Significance
Calorie intake exceeding energy requirements is the chief culprit in the obesity epidemic affecting the United States and other countries. However, genetic factors may also contribute to the etiology of obesity. This study demonstrates that ankyrin-B (AnkB) deficiency in adipose tissue (AT) causes cell-autonomous adiposity, rendering mice more susceptible to becoming obese with age or when fed a high-fat diet and causing other metabolic complications. Loss of AnkB in AT increases glucose uptake and lipogenesis as a result of deficient clathrin-mediated GLUT4 endocytosis. We also show that AnkB mutations present in millions of Americans cause similar cellular metabolic impairments.
Keywords: ankyrin-B, Glut4, clathrin, obesity, membrane transport
Abstract
Obesity typically is linked to caloric imbalance as a result of overnutrition. Here we propose a cell-autonomous mechanism for adiposity as a result of persistent cell surface glucose transporter type 4 (GLUT4) in adipocytes resulting from impaired function of ankyrin-B (AnkB) in coupling GLUT4 to clathrin-mediated endocytosis. Adipose tissue-specific AnkB-KO mice develop obesity and progressive pancreatic islet dysfunction with age or high-fat diet (HFD). AnkB-deficient adipocytes exhibit increased lipid accumulation associated with increased glucose uptake and impaired endocytosis of GLUT4. AnkB binds directly to GLUT4 and clathrin and promotes their association in adipocytes. AnkB variants that fail to restore normal lipid accumulation and GLUT4 localization in adipocytes are present in 1.3% of European Americans and 8.4% of African Americans, and are candidates to contribute to obesity susceptibility in humans.
The United States and other countries enjoying Western lifestyles have experienced a marked increase in obesity in the past 40 y. Although this epidemic has occurred in parallel with increased prevalence of overnutrition and sedentary lifestyles, numerous familial studies also provide strong evidence for heritable contributions to obesity (1). More than 100 genetic loci have been linked to obesity, although their effect size is small and the mechanisms are not clear (2).
Recently, ANK2 encoding 220-kDa ankyrin-B (AnkB), a member of the ankyrin family of membrane adaptors, has been proposed as an obesity susceptibility gene based on studies of mice bearing a human AnkB R1788W variant that is enriched in people with type 2 diabetes (3–5). AnkBR1788W/R1788W mice developed age-dependent obesity and insulin resistance (IR) without detectable differences in food consumption or metabolic activity (3). Young AnkB mutant mice displayed increased glucose consumption during hyperinsulinemic euglycemic clamp studies, which also revealed increased glucose uptake in white adipose tissue (WAT) and skeletal muscle (SKM). Moreover, differentiated adipocytes from AnkB mutant mice exhibited increased levels of plasma membrane (PM) GLUT4 and glucose uptake as well as increased lipid accumulation. Thus, we hypothesized that obesity in AnkB mutant mice results from increased glucose uptake by WAT, whereby elevated GLUT4 is known to promote lipogenesis (6, 7). However, AnkB is expressed in the hypothalamus, and a small but sustained increase in appetite could not be rigorously excluded (8, 9). Moreover, it was not evident how loss of AnkB function, which conventionally restricts PM proteins within domains, could increase GLUT4 levels on the PM of adipocytes and SKM.
Here, we present direct evidence for a cell-autonomous mechanism for obesity as a result of gain of GLUT4 function through AnkB deficiency. We find that KO of AnkB solely in adipose tissue (AT) in mice is sufficient to promote adiposity with IR. Moreover, we report a role for AnkB as a direct adaptor between GLUT4 and clathrin, which can explain increased cell surface GLUT4 and glucose uptake in AnkB-deficient cells. Finally, we identify human AnkB variants expressed in millions of Americans that exhibit loss of activity in restoring normal lipid accumulation, glucose uptake, and PM GLUT4.
Results
Conditional KO of AnkB in AT Results in Age-Dependent Obesity and Metabolic Dysregulation.
AnkBR1788W/R1788W mice exhibit a complex metabolic syndrome (MS) with age- and diet-dependent obesity and IR combined with impaired insulin secretion caused by loss of IP3 receptor function in pancreatic β-cells (3). Reduced insulin secretion is partially compensated in young animals by increased basal glucose uptake by SKM and WAT, but IR emerges as animals age and gain weight (3). Consistent with this complexity, AnkB is expressed in multiple tissues involved in metabolism, including pancreatic β-cells, SKM, WAT, liver parenchymal cells, and the nervous system (3). To distinguish the specific contribution of AT to AnkB MS, we selectively eliminated AnkB expression in AT by crossing AnkB floxed mice with mice expressing adiponectin-Cre in AT (ADIPOQ-Cre; Fig. 1A).
