Significance
The contribution of individual effectors to Legionella pneumophila virulence has not been systematically examined. This study employed a parallel high-throughput transposon insertion sequencing technique called INSeq to probe the L. pneumophila effector repertoire and identified multiple effectors that contribute to virulence in several host organisms, including an animal model of Legionnaires’ disease. Importantly, this study demonstrates that effector proteins contribute to host virulence both positively and negatively by controlling intracellular replication and influencing host immune responses, which demonstrates that the subtle alterations in the effector repertoire of a single L. pneumophila strain can greatly impact host pathogenicity.
Keywords: type IV secretion, transposon insertion sequencing, bacterial effectors
Abstract
Legionella pneumophila is the causative agent of a severe pneumonia called Legionnaires’ disease. A single strain of L. pneumophila encodes a repertoire of over 300 different effector proteins that are delivered into host cells by the Dot/Icm type IV secretion system during infection. The large number of L. pneumophila effectors has been a limiting factor in assessing the importance of individual effectors for virulence. Here, a transposon insertion sequencing technology called INSeq was used to analyze replication of a pool of effector mutants in parallel both in a mouse model of infection and in cultured host cells. Loss-of-function mutations in genes encoding effector proteins resulted in host-specific or broad virulence phenotypes. Screen results were validated for several effector mutants displaying different virulence phenotypes using genetic complementation studies and infection assays. Specifically, loss-of-function mutations in the gene encoding LegC4 resulted in enhanced L. pneumophila in the lungs of infected mice but not within cultured host cells, which indicates LegC4 augments bacterial clearance by the host immune system. The effector proteins RavY and Lpg2505 were important for efficient replication within both mammalian and protozoan hosts. Further analysis of Lpg2505 revealed that this protein functions as a metaeffector that counteracts host cytotoxicity displayed by the effector protein SidI. Thus, this study identified a large cohort of effectors that contribute to L. pneumophila virulence positively or negatively and has demonstrated regulation of effector protein activities by cognate metaeffectors as being critical for host pathogenesis.
Bacteria of the genus Legionella are inhabitants of fresh water and soil environments where they have evolved the capacity to replicate in a diverse number of protozoan hosts. Although there are over 40 species of Legionella, human infections that progress to a severe pneumonia called Legionnaires’ disease are most often caused by L. pneumophila (1). Legionnaires’ disease results from inhalation of Legionella-contaminated aerosols and subsequent bacterial replication within alveolar macrophages. Bacterial replication occurs in a specialized Legionella-containing vacuole (LCV) that evades fusion with lysosomes and associates intimately with the host endoplasmic reticulum (ER) (2, 3). Formation of the LCV and intracellular bacterial replication is dependent on the Dot/Icm type IV secretion system (T4SS) (4, 5), which translocates bacterial effector proteins into the host cell where they subvert normal host processes to promote pathogen replication (6). The Philadelphia-1 strain of L. pneumophila was isolated from the eponymous Legionnaires’ disease outbreak that occurred in 1976 (7), and this strain has been shown to encode over 300 different effector proteins (8).
Genome sequencing studies have demonstrated a high degree of plasticity in the effector repertoires encoded by between different strains of L. pneumophila and different Legionella species (9). How the effector repertoire influences host virulence remains poorly understood. Initial forward genetic screens aimed at identifying avirulent mutants of L. pneumophila were successful in identifying essential components of the Dot/Icm system, but these screens did not identify effector proteins translocated by the Dot/Icm system (10, 11). It is appreciated that most effectors are not essential for intracellular replication (12), which is why the genes encoding effector proteins that are important for virulence were difficult to identify by standard screening strategies that assess intracellular replication using binary assays that measure plaque formation or destruction of host cell monolayers (10, 13). Thus, new approaches are required to systematically assess the contribution of individual L. pneumophila effector proteins during infection.
High-throughput sequencing (HTS)-based phenotypic screening of bacterial transposon (Tn) mutants has become a powerful technique to assess the contribution of individual genes to bacterial fitness during host colonization (14). Techniques such as insertion sequencing (INSeq) (15) and Tn sequencing (TNSeq) (16) are massively parallel HTS techniques that enable determination of relative fitness of individual Tn mutants within a mixed population. These techniques have been used to generate whole-genome mutant populations to identify genes that contribute to virulence of several clinically important bacterial pathogens such as Campylobacter jejuni, Haemophilus influenza, Acinetobacter baumannii, and Pseudomonas aeruginosa (15, 17–20). However, traditional whole-genome screening approaches are susceptible to population bottlenecks, which result in stochastic changes in mutant abundance that are unrelated to fitness (21). In most animal models of Legionnaires’ disease, these bottlenecks would likely occur using populations containing more than 1,000 different mutants, which complicates using whole-genome INSeq approaches for assessing the contribution of effector proteins in host pathogenicity.
To circumvent the challenges associated with whole-genome mutant screening, INSeq technology was used to sequence an arrayed L. pneumophila Tn mutant library and determine where individual Tn insertion mutants were located in the arrayed library. From these data, mutants deficient in individual effector genes were clonally isolated to generate an effector mutant pool (EMP) that was used to assess the fitness of individual effector mutants using both a mouse model of Legionnaires’ disease and cultured host cells. This systematic analysis revealed distinct virulence phenotypes for individual effector mutants and a complex relationship between the L. pneumophila effector gene repertoire and host virulence.
Results
Generation of the L. pneumophila EMP.
To produce a pool of L. pneumophila mutants where specific effector genes were inactivated, an arrayed Tn mutant library was generated and Tn insertion sites were mapped using INSeq technology. Briefly, L. pneumophila mutants were generated using a Mariner-based Tn engineered to confer chloramphenicol resistance. Individual mutants were arrayed in 96-well plates until over 10,000 individual mutants were obtained. Combinatorial pooling and INSeq analysis were used to identify all Tn insertion sites and the location of each mutant in the arrayed library (22) (SI Materials and Methods).
