Abstract
Optical methods to interrogate membrane potential changes in neurons promise to revolutionize our ability to dissect the activity of individual cells embedded in neural circuits underlying behavior and sensation. A number of voltage imaging strategies have emerged in the last few years. This Perspective discusses developments in both small molecule and genetically-encoded fluorescent indicators of membrane potential. We survey recent advances in small molecule fluorescent indicators that rely on photoinduced electron transfer (PeT) to sense voltage as well as refinements of voltage-sensitive fluorescent proteins and new opsin-based strategies for monitoring voltage changes. We compare the requirements of fluorescent voltage indicators to those for more canonical Ca2+ sensing as a way to illuminate the particular challenges associated with voltage imaging.
Graphical abstract
Introduction
Neuronal membrane voltage changes drive signaling in the brain and are a key component of the emergent properties of the human experience. Despite the important of membrane voltage to neurobiology, most of the knowledge we have about the electrophysiological behavior of neurons arises from electrode-based methods, which usually involve the observation of single neurons at a time. Deeper understanding of neural circuits requires the ability to make simultaneous measurements of large numbers of neurons without compromising temporal or spatial resolution. A popular method for interrogating neuronal circuit behavior is calcium imaging; however, it remains an indirect measure of neuronal activity complicated by the slow intrinsic kinetics of calcium transients relative to underlying membrane potential dynamics. While small-molecule and genetically-encoded calcium sensors have transformed our ability to indirectly measure neuronal activity,1 deconvolution of detected calcium transients into the underlying voltage changes remains a difficult problem.
Direct fluorescence imaging of voltage presents an attractive method for studying neural circuits, complementary to traditional electrode-based methods and imaging modalities that rely on Ca2+ fluxes. The field of voltage imaging with fluorescent indicators spans nearly four decades. There has been a recently flurry of activity in the development of new methods to optically monitor voltage. These new indicators, both small molecule and genetically-encoded, have made strides towards realizing the potential of voltage imaging. In this perspective, we provide a brief overview of recent chemical and genetic strategies employed for voltage imaging. We then discus universal constraints on optical voltage determination that apply to all sensor designs, with the hope of providing a perspective on upcoming challenges.
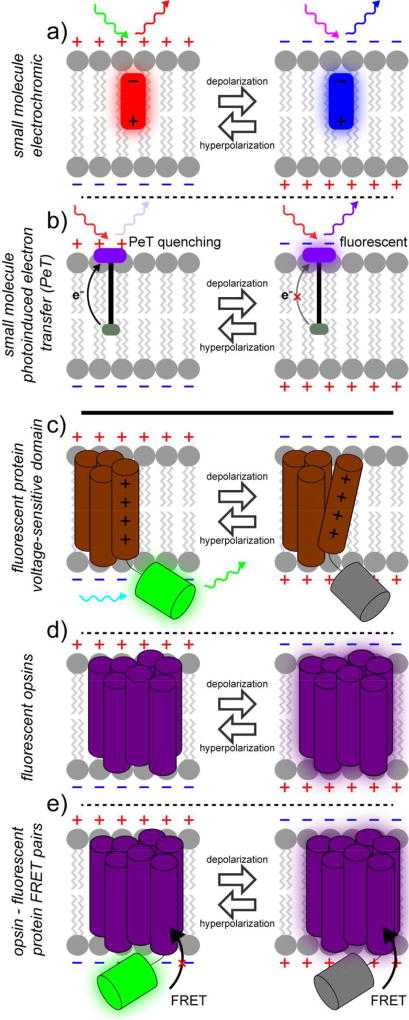
Pioneering work in the 1970s identified several different classes of small molecules that possessed voltage-sensitive optical properties, and this work is extensively reviewed elsewhere.2–4 Small molecule voltage-sensitive fluorescent indicators generally fall into two classes: electrochromic-type dyes (Figure 1a), which possess response speeds sufficient to track to action potentials, but with low sensitivity;5 and oxonols, which have larger fractional fluorescence responses to voltage, but respond so slow as to prohibit visualization of discrete action potentials.6 Recent work in our lab has employed photoinduced electron transfer (PeT) as a voltage sensitive trigger that achieves both fast (sub-millisecond response times) and sensitive (>60% ΔF/F per 100 mV) monitoring of membrane potential dynamics.
Figure 1.
