Abstract
Herpes simplex virus 2 (HSV-2) is the leading cause of genital herpes and increases the risk of HIV infection, but there is no effective vaccine. A replication-defective HSV-2 mutant virus, dl5-29, is effective in animal models and has been in a phase I trial. Previous studies have shown that dl5-29 gives higher antibody responses and better protection when inoculated intramuscularly (IM) as compared with subcutaneously (SC). However, the basis for this effect has not been defined. We confirmed that IM inoculation of dl5-29 is more immunogenic and provides better protection than SC inoculation. IM inoculation of HSV-2 strains produced higher levels of a luciferase transgene than SC inoculation, as measured by intravital bioluminescence imaging. Intramuscular immunization also showed better protection against infection with a highly pathogenic African HSV-2, demonstrating that this single vaccine can be efficacious against HSV-2 strains from different geographic regions.
Introduction
Herpes simplex virus 2 (HSV-2) is the predominant cause of genital herpes infections worldwide and increases the risk of acquisition and transmission of HIV (Freeman et al., 2006; Roizman, Knipe, and Whitley, 2013; Wald et al., 2001). It is an incurable sexually transmitted disease that can result in serious complications for newborns and immunocompromised patients. After initial infection of mucosal epithelial cells, HSV-2 spreads to sensory neurons in which it establishes a latent infection. Antiviral treatment can block viral replication and reduce symptoms during symptomatic disease. However, these drugs can neither prevent de novo infection or reactivation nor clear the latent virus. A few vaccines have been tested clinically in the last decade, some of which have shown to be safe, but none have succeeded in effectively preventing or treating HSV-2. New approaches are being tested to find safe and effective herpes vaccines. Some studies have used one or more HSV-2 glycoproteins as subunit vaccines to elicit neutralizing antibodies. Other approaches emphasize the inclusion of a few antigens proposed to induce cellular responses as well (Skoberne et al., 2013). Alternatively, a replication-defective HSV-2 virus, dl5-29, was constructed that completes only part of the herpes lytic cycle. In normal cells, this virus produces many HSV-2 proteins and, hence, many antigens. However, dl5-29 will not spread to other cells, thus providing the safety of subunit vaccines and, potentially, the immunogenicity of a live virus (Da Costa et al., 2000; Da Costa et al., 1997). A glycoprotein D-null mutant virus has been proposed to protect against HSV-2 challenge through ADCC mechanisms (Petro et al., 2015). Various studies have tested vaccine candidates by injection through different routes, most commonly intranasal, subcutaneous, intramuscular, or intradermal. Although some studies have shown that intranasal or intravaginal vaccination may elicit mucosal immunity and robust protection against HSV-2 (Jones, Taylor, and Knipe, 2000; Morrison, Da Costa, and Knipe, 1998; Shin and Iwasaki, 2012; Wu et al., 2009), these approaches raise some safety concerns or could prove impractical to deliver. A number of groups have reported that their immunogens show better efficacy against HSV utilizing intramuscular delivery (Awasthi et al., 2012; Delagrave et al., 2012; Diaz and Knipe, 2016; Wang et al., 2012). In particular, Delagrave et al. showed that intramuscular delivery of dl5-29 elicited higher antibody titers and better general protection from genital herpetic disease than subcutaneous or intradermal delivery (Delagrave et al., 2012). In this study, we compare side-by-side the effects of subcutaneous and intramuscular routes of immunization on the efficacy of dl5-29 and examine the mechanisms of the enhanced efficacy of the IM route.
Results
HSV-2 dl5-29 virus protects better against genital herpes when administered intramuscularly
Previous studies have reported that IM immunization gives better protection against genital disease than other routes of inoculation (Delagrave et al., 2012; Diaz and Knipe, 2016; Awasthi et al., 2012). We wished to confirm this and to determine the mechanism of this effect; thus, we first compared IM versus SC immunization with HSV-2 dl5-29 in our mouse infection system (Morrison, Da Costa, and Knipe, 1998). Mice were primed and boosted with HSV-2 dl5-29 virus by the SC or IM routes and then challenged at day 54 with 50 LD50 of the wild type (WT) HSV-2 G strain virus (LD50 = 6×103 PFU) (Dudek et al., 2011). Mock-immunized animals showed increasing degrees of disease over time after challenge with 100% of the animals becoming paralyzed during the first week (Figure 1A). All vaccinated animals showed reduced genital disease compared to the control group, and we detected virtually no genital disease in animals that had received intramuscular immunization (Figure 1A). Significant differences in protection from disease were observed for the two routes of immunization (p=0.0005, two-way ANOVA). An important aspect of herpetic infection is viral shedding because infectious viruses can be shed from patients with no visible signs of disease. Therefore, it would be desirable for an HSV-2 vaccine to not only protect from disease but also reduce viral shedding (Bonneau et al., 1993). SC immunization reduced viral shedding when compared to mock-vaccinated animals; however, animals in this group still shed a significant amount of virus during the first week (Figure 1B). Furthermore, IM immunization consistently reduced viral shedding with no detectable virus by day 6 (Figure 1B, p<0.0001 all three groups, two-way ANOVA). Mice infected intravaginally with high doses of wild-type HSV-2 as challenge, suffered from neurological disease, including hind limb paralysis. Although SC immunization reduced paralysis, IM immunization with dl5-29 showed higher levels of protection from paralytic disease (Figure 1C, p=0.0006 by the Mantel-Cox log rank test).