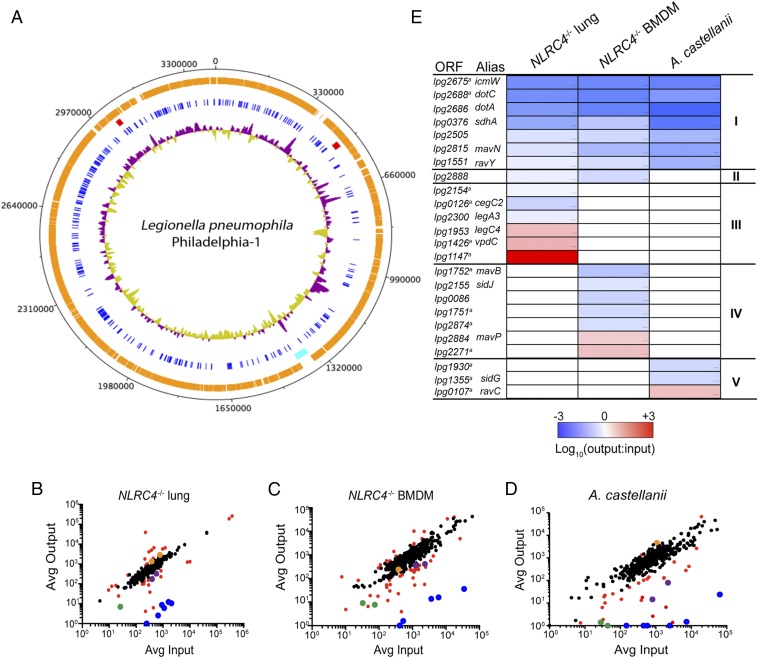
Sequencing results identified 10,163 independent insertion events, and these data were used to determine the location of the mutants in the arrayed library. PCR analysis and phenotypic analysis were used to validate the predicted location of multiple mutants within the arrayed library (SI Materials and Methods). Genome coverage did not appear to be affected by GC content, and no insertions mapped to the lvh region because Lp01-derived strains of L. pneumophila Philadelphia-1 have a chromosomal deletion that eliminated this locus (23) (Fig. 1A). Tn insertions were identified in the coding regions of 297 of the 315 genes predicted to encode effector proteins (Fig. 1A and Dataset S1). An EMP was generated by isolating individual effector mutants from the arrayed library and combining them into a single pool. Several mutants having Tn insertions in genes encoding Dot/Icm secretion system components were added to the EMP to serve as internal controls for mutants that should display severe fitness defects in both the mouse model of infection and in cultured host cells. The EMP also contained mutants in the flaA gene, which are deficient in production of flagellin (Dataset S2). The flaA::Tn mutants served as controls for bacteria capable of escaping detection of flagellin by the host NAIP5/NLRC4 inflammasome, which restricts intracellular replication of L. pneumophila in mouse macrophages (24–26). When possible, two different insertion mutants deficient in an effector were added to the EMP so that fitness changes identified by INSeq analysis could be validated independently.
Fig. 1.
Generation and screening of a Dot/Icm EMP. (A) Schematic diagram of the L. pneumophila genome (black). Arrayed library Tn insertions (orange) and Dot/Icm effector Tn insertions (blue) are indicated by tick marks. The lvh locus (cyan) and dot/icm loci (red) are shown. GC skewing (G−C/G+C) is shown in purple (>0) and mustard (<0). (B–D) Average normalized read counts for each insertion from INSeq analysis from input and output EMP libraries obtained from NLRC4−/− mice (n = 10) (B), NLRC4−/− BMDMs (n = 8) (C), and A. castellanii (n = 8) (D). Tn mutants with significant fitness differences are shown in red (q ≤ 0.05), dot/icm::Tn mutants are blue, lpg2505::Tn mutants are green, ravY::Tn mutants are cyan, and legC4::Tn mutants are orange. (E) Heat map showing log output:input ratios >1 (red), <1 (blue), and nonsignificant (white) of mutants with significant (q ≤ 0.05) fitness differences in NLRC4−/− lung, NLRC4−/− BMDMs, and A. castellanii. Mutants were categorized into universal defect (I), mammalian-specific defect (II), lung-specific phenotypes (III), BMDM-specific phenotypes (IV), and amoeba-specific phenotypes (V). aGenes with only one representative mutant in the EMP. Statistical analyses were performed as described in SI Materials and Methods.
The final EMP contained 528 isogenic L. pneumophila strains having independent Tn insertions that could be quantitated in parallel using INSeq. This was demonstrated by culturing the EMP axenically and sequencing the input and output populations. These data show the distribution of each mutant in the library did not change significantly after axenic cultivation (Fig. S1). Thus, any changes in the distribution of a mutant following cultivation in either the mouse model of infection or in cultured host cells would indicate that the fitness of the mutant has been altered by the host environment.
INSeq Analysis of the EMP Identifies Factors That Control L. pneumophila Virulence.
A mouse model of Legionnaires’ disease was used to determine whether individual mutants with known virulence phenotypes could be identified after INSeq analysis of the EMP. Specifically, the EMP was screened after intranasal inoculation of C57BL/6 (WT) mice and NLRC4−/− mice. INSeq was used to profile the output EMP population after 48 h of infection, which is when replication of L. pneumophila peaks in the lungs of mice (27). The distribution of mutants in the output populations was compared with the input populations (Fig. 1B and Fig. S2).