Fluorescent voltage indicators. Numerous strategies for optically sensing voltage changes in living cells exist. Small molecule fluorophore platforms (upper panels) include electrochromic dyes (a) and photo-induced electron transfer (PeT) approaches (b). Genetically encoded strategies (lower panels) include fusions of fluorescent proteins to voltage-sensing domains (c), use of opsins (d), and hybrid opsin – fluorescent protein pairs (e). [note: single column figure]
In contrast, genetically-encoded strategies were first employed about 20 years ago. The first genetically-encoded voltage sensitive fluorescent proteins were fusions of native ion channels or voltage sensing domains and fluorescent proteins. The first indicators were in principal voltage sensitive, but suffered from severe trafficking defects that prohibited efficient expression at the cell surface. Poor membrane targeting limited their utility for detecting action potentials in neurons. Recent iterations have improved trafficking, kinetics and sensitivity. A complementary addition to the genetically encoded toolkit makes use of light-sensitive opsins. This is a new mechanism for sensing voltage and has helped to reinvigorate the field of voltage sensing by enabling opsin or opsin-fluorescent protein hybrid voltage sensing strategies. Comprehensive reviews of voltage-sensitive fluorescent proteins are available.7–10 Below, we will first discuss recent dye-based voltage-sensing strategies followed by new protein-based strategies.
Small-molecule fluorescent voltage indicators
Our lab has been exploring photoinduced electron transfer (PeT) as a platform for optical voltage sensing. The general scaffold of these PeT-based voltage sensors, or VoltageFluors, consists of a fluorescent reporter molecule covalently linked to an electron-rich molecular wire-donor moiety that quenches the fluorescent reporter via photoinduced electron transfer (PeT). The rate of PeT is sensitive to the presence of an external electric field, enabling it to be used as a voltage-sensitive mechanism of fluorescence quenching. By orienting the dye in the membrane so that the electron-rich donor is intercalated into the plasma membrane, the resting membrane potential accelerates the rate of PeT, quenching the fluorophore. Depolarization inhibits PeT, resulting in an increase in fluorescence (Figure 1b).
The use of PeT as a voltage-sensing mechanism affords unique opportunities important for voltage imaging. In the context of a VoltageFluor, the rate of PeT must occur within the nanosecond lifetime of the fluorophore excited state. This makes PeT processes approximately 6 orders of magnitude faster than an action potential and allows VF dyes to effectively resolve AP kinetics. Additionally, PeT modulates the fluorescence efficiency of the dye in a voltage-dependent fashion, making all of the emitted photons voltage-sensitive and useful for imaging. Finally, moving an electron over a relatively long distance (approximately half-way across a typical 4 nm plasma membrane), results in high sensitivity, without sacrificing response kinetics. We control the orientation of VoltageFluors dyes in the membrane using a combination of a hydrophobic molecular wire coupled with an anionic anchoring group, usually a sulfonate11 or a tertiary amide.12 After bath application of the VoltageFluor, the hydrophobic wire partitions into the plasma membrane, but the water-soluble anchor prevents the dye from crossing the membrane, ensuring that the dye is uniformly oriented in the outer leaflet of the plasma membrane.
Sensitivity of VF dyes can be improved by tuning the relative redox potentials of the donor/acceptor pairs13 or by improving dye orientation in the plasma membrane.14 Because the reporter (fluorescent dye) is chemically orthogonal to the voltage sensing domain (molecular wire/aniline) the VF scaffold enables ready exchange the fluorophore acceptor to access a wide range of the visible and near-infrared portions of the electromagnetic spectrum,12, 15 as well as optimized VoltageFluors for two-photon voltage imaging.16 Although VoltageFluors are bright, sensitive, and employ a fast and non-disruptive mechanism of voltage sensing, their pan-membrane localization introduces a source of background noise in heterogeneous samples, limiting sensitivity.13, 16 We have developed a small-molecule photoactivatable optical sensor of transmembrane potential, or SPOT, a photocaged voltage sensor based on the first generation VoltageFluor molecules that enables cellular contrast via a photoactivation mechanism.17 Other strategies we are exploring involve the use of enzymatically cleavable masking groups and self-labeling enzyme/ligand pairs to target VoltageFluors to specific neuronal subtypes.
Fluorescent protein-based voltage indicators
A voltage-sensitive fluorescent protein was first reported in 1997, when Iscaoff and Siegel fused the green fluorescent protein (GFP) to the Shaker potassium channel.18 Although the kinetics of the optical response to voltage were too slow to enable action potential tracking, this study showed that membrane potential-induced conformational changes could alter the fluorescence of GFP. In 2001, Knopfel and co-workers showed that voltage sensitivity could be achieved by using just the voltage-sensing domain of a potassium channel and coupled this movement to changes in FRET efficiency between cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP).19 In 2002, Pieribone fused GFP to a sodium channel to achieve fast kinetics.20 This first generation of voltage-sensitive fluorescent proteins represent an important first step, but combinations of slow response kinetics, poor membrane localization, and/or low sensitivity hindered their wide-spread use.