Figure 1.
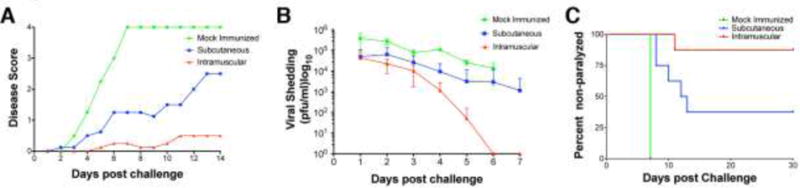
Comparison of protection conferred by intramuscular (IM) versus subcutaneous (SC) immunization with HSV-2 dl5-29 replication-defective mutant virus. Mice were immunized with HSV-2 dl5-29 virus by the indicated routes or mock-immunized and later challenged by intravaginal infection with 50 LD50 of HSV-2 strain G virus. A) Disease scores in immunized and control groups of mice. B) Viral shedding into the vaginal cavity in control and immunized groups of mice. C) Overall survival at 30 d post intravaginal challenge in the control and immunized groups of mice. Shown is one representative experiment of two independent experiments with 10 mice per vaccine group and 5 mice in the mock-immunized control. One animal in the IM group died for reasons unrelated to herpetic disease.
IM immunization with HSV-2 dl5-29 elicits stronger cellular immune responses than SC immunization
To determine the basis for the enhanced protection in the IM-immunized mice, we measured cellular and antibody responses to HSV-2 antigens. The H2Kb-restricted peptide SSIEFARL of HSV glycoprotein B acts as an immunodominant epitope in C57Black/6 mice and provides a useful probe of CD8+ T cell responses to HSV antigens (Bonneau et al., 1993). After inoculation with HSV-2 vaccine vectors, T lymphocytes proliferate with CD8+ cells peaking at approximately 7 d after injection (Dudek, Mathews, and Knipe, 2008). At 1 week after priming mice with the HSV-2 dl5-29 virus either SC or IM, we collected peripheral blood from each animal via tail bleed. Total leukocytes were stained, and CD8+/ SSIEFARL-pentamer double-positive T lymphocytes were quantified by flow cytometry. Subcutaneous immunization elicited robust proliferation of HSV gB-specific CD8+ lymphocytes; however, the same dose given intramuscularly generated a significantly higher number of CD8, gB pentamer double-positive lymphocytes (t-test p = 0.0214) (Figure 2A). At 4 weeks after priming, we boosted the animals with a second dose of dl5-29 virus. One week later, the animals were sacrificed, spleens were harvested, and total leukocytes were collected. Equal numbers of cells were re-stimulated with synthetic SSIEFARL peptide. After standard intracellular cytokine staining (ICS), we quantified the number of CD8+ lymphocytes that produced interferon-γ. Once again, intramuscular immunization elicited a significantly higher cellular response (t-test p = 0.0027) (Figure 2B).
Figure 2.
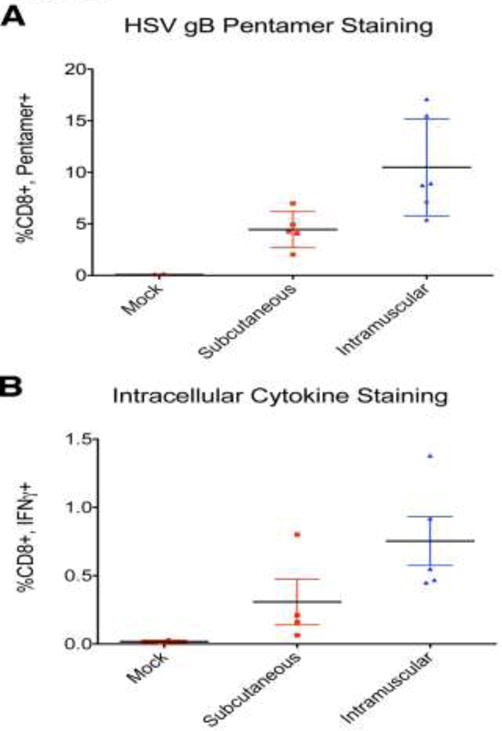
Comparison of HSV-specific cellular responses after intramuscular versus subcutaneous immunization. A) HSV-specific CD8+ T cell responses as assayed by pentamer staining. PBMCs were collected at 7 d after the first dose of dl5-29 and stained with gB-specific pentamers. B) HSV-specific CD8+ T cell responses as assayed by intracellular cytokine staining. Splenocytes were isolated at 7 d after the booster injection and were incubated with the gB peptide SSIEFARL and analyzed by intracellular cytokine staining. Shown is one of two independent experiments with 5 mice per group.