There was a significant increase in the proportion of flaA::Tn mutants in the lungs of C57BL/6 mice in the output population at 48 h, which indicated that the flagellin-deficient mutant had a competitive advantage over other mutants in the EMP (Fig. S2 and Dataset S3). By contrast, flaA::Tn mutants did not display a competitive advantage in NLRC4-deficient mice (Dataset S3). Thus, INSeq analysis of the EMP successfully determined the competitive advantage flaA::Tn mutants have in escaping flagellin-mediated activation of the NAIP5/NLRC4 inflammasome. Using C57BL/6 mice, there was a significant decrease in the output population at 48 h for mutants with Tn insertions in genes encoding essential Dot/Icm components (Fig. S2 and Dataset S3). The decrease in the proportion of Dot/Icm-deficient mutants in the output population collected from the lungs of NLRC4-deficient mice at 48 h was more pronounced. This is because the NLRC4-deficient mouse is a highly permissive host for L. pneumophila intracellular replication, and the dramatic increase in the number of replication-competent mutants in the output population will contribute to the difference in sequence reads compared with dot and icm mutants that are replication-deficient (Fig. 1B and Dataset S3). These data indicate that INSeq analysis of the EMP was able to detect mutants with intracellular replication defects and was sensitive enough to quantify the magnitude of the defects using mice that varied in their permissiveness for L. pneumophila replication. Thus, using the EMP in conjunction with INSeq analysis was successful at identifying and quantifying the predicted gene-for-gene relationships between bacterial and host factors that control intracellular replication of L. pneumophila in the mouse model of Legionnaires’ disease.
INSeq Analysis Reveals Effector Mutant Virulence Phenotypes.
To determine the contribution of individual effectors to L. pneumophila virulence, the INSeq data obtained from the analysis of the EMP in mice was compared with INSeq data obtained by ex vivo passaging of the EMP through bone marrow-derived macrophages (BMDMs) from NLRC4-deficient mice and the protozoan host Acanthamoeba castellanii (Fig. 1 C and D). Mutants were defined as having significant fitness differences based on statistical analysis of INSeq data (q ≤ 0.05; see SI Materials and Methods). Hierarchical clustering analysis was used to assign individual mutants to distinct categories (class I–V) based on phenotypes displayed in the cell culture and in vivo screens (Fig. 1E). In addition to mutants deficient in the Dot/Icm system, multiple effector mutants were included in the class I category, which represents mutants that display intracellular replication defects in both mammalian and amoeba hosts (Fig. 1E). Included in this group were mutants having Tn insertions in mavN and sdhA, which encode effectors that have been shown previously to be important for L. pneumophila intracellular replication (28–32) (Fig. 1E and Dataset S3). Identification of these mutants in the INSeq analysis provided further evidence that this technology was successful in evaluating the role of effectors in virulence. Importantly, virulence phenotypes were detected for a large number of uncharacterized effector mutants, which suggested that INSeq analysis identified fitness defects displayed by effector mutants that were not discovered in traditional forward genetic screens. The high-confidence hits shown in Fig. 1E, which represent genes where all of the insertions in that particular gene in the EMP displayed the phenotype of interest, revealed several different categories of virulence phenotypes. These included effector mutants that displayed fitness differences in either mammalian or protozoan hosts as well as effector mutants that displayed a virulence phenotype only in the context of the Legionnaires’ disease pulmonary infection model.
LegC4 Enhances Pulmonary Clearance of L. pneumophila.
Validation studies were conducted to test whether virulence phenotypes observed by INSeq analysis of individual mutants in the EMP were the result of a loss-of-function mutation affecting the corresponding effector protein. A virulence phenotype of particular interest was that displayed by the two independent legC4::Tn mutants, where these mutants had a significant fitness advantage in the lungs of NLRC4−/− mice but not in A. castellanii or BMDMs (Fig. 1E and Dataset S3). These data suggested that LegC4 function might decrease L. pneumophila virulence in the context of a cellular immune response in the lung. Indeed, an isolated legC4::Tn mutant replicated to higher levels (sixfold) in the lungs of NLRC4−/− mice compared with the parental strain of L. pneumophila (Fig. 2A, P < 0.01). A strain containing a clean deletion of legC4 (∆legC4) displayed a similar increase in the bacterial burden in the lungs of NLRC4−/− mice compared with wild-type L. pneumophila (Fig. 2A, P < 0.01). Competitive index (CI) studies, where mice are infected with a 1:1 ratio of mutant and wild-type L. pneumophila, also revealed that the legC4::Tn mutant had a fitness advantage (CI > 1) over the parental strain of L. pneumophila in vivo (Fig. 2B). Importantly, complementation of the legC4::Tn mutation with a plasmid-encoded allele of legC4 abrogated the fitness advantage displayed by the legC4::Tn mutant. Indeed, this legC4::Tn mutant strain overproducing LegC4 from a plasmid displayed a fitness defect compared with the nonmutant strain containing the vector alone (Fig. 2C). Thus, expression of legC4 attenuates L. pneumophila virulence in a mouse model of Legionnaires’ disease.
Fig. 2.
LegC4 function attenuates L. pneumophila replication in the mouse lung. (A) Enumeration of WT, legC4::Tn, or ∆legC4 from lungs of NLRC4−/− mice. (B) CI of legC4::Tn versus WT from the lungs of NLRC4−/− mice. (C) CI of legC4::Tn (pV) or legC4::Tn (plegC4) versus WT (pV) in the lungs of NLRC4−/− mice. Each symbol represents an individual animal, and asterisks denote statistical significance by Mann–Whitney U test (*P < 0.05, **P < 0.01). (D) ELISA for IL-12 p40 secretion from NLRC4−/− BMDMs infected with the indicated strains. Data are presented as mean ± SD, and asterisks denote statistical significance (**P < 0.01; n.s., not significant). Data are representative of at least two independent experiments.