In 2007, Knopfel and co-workers discovered that the use of the voltage-sensing domain from the voltage-sensitive phosphatase (VSP) from Ciona intestinalis improved membrane localization, response kinetics, and sensitivity.21 Most of the recently-deployed FP-only strategies (Figure 1c) make use of the C. intestinalis VSP domain (CiVSD). Pieribone, Cohen, and co-workers demonstrated that insertion of super-ecliptic pHluorin to an intracellular loop of the CiVSD, results in a voltage-sensitive fluorescent protein. This construct, ArcLight, displays high sensitivity to membrane potential changes and sufficient kinetics and brightness to detect action potentials in neurons.22 Although the precise molecular mechanisms by which CiVSD transduces fluorescence changes in FPs remain incompletely characterized, for the case of ArcLight, depolarization causes a turn-off response. ArcLight has been used in vivo for dissecting neural circuits underlying odor perception in the fly.23 Recently, Pieribone and co-workers uncovered mutations in ArcLight which reverses the polarity of response. This new indicator, Marina, gives fluorescence increases upon depolarization and maintains the response magnitude and kinetics of the parent ArcLight.24
Use of non-canonical FPs, such as super-ecliptic pHluorin, have proved a useful strategy for generating voltage-sensitive fluorescent proteins. Another example is the use of circularly permuted GFPs (cpGFPs). A screen of CiVSD and cpGFP insertions yielded ElectricPk, which had exceptional kinetics and good trafficking to plasma membranes. This study showed that cpGFPs and CiVSDs enable fast detection of voltage changes in an FP-only sensor.25 Building off of this work, Lin and co-workers described the development of ASAP, in which cpGFP is inserted into an extracellular loop of the CiVSD. The ASAP family of indicators maintains the rapid kinetics and turn-off response of the ElectricPk construct while improving voltage sensitivity by an order of magnitude. This enables detection of action potentials in single trials in cultured neurons. Improved versions of ASAP have been employed in intact flies.26 Insertion of circularly-permuted mApple (cpmApple), a red-fluorescent protein, into an intracellular loop of CiVSD enabled the development of FlicR1 (fluorescent indicator for voltage imaging red), which provides a turn-on response to action potentials and retains good response magnitude and kinetics.27
Opsin-based voltage indicators
Genetically encoding sensors of transmembrane potential typically rely on the fusion of a fluorescent protein (or two) to an ion channel or voltage sensing domain. A more recent approach uses the natural electrochromism of bacteriorhodopsins. Light-gated ion pumps and channels find wide application in neurobiology for optical activation or silencing of neurons, since cells which express these membrane-bound proteins now pass ions in response to light, thereby changing their membrane potential.28 In 2011, Cohen’s group showed that these opsin proteins could be “run in reverse,” that is, changes in membrane potential causes a change in their fluorescence.29, 30 The reversible electric field-induced deprotonation event in bacteriorhodopsins modulates their fluorescence and makes these proteins an attractive candidate for genetically-encoded voltage indicators (Figure 1d). Cohen and coworkers developed the Arch system, based on the light-gated proton pump Archaerhodopsin 3 (Arch),29 and showed that the weak fluorescence of the all-trans-retinal (ATR) cofactor of Arch was modulated by changes in membrane potential. By deactivating the proton pumping mechanism with a single amino acid mutation (D95N) and taking advantage of the improved eukaryotic membrane localization of Arch 3, Arch(D95N) can effectively report on voltage changes in cells without the induction of a photocurrent. Despite the high voltage sensitivities and turn-on responses of rhodopsin-based voltage indicators, they require high light power to achieve sufficient fluorescence, as a result of their complex photocycle.31 Mutational screens identified a few mutations that improve brightness32 (Archer, or Arch with enhanced radiance) or kinetics,33 relative to Arch(D95N). Focused screening to improve brightness and reduce photocurrent, while retaining the fast kinetics of Arch resulted in the development of QuasAr1 and QuasAr2 (Quality superior to Arch).34 With improvements in brightness and further bathochromic excitation shifts, QuasAr1 can be used in conjunction with optogenetic actuators to faithfully report neuronal action potentials. More recently, synthetic analogs of ATR, merocyanine retinals (MCR), coupled with evolved Arch mutants with binding preferences for MCR over ATR provide access to brighter and red-shifted variants of Arch.35 The most promising Arch mutant, Mero-6, displays strong, membrane-associated, near-infrared fluorescence in E. coli expressing Mero-6 and treated with MCR. Trafficking to the plasma membrane in eukaryotic cells is poor relative to E. coli. Despite this, the Mero-6/MCR pair displays some voltage sensitivity and may be a promising platform for future development.