Total anti-HSV-2 antibody levels are higher after IM than after SC immunization
By 3 weeks after immunization of BALB/c mice with dl5-29 virus, humoral responses to HSV-2 are detectable (Da Costa et al., 2000). When virus stocks prepared from infected cell supernatants were used as the immunogen, the subcutaneous route produced antibody levels barely above those of the mock-immunized animals (Figure 3A). In contrast, intramuscular injection yielded higher levels of total anti-HSV antibodies (Figure 3A, t-test p=0.0289). At day 37, 9 days after booster injection, BALB/c mice showed a robust humoral response against HSV-2 after both subcutaneous and intramuscular vaccination; however, the latter elicited much higher levels of anti-HSV antibodies (Figure 3B, t-test p<0.0001). C57Bl/6 mice were available for humoral response assessment only at 3 weeks post priming because they were sacrificed for spleen harvesting a week after boosting. Their genetic background differs from that of the BALB/c strain, and we measured higher levels of total anti-HSV antibody in the vaccinated groups compared to the mock-immunized mice at 21 d post infection. In these animals as well, intramuscular vaccination elicited higher humoral responses (Figure 3C, t-test p=0.0204).
Figure 3.
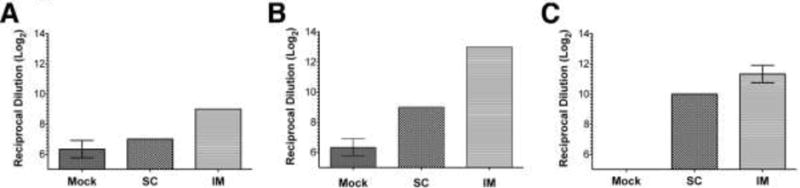
Measurement of HSV-specific antibodies in BALB/c and C57Black6 mice immunized by IM or SC injection. A) ELISA measurements of HSV-specific antibodies from BALB/c mice, 21 d after priming. B) Antibody levels of BALB/c mice, 7 d after boosting. C) C57Black6 mice, 21 d after priming. BALB/c experiments included 10 mice per group. C57Black6 included 5 mice per group. One representative experiment of two independent experiments is shown. Values where error bars are not present showed identical replicates.
Virus-encoded proteins are expressed in vivo from replication-defective HSV-2 strains
To test if the levels of viral gene expression were different by the two routes of immunization, we constructed an HSV-2 replication-defective viral strain, HSV-2 5BLuciferase, that expressed firefly luciferase driven by an HSV IE promoter as described in Materials and Methods, and we measured luciferase expression by intravital imaging. Following injection of HSV-2 5BLuciferase virus into mice, we observed expression of luciferase by 5 h post injection at both IM and SC sites of inoculation but not immediately after injection (Figure 4). The lack of luminescence immediately after inoculation ruled out the possibility that the signals observed were not due to luciferase enzyme being injected with the vector and immediately internalized by cells. Most importantly, IM vaccination resulted in higher levels of luciferase expression (Figure 4A). In animals injected by the SC route, luciferase expression was lower and quickly dropped to levels close of the mock-vaccinated mice. In contrast, IM vaccination resulted in higher luciferase activity that persisted longer at the site of injection (p<0.0001, two-way ANOVA). However, because this vector did not replicate in vivo, we found no detectable luciferase activity by 3 days after injection (Figures 4A and B). Although we cannot rule out the possibility that infection occurs at low levels at other anatomical sites, we detected luciferase activity localized exclusively at the site of injection.
Figure 4.
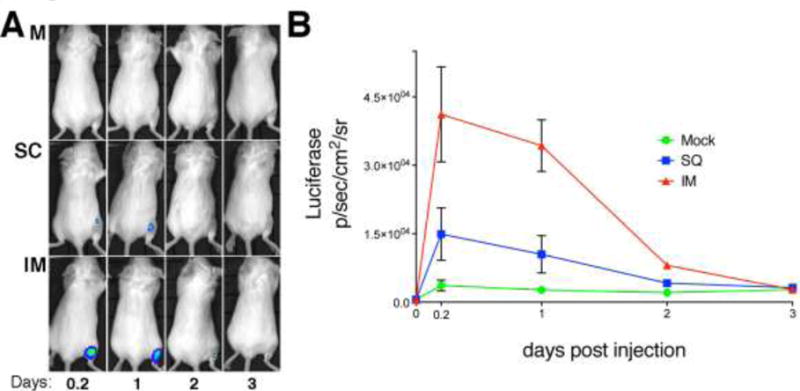
Levels of viral transgene expression in mice immunized by intramuscular versus subcutaneous routes. A) In vivo imaging of BALB/c mice inoculated either SC or IM with a replication-defective HSV-2 encoding firefly luciferase expressed from a viral IE gene promoter. Images shown are of a representative mouse from one time point of each group from one of two independent experiments. B) Quantification of the luciferase activity expressed as average radiance (photons/second/ squared cm/steradian). Number of mice: 5 per group.
Having observed that IM inoculation gives higher virus-encoded gene expression, we wanted to define the location of protein expression following IM inoculation. For immunohistological studies, we used the HSV-2 replication-defective 5BLacZ recombinant virus that expresses β-galactosidase fused to a portion of the ICP8 protein (Da Costa et al., 1997). Following injection of the HSV-2 5BLacZ virus into the gastrocnemius muscle, we observed β-galactosidase activity in muscle tissue and inflammatory cells. When sections of fixed muscle were stained with X-gal, a distinctive blue staining was detected in the injected muscle, and histological analysis showed that β-galactosidase activity was present in muscle fibers and inflammatory cells (Figure 5). This supported the idea that viral antigens were synthesized de novo at the site of intramuscular injection and therefore persisted after the time of vaccination. In the case of subcutaneous vaccination, we detected no β-galactosidase activity at or near the site of injection (results not shown). This suggested that the viral vaccine when delivered by the SC route was cleared and potentially processed by the immune system before the infection could be efficiently established.