Intracellular replication of legC4 mutant bacteria was assessed using isolated BMDMs and A. castellanii to determine if LegC4 function stimulates a cell-autonomous immune defense pathway capable of restricting L. pneumophila intracellular replication. Intracellular replication of the legC4::Tn mutant over 72 h was similar to the parental strain of L. pneumophila in NLRC4−/− BMDMs (Fig. S3A). In A. castellanii, the legC4::Tn mutant displayed a modest defect in replication at 24 h, but no defect in replication was detected at later times (Fig. S3B). Thus, LegC4 function does not attenuate replication of L. pneumophila in isolated host cells, which suggests that LegC4 is detrimental to L. pneumophila virulence only in the context of animal infection.
Proinflammatory cytokines stimulate clearance of L. pneumophila in vivo (33). Thus, the in vivo-specific phenotype observed for legC4 mutant bacteria could result from enhanced production of inflammatory cytokines from infected macrophages that could enhance clearance by bystander immune cells such as neutrophils. Interleukin (IL)-12 is a proinflammatory cytokine that promotes clearance of L. pneumophila in vivo but does not contribute to cell-autonomous defense when macrophages are cultured ex vivo (34). To determine if LegC4 function may influence a proinflammatory response by infected cells, BMDMs cultured ex vivo were infected with wild-type or legC4 mutant L. pneumophila, and IL-12 levels were measured. Despite similar levels of intracellular replication, significantly less IL-12 was secreted by macrophages infected with the legC4::Tn mutant compared with BMDMs infected with wild-type L. pneumophila (Fig. 2D, P < 0.01). IL-12 production was similar when BMDMs infected with the complemented legC4::Tn (plegC4) strain were compared with wild-type L. pneumophila (Fig. 2D). Thus, L. pneumophila producing a functional LegC4 protein may be less virulent for animals because this effector has the capacity to enhance a proinflammatory response that stimulates cellular immunity.
The Effectors Lpg0086, Lpg2505, and RavY Are Important for L. pneumophila Intracellular Replication.
Tn insertions in several uncharacterized effector genes resulted in decreased L. pneumophila intracellular replication. Loss-of-function mutations in the genes lpg0086, lpg2505, and ravY resulted in fitness defects in BMDMs cultured ex vivo (Fig. 1E, class I and III), which suggested these mutants may have intracellular replication defects. Additionally, lpg2505::Tn and ravY::Tn strains displayed growth defects in A. castellanii and the mouse model of Legionnaires’ disease. Intracellular replication of lpg0086::Tn, lpg2505::Tn, and ravY::Tn mutants was measured in both BMDMs and A. castellanii. Consistent with the class I phenotype observed in the EMP screens, the isolated lpg2505::Tn and ravY::Tn mutants were attenuated for intracellular replication in BMDMs and A. castellanii (Fig. 3A and Fig. S4 A and B). The lpg0086::Tn mutant had a significant intracellular replication defect in BMDMs, and intracellular replication of lpg0086::Tn was enhanced significantly upon introduction of the wild-type lpg0086 gene on a plasmid (Fig. S5). By contrast, the lpg0086::Tn mutant did not have an intracellular replication defect in A. castellanii or a fitness defect in mice, which validates the class III phenotype displayed by this mutant (Fig. S5). After 72 h of infection, L. pneumophila strains mutated in lpg2505 or ravY displayed roughly a 2-log decrease in cfu counts compared with the isogenic wild-type strain (Fig. 3). In-frame deletions in lpg2505 or ravY were created in the parental strain of L. pneumophila to further validate the growth defects observed for the Tn insertion mutants. Indeed, the ∆lpg2505 and ∆ravY mutants displayed intracellular replication defects, which were complemented when the plasmid-encoded allele encoding wild-type effector protein was introduced in trans to the respective chromosomal deletion mutant (Fig. 3 B and C and Fig. S4 C and D).
Fig. 3.
Lpg2505 and RavY are effectors important for intracellular replication. (A) Growth of WT, lpg2505::Tn, ravY::Tn, and dotA::Tn mutant strains in NLRC4−/− BMDM. (B) Growth of WT, ∆lpg2505 (pV), ∆lpg2505 (plpg2505), and dotA::Tn in NLRC4−/− BMDM. (C) Growth of WT ∆ravY (pV), ∆ravY (pravY), or dotA::Tn in NLRC4−/− BMDM. Asterisks denote statistical significance by Student’s t test (**P < 0.01). (D) CI of ∆lpg2505 (pV) or ∆lpg2505 (plpg2505) versus WT in the lungs of NLRC4−/− mice at 48 h postinfection. (E) CI of WT versus ∆ravY (pV) or ∆ravY (pravY) in the lungs of NLRC4−/− mice at 48 h postinfection. Each point represents a single mouse and data shown are mean ± SD. Asterisks denote statistical significance by Mann–Whitney U test (**P < 0.01). Data are representative of at least two independent experiments.
CI studies confirmed the fitness defects displayed by the lpg2505::Tn and ravY::Tn mutants in the INSeq analysis. Specifically, the parental strain of L. pneumophila outcompeted the ∆lpg2505 mutant and the ∆ravY mutant, but not the complemented strains, in the lungs of NLRC4−/− mice (Fig. 3 D and E). Thus, RavY and Lpg2505 are effectors important for L. pneumophila intracellular replication and host virulence, further indicating that INSeq screening of the EMP was successful in identifying novel L. pneumophila effectors that contribute to host pathogenesis.
Lpg2505 Is a Metaeffector That Regulates SidI-Mediated Cytotoxicity.