Dual opsin-fluorescent protein strategies
In addition to improving the intrinsic brightness of the opsin indicators, another solution is to couple opsin voltage indicators with a brighter reporter via energy transfer (Figure 1e). Decoupling the voltage sensing component (opsin) from the optical reporter (fluorescent protein), conceptually similar to the sensor/reporter decoupling in VoltageFluor dyes, allows for flexibility in choosing both the voltage sensor and optical reporter. This strategy was initially used to investigate the photocycle of opsins,36 because changes in the absorption spectrum of the opsin alter the efficiency of fluorescence resonance energy transfer (FRET) from a nearby fluorophore. Changes in FRET efficiency indicate changes in the absorption spectrum of the opsin. If the spectrum of the opsin is altered by changes in transmembrane potential, the fluorescence of the FRET donor provides a convenient signal for monitoring voltage.37 This approach, variously described as FRET-opsin38 or electrochromic FRET (eFRET),39 has been implemented across a number of opsins—including Arch,37 QuasAR (Arch parent),39 Mac (L. maculans),38 and Ace (A. acetabulum)40—paired with assorted fluorescent proteins—mOrange2,39 mCitrine,38 or mNeon.40 The proposed mechanism is that depolarization of the plasma membrane results in an increase in the absorption spectrum of the opsin, making it a better FRET acceptor and quenching fluorescence from the fluorescent protein donor. This results in a fluorescence decrease upon membrane depolarization – whereas the opsin fluorescence itself increases upon depolarization. FRET-opsin or eFRET efficiency is governed by the usual FRET parameters: both distance and spectral overlap between donor and acceptor influence FRET efficiency. Recently, a FRET-opsin hybrid, Ace2-mNeon, was deployed to optically record spikes in intact mouse and fly brains.40 The improved performance of Ace2-mNeon pair is, in part, aided by the hypsochromic spectral shift of the Ace chromophore relative to Mac, providing enhanced overlap with the bright fluorescent protein, mNeon.41 Matching rhodopsins (and mutants) with desirable photocycle kinetics and spectral overlap with fluorescent reporters may be source of enhanced sensitivity and/or brightness. Enhancing trafficking to cell membrane and substantially reducing photocurrents while maintaining response kinetics offer pathways for improvement of these indicators.
Universal challenges for voltage imaging
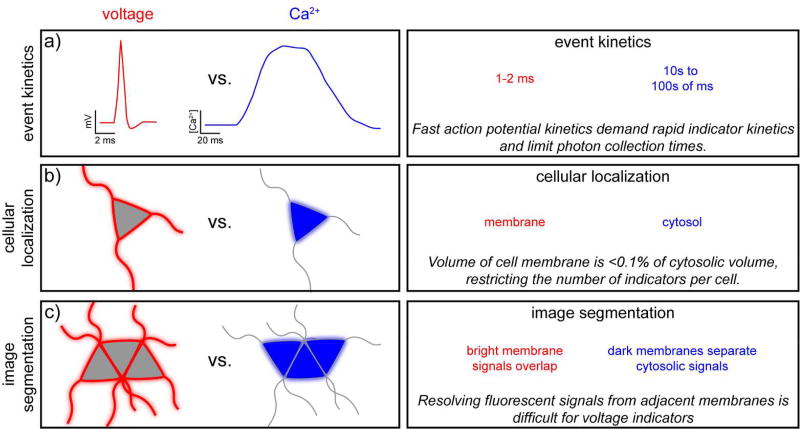
Whether chemically-synthesized or genetically encoded, optical voltage determination faces inherent challenges. We outline a few of these considerations below and compare them to considerations for fluorescent Ca2+ indicators (Figure 2), which are the most prevalent functional imaging modality in neurobiology.
Figure 2.
Design challenges encountered in the development of fluorescent voltage indicators. Constraints for the design of fluorescent voltage indicators (red) are contrasted against the requirements of fluorescent Ca2+ indicators (blue). [note: double column figure]
The first consideration is the speed of the biological event of interest: the neuronal action potential (Figure 2a). The electric field reorientation that occurs during an action potential occurs on timescale of one to two milliseconds with the fastest component, the rising phase, occurring over the course of approximately 250 µs.42 Therefore the response kinetics of a voltage indicator must be able to resolve changes on the sub-millisecond timescale. Achieving sufficiently fast response speed while maintaining high sensitivity is the primary hurdle for voltage indicators. While neuronal action potentials have a fleeting duration, on the order of several milliseconds, Ca2+ concentration increases can persist for 10s to 100s of milliseconds inside of a neuron. The consequences of this are several-fold. First, image acquisition must be fast, on the order of 1000 Hz to accurately sample action potentials. Acquisition speeds for Ca2+ imaging can be in the 1 to 10 Hz range. Relative to Ca2+ indicators, voltage indicators therefore have less time to deliver photons to the imaging detector, making each measurement inherently noisier. To compensate, voltage indicators need to have higher inherent brightness than Ca2+ indicators. One cannot bypass this restriction by acquiring images at lower rates, because slower sampling frequencies will result in “missed” events. Achieving fast acquisition speeds (>1 kHz) while maintaining a large field of view is a related hurdle faced uniquely by voltage imaging—more detailed reviews of the quantitative aspects of signal detection for imaging43 and voltage imaging,44 in particular are available. For imaging modalities well-suited for thick tissues, like raster-scanning two-photon microscopy, acquiring large fields of view at fast rates is difficult for voltage imaging, but less problematic for Ca2+ imaging. A number of promising strategies are pushing the limits of acquisition rate during two-photon imaging.45 Future progress in this area will be required to capitalize on the full potential of voltage imaging.