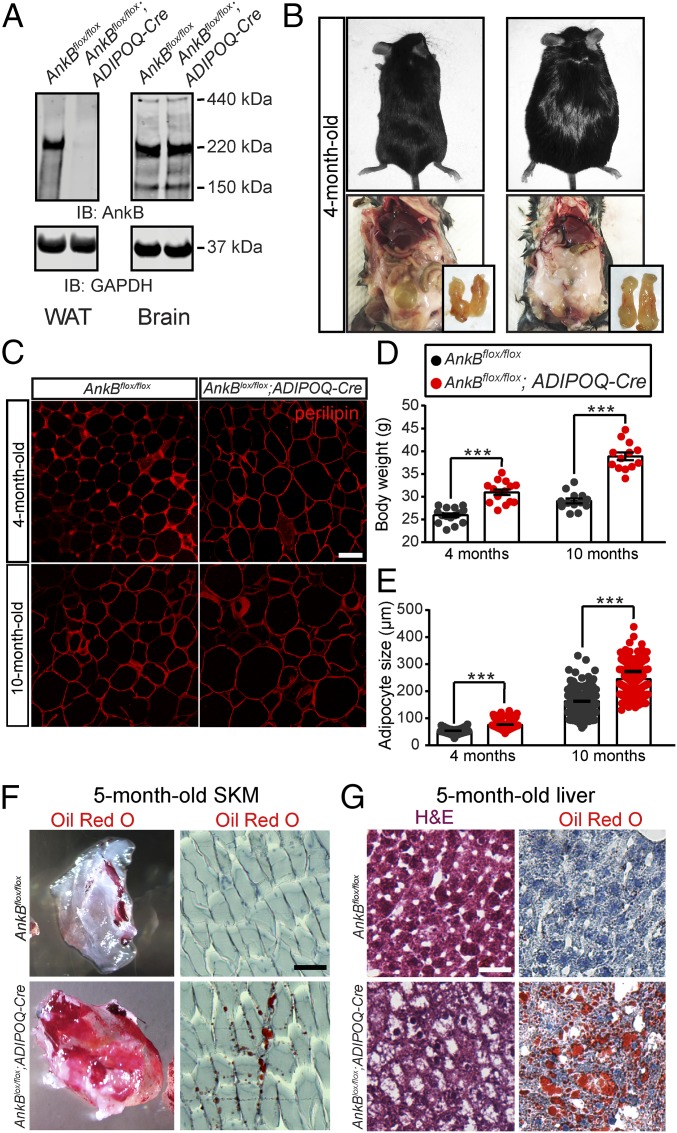
Fig. 1.
AnkB deficiency in AT causes age-dependent obesity and ectopic lipid accumulation in mice. (A) AnkB and GAPDH protein levels in WAT and brain of 4-mo-old mice. (B) Four-month-old control and AnkB KO male mice. (C) eWAT sections from 4-mo-old and 10-mo-old mice stained for perilipin. (Scale bar, 20 µm.) (D) Body weight. (E) eWAT cell size. (F, Left) Decellularized tibialis anterior muscle sample from 5-mo-old mice and (F, Right) section stained with Oil Red O. (G) H&E and Oil Red O staining of liver sections of 5-mo-old mice. (Scale bar, 100 µm.) Data represent mean ± SEM (n = 8–13 mice) for one experiment (***P < 0.001, unpaired t test). Results are representative of three independent experiments.
Four-month-old AnkB KO (AnkBflox/flox;ADIPOQ-Cre) mice exhibited increased body weight, fat pad mass, and epididymal WAT (eWAT) size (Fig. 1 B–E), which was exacerbated with age (Fig. 1 C–E). Increased adiposity correlated with up-regulation of adipogenic and de novo lipogenesis genes in WAT of AnkB KO mice (Fig. S1). Changes in body composition were not caused by changes in motor activity (Fig. S2A) or food consumption (Fig. S2B). However, AnkB KO mice had lower energy expenditure (EE) relative to total body weight and lower respiration quotient (Fig. S2 D–G), accompanied by whitening of brown AT (BAT; Fig. S3A). AnkB and uncoupling protein 1 (Ucp1) levels were reduced in BAT of AnkB KO mice (Fig. S3 B–D), although the amount of BAT did not change relative to controls (Fig. S3E). The potentially reduced thermogenic activity resulting from impaired BAT function might contribute to the lower levels of EE. These mice also exhibited other signs of increased fat mass, including elevation in circulating levels of leptin and reduced adiponectin (Fig. S4 A and C).
Consistent with increased adipose mass, AnkB KO mice showed secondary age-dependent signs of IR and increased inflammation. AnkB KO mice developed mild hyperglycemia and oral glucose intolerance during an oral glucose tolerance test (OGTT) with age (Fig. 2 A and B and Fig. S4 F and H). Older AnkB KO mice also developed IR, as demonstrated by reduced response to insulin stimulation during an insulin tolerance test (ITT; Fig. 2C and Fig. S4 G and I), reduced suppression of gluconeogenic genes by the liver in response to insulin (Fig. S5A), and impaired activation of the insulin signaling pathway in WAT, SKM, and liver (Fig. 3 C–F and H and Fig. S5 B and C). These mice also acquired pancreatic dysfunction, including enlarged pancreatic islets (Fig. 2D) and hyperinsulinemia (Fig. 2E), likely reflecting the acute expansion of β-cells associated with the onset of IR (10). Finally, 4-mo-old AnkB KO mice showed increases in basal lipolysis (Fig. 2F) and circulating levels of nonesterified free fatty acids (NEFAs; Fig. S4B). Elevated serum NEFA led to ectopic lipid infiltration in SKM and liver (Fig. 1 F and G) and contributed to systemic inflammation, as shown by significant macrophage infiltration in WAT (Fig. S4 D and E) and elevations in proinflammatory cytokines TNF-α and IL-6 (Fig. 2G).
Fig. 2.