Figure 5.
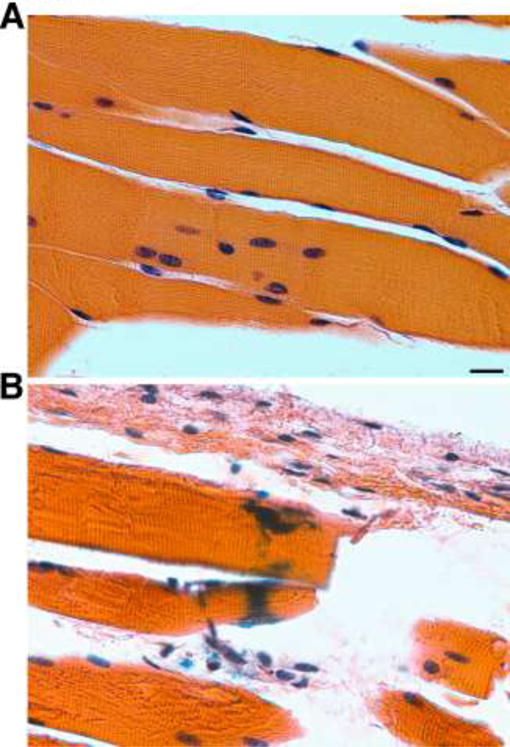
Detection of β-galactosidase following IM inoculation of the replication-deficient HSV-2 5BLacZ virus in muscle syncytia and inflammatory cells at the site of injection 24 h post vaccination. A) Mock-infected. B) IM infected with HSV-2 5BLacZ. All paraffin-embedded sections were stained with X-gal and H&E. Scale bar: 10 μm.
Viral proteins are expressed at the site of intramuscular injection of replication-defective HSV-2 viruses
To detect viral proteins at or around the site of IM injection, we used a combination of two replication-defective viruses, 5BLacZ and dl5, to increase the amount of antigens that could be detected and also to facilitate the location of the injection site by X-gal staining. After IHC analysis, we observed HSV-2 proteins at the site of injection at 24 h post injection (Figure 6). We observed viral antigens in syncytial muscle cells and possibly infiltrating inflammatory cells, showing that there was expression of authentic viral proteins from a replication-defective virus at the site of IM injection.
Figure 6.
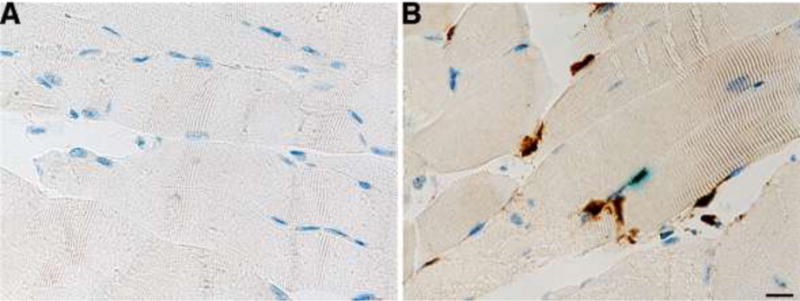
Expression of viral proteins at the site of IM injection. Mouse muscle tissues were stained with X-gal to reveal the site of injection prior to IHC and anti-HSV2 antibody detection with HRP+DAB. Mock infected (A) or infected with a combination of HSV-2 5BLacZ+dl5 (B). Nuclei are stained with hematoxylin. Scale bar: 10 μm.
IM vaccination with dl5-29 virus provides better protection against South African HSV-2
Previous studies had shown that dl5-29 offers better protection against HSV-2 strains isolated in the United States than against those isolated in South Africa (Dudek et al., 2011). Our results described above had demonstrated that the immunogenicity of dl5-29 could be improved by IM delivery; thus, we compared the two routes of immunization in the context of intravaginal challenge with the South African HSV-2 SD90-3P virus (Colgrove et al., 2014; Dudek et al., 2011). We observed greatly diminished disease severity in the IM vaccinated group as compared to the SC immunized animals (Figure 7, panels A and C, p=0.0009 two-way ANOVA IM vs SQ), with disease scores similar to those observed after challenge with a U.S. HSV-2 strain (Dudek et al., 2011). Interestingly, viral shedding into the vaginal cavities was also reduced in the IM group, with SD90-3P being cleared past 5 to 6 d after challenge (Figure 7, panels B and D, p<0.0001 two-way ANOVA IM vs SQ). Titers of shed South African virus were comparable to those of US challenge virus after IM delivery of dl5-29 (Dudek et al., 2011).
Figure 7.
