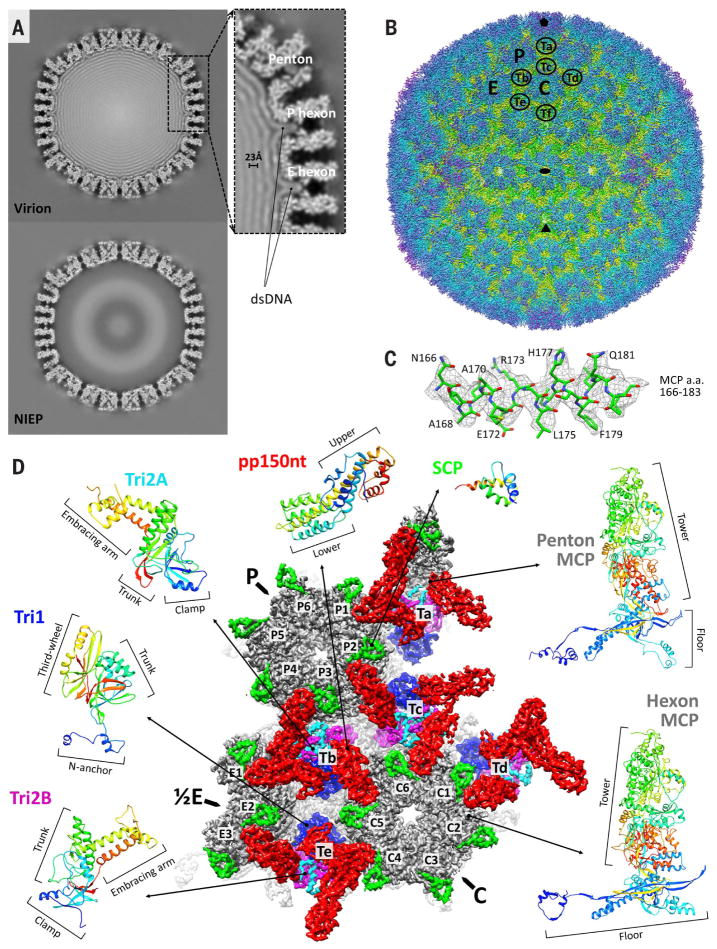
Fig. 1. CryoEM reconstruction and atomic modeling of HCMV.
(A) Central slices of HCMV virion (top) and noninfectious enveloped particle (NIEP, bottom) reconstructions at 15 Å. The inset shows a 23-Å dsDNA interlayer distance and dsDNA density within hexon channels. (B) Radially colored HCMV reconstruction at 3.9-Å resolution viewed along a twofold axis. Fivefold, threefold, and twofold axes are denoted by a pentagon, triangle, and oval, respectively. P (peripentonal), C (center), and E (edge) denote hexons and Ta to Tf denote triplexes that together contribute to an asymmetric unit. (C) Density map (mesh) and atomic model of an MCP helix illustrate side chain features. A, alanine; E, glutamic acid; F, phenylalanine; H, histidine; L, leucine; N, asparagine; Q, glutamine; R, arginine; a.a., amino acids. (D) Asymmetric unit colored by protein subunit type. MCPs (gray) make up pentons and hexons. Triplexes are heterotrimers composed of Tri1 (blue) and a Tri2A (cyan)–Tri2B (magenta) dimer. SCPs (bright green) bind to all MCPs, whereas pp150 tegument proteins (red) cluster above triplexes. Rainbow ribbon models show individual proteins and conformers (blue N terminus through green and yellow to red C terminus). pp150nt, N-terminal one-third of pp150.