Increased adiposity triggers metabolic dysregulation in AnkB KO mice. (A) Basal glucose levels. (B and C) Area under the curve for blood glucose levels during OGTT (B) or ITT (C). (D) Pancreatic islets stained for AnkB. (Scale bar, 20 μm.) (E) Serum insulin levels 30 min after oral glucose administration. (F) Glycerol release during basal lipolysis of WAT explants. (G) Serum TNF-α and IL-6 levels. Data represent mean ± SEM (n = 8 mice; **P < 0.01 and ***P < 0.001, unpaired t test). Results are representative of three independent experiments.
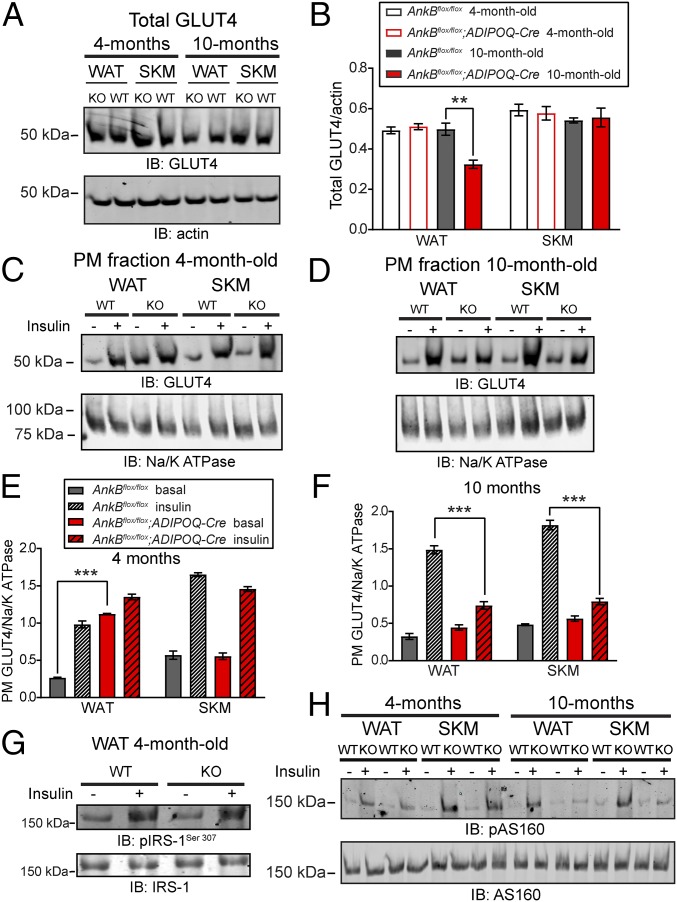
Fig. 3.
Persistent GLUT4 association with WAT PM of AnkB KO mice. (A and B) Immunoblots (A) and quantification (B) of total GLUT4 levels in WAT and SKM. (C–F) Immunoblots (C and D) and quantification (E and F) of PM-associated GLUT4 levels in WAT and SKM under basal conditions and following insulin stimulation. (G) Immunoblots of phosphorylated insulin receptor substrate-1 (pIRS-1Ser307) levels in WAT from 4-mo-old mice. (H) Immunoblots of phosphorylated AS160 (p-AS160Thr642) levels in WAT and SKM. Data represent mean ± SEM (n = 5 mice; ***P < 0.001, unpaired t test). Results are representative of three independent experiments.
Knock-in mice carrying the human disease-linked R1788W and L1622I AnkB variants develop early-onset obesity and MS under HFD conditions (3). AnkB KO mice challenged with an eucaloric HFD were more prone to increases in body weight (Fig. S6A) and eWAT size (Fig. S6 B and C). HFD-fed AnkB KO mice also showed higher fasting glucose levels and significant oral glucose intolerance (Fig. S6 D and E). Furthermore, systemic inflammation and IR phenotypes were more severe in AnkB KO animals than in controls (Fig. S6 F–H). These results demonstrate that specific loss of AnkB in AT is sufficient to recapitulate metabolic consequences of global AnkB mutation, including obesity, susceptibility to HFD, increased lipid infiltration in peripheral tissues, and systemic metabolic dysregulation. Moreover, these effects occur in the absence of increased food consumption. Together, these observations support the interpretation that adiposity in AnkB mutant mice results from cell-autonomous changes in adipocytes.
Age-Dependent Changes in Cell Surface GLUT4 in WAT and SKM from AnkB-Deficient Mice.
AnkBR1788W/R1788W mice exhibit increased glucose uptake by SKM and WAT as well as elevated levels of GLUT4 in the PM fractions in these tissues (3). Therefore, we evaluated the levels of GLUT4 in PM fractions of SKM and WAT before and after insulin stimulation. Total GLUT4 levels were similar in WAT and SKM of 4-mo-old control (AnkBflox/flox) and AnkB KO mice (Fig. 3 A and B). In contrast, GLUT4 was robustly associated with WAT PM from 4-mo-old AnkB KO mice (fourfold higher than in controls) under basal conditions, and it maintained its PM localization after insulin treatment (Fig. 3 C and E). We detected no changes in cell surface GLUT4 in SKM at baseline or following activation of insulin signaling between the two groups of 4-mo-old mice (Fig. 3 C and E). Levels of phosphorylated IRS-1 (Ser307) and AS160 (Thr642) were similar in WAT and in SKM of young control and AnkB KO mice under basal and insulin-stimulated (IS) conditions (Fig. 3 G and H). Thus, increased basal PM GLUT4 in 4-mo-old AnkB KO mice is restricted to WAT and is not a result of increased activation of insulin signaling. In line with the development of age-dependent obesity and systemic IR, GLUT4 was no longer significantly elevated at the basal state in WAT PM fractions of 10-mo-old AnkB KO mice (Fig. 3 D and F). Ten-month-old AnkB KO mice had a 37% reduction in total GLUT4 levels in WAT (Fig. 3 A and B), consistent with IR. We observed impaired response to insulin in WAT and SKM of older AnkB KO mice, as measured by decreased GLUT4 translocation to the PM (Fig. 3 D and F) and decreased pAS160Thr642 levels (Fig. 3H).