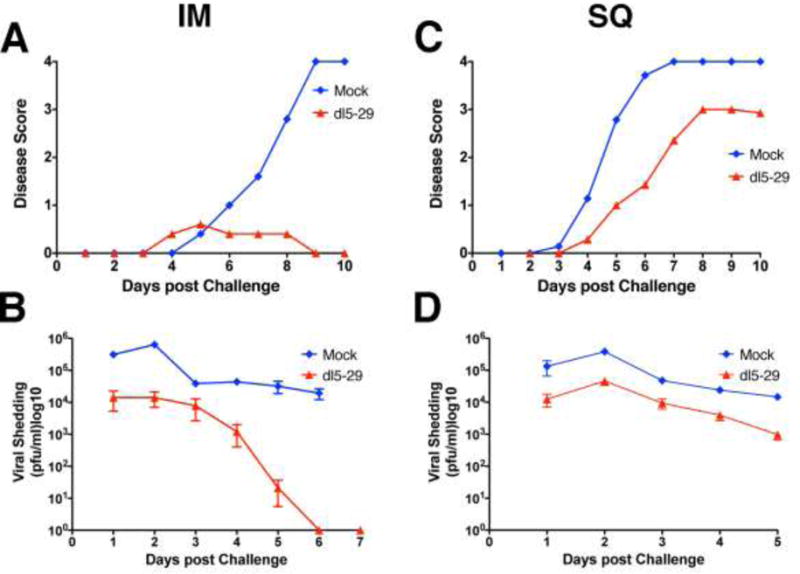
Comparison of IM and SC immunization with dl5-29 for protection against the highly pathogenic HSV-2 SD90 virus. BALB/c mice were immunized with HSV-2 dl5-29 or mock-immunized by the IM or SC routes and challenged with the highly pathogenic South African HSV-2 strain SD90 virus. Shown are disease scores (A) and viral shedding (B) for the IM immunization versus SC immunization (C and D).
Discussion
In this study, we have confirmed previous reports that IM immunization with genital herpes vaccines gives better protection against genital infection by providing better survival, reduced viral shedding, and reduced disease and increased antibody responses (Awasthi et al., 2012; Delagrave et al., 2012; Diaz and Knipe, 2016). We have extended those results by showing that IM immunization with dl5-29 gives higher CD8+ T cell responses. Furthermore, we have shown that IM immunization gives higher levels of virus-encoded proteins and excellent protection against a highly pathogenic African HSV-2 strain. These results have implications for the mechanisms of immunization by this replication-defective HSV-2 strain and for the design of genital herpes vaccines.
Mechanisms of increased IM immunogenicity
Higher levels of protection and increased immunogenicity could be due to 1) higher levels of antigen expression; 2) prolonged expression of antigens; and/or 3) different antigen-presenting cells or different cytokine induction. In this study, we tested the first and second hypotheses, and we found that expression of proteins from an HSV-2 replication-defective recombinant virus was higher following intramuscular inoculation but not necessarily for longer periods of time. More sensitive and more frequent measurements of the kinetics of protein expression may show differences in the persistence of virus-encoded proteins, but our results using luciferase imaging show that this enzymatic level was at the level of detection by 3 d post infection. Nevertheless, immunological epitopes may persist as fragments of the protein. In total, we conclude that higher levels of antigen expression are at least part of the explanation for the increased immunogenicity.
There are a number of possible explanations for higher viral protein expression following IM inoculation of virus. Inoculation into solid tissue may simply guarantee that more cells are infected. Second, infection of syncytial muscle cells, as demonstrated in our study, would allow the replication-defective mutant virus to access a large multi-nucleated cell where transcription of viral genomes could take place in many nuclei. In most normal cells, a replication-defective mutant virus could only infect a limited number of cells, but infection of a muscle syncytium would provide access for the replication-defective virus to multiple cell nuclei where different viral genomes could target to express viral genes. Third, epigenetic silencing mechanisms may be less active in muscle cells, allowing enhanced viral gene expression. Adult muscle is considered to be “extremely plastic” (Baar, 2010), meaning that the tissue can respond rapidly to physiological changes by altering gene expression. This is thought to be accomplished by epigenetic regulation of muscle cell genes (Baar, 2010). Muscle activity promotes histone acetylation in muscle cells (McGee and Hargreaves, 2011), so muscle cells may provide a situation for less silencing than other cell types and allow more viral gene expression. In fact, exercise has been shown to increase histone acetylation (McGee and Hargreaves, 2011), so exercise of the muscle to be injected might further increase immunogenicity of a DNA virus vaccine that is subject to epigenetic regulation. Further studies are needed to compare the chromatin states of the viral genome in muscle cells versus other cell types.
IM inoculation has also been used effectively for subunit vaccines, a situation where, unlike injection of our replication defective vaccine, antigen expression would not be a factor. Therefore, it is likely that immunological mechanisms also differ between the various routes of immunization. It will be informative to determine the type of dendritic cells that pick up antigen in muscle tissue versus SC immunization and migrate to the draining lymph node. This could define further factors that define the immunogenicity of IM immunization.