Bioinformatic analysis indicated that lpg2505 is encoded downstream of the effector gene sidI in a predicted operon (35, 36) (Fig. 4A). SidI inhibits host cell protein synthesis and is toxic when produced ectopically in eukaryotic cells (37). Several L. pneumophila effectors have been characterized that have biochemical functions that modulate the activity of other effector proteins after delivery of the proteins into the host cell (38, 39). These modulating effectors have been called metaeffectors. Because many characterized L. pneumophila metaeffectors are encoded near the effector protein they modulate (38), the possibility that Lpg2505 functions as an metaeffector that regulates SidI function was tested. Consistent with Lpg2505 being an effector that could modulate SidI-mediated toxicity, the sidI::Tn mutants in the EMP did not display a fitness defect in the INSeq analysis even though these mutants were likely to be defective in lpg2505 expression. In addition, previous studies demonstrated that a loss-of-function mutation in the sidI gene did not affect L. pneumophila replication in cultured host cells (37). This suggested that lpg2505 might be essential for intracellular replication only if a functional SidI protein was produced.
Fig. 4.
Lpg2505 is a metaeffector that inhibits SidI toxicity. (A) Schematic diagram of the putative operon encoding sidI and lpg2505. (B) Growth of WT, ∆lpg2505, ∆operon, and dotA::Tn in NLRC4−/− BMDM over 72 h. (C) Growth of WT, ∆lpg2505, ∆operon::sidIR453P, and dotA::Tn in NLRC4−/− BMDM over 72 h. Data shown are mean ± SD, and asterisks denote statistical significance by Student’s t test (**P < 0.05). (D) Yeast expressing vector, lpg2498, lpg2505, lpg2508, or lpg2509 were transformed with vector, sidI, lpg2505, lpg2508, or left untransformed, and growth was assessed on selective media. Data are representative of at least two independent experiments.
To determine if the virulence defect displayed by the ∆lpg2505 mutant required SidI function, intracellular replication of the ∆lpg2505 mutant was compared with an isogenic mutant where both sidI and lpg2505 were deleted (∆operon). Indeed, the ∆operon mutant did not have a detectible intracellular replication defect, which indicates the elimination of sidI suppressed the intracellular replication defect resulting from a lpg2505 loss-of-function mutation (Fig. 4B).
The mutant SidIR453P protein has a single amino acid substitution that eliminates the cytotoxic effect of SidI on eukaryotic host cells (37). To address whether Lpg2505 function is important for neutralizing SidI-mediated cytotoxicity during L. pneumophila infection, allelic exchange was used to replace the wild-type sidI gene with the mutant sidIR453P allele in the ∆lpg2505 mutant. The sidIR453P, ∆lpg2505 mutant did not have an intracellular replication defect (Fig. 4C), which indicates that Lpg2505 is important for virulence in strains producing a cytotoxic SidI protein. Lastly, the ability of Lpg2505 to suppress the toxic activity of SidI was tested in yeast. Consistent with previous studies (37), expression of a functional SidI protein was toxic to yeast and interfered with colony formation on agar plates (Fig. 4D). SidI toxicity was suppressed when Lpg2505 was coexpressed in yeast, but expression of other effector proteins encoded nearby did not suppress SidI cytotoxicity (Fig. 4D). Thus, Lpg2505 regulates SidI activity to prevent host damage that is detrimental to L. pneumophila intracellular infection and limits virulence.
Discussion
This study utilized INSeq technology to generate and screen a targeted pool of effector mutants for virulence phenotypes in cultured host cells and the mouse model of Legionnaires’ disease. Compared with whole-genome screening approaches, the use of a targeted mutant library decreased the chances of population bottlenecks and enabled identification of mutants with significant virulence phenotypes. Importantly, loss-of-function mutations in multiple effector genes resulted in high-confidence hits where significant fitness differences in at least one of the host screens was observed for all of the individual mutants in the EMP. This screen also identified a large collection of effector genes where one of two independent Tn insertions displayed significant fitness phenotypes (Dataset S3). Thus, INSeq analysis identified effector mutants with replication and virulence phenotypes that were not easily detected using more traditional screening methods for intracellular replication.
Many of the previous screens to identify genes important for L. pneumophila virulence have used death of the host cell as an indicator of intracellular replication (10, 13), which is problematic because mutants that replicate less efficiently but still cause host toxicity would elude detection. By contrast, sequence reads provided by this INSeq approach correlate with the intracellular replication capacity of each mutant in the pool so that effector mutants having intracellular replication defects that were not as severe as the defects displayed by dot or icm mutants were identified. Additional studies will be necessary to validate the virulence phenotypes associated with the uncharacterized effector mutants listed in Fig. 1E and Dataset S3. For example, lpg1751::Tn mutants and lpg1752::Tn mutants both displayed intracellular growth defects in BMDMs, but these two genes are predicted to be encoded in an operon. Thus, it is possible that the phenotype displayed by the lpg1751::Tn mutants results from polar effects on the expression of lpg1752. There were also examples, such as the lpg0717::Tn mutant, where the effector mutant added to the EMP had multiple insertions so that the virulence phenotype displayed by these mutants requires additional validation to ensure that the phenotype is the result of an effector loss-of-function.
Although most of the effector mutants in the EMP that were defective for intracellular replication in cell culture also displayed fitness defects in NLRC4−/− mice, there were exceptions where replication defects were detected using cultured host cells but these defects were not apparent in the animal model of disease (Fig. 1E), which was a phenotype displayed by the lpg0086::Tn mutant. This likely reflects a difference in sensitivity between the two assays. Using cultured host cells, it is typical to observe a 3-log increase in bacterial numbers resulting from intracellular replication over a 72-h assay, whereas in the mouse model of disease the increase in bacterial numbers is typically only 1-log because innate immune defenses that limit bacterial replication and dissemination in the lung are activated within the first 24 h of infection (Dataset S3). Thus, lower levels of expansion of the EMP in the lungs of infected mice likely prevent detection of virulence defects that are caused by subtle differences in the levels of intracellular replication.