The second universal constraint is indicator localization (Figure 2b). Ca2+ indicators function by translating the rise in intracellular Ca2+ concentration into changes in fluorescence intensity or color. Mediated through ionotropic receptors, voltage-gated Ca2+ channels or release from internal stores, cytosolic Ca2+ concentration can increase by one to two orders of magnitude over their 50–100 nM resting concentration. Ca2+ indicators work best when localized to cytosolic compartments. In contrast, voltage indicators must localize to the plasma membrane in order to properly sense voltage. Improperly localized voltage indicator erodes sensitivity by raising the level of background fluorescence from non-response indicator. However, even given perfect localization, the total amount of voltage indicator that can “fit” in the volume of the plasma membrane on the order of <0.1% of the amount of Ca2+ indicator that can fit in the cytosol (based on the relative volumes of plasma membrane and cytosol for a perfectly spherical cell with a 10 µm radius and 4 nm plasma membrane). This restriction places a further design challenge on voltage indicators if they are to match the performance of a typical Ca2+ indicator.
Finally, image analysis and segmentation must be considered for voltage imaging (Figure 2c). One aspect of the appeal of optical indicators is the ability to record from multiple neurons simultaneous. Ca2+ indicator-stained neurons appear as bright islands, segmented by the plasma membrane of adjacent cells. Voltage indicators, in contrast, give a “chicken wire” staining pattern when cell bodies abut one another. Optically resolving two adjacent membranes using just their fluorescent signals is difficult and presents a unique challenge that must be solved by all optical voltage-sensing modalities. In areas like the CA1 region of the hippocampus, where cell bodies are positioned in ordered arrays, this represents a particular challenge. In brain regions like the cortex, where cell bodies can be more sparsely scattered, this may be less of an issue. Nonetheless, registering signals from hundreds of neurons with cytosolic Ca2+ indicators is challenging and the problem is compounded with voltage imaging, where fluorescence signals overlap in space.
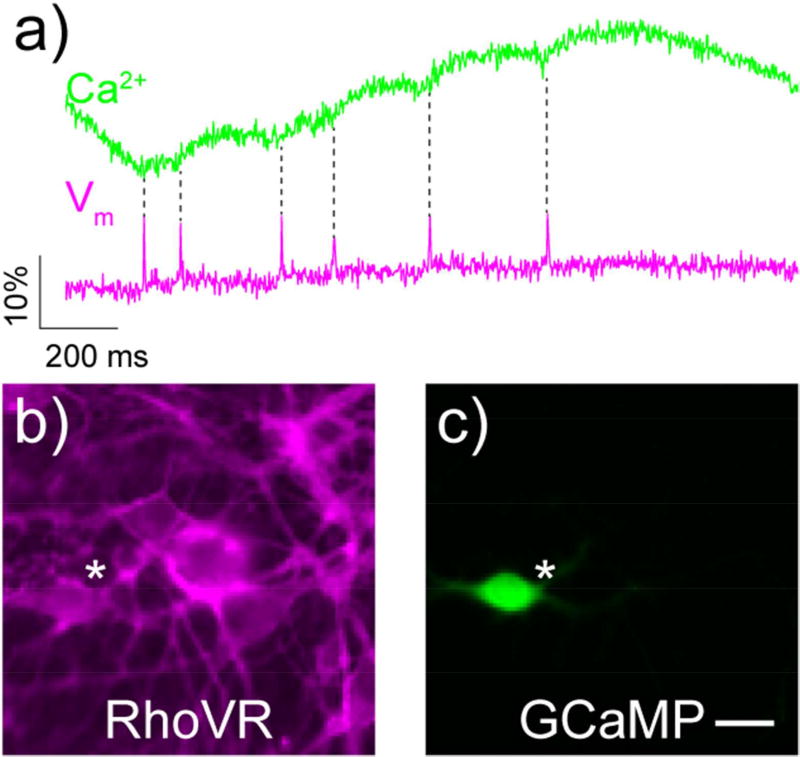
Overall, compared to Ca2+ imaging, voltage imaging requires acquiring data at 100–1000 times faster speeds, resulting in 100 to 1000-fold fewer photons collected per cycle. Voltage imaging must be done with concentrations of indicators limited to just the plasma membrane, resulting in 1000-fold fewer indicators per cell. Finally, because fluorescent signals from voltage indicators come from the plasma membrane rather than cytosol, segmentation of images and resolving individual cells is more difficult than for cytosolic Ca2+ indicators. Despite having to do “more with less”, voltage indicators can achieve good success in detecting single action potentials. For example, the red voltage indicator developed in our lab, RhoVR 1 shows excellent signal to noise and good ΔF/F, in addition to far superior kinetics for single action potentials when compared to the genetically encoded Ca2+ indicator GCaMP (Figure 3).12
Figure 3.