Implications for vaccine design
We had previously observed that African HSV-2 isolates were more difficult to protect against than U.S. isolates using the SC route for immunization (Dudek et al., 2011). This raised the possibility that there were different clades or strains of HSV-2 in various geographical areas that might require different vaccines or different genetic backbones for the vaccine virus. Because we had shown that IM immunization gave better protection than SC immunization against a U.S. challenge virus, we hypothesized that IM immunization might provide better protection against the African HSV-2 strains as well. Indeed, we observed that IM immunization with dl5-29 gave better protection against HSV-2 SD90 challenge infection than the historical data using SC immunization. This protection was equivalent to that seen using U.S. challenge viruses, arguing that a different vaccine strain is not required for use against African strains. Accumulating data on HSV-2 sequence diversity are consistent with this. Genome sequences of 34 HSV-2 strains from around the world have shown less than 0.4% nucleotide divergence across the genome (Kolb et al., 2015: Newman et al., 2015); therefore, HSV-2 is very highly conserved. These genomic sequence results support the idea that IM immunization with HSV-2 dl5-29 should be capable of inducing protective immunity against HSV-2 strains from around the world.
Methods
Cells and Viruses
Vero cells (ATCC CCL-81) were used for propagation and titration of WT virus stocks by plaque assay (Spang, Godowski, and Knipe, 1983). The V529 complementing cell line (Da Costa et al., 2000) was used for propagation and titration of replication-defective viruses. The HSV-2 5BlacZ replication-defective mutant virus was derived from HVS-2 186syn+-1 virus (Da Costa et al., 1997). The 5BlacZ strain contains a UL29-lacZ gene fusion cassette in place of the essential UL29 ORF, driven by the promoter of the early UL29 gene. The dl5 virus is a replication-defective HSV-2 lacking UL5 but expressing UL29 (Da Costa et al., 2000). The HSV-2 dl5-29 replication-defective mutant virus was also generated from 186syn+ by deletion of the essential ORFs UL5 and UL29 (Da Costa et al., 2000; Da Costa et al., 1997). The HSV-2 5Bluciferase recombinant virus was derived from 5BlacZ virus and generated in our laboratory by replacing the UL29-lacZ gene fusion with a firefly luciferase expression cassette driven by the HSV-1 ICP22/ICP47 (IE4/5) gene promoter (Yamamoto et al., 2006). The low passage HSV-2 strain G virus, isolated in Chicago in the 1960s (Ejercito, Kieff, and Roizman, 1968), was obtained from Bernard Roizman and passaged <4 times in our laboratory. The HSV-2 SD90-3P virus derived from the SD90 virus, which was isolated from genital ulcer swab samples from an STD clinic in Carletonville, South Africa (Lai et al., 2003). Viral cultures of SD90 were originally grown at the CDC, and extracellular samples were transferred to Harvard Medical School. The viruses were passed 3 times on Vero cells in our lab to prepare stocks. The SD90-3P is a clonally derived virus derived from SD90 through three plaque purifications (Colgrove et al., 2014; Dudek et al., 2011). For immunizations, virus was purified from cell culture supernatant. Briefly, infected cells were cultured at 34°C until CPE was observed. Heparin was added to a final concentration of 50 μg/ml, and cells were transferred to 37°C for 6 h. Supernatant was collected and clarified by spinning at low speed (500 g for 10 min). Pellets were discarded, and medium containing virus was centrifuged at 20,000g for 90 min at 4°C. Pellets were resuspended in 5% glycerol in PBS.
For vaginal challenges, virus was used as infected cell lysate. Infected cells were incubated at 34°C until full CPE was observed. Cells were detached from flask by shaking or using a cell scraper and pelleted by low speed centrifugation. Supernatant was discarded and pellets resuspended in a 1:1 mix of medium and sterile non-fat milk. Cells were frozen/thawed once and sonicated for a total of 90 sec. Preparation was further clarified by low speed centrifugation.
Animal Studies
Animal housing and experiments were conducted according to protocols approved by the Harvard Medical Area Standing Committee on Animals.
Immunization and Challenge Studies
Five-week-old female BALB/c mice (Taconic Farms) were immunized twice, subcutaneously (SC) in the rear flank or intramuscularly (IM) in the gastrocnemius muscle, four weeks apart (day 0 and day 28), with 1×105 plaque-forming units (PFU) of supernatant dl5-29 virus in a 50 μl volume of PBS. At days 48 and 55, mice were injected subcutaneously in the scruff of the neck with 3 mg of Depo-Provera in a 100 μl volume. At 4 weeks after the second immunization (day 56), the vaginal cavities were pre-swabbed with a wet polyester swab, and mice were then challenged by intravaginal infection with a dose of wild-type HSV-2 G or SD90-3P strains equivalent to 50 times the LD50 (3.0×105 or 1.5×105 PFU respectively, as cell lysate preparation) in a 10 μl volume using a micropipettor as described previously (Morrison, Da Costa, and Knipe, 1998).
Assay of Acute Infection
On days 1–7 postinfection, the vaginal cavities of the mice were swabbed twice with pre-wetted polyester swabs. Each swab was placed in 1 ml of assay medium (DMEM, 0.1% glucose, 1% FCS, 5% glycerol) and stored at −80°C. Viral titers were determined by standard plaque assay on Vero cells.
Clinical Observations
Wild-type HSV-2 infected mice were observed daily on days 1–14 for signs of genital lesions and systemic illness. The severity of disease was scored as previously defined: 0 = no sign of disease; 1 = slight genital erythema and edema; 2 = moderate genital inflammation; 3 = purulent genital lesions; 4 = hind-limb paralysis (Morrison, Da Costa, and Knipe, 1998). Mice were euthanized with CO2 at the first sign of paralysis.