There were significant advantages to screening the EMP in a mouse model of Legionnaires’ disease. Testing 300 different effector mutants of Legionella individually using an animal model of disease is impractical, which is why there has not been a comprehensive analysis of the role individual effectors play in disease. In this study, it was demonstrated that INSeq screening of the EMP in a mouse model of Legionnaires’ disease enabled the fitness of over 500 different mutants to be measured individually in parallel in a single animal. Although INSeq technology was used here to address the fitness of mutants deficient in Dot/Icm-translocated effector proteins, insertion site mapping data obtained from the arrayed library will now enable the rapid construction of other mutant pools that could be used to evaluate virulence phenotypes for L. pneumophila defective in other pathways, such as mutants defective in type II secreted proteins or mutants defective in metabolic pathways that could be important for intracellular survival.
Another advantage of using INSeq technology to screen the EMP in an animal model of disease was that fitness advantage or cost of an individual effector could be assessed in the context of a complex cellular immune response. Accordingly, there were categories of effector mutants that had fitness defects or fitness advantages that were revealed only in the mouse model of Legionnaires’ disease. The legC4::Tn mutant was selected for further analysis to validate an effector mutant virulence phenotype that was revealed only in the animal infection model. Independent Tn insertion mutants and a strain containing a clean deletion of the legC4 gene confirmed that L. pneumophila deficient in the effector LegC4 had a competitive advantage over other L. pneumophila strains in the mouse model of infection. The observation that the legC4 mutant did not have a replication advantage in cultured macrophages but did replicate to higher levels in the lungs of mice infected indicates that this mutant is not simply a “cheater” receiving a benefit from other L. pneumophila in the EMP. The modest replication defect observed for the legC4 mutant in A. castellanii implicates this effector as having a beneficial role during L. pneumophila infection of protozoan hosts in their natural environment, but the activity of LegC4 is detrimental when these bacteria enter the lungs of the mammalian host. The observation of decreased IL-12 production by macrophages infected with the legC4 mutant suggests that LegC4 function may enhance innate immune detection of L. pneumophila, which could accelerate clearance through the recruitment of neutrophils to the infected area and/or enhance clearance through IFNγ production by natural killer (NK) cells (40, 41). As L. pneumophila is an accidental pathogen of humans and rarely transmits person-to-person, effectors with functions that attenuate bacterial replication in the context of a cellular immune response have likely not been selected against over time. Validation of the legC4::Tn mutant phenotype revealed by INSeq analysis demonstrates the utility of this approach in identifying virulence phenotypes displayed by effector-deficient L. pneumophila during infection of a mammalian host.
INSeq analysis revealed that previously uncharacterized effectors, such as RavY and Lpg2505, were essential for L. pneumophila virulence. Interestingly, RavY is an effector that is highly conserved in all strains of L. pneumophila but does not appear to be encoded in many other Legionella species (9). Thus, RavY is a candidate virulence factor that contributes to the enhanced virulence potential displayed by L. pneumophila for humans.
The metaeffector paradigm, where the primary function of an effector is to counteract the specific activity of another effector after both have been translocated into the host cell, was established for L. pneumophila when the function of the ubiquitin ligase LubX was shown to target the effector protein SidH for degradation by the host proteasome (39). In this example, the metaeffector interacts directly with the effector it modulates. There are also metaeffectors that do not interact directly with other effectors but instead balance the activity of the effector by reversing a modification to a host protein mediated by that effector. An example is the de-AMPylation activity displayed by SidD, which reverses DrrA(SidM)-mediated AMPylation of the host protein Rab1 (42, 43). It is now appreciated that metaeffectors are used frequently to modulate the activity of other effectors in the host cell (31, 39, 42–45) and a recent study that used yeast to identify effector two-hybrid interactions and suppression of host cytotoxicity identified a large cohort of effector–metaeffector pairs (38). The metaeffector SidJ has also been implicated in virulence, and recent data indicate that SidJ reverses phosphoribosyl-linked protein ubiquitination of host proteins mediated by the SidE family of effectors (46, 47). The SidI–Lpg2505 effector–metaeffector pair that was revealed here using INSeq screening of the EMP, however, eluded detection in these previous studies, which provides additional support for the power of this unbiased approach in revealing new effector activities.
In summary, INSeq analysis of a targeted L. pneumophila Tn mutant library provided a quantitative assessment of fitness defects resulting from inactivation of potential virulence determinants. Using a targeted EMP in conjunction with screens in both animal and cultured host cells, it was revealed that inactivation of a single effector has the potential to influence virulence of L. pneumophila both positively and negatively. Thus, addressing the role of effectors through systematic analysis of mutant phenotypes revealed a complex interrelationship of effector activities that ultimately impact the ability of L. pneumophila to cause disease. Determining the biochemical function of effectors with virulence phenotypes that were revealed in this analysis will further our understanding of how L. pneumophila is able to promote human disease and how effector proteins contribute to innate immune control of L. pneumophila in healthy hosts. Lastly, INSeq mapping data obtained for the arrayed library can be used to assemble additional libraries to address other functional classes of genes in L. pneumophila, which include genes regulating metabolic pathways or additional secretion pathways.
Materials and Methods
Bacterial Strains and Culture Conditions.
L. pneumophila serogroup-1 strains were cultured on supplemented charcoal N-(2-Acetamido)-2-aminoethanesulfonic acid (ACES)-buffered yeast extract (CYE) and grown at 37 °C as described (48, 49). Liquid cultures were grown at 37 °C in supplemented ACES-buffered yeast extract (AYE) as described. All L. pneumophila were grown in the presence of 100 µg·mL−1 streptomycin. When necessary, media were supplemented with 5 µg·mL−1 chloramphenicol (Tn mutants), 10 µg·mL−1 chloramphenicol (plasmid maintenance), 10 µg·mL−1 kanamycin (allelic exchange), or 25 µg·mL−1 kanamycin (plasmid maintenance). Plasmids used in this study are listed in Table S1. For molecular cloning and strain construction, please refer to SI Materials and Methods.