Comparison of voltage and Ca2+ imaging in neurons. a) Ca2+ (green) and voltage (magenta) responses to single, spontaneously-arising action potentials in the same rat hippocampal neuron, as recorded by GCaMP6s (green) and the voltage-sensitive dye, RhoVR 1 (magenta). Panels b and c depict the neuron (white star) analyzed in part a. Scale bar is 20 µm. Figure adapted from Deal, et al., 2017. [note: single column figure]
In addition to the universal constraints or design challenges of all voltage indicators, there are some specific considerations related to particular methods of voltage sensing. First, because voltage indicators must reside in the plasma membrane to sense voltage, it is important to consider delivery routes for a particular indicator to the plasma membrane. For small molecule approaches, this means developing amphipathic indicators that can exist in aqueous environments en route to the cell of interest, reside in the hydrophobic cell membrane environment, and avoid internalization once reaching the membrane of interest. VoltageFluor dyes do both of these well, and bath application of VF dyes gives membrane localized fluorescence.12, 14 However, small molecule voltage indicators display poor selectivity for particular cell types and stain all membranes, making cellular resolution difficult.13, 16 We have developed photoactivation methods to address the problem of contrast,17 and development of complementary approaches with genetically encoded components is underway in our lab.
Genetically-encoded voltage indicators offer cell-type specificity but face similar challenges in proper targeting to the cell membrane. Indeed, the very first generation of fluorescent protein- and opsin-based voltage indicators suffered from poor membrane trafficking in mammalian cells, limiting their performance.19, 30 Trafficking to the plasma membrane has improved with both the use of voltage sensing domains from CiVSD and targeting sequences that can, in some cases, improve membrane trafficking. Poor trafficking of genetically encoded voltage sensitive fluorescent proteins results, at best, in intracellular fluorescence that is not voltage sensitive and, at worst, in indicators that do not function in mammalian cells.30 Optimal trafficking signals remain an empirically determined choice and improvements in this area are needed.
Second, with any indicator, care must be taken that use of an indicator does not alter the observed event itself. Ca2+ indicators can buffer native Ca2+ fluxes. All voltage indicators can, depending on the mechanism of sensing, change the excitability of neurons in a number of ways. Added capacitance coming from charges moving within the membrane on the same time scale as biological charges perturbs the intrinsic properties of the neuron under study—this is most problematic for “slow response” dyes6 and is not encountered for electrochromic or PeT-based dyes. For genetically encoded indicators, the gating charges on the voltage sensing domains (VSDs) used in fluorescent protein-based indicators could also increase capacitance. If high concentrations of electrochromic or oxonol indicators are required, this can alter neuronal responses by potentiating the GABA-A receptor.46 Phototoxicity—often through generation of singlet oxygen and other reactive oxygen species—can compromise membrane integrity. Another perturbation specific to opsin-based approaches is the generation of a photocurrent. For sensing methodologies based on opsins, removal of the native light-induced charge transport function of the proteins must be mitigated while maintaining voltage sensitivity and response kinetics. For each of the approaches discussed above, these disruptive properties must be considered during any imaging application.
Summary/Outlook
In summary, voltage imaging presents a unique opportunity to peer into the inner workings of neural systems with unprecedented spatial and temporal resolution. Yet, despite the clear promise of voltage imaging, widespread application of voltage imaging lags behind techniques like Ca2+ imaging. This gap is due, in part, to a lack of methods that can faithfully record neuronal action potentials with the required speed and sensitivity. As discussed in this Perspective, the requirements for voltage imaging are more demanding than the requirements for Ca2+ imaging. Voltage indicators must respond orders of magnitude more quickly and occupy a small fractional volume of cell bodies. These constraints place sharp demands on fluorescent voltage indicators, which must be faster, brighter, and more sensitive than corresponding Ca2+ indicators. Despite these challenges, in the last few years, several complementary approaches have emerged, offering new promise for voltage imaging. Small molecule PeT-based indicators, fluorescent protein-CiVSD fusions, voltage-sensitive opsins, and opsinfluorescent protein hybrid all address aspects of the challenges outlined above. In future years, these approaches, and others, such as the use of nanodiamonds47 or nanoparticles48 embedded in plasma membranes, will need to address the pitfalls outlined here in order to reach the full potential of voltage imaging.