Assessment of cellular immunity
Six-week-old female C57Bl/6 mice (Taconic Farms or Charles River) were immunized twice, SC or IM as above, but with 1×106 PFU of supernatant dl5-29 virus in 50 μl of sterile PBS.
MHC-I Pentamer Staining of HSV gB-Specific CD8+ T-cells. Whole blood samples, approximately 100 μl, were collected via tail vein bleed. Red blood cells were lysed using RBC Lysis Buffer (eBioscience, Cat# 00-4300-54 San Diego, CA). White blood cells were pelleted and then resuspended in 20 μl PBS, 1% BSA, and incubated with 1 μl of HSV gB SSIEFARL, specific MHC-I pentamers (Proimmune Ltd, Oxford, United Kingdom) at room temperature for 15 min. Cells were washed and incubated with of 1μg of anti-CD16/CD32 antibodies (Fc Block, BD Biosciences) at 4°C for 10 min. Cells were washed, labeled by incubation with the R-PE fluorophore (ProImmune), and stained with antibodies against murine CD8α at 4°C for 30 min. Cells were washed, and samples were analyzed by flow cytometry. Results were expressed as the percentage of CD8α positive cells that stain positive for the gB-specific MHC-I pentamer.
Intracellular Cytokine Staining. Splenocytes from immunized C57Bl/6 mice were harvested on day 34. To analyze the CD8+ cellular immune response, 2×106 cells were incubated with anti-mouse CD28 antibody (2 μg) (BDBioscience) and the H2Kb-specific HSV gB SSIEFARL peptide (10 ng/ml) in a 37°C, 5% CO2 humidified incubator for 2 h, at which point Brefeldin A was added, and cells were incubated further for 4 h. Cells were washed in PBS-5mM EDTA and labeled with LIVE/DEAD fixable stain (Life Technologies Cat # 34957). After thorough washing with PBS-1% BSA-5mM EDTA, cells were stained with antibodies specific for murine CD3 and CD8α for 1 h, washed, then fixed with 1% formaldehyde in PBS, permeabilized in PBS + 0.2% saponin and stained with antibodies for IFN-γ for 16 h at 4°C. Samples were analyzed via flow cytometry gating on live CD3+ cells. The results were expressed as the percentage of cells staining positively for CD8α that also stain positive for IFN-γ when specifically stimulated with the gB peptide. All flow cytometry data were analyzed with FlowJo (FlowJo, Ashland OR).
Assessment of humoral immunity
At 1 week after each vaccine dose, mice were bled from the tail vein, and the sera were separated in BD Microtainer tubes (BD, Ref# 365956). Total anti-HSV-2 antibody titers were determined by ELISA in 96-well flat-bottomed plates (Nunc, MaxiSorp) coated with 50 ng/well of purified HSV-2 (Advanced Biotech, Cat# 10-146-000) in 50 μl of carbonate-bicarbonate buffer pH 9.6. Bound immunoglobulin was detected by alkaline phosphatase-conjugated rabbit anti-mouse antibody followed by p-Nitrophenyl phosphate (Sigma, Cat# A4312, N2770) and measuring absorbance at 405nm. The endpoint titer was determined according to Frey et al. (Frey, Di Canzio, and Zurakowski, 1998).
Intravital bioluminescence imaging
Female BALB/c mice (Taconic Farms) were inoculated with 1×105 PFU of extracellular HSV-2 5BLuciferase virus either SC or IM as above. Negative control animals were injected with PBS. At the times indicated, mice were anesthetized with isoflurane, and D-luciferin (150 mg/Kg, in 150 μl, Promega Cat. #P1043) was injected SC in the scruff of the neck. Animals were imaged in an in vivo imaging system (IVIS) Lumina LT (Perkin Elmer) connected to a gas anesthesia system for 20–30 minutes to capture luminescence signal at its peak. Bioluminescence was quantified with Living Image imaging software (Perkin Elmer) and expressed as normalized radiance (photons/second/cm2/steradian).
Histochemistry and X-gal staining
Female CD-1 mice (Charles River) were immunized with 1×105 PFU of extracellular HSV-2 5BLacZ or a mix of 5BLacZ + dl5 as before. At 16 h post vaccination, mice were sacrificed by CO2 asphyxiation, and the rear legs (including the skin in the rear flank for the SC route) were detached from the carcass and fixed (including the bone) in EM grade paraformaldehyde (EMS Cat# RT15700) on ice for 2 h. After rinsing in PBS, the gastrocnemius muscle was dissected from the leg and sliced with a surgical blade into pieces no more than 1–2 mm thick. Tissue was stained with HistoMark X-gal (KPL Cat# 54-13-00) for 3 h at 37°C. The stained muscle was then extensively rinsed in PBS and placed in 70% ethanol. Non-infected tissue was processed in the same way as the HSV-2 5BLacZ infected muscle. Samples were paraffin embedded, sectioned, and stained with a rabbit polyclonal anti-HSV-2 antibody (Abcam, Cat. # 9534) or H&E at the Dana-Farber/Harvard Cancer Center Specialized Histopathology Services Core Facility. Color images were acquired with a Nikon Eclipse Ti microscope using the 20× and 40× objectives and a Nikon Digital Sight DS-Fi1 camera.
Statistical analysis
The indicated statistical tests were performed with GraphPad Prism statistical software.