Increased PM GLUT4 and Glucose Uptake in AnkB KO Differentiated Adipocytes.
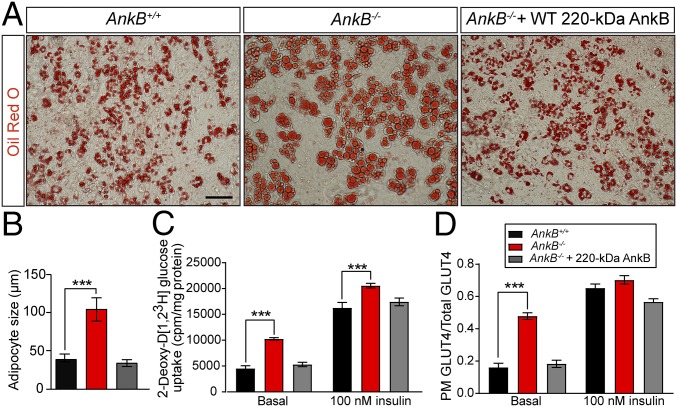
To study increased PM GLUT4 and lipogenesis in AnkB-deficient WAT under controlled conditions, we differentiated mouse embryonic fibroblasts (MEFs) into adipocytes (3) (Fig. 4A). AnkB KO MEFs (AnkB−/−) exhibited enlarged lipid droplets and increased glucose uptake (Fig. 4 A–C). We assessed GLUT4 localization in AnkB KO (AnkB−/−) adipocytes by using a GLUT4-GFP construct containing an exofacial Myc epitope (3, 11) (Fig. S7A). At steady state, most GLUT4 molecules were intracellular in control (AnkB+/+) adipocytes, as measured by the ratio of PM myc-GLUT4 to total GLUT4-GFP signal (Fig. 4D, “basal,” and Fig. S7B). In contrast, PM GLUT4 was threefold higher in AnkB−/− adipocytes at rest (Fig. 4D and Fig. S7B). The cellular adiposity and GLUT4 localization phenotypes can be rescued by WT 220-kDa AnkB, but not by expression of R1788W or L1622I AnkB variants, which lead to increased adiposity in knock-in mice and differentiated adipocytes (3) (Figs. 4 A and C and 5 and Fig. S8A). These results are consistent with the in vivo findings (Figs. 1 C and E and 3 C and E), and support the idea that AnkB deficiency causes adiposity on a cell-autonomous basis.
Fig. 4.
AnkB KO adipocytes show cell-autonomous increases in lipid accumulation associated with persistent PM GLUT4 localization and elevations in glucose uptake. (A) Oil Red O staining of control (AnkB+/+), AnkB-null (AnkB−/−), and WT AnkB rescued AnkB−/− adipocytes (day 8). (Scale bar, 50 μm.) (B) Lipid droplet size. (C and D) Effects of loss of AnkB on glucose uptake (C) and PM GLUT4 localization at basal and insulin stimulated states (D). Data represent mean ± SEM (n = 100 adipocytes for one of three independent experiments; one-way ANOVA with Tukey posttest).
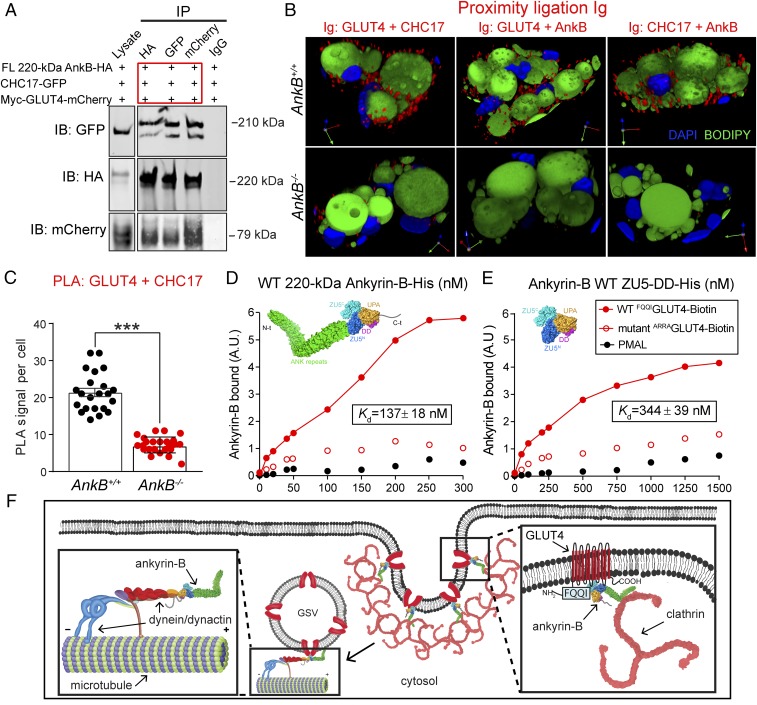
Fig. 5.