Mice.
Six-week-old female C57BL/6 mice were purchased from the Jackson Laboratories. C57BL/6 NLRC4−/− mice were obtained from Richard Flavell, Yale School of Medicine, New Haven, CT. All procedures using mice were performed with approval of the Yale Institutional Animal Use and Care Committee and in accordance with the Animal Welfare Act.
Arrayed Tn Mutant Library Production.
Electrocompetent L. pneumophila were transformed with pSRS_CM1, plated on selective media, and grown at 37 °C. pSRS_CM1 encodes an MmeI-modified Mariner Tn and requires pir from phage lambda (λpir) to replicate. Since L. pneumophila lacks λpir, any resistant colonies resulted from incorporation of the Tn into the chromosome, which was confirmed by Southern blot analysis. Single colonies of chloramphenicol-resistant L. pneumophila were patched on 48-well agar plates and grown at 37 °C for 48 h. Chloramphenicol-resistant bacteria were resuspended in sterile d2H2O, transferred into a sterile 96-well plate containing 2× freezing medium (4% peptone, 10% glycerol), and stored at −70 °C. This procedure was repeated until 106 96-well plates were stocked.
The library of L. pneumophila Tn mutants was arrayed as previously described (22). An EpMotion 5075 robot was used as previously described with indicated programs to generate 24 pools of strains, in which each strain was placed in a unique subset of pools (22). INSeq libraries were generated from each of the 24 pools using a different 6-bp barcode for each pool as described (22). Adapter sequences are listed in Dataset S4. Adapter-ligated libraries were normalized to 10 nM, and equal volumes were combined before sequencing on an Illumina HiSeq 2500 (Yale Center for Genome Analysis, Yale University). Data were analyzed via a data analysis package described previously and modified to align reads to the L. pneumophila Philadelphia-1 genome (22). A total of 10,164 mutants were mapped to wells of the library (Dataset S1), and mapping was validated by PCR and NaCl sensitivity and found to be accurate (10).
Generation of the EMP.
The EMP was generated by isolation and subsequent pooling of individual clones from the arrayed library as described in SI Materials and Methods.
Production of INSeq Libraries.
BMDMs from C57BL/6 NLRC4−/− mice and A. castellanii were derived and cultured as described in SI Materials and Methods. Infections were performed by growing the EMP on CYE media for 72 h. Bacteria were scraped from the plate and grown overnight in liquid AYE media until the culture reached OD600 = 3.2–3.8. A sample of this inoculum was plated into CYE and used to generate input library gDNA. BMDMs and A. castellanii were infected as described in SI Materials and Methods. Bacteria were harvested and plated on CYE from cell lysates at 48 h postinfection and used to generate output library gDNA. INSeq library preparation and data analysis were performed as described (15, 22) (SI Materials and Methods).
Six- to 8-wk-old female NLRC4−/− or wild-type C57BL/6 mice were infected with the EMP for INSeq analysis. The EMP was prepared as described above, and mice were infected by the intranasal route at 5 × 106 bacteria per animal. Input libraries were prepared as described above, and output libraries were prepared from the lungs of mice following 48 h of infection (SI Materials and Methods). INSeq library preparation and data analysis were performed as described (15, 22) (SI Materials and Methods).
Please refer to SI Materials and Methods for more information regarding infection of mice, BMDM and A. castellanii growth curves, ELISA, yeast experiments, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Dr. Richard Flavell for providing NLRC4−/− mice and Aline Gozzi for technical assistance in library preparation. This work was supported by NIH Grants AI041699 and AI048770 (to C.R.R.) and GM118159 (to A.L.G.), a CIHR postdoctoral fellowship (to S.R.S.), the China Scholarship Council (L.L.), and the Burroughs Wellcome Fund (A.L.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708553114/-/DCSupplemental.
References
- 1.Newton HJ, Ang DKY, van Driel IR, Hartland EL. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev. 2010;23:274–298. doi: 10.1128/CMR.00052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwitz MA. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 5.Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- 7.Fraser DW, et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 8.Burstein D, et al. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 2009;5:e1000508. doi: 10.1371/journal.ppat.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein D, et al. Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires. Nat Genet. 2016;48:167–175. doi: 10.1038/ng.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadosky AB, Wiater LA, Shuman HA. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor TJ, Adepoju Y, Boyd D, Isberg RR. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc Natl Acad Sci USA. 2011;108:14733–14740. doi: 10.1073/pnas.1111678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews HL, Vogel JP, Isberg RR. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Opijnen T, Camilli A. Transposon insertion sequencing: A new tool for systems-level analysis of microorganisms. Nat Rev Microbiol. 2013;11:435–442. doi: 10.1038/nrmicro3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman AL, et al. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009;6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Opijnen T, Bodi KL, Camilli A. Tn-seq: High-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods. 2009;6:767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skurnik D, et al. A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog. 2013;9:e1003582. doi: 10.1371/journal.ppat.1003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang N, Ozer EA, Mandel MJ, Hauser AR. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio. 2014;5:e01163–e14. doi: 10.1128/mBio.01163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao B, Lara-Tejero M, Lefebre M, Goodman AL, Galán JE. Novel components of the flagellar system in epsilonproteobacteria. MBio. 2014;5:e01349–e14. doi: 10.1128/mBio.01349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SM, Bernui M, Shen H, Akerley BJ. Genome-wide fitness profiling reveals adaptations required by Haemophilus in coinfection with influenza A virus in the murine lung. Proc Natl Acad Sci USA. 2013;110:15413–15418. doi: 10.1073/pnas.1311217110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel S, Abel zur Wiesch P, Davis BM, Waldor MK. Analysis of bottlenecks in experimental models of infection. PLoS Pathog. 