References
- 1.Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Loew LM. Design and Use of Organic Voltage Sensitive Dyes. In: Canepari M, Zecevic D, Bernus O, editors. Membrane Potential Imaging in the Nervous System and Heart. Springer-Verlag Berlin; Berlin: 2015. pp. 27–53. [Google Scholar]
- 3.Miller EW. Small molecule fluorescent voltage indicators for studying membrane potential. Curr Opin Chem Biol. 2016;33:74–80. doi: 10.1016/j.cbpa.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braubach O, Cohen LB, Choi Y. Historical Overview and General Methods of Membrane Potential Imaging. In: Canepari M, Zecevic D, Bernus O, editors. Membrane Potential Imaging in the Nervous System and Heart. Springer International Publishing; Cham: 2015. pp. 3–26. [DOI] [PubMed] [Google Scholar]
- 5.Fluhler E, Burnham VG, Loew LM. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985;24:5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez JE, Tsien RY. Voltage sensing by fluorescence resonance energy transfer in single cells. Biophys J. 1995;69:1272–1280. doi: 10.1016/S0006-3495(95)80029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inagaki S, Nagai T. Current progress in genetically encoded voltage indicators for neural activity recording. Curr Opin Chem Biol. 2016;33:95–100. doi: 10.1016/j.cbpa.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nat Neurosci. 2016;19:1142–1153. doi: 10.1038/nn.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Zou P, Cohen AE. Voltage imaging with genetically encoded indicators. Curr Opin Chem Biol. 2017;39:1–10. doi: 10.1016/j.cbpa.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang HH, St-Pierre F. Genetically Encoded Voltage Indicators: Opportunities and Challenges. J Neurosci. 2016;36:9977–9989. doi: 10.1523/JNEUROSCI.1095-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB, Jr, Tsien RY. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc Natl Acad Sci U S A. 2012;109:2114–2119. doi: 10.1073/pnas.1120694109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deal PE, Kulkarni RU, Al-Abdullatif SH, Miller EW. Isomerically Pure Tetramethylrhodamine Voltage Reporters. J Am Chem Soc. 2016;138:9085–9088. doi: 10.1021/jacs.6b05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodford CR, Frady EP, Smith RS, Morey B, Canzi G, Palida SF, Araneda RC, Kristan WB, Jr, Kubiak CP, Miller EW, Tsien RY. Improved PeT molecules for optically sensing voltage in neurons. J Am Chem Soc. 2015;137:1817–1824. doi: 10.1021/ja510602z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni RU, Yin H, Pourmandi N, James F, Adil MM, Schaffer DV, Wang Y, Miller EW. A Rationally Designed, General Strategy for Membrane Orientation of Photoinduced Electron Transfer-Based Voltage-Sensitive Dyes. ACS Chem Biol. 2017;12:407–413. doi: 10.1021/acschembio.6b00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YL, Walker AS, Miller EW. A Photostable Silicon Rhodamine Platform for Optical Voltage Sensing. J Am Chem Soc. 2015;137:10767–10776. doi: 10.1021/jacs.5b06644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni RU, Kramer DJ, Pourmandi N, Karbasi K, Bateup HS, Miller EW. Voltage-sensitive rhodol with enhanced two-photon brightness. Proc Natl Acad Sci U S A. 2017;114:2813–2818. doi: 10.1073/pnas.1610791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grenier V, Walker AS, Miller EW. A Small-Molecule Photoactivatable Optical Sensor of Transmembrane Potential. J Am Chem Soc. 2015;137:10894–10897. doi: 10.1021/jacs.5b05538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel MS, Isacoff EY. A genetically encoded optical probe of membrane voltage. Neuron. 1997;19:735–741. doi: 10.1016/s0896-6273(00)80955-1. [DOI] [PubMed] [Google Scholar]
- 19.Sakai R, Repunte-Canonigo V, Raj CD, Knopfel T. Design and characterization of a DNA-encoded, voltage-sensitive fluorescent protein. Eur J Neurosci. 2001;13:2314–2318. doi: 10.1046/j.0953-816x.2001.01617.x. [DOI] [PubMed] [Google Scholar]
- 20.Ataka K, Pieribone VA. A genetically targetable fluorescent probe of channel gating with rapid kinetics. Biophys J. 2002;82:509–516. doi: 10.1016/S0006-3495(02)75415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimitrov D, He Y, Mutoh H, Baker BJ, Cohen L, Akemann W, Knopfel T. Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS One. 2007;2:e440. doi: 10.1371/journal.pone.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012;75:779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao G, Platisa J, Pieribone VA, Raccuglia D, Kunst M, Nitabach MN. Genetically targeted optical electrophysiology in intact neural circuits. Cell. 2013;154:904–913. doi: 10.1016/j.cell.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platisa J, Vasan G, Yang A, Pieribone VA. Directed Evolution of Key Residues in Fluorescent Protein Inverses the Polarity of Voltage Sensitivity in the Genetically Encoded Indicator ArcLight. ACS Chem Neurosci. 2017;8:513–523. doi: 10.1021/acschemneuro.6b00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett L, Platisa J, Popovic M, Pieribone VA, Hughes T. A fluorescent, genetically-encoded voltage probe capable of resolving action potentials. PLoS One. 2012;7:e43454. doi: 10.1371/journal.pone.0043454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang HH, St-Pierre F, Sun X, Ding X, Lin MZ, Clandinin TR. Subcellular Imaging of Voltage and Calcium Signals Reveals Neural Processing In Vivo. Cell. 2016;166:245–257. doi: 10.1016/j.cell.