AnkB is required for GLUT4 association with clathrin in adipocytes. (A) Immunoprecipitation studies show the formation of a GLUT4–AnkB–CHC17 ternary complex. (B) PLA reveals in situ interaction at the nanoscale resolution between indicated protein pairs. (C) Quantification of PLA-based GLUT4–CHC17 association in adipocytes. (D and E) Direct binding of FL 220-kDa AnkB-His (D) and the ZU5-DD AnkB-His construct (E) to GLUT4 using purified proteins and biotinylated N-terminal GLUT4 peptides. Data represent mean ± SEM (n = 22–25 cells; ***P < 0.001, unpaired t test). Results are representative of three independent experiments. (F) Proposed model of AnkB-mediated internalization of GLUT4 in adipocytes. AnkB’s dual association with GLUT4 and CHC17 promote GLUT4 retrieval from the PM. AnkB also recruits the dynein/dynactin motor complex to PtdIns(3)P-positive endosomes, which is critical for microtubule-based retrograde transport of GSVs to the perinuclear GLUT4 compartment.
AnkB Is a Clathrin Adaptor Required for GLUT4 Endocytosis in Adipocytes.
Low levels of PM GLUT4 under basal conditions are maintained by a faster rate of GLUT4 endocytosis relative to reexocytosis (12, 13). We have previously reported that AnkB R1788W and L1622I mutant adipocytes exhibit reduced rates of GLUT4 internalization (3). To test whether a slower GLUT4 internalization rate was responsible for the elevation in PM GLUT4 in AnkB KO adipocytes, we monitored GLUT4 internalization in differentiated adipocytes stably expressing myc-GLUT4-GFP (Fig. S7A). In control adipocytes, insulin stimulation markedly increased GLUT4 PM localization (100 nM insulin; Fig. 4D and Fig. S7B), which decreased to nonstimulated values 30 min after insulin treatment (Fig. S7B, “insulin + 30 min int”, and Fig. S7D), corresponding to a t1/2 of GLUT4 internalization of 9 min. In contrast, the internalization rate of myc-GLUT4-GFP was substantially slower in AnkB−/− adipocytes (t1/2 of 25 min; Fig. S7 B and D). Expression of WT 220-kDa AnkB-BFP in AnkB−/− adipocytes rescued the deficits in internalization of GLUT4 (t1/2 = 11 min; Fig. S7 B and D).
AnkB may directly couple GLUT4 to clathrin, which promotes GLUT4 endocytosis (12, 13), based on two observations. First, AnkB coimmunoprecipitates with GLUT4 in vivo (3). Second, the N-terminal domain of clathrin heavy chain (CHC17) binds with high affinity to the highly conserved ANK repeat domain, also referred to as the membrane binding domain (MBD), of ankyrin-R (14). We have directly demonstrated an interaction between full-length (FL) AnkB and CHC17 through coimmunoprecipitation (co-IP) experiments from COS7 cell homogenates expressing HA-CHC17 and GFP-tagged AnkB (Fig. 5A and Fig. S9A). Moreover, AnkB associates with CHC17 through its MBD (Fig. S9A). Finally, GLUT4 and CHC17 individually coimmunoprecipitate (Fig. S9 A and B) and colocalize (Fig. S9 C and D) with AnkB and coimmunoprecipitate in a ternary complex with AnkB when all three proteins are coexpressed in COS7 cells (Fig. 5A).
We hypothesized that AnkB facilitates GLUT4 retrieval from the PM through coupling GLUT4 to CHC17. To evaluate an AnkB-dependent interaction between GLUT4 and CHC17 in adipocytes, we conducted proximity ligation essays (PLAs) for GLUT4 and CHC17, GLUT4 and AnkB, and CHC17 and AnkB in WT and AnkB−/− adipocytes (Fig. 5 B and C). PLA revealed in situ nanoscale interactions (40 nm) between GLUT4 and CHC17, GLUT4 and AnkB, and AnkB and CHC17 in WT adipocytes (Fig. 5B). Remarkably, PLA signals for all three pairs of proteins were markedly reduced in AnkB−/− cells (Fig. 5C). AnkB is therefore required for GLUT4 localization to clathrin-enriched zones in adipocytes (Fig. 5 B and C). Consistent with the PLA results, endogenous CHC17 and GLUT4 strongly colocalize in immunofluorescence images in WT but not in AnkB−/− adipocytes (Fig. S9 E and F).
We next sought to identify sites of AnkB interaction with GLUT4. Co-IP experiments revealed that GLUT4 associates with FL AnkB and its ZU5-DD supermodule, but not the AnkB MBD (Fig. S9B). GLUT4 contains an N-terminal F5QQI8 motif that regulates its localization by facilitating GLUT4 interaction with the endocytic machinery located at the cell surface and by promoting GLUT4 intracellular sequestration (12, 13). Moreover, GLUT4 requires its F5QQI8 motif to interact with AnkB in immunoprecipitation assays (3). Therefore, we assessed whether the N-terminal GLUT4 peptide interacts with AnkB by using purified histidine-tagged AnkB proteins and biotinylated GLUT4 peptides containing the first 24 aa, including the WT F5QQI8 sequence or a mutant A5RRA8 version (Fig. 5D). We found that the WT N-terminal GLUT4 peptide associated with FL WT 220-kDa AnkB-His with high affinity (Kd = 137 ± 18 nM; Fig. 5D) and with the AnkB ZU5-DD supermodule with a lower Kd of 344 ± 39 nM (Fig. 5E). In contrast, the A5RRA8 mutant GLUT4 peptide exhibited negligible binding to FL AnkB or its ZU5-DD supermodule (Fig. 5 D and E). This demonstrates AnkB interaction with a membrane-spanning protein independent of its MBD. The lower Kd of the ZU5-DD supermodule compared with FL AnkB indicates that additional contacts, possibly with the MBD, are required for full affinity (Fig. 5 B and C). It will be important in future studies to fine-map the GLUT4 binding site of the ZU5-DD supermodule.