2015;11:e1004823. doi: 10.1371/journal.ppat.1004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman AL, Wu M, Gordon JI. Identifying microbial fitness determinants by insertion sequencing using genome-wide transposon mutant libraries. Nat Protoc. 2011;6:1969–1980. doi: 10.1038/nprot.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samrakandi MM, Cirillo SLG, Ridenour DA, Bermudez LE, Cirillo JD. Genetic and phenotypic differences between Legionella pneumophila strains. J Clin Microbiol. 2002;40:1352–1362. doi: 10.1128/JCM.40.4.1352-1362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamboni DS, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 25.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascarenhas DPA, Pereira MSF, Manin GZ, Hori JI, Zamboni DS. Interleukin 1 receptor-driven neutrophil recruitment accounts to MyD88-dependent pulmonary clearance of Legionella pneumophila infection in vivo. J Infect Dis. 2015;211:322–330. doi: 10.1093/infdis/jiu430. [DOI] [PubMed] [Google Scholar]
- 28.Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci USA. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaac DT, Laguna RK, Valtz N, Isberg RR. MavN is a Legionella pneumophila vacuole-associated protein required for efficient iron acquisition during intracellular growth. Proc Natl Acad Sci USA. 2015;112:E5208–E5217. doi: 10.1073/pnas.1511389112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Luo Z-Q. The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect Immun. 2007;75:592–603. doi: 10.1128/IAI.01278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havey JC, Roy CR. Toxicity and SidJ-mediated suppression of toxicity require distinct regions in the SidE family of Legionella pneumophila effectors. Infect Immun. 2015;83:3506–3514. doi: 10.1128/IAI.00497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong KC, Sexton JA, Vogel JP. Spatiotemporal regulation of a Legionella pneumophila T4SS substrate by the metaeffector SidJ. PLoS Pathog. 2015;11:e1004695. doi: 10.1371/journal.ppat.1004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archer KA, Roy CR. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires’ disease. Infect Immun. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brieland JK, Remick DG, LeGendre ML, Engleberg NC, Fantone JC. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect Immun. 1998;66:65–69. doi: 10.1128/iai.66.1.65-69.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dam P, Olman V, Harris K, Su Z, Xu Y. Operon prediction using both genome-specific and general genomic information. Nucleic Acids Res. 2007;35:288–298. doi: 10.1093/nar/gkl1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao F, Dam P, Chou J, Olman V, Xu Y. DOOR: A database for prokaryotic operons. Nucleic Acids Res. 2009;37:D459–D463. doi: 10.1093/nar/gkn757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen X, et al. Targeting eEF1A by a Legionella pneumophila effector leads to inhibition of protein synthesis and induction of host stress response. Cell Microbiol. 2009;11:911–926. doi: 10.1111/j.1462-5822.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbanus ML, et al. Diverse mechanisms of metaeffector activity in an intracellular bacterial pathogen, Legionella pneumophila. Mol Syst Biol. 2016;12:893. doi: 10.15252/msb.20167381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubori T, Shinzawa N, Kanuka H, Nagai H. Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog. 2010;6:e1001216. doi: 10.1371/journal.ppat.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spörri R, Joller N, Albers U, Hilbi H, Oxenius A. MyD88-dependent IFN-gamma production by NK cells is key for control of Legionella pneumophila infection. J Immunol. 2006;176:6162–6171. doi: 10.4049/jimmunol.176.10.6162. [DOI] [PubMed] [Google Scholar]
- 41.Brown AS, et al. Cooperation between monocyte-derived cells and lymphoid cells in the acute response to a bacterial lung pathogen. PLoS Pathog. 2016;12:e1005691. doi: 10.1371/journal.ppat.1005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan Y, Luo Z-Q. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature. 2011;475:506–509. doi: 10.1038/nature10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neunuebel MR, et al. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science. 2011;333:453–456. doi: 10.1126/science.1207193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukherjee S, et al. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 2011;477:103–106. doi: 10.1038/nature10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goody PR, et al. Reversible phosphocholination of Rab proteins by Legionella pneumophila effector proteins. EMBO J. 2012;31:1774–1784. doi: 10.1038/emboj.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu J, et al. A unique deubiquitinase that deconjugates phosphoribosyl-linked protein ubiquitination. Cell Res. 2017;27:865–881. doi: 10.1038/cr.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu J, et al. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature. 2016;533:120–124. doi: 10.1038/nature17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feeley JC, et al. Charcoal-yeast extract agar: Primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito A, Rolfe RD, Edelstein PH, Finegold SM. Comparison of liquid growth media for Legionella pneumophila. J Clin Microbiol. 1981;14:623–627. doi: 10.1128/jcm.14.6.623-627.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo Z-Q, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci USA. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagai H, Roy CR. The DotA protein from Legionella pneumophila is secreted by a novel process that requires the Dot/Icm transporter. EMBO J. 2001;20:5962–5970. doi: 10.1093/emboj/20.21.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. doi: 10.1111/j.1365-2958.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 53.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Case CL, Roy CR. Analyzing caspase-1 activation during Legionella pneumophila infection in macrophages. Methods Mol Biol. 2013;954:479–491. doi: 10.1007/978-1-62703-161-5_29. [DOI] [PubMed] [Google Scholar]
- 55.Ivanov SS, Roy CR. Pathogen signatures activate a ubiquitination pathway that modulates the function of the metabolic checkpoint kinase mTOR. Nat Immunol. 2013;14:1219–1228. doi: 10.1038/ni.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choy A, et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moffat JF, Tompkins LS. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.