A. doi: 10.1378/chest.1990041. [DOI] [Google Scholar]
- 17.Fogarty C, Kerwin E, Dunn K, Singh D, Tutuncu A. The GOLDEN-1 study: safety and bronchodilatory effects of nebulized glycopyrrolate (EP-101) using high efficiency nebulizer in patients with COPD. Eur Respir J. 2012;40(Suppl 56):2194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to proprietary ownership by Sunovion Pharmaceuticals Inc., but are available from the corresponding author on reasonable request.