Finally, we asked whether AnkB deficiency perturbs global clathrin-mediated endocytosis in adipocytes. By using a biotinylation assay, we found that the rate and extent of internalization of biotinylated cell surface proteins were similar between control and AnkB−/− adipocytes (Fig. S9 G and H). Together, our findings identify AnkB as a specialized adaptor that specifically promotes GLUT4 interaction with clathrin and is required for efficient retrieval of GLUT4 from the PM in adipocytes.
AnkB Promotes GLUT4 Internalization in Adipocytes Through Interaction with Dynactin and Phosphatidylinositol 3-Phosphate Lipids.
AnkB promotes intracellular transport through coupling dynactin to organelles containing phosphatidylinositol 3-phosphate [PtdIns(3)P] lipids (15, 16). By using rescue experiments in AnkB−/− adipocytes, we tested whether perturbations of the interactions between AnkB and the retrograde transport machinery or endocytic cargos affected GLUT4 internalization. The normal intracellular distribution of GLUT4 in differentiated AnkB+/+ adipocytes under basal conditions is dramatically shifted to favor GLUT4 association with the PM in AnkB−/− cells (Fig. S10 A and B). Although rescue experiments with WT 220-kDa AnkB restored normal basal GLUT4 distribution (Fig. S10 A and B), neither the DD1320AA AnkB mutant (Fig. S10C), which is unable to bind to the dynactin-4 subunit of the dynactin complex (15, 16), nor the R1194E AnkB mutant (Fig. S10C), which eliminates binding of AnkB to PtdIns(3)P lipids/cargo (15, 17), could rescue normal partitioning of PM to cytosolic GLUT4 (Fig. S10 A and B). These results suggest that AnkB is an essential facilitator of GLUT4 microtubule-based endocytic transport in addition to functioning as a clathrin adaptor.
A Subset of AnkB Cardiac Arrhythmia Variants Exhibit a Metabolic Phenotype in Differentiated Adipocytes.
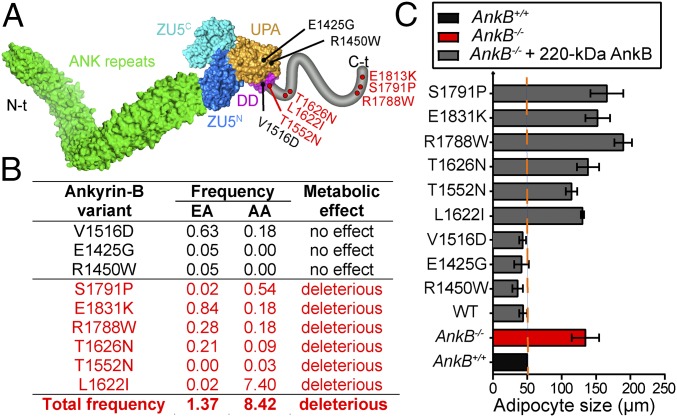
Multiple human variants of AnkB are associated with cardiac arrhythmia and result in loss of function in restoring normal calcium dynamics and rhythmic beating in AnkB KO neonatal cardiomyocytes (18, 19). We assessed the effect of these mutations on the ability of AnkB to rescue metabolic abnormalities of AnkB−/− differentiated adipocytes (Fig. 6 and Figs. S7 B–D and S8). Intriguingly, we found that a subset of AnkB variants, all located within the C-terminal unstructured regulatory domain (Fig. 6A), failed to restore lipid levels as well as reduce glucose uptake and lower PM GLUT4 (Fig. 6 B and C and Figs. S7 B–D and S8). Therefore, AnkB variants with impaired function in adipocytes, cumulatively present in 1.3% of European Americans and 8.4% of African Americans, are candidates to contribute to obesity susceptibility in humans.
Fig. 6.
Human AnkB mutations cause cell-autonomous increases in lipid accumulation in differentiated adipocytes. (A) AnkB structure showing the sites of disease-associated variants. Putative pathogenic AnkB variants within the C-terminal domain are shown in red. (B) Variant frequency in Americans of European (EA) and African (AA) descent. Predicted functional effect of these variants on adipocytes is indicated. (C) Lipid droplet size. Data represent mean ± SEM (n = 100 cells for one of three independent experiments).