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelfattah AS, Farhi SL, Zhao Y, Brinks D, Zou P, Ruangkittisakul A, Platisa J, Pieribone VA, Ballanyi K, Cohen AE, Campbell RE. A Bright and Fast Red Fluorescent Protein Voltage Indicator That Reports Neuronal Activity in Organotypic Brain Slices. J Neurosci. 2016;36:2458–2472. doi: 10.1523/JNEUROSCI.3484-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods. 2011;9:90–95. doi: 10.1038/nmeth.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kralj JM, Hochbaum DR, Douglass AD, Cohen AE. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011;333:345–348. doi: 10.1126/science.1204763. [DOI] [PubMed] [Google Scholar]
- 31.Maclaurin D, Venkatachalam V, Lee H, Cohen AE. Mechanism of voltage-sensitive fluorescence in a microbial rhodopsin. Proc Natl Acad Sci U S A. 2013;110:5939–5944. doi: 10.1073/pnas.1215595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flytzanis NC, Bedbrook CN, Chiu H, Engqvist MK, Xiao C, Chan KY, Sternberg PW, Arnold FH, Gradinaru V. Archaerhodopsin variants with enhanced voltage-sensitive fluorescence in mammalian and Caenorhabditis elegans neurons. Nat Commun. 2014;5:4894. doi: 10.1038/ncomms5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong Y, Li JZ, Schnitzer MJ. Enhanced Archaerhodopsin Fluorescent Protein Voltage Indicators. PLoS One. 2013;8:e66959. doi: 10.1371/journal.pone.0066959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, Saulnier JL, Boulting GL, Straub C, Cho YK, Melkonian M, Wong GK, Harrison DJ, Murthy VN, Sabatini BL, Boyden ES, Campbell RE, Cohen AE. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods. 2014;11:825–833. doi: 10.1038/nmeth.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herwig L, Rice AJ, Bedbrook CN, Zhang RK, Lignell A, Cahn JK, Renata H, Dodani SC, Cho I, Cai L, Gradinaru V, Arnold FH. Directed Evolution of a Bright Near-Infrared Fluorescent Rhodopsin Using a Synthetic Chromophore. Cell Chem Biol. 2017;24:415–425. doi: 10.1016/j.chembiol.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayraktar H, Fields AP, Kralj JM, Spudich JL, Rothschild KJ, Cohen AE. Ultrasensitive measurements of microbial rhodopsin photocycles using photochromic FRET. Photochem Photobiol. 2012;88:90–97. doi: 10.1111/j.1751-1097.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen AE, Kralj JM, Douglass AD. Optogenetic probes for measuring membrane potential, Google Patents 2012 [Google Scholar]
- 38.Gong Y, Wagner MJ, Zhong Li J, Schnitzer MJ. Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors. Nat Commun. 2014;5:3674. doi: 10.1038/ncomms4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou P, Zhao Y, Douglass AD, Hochbaum DR, Brinks D, Werley CA, Harrison DJ, Campbell RE, Cohen AE. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat Commun. 2014;5:4625. doi: 10.1038/ncomms5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong Y, Huang C, Li JZ, Grewe BF, Zhang Y, Eismann S, Schnitzer MJ. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science. 2015;350:1361–1366. doi: 10.1126/science.aab0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaner NC, Lambert GG, Chammas A, Ni Y, Cranfill PJ, Baird MA, Sell BR, Allen JR, Day RN, Israelsson M, Davidson MW, Wang J. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat Methods. 2013;10:407–409. doi: 10.1038/nmeth.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952;116:473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilt BA, Fitzgerald JE, Schnitzer MJ. Photon shot noise limits on optical detection of neuronal spikes and estimation of spike timing. Biophys J. 2013;104:51–62. doi: 10.1016/j.bpj.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sjulson L, Miesenbock G. Optical recording of action potentials and other discrete physiological events: a perspective from signal detection theory. Physiology (Bethesda) 2007;22:47–55. doi: 10.1152/physiol.00036.2006. [DOI] [PubMed] [Google Scholar]
- 45.Ji N, Freeman J, Smith SL. Technologies for imaging neural activity in large volumes. Nat Neurosci. 2016;19:1154–1164. doi: 10.1038/nn.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mennerick S, Chisari M, Shu HJ, Taylor A, Vasek M, Eisenman LN, Zorumski CF. Diverse voltage-sensitive dyes modulate GABAA receptor function. J Neurosci. 2010;30:2871–2879. doi: 10.1523/JNEUROSCI.5607-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karaveli S, Gaathon O, Wolcott A, Sakakibara R, Shemesh OA, Peterka DS, Boyden ES, Owen JS, Yuste R, Englund D. Modulation of nitrogen vacancy charge state and fluorescence in nanodiamonds using electrochemical potential. Proc Natl Acad Sci U S A. 2016;113:3938–3943. doi: 10.1073/pnas.1504451113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park K, Deutsch Z, Li JJ, Oron D, Weiss S. Single molecule quantum-confined Stark effect measurements of semiconductor nanoparticles at room temperature. ACS Nano. 2012;6:10013–10023. doi: 10.1021/nn303719m. [DOI] [PMC free article] [PubMed] [Google Scholar]