Highlights.
Intramuscular immunization with HSV-2 is more protective than subcutaneous immunization.
IM immunization yields higher humoral and cellular responses.
IM immunization gives higher expression of viral proteins.
IM immunization provides protection against highly virulent clinical isolates of HSV-2.
Acknowledgments
We thank Jeho Shin for technical assistance and Patrick T. Waters for assistance in preparation of the manuscript. This research was supported by grant AI057552 to DMK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awasthi S, Zumbrun EE, Si H, Wang F, Shaw CE, Cai M, Lubinski JM, Barrett SM, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. Live attenuated herpes simplex virus 2 glycoprotein E deletion mutant as a vaccine candidate defective in neuronal spread. J Virol. 2012;86:4586–98. doi: 10.1128/JVI.07203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K. Epigenetic control of skeletal muscle fibre type. Acta Physiol (Oxf) 2010;199:477–87. doi: 10.1111/j.1748-1716.2010.02121.x. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- Colgrove R, Diaz F, Newman R, Saif S, Shea T, Young S, Henn M, Knipe DM. Genomic sequences of a low passage herpes simplex virus 2 clinical isolate and its plaque-purified derivative strain. Virology. 2014;450–451:140–5. doi: 10.1016/j.virol.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa X, Kramer MF, Zhu J, Brockman MA, Knipe DM. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J Virol. 2000;74:7963–71. doi: 10.1128/jvi.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa XJ, Bourne N, Stanberry LR, Knipe DM. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital disease. Virology. 1997;232:1–12. doi: 10.1006/viro.1997.8564. [DOI] [PubMed] [Google Scholar]
- Delagrave S, Hernandez H, Zhou C, Hamberger JF, Mundle ST, Catalan J, Baloglu S, Anderson SF, DiNapoli JM, Londono-Hayes P, Parrington M, Almond J, Kleanthous H. Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model. PLoS One. 2012;7:e46714. doi: 10.1371/journal.pone.0046714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FM, Knipe DM. Protection from genital herpes disease, seroconversion and latent infection in a non-lethal murine genital infection model by immunization with an HSV-2 replication-defective mutant virus. Virology. 2016;488:61–7. doi: 10.1016/j.virol.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek T, Mathews LC, Knipe DM. Disruption of the U(L)41 gene in the herpes simplex virus 2 dl5-29 mutant increases its immunogenicity and protective capacity in a murine model of genital herpes. Virology. 2008;372:165–75. doi: 10.1016/j.virol.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis. 2011;203:1434–41. doi: 10.1093/infdis/jir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effect on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221:35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- Kolb AW, Larsen IV, Cuellar JA, Brandt CR. Genomic, phylogenetic, and recombinational characterization of herpes simplex virus 2 strains. Journal of Virology. 2015;89:6427–6434. doi: 10.1128/JVI.00416-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W, Chen CY, Morse SA, Htun Y, Fehler HG, Liu H, Ballard RC. Increasing relative prevalence of HSV-2 infection among men with genital ulcers from a mining community in South Africa. Sex Transm Infect. 2003;79:202–7. doi: 10.1136/sti.79.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL, Hargreaves M. Histone modifications and exercise adaptations. J Appl Physiol (1985) 2011;110:258–63. doi: 10.1152/japplphysiol.00979.2010. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- Newman RM, Lamers SL, Weiner B, Ray SC, Colgrove RC, Diaz F, Jing L, Wang K, Saif S, Young S, Henn M, Laeyendecker O, Tobian AA, Cohen JI, Koelle DM, Quinn TC, Knipe DM. Genome Sequencing and Analysis of Geographically Diverse Clinical Isolates of Herpes Simplex Virus 2. J Virol. 2015;89:8219–32. doi: 10.1128/JVI.01303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, Sengupta M, Herold BC, Jacobs WR. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife. 2015;4 doi: 10.7554/eLife.06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley RJ. Herpes Simplex Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 1823–1897. [Google Scholar]
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–7. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, Lundberg A, Larson S, Bravo FJ, Bernstein DI, Flechtner JB, Long D. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J Virol. 2013;87:3930–42. doi: 10.1128/JVI.02745-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang AE, Godowski PJ, Knipe DM. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J Virol. 1983;45:332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Langenberg AG, Link K, Izu AE, Ashley R, Warren T, Tyring S, Douglas JM, Jr, Corey L. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA. 2001;285:3100–3106. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- Wang K, Kappel JD, Canders C, Davila WF, Sayre D, Chavez M, Pesnicak L, Cohen JI. A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM. J Virol. 2012;86:12891–902. doi: 10.1128/JVI.01055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Navarro F, Lal A, Basar E, Pandey RK, Manoharan M, Feng Y, Lee SJ, Lieberman J, Palliser D. Durable protection from Herpes Simplex Virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell Host Microbe. 2009;5:84–94. doi: 10.1016/j.chom.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Deckter LA, Kasai K, Chiocca EA, Saeki Y. Imaging immediate-early and strict-late promoter activity during oncolytic herpes simplex virus type 1 infection and replication in tumors. Gene Ther. 2006;13:1731–6. doi: 10.1038/sj.gt.3302831. [DOI] [PubMed] [Google Scholar]