Discussion
In this study, we identify a cell-autonomous mechanism that causes obesity in mice with KO of AnkB targeted to AT. These mutant mice developed age- and diet-dependent obesity and IR associated with progressive systemic lipotoxicity and inflammation, which affected the pancreas, SKM, liver, and BAT. In addition to the WAT-autonomous mechanism described here, BAT dysfunction may also contribute to the observed metabolic phenotype. It is not possible from our present data to distinguish the relative contributions of WAT hypertrophy vs. BAT disfunction. We demonstrated that AnkB deficiency in WAT resulted in increased glucose uptake as a result of persistent association of GLUT4 with the PM, which is associated with increased lipid accumulation. We found that AnkB binds directly to GLUT4 and CHC17 and promotes their association in adipocytes. Therefore, we propose that AnkB is a clathrin adaptor, and that impaired function of AnkB in coupling GLUT4 to CHC17 causes inefficient retrieval of GLUT4 from the cell surface and associated elevated glucose uptake and lipogenesis (Fig. 5F). We further found that AnkB mutations that prevent its interaction with dynactin and PtdIns(3)P also abolish the ability of AnkB to rescue GLUT4 trafficking (Fig. 5F). Taken together, these results revealed that AnkB, through its interactions with GLUT4, CHC17, the dynactin complex, and PtdIns(3)P, is essential for normal GLUT4 distribution and glucose uptake in adipocytes. Finally, we highlighted the potential public health relevance of this role of AnkB in AT biology with the finding that multiple AnkB human variants, cumulatively present in millions of Americans, failed to restore normal lipid accumulation and GLUT4 localization in adipocytes (Fig. 6 and Figs. S7 and S8).
Our findings, considered together with reports that overexpression of GLUT4 in AT promotes lipogenesis (6, 7), highlight the importance of retrieval of GLUT4 from the PM in maintaining metabolic balance. The AP-2 complex is a clathrin adaptor known to coordinate GLUT4’s packaging into endocytic vesicles in adipocytes (20, 21). Silencing of AP-2 in adipocytes reduced GLUT4 internalization only in IS cells, but GLUT4 still colocalized with clathrin puncta and was internalized through clathrin-mediated endocytosis in the basal state (22). In adipocytes, GLUT4 internalization occurs through a clathrin-dependent mechanism during the IS state (13) or independently of clathrin (23) during basal conditions. How these mechanisms are interchanged and how each route sorts GLUT4 in the endosomal system is not clear.
Humans, but not mice, express another clathrin heavy-chain isoform, CHC22, which is 85% homologous with CHC17, including conservation of the N-terminal portion known to mediate the interaction of CHC17 with ankyrins (14). Although these two clathrin isoforms are expressed in human SKM and adipocytes (24), they bind to different adaptors and cargo involved in GLUT4 transport, and have been suggested to mediate different steps of GLUT4 membrane traffic (25). Therefore, it will be important to assess whether AnkB associates with CHC22 to regulate of CHC22-mediated formation of GLUT4 storage vesicles (GSVs) and GLUT4 endosomal sorting.
A mouse model overexpressing GLUT4 in AT (AG4OX) showed increased localization of GLUT4 at the PM with resulting elevation in glucose uptake and increased adiposity (6, 7). In contrast to the AnkB KO mice we described here earlier, AG4OX mice do not develop IR. Whereas persistent GLUT4 at PM without elevation of total GLUT4 in WAT in AnkB KO mice results in hypertrophic obesity, overexpression of GLUT4 in AG4OX mice leads to hyperplastic obesity. As we showed here earlier, persistent AT hypertrophy and increased lipolysis can result in lipid infiltration in other metabolic organs and triggers a systemic inflammatory response that eventually leads to IR (26). On the contrary, obesity caused by hyperplastic AT expansion has milder metabolic consequences. However, AG4OX mice are not protected from glucose intolerance when fed an HFD (27). Thus, regulation of GLUT4 expression and dynamics may offer therapeutic benefits, provided the effects on lipogenesis are controlled.
We had previously reported that knock-in mice carrying the human cardiac disease-linked R1788W or L1622I AnkB variants have reduced expression of AnkB in WAT and develop obesity and MS under age and HFD conditions (3). The present study suggests that the metabolic dysregulation developed by these animals is partly caused by a cell-autonomous increase in adiposity that develops with age or HFD. By using cellular essays, we reproduced the impaired GLUT4 dynamics, high glucose uptake, high lipid accumulation, and adipocyte hypertrophy observed in those animals. Importantly, by using these in vitro essays, we identified four additional human cardiac disease-linked variants exhibiting similar cellular metabolism impairments that are therefore likely to also result in MS in mice and humans. It is important to note that AnkB is expressed in all metabolic organs, and AnkB deficiency can also cause intrinsic defects in pancreatic β-cells (3, 4). Therefore, it will be important to understand the individual metabolic roles of AnkB in these other tissues, along with the resulting inherent effects of AnkB deficiencies and how they affect the metabolic organ cross-talk. This, in turn, can be directly relevant to the 6.5 million Americans bearing potentially deleterious AnkB variants.
Materials and Methods
Detailed materials and methods, including statistical analysis, are described in SI Materials and Methods. Experiments were performed in accordance with the guidelines for animal care of the institutional animal care and use committee at Duke University and University of North Carolina at Chapel Hill. AnkB KO mice were generated by breeding AnkBflox/flox mice (28) with ADIPOQ-Cre mice. Studies were conducted in 4-, 5-, or 10-mo-old male mice housed at 22 ± 2 °C on a 12-h-light/12-h-dark cycle and fed ad libitum regular chow and water. For HFD studies, 5-wk-old male mice were fed ad libitum control diet or HFD for a period of 16 wk. Tolerance tests, measurement of plasma metabolites, membrane fractionation assays, MEF cultures and differentiation into adipocytes, and assessment of GLUT4 cellular distribution and glucose uptake were performed as previously described (3).
Supplementary Material
Acknowledgments
We thank Dr. Peter Mohler for the gift of the AnkB conditional KO mice and Erica Robinson, Janell Hostettler, and Hancheng Mao for technical assistance. This study received funding from the Howard Hughes Medical Institute, a George Barth Geller endowed professorship, and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30DK056350 (to the University of North Carolina Nutrition Obesity Research Center).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708865114/-/DCSupplemental.
References
- 1.Locke AE, et al. LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chesi A, Grant SF. The genetics of pediatric obesity. Trends Endocrinol Metab. 2015;26:711–721. doi: 10.1016/j.tem.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenzo DN, et al. Ankyrin-B metabolic syndrome combines age-dependent adiposity with pancreatic β cell insufficiency. J Clin Invest. 2015;125:3087–3102. doi: 10.1172/JCI81317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healy JA, et al. Cholinergic augmentation of insulin release requires ankyrin-B. Sci Signal. 2010;3:ra19. doi: 10.1126/scisignal.2000771. [DOI] [PubMed] [Google Scholar]
- 5.Bennett V, Lorenzo DN. An adaptable spectin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr Top Membr. 2016;77:143–184. doi: 10.1016/bs.ctm.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd PR, et al. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- 7.Tozzo E, Shepherd PR, Gnudi L, Kahn BB. Transgenic GLUT-4 overexpression in fat enhances glucose metabolism: Preferential effect on fatty acid synthesis. Am J Physiol. 1995;268:E956–E964. doi: 10.1152/ajpendo.1995.268.5.E956. [DOI] [PubMed] [Google Scholar]
- 8.Lee EB, Ahima RS. Alteration of hypothalamic cellular dynamics in obesity. J Clin Invest. 2012;122:22–25. doi: 10.1172/JCI61562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linnemann AK, Baan M, Davis DB. Pancreatic β-cell proliferation in obesity. Adv Nutr. 2014;5:278–288. doi: 10.3945/an.113.005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lizunov VA, et al. Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice. Am J Physiol Endocrinol Metab. 2012;302:E950–E960. doi: 10.1152/ajpendo.00466.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou JC, Pessin JE. Ins (endocytosis) and outs (exocytosis) of GLUT4 trafficking. Curr Opin Cell Biol. 2007;19:466–473. doi: 10.1016/j.ceb.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–3061. doi: 10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 14.Michaely P, Kamal A, Anderson RG, Bennett V. A requirement for ankyrin binding to clathrin during coated pit budding. J Biol Chem. 1999;274:35908–35913. doi: 10.1074/jbc.274.50.35908. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo DN, et al. A PIK3C3-ankyrin-B-dynactin pathway promotes axonal growth and multiorganelle transport. J Cell Biol. 2014;207:735–752. doi: 10.1083/jcb.201407063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayalon G, et al. Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J Biol Chem. 2011;286:7370–7378. doi: 10.1074/jbc.M110.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu F, et al. Ankyrin-B is a PI3P effector that promotes polarized α5β1-integrin recycling via recruiting RabGAP1L to early endosomes. eLife. 2016;5:e20417. doi: 10.7554/eLife.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohler PJ, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 19.Mohler PJ, et al. Defining the cellular phenotype of “ankyrin-B syndrome” variants: Human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007;115:432–441. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hasani H, et al. Roles of the N- and C-termini of GLUT4 in endocytosis. J Cell Sci. 2002;115:131–140. doi: 10.1242/jcs.115.1.131. [DOI] [PubMed] [Google Scholar]
- 21.Bernhardt U, Carlotti F, Hoeben RC, Joost HG, Al-Hasani H. A dual role of the N-terminal FQQI motif in GLUT4 trafficking. Biol Chem. 2009;390:883–892. doi: 10.1515/BC.2009.095. [DOI] [PubMed] [Google Scholar]
- 22.Blot V, McGraw TE. GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. EMBO J. 2006;25:5648–5658. doi: 10.1038/sj.emboj.7601462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klip A, Ramlal T, Young DA, Holloszy JO. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987;224:224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- 24.Vassilopoulos S, et al. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science. 2009;324:1192–1196. doi: 10.1126/science.1171529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esk C, Chen CY, Johannes L, Brodsky FM. The clathrin heavy chain isoform CHC22 functions in a novel endosomal sorting step. J Cell Biol. 2010;188:131–144. doi: 10.1083/jcb.200908057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Gnudi L, Tozzo E, Shepherd PR, Bliss JL, Kahn BB. High level overexpression of glucose transporter-4 driven by an adipose-specific promoter is maintained in transgenic mice on a high fat diet, but does not prevent impaired glucose tolerance. Endocrinology. 1995;136:995–1002. doi: 10.1210/endo.136.3.7867610. [DOI] [PubMed] [Google Scholar]
- 28.Smith SA, et al. Dysfunction in the βII spectrin-dependent cytoskeleton underlies human arrhythmia. Circulation. 2015;131:695–708. doi: 10.1161/CIRCULATIONAHA.114.013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biltz NK, Meyer GA. A novel method for the quantification of fatty infiltration in skeletal muscle. Skelet Muscle. 2017;7:1. doi: 10.1186/s13395-016-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.