Significance
Many patients with colorectal cancer die because of metastases in distant organs such as the liver and lungs, rather than from the primary tumor. A better molecular understanding of colorectal cancer has allowed for improved patient prognosis and the launching of precision medicine for treating metastatic colorectal cancer. Here we demonstrate that a deficiency of ribosomal S6 kinase 2 (RSK2) can result in dramatically decreased IFNγ secretion through an inappropriate phosphorylation status of T-bet, a modulator of IFNγ expression. Decreased IFNγ levels can lead to immune suppression, accelerating colon cancer-mediated liver and lung metastasis. We found that RSK2-mediated phosphorylation of T-bet at serines 498 and 502 is required for the inhibition of colon cancer metastasis and growth, through a positive regulation of RSK2/T-bet/IFNγ signaling.
Keywords: RSK2, T-bet, IFN gamma, metastasis, growth
Abstract
Metastasis is a major cause of cancer-related deaths. Approximately 80% of patients with colorectal cancer develop liver metastasis and 20% develop lung metastasis. We found that at different stages of colon cancer, IFNγ secretion from peripheral blood mononuclear cells was decreased compared with healthy controls. The ribosomal S6 kinase (RSK) family of kinases has multiple cellular functions, and we examined their roles in this observed IFNγ decrease. Flow cytometry analysis of wild-type (WT) and RSK2 knockout (KO) mice revealed significantly lower levels of IFNγ in the RSK2 KO mice compared with the WT mice. Since IFNγ is a component of immunity, which contributes to protection against metastatic carcinomas, we conducted a colon cancer liver metastasis experiment. We found significantly greater metastasis in RSK2 KO mice compared with WT mice. Transcription factor T-bet can directly activate Ifnγ gene transcription. In vitro kinase assay results showed that RSK2 phosphorylated T-bet at serines 498 and 502. We show that phosphorylation of T-bet by RSK2 is required for IFNγ expression, because knockdown of RSK2 expression or overexpression of mutant T-bet reduces IFNγ mRNA expression. To verify the function of the phosphorylation sites, we overexpressed a constitutively active mutant T-bet (S498E/S502E) in bone marrow. Mutant T-bet restored the IFNγ mRNA levels and dramatically reduced the metastasis rate in these mice. Overall, these results indicate that phosphorylation of T-bet is required for the inhibition of colon cancer metastasis and growth through a positive regulation of RSK2/T-bet/IFNγ signaling.
Metastasis is a major cause of death for cancer patients (1). Liver metastases are cancerous tumors that have spread from another part of the body to the liver. Most cases of liver metastases develop from colon or rectal cancers; in fact, ∼80% and 20% of patients with colorectal cancer develop liver and lung metastasis, respectively (2, 3). Cancer metastasis is accelerated through immunosuppression (4); however, the interaction between immune cells and cancer cells in the context of metastasis remains incompletely understood.
Ribosomal S6 kinase 2 (RSK2) is a serine/threonine kinase with two catalytic domains, and simultaneous mutation of both adenosine triphosphate (ATP)-binding sites abrogates kinase activity (5). RSK family kinases exhibit multiple cellular functions when activated by growth factors, peptide hormones, or neurotransmitters (6). They can regulate gene expression by phosphorylating transcription factors (7). Finally, loss-of-function mutations of RSK2 in humans causes Coffin–Lowry syndrome (CLS), an X-linked form of mental retardation associated with delayed bone age, belated closure of cranial fontanels, and short stature (8–10). Although the RSK family of proteins has been extensively studied, little is known regarding their role in the immune system.
T-bet (encoded by Tbx21) is an immune cell-specific member of the T-box family of transcription factors (11). T-bet undergoes posttranslational protein modifications and can determine cell fate by exerting direct stimulatory or indirect inhibitory activity on target gene expression (12). T-bet can directly regulate and activate Ifnγ gene transcription (13). IFNγ is produced in CD4, CD8, and natural killer (NK) cells (14). In response to IFNγ, T-bet is induced and is involved in remodeling the chromatin of the Ifnγ gene and stabilization of the IFNγ protein (15). Among the many factors produced by Th1 and CD8+ T cells, IFNγ is the most significant cytokine, with a role in preventing and suppressing the development of cancers (16). In addition, IFNγ produced in CD4+ and CD8+ T cells plays an important role in inhibiting and killing tumor cells and impeding growth (17).
In this study, we have demonstrated that RSK2 phosphorylates T-bet and promotes T-bet regulation of IFNγ mRNA expression levels. Moreover, our results indicate that RSK2 knockout (KO) mice exhibit down-regulation of IFNγ and a higher rate of colon cancer liver metastasis compared with wild-type (WT) mice. Overall, these findings suggest that T-bet is phosphorylated by RSK2, and that phosphorylation of serines 498 and 502 of T-bet is required for inhibition of colon cancer metastasis and growth through a positive regulation of RSK2/T-bet/IFNγ signaling.
Results
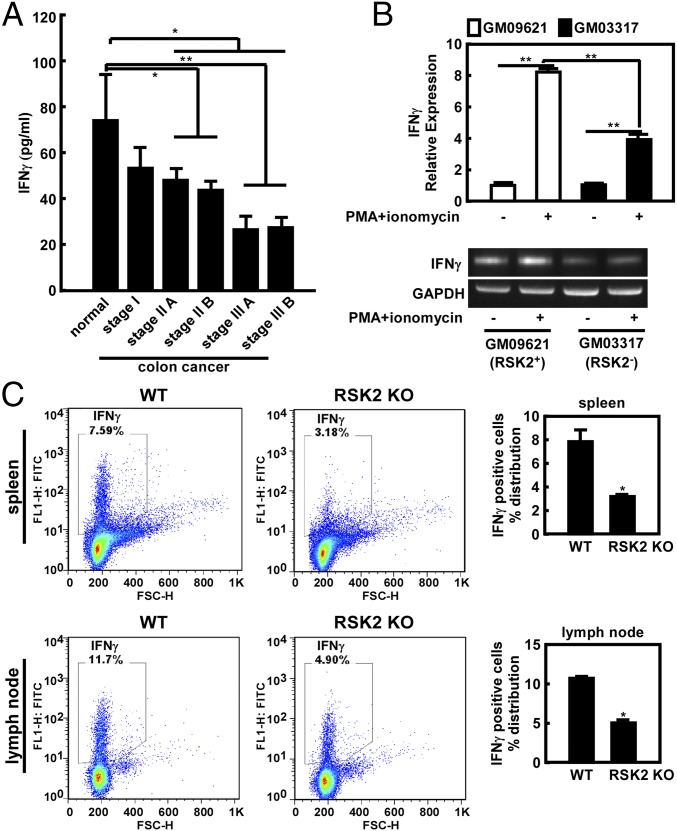
IFNγ Levels Spontaneously Decreased in Peripheral Blood Mononuclear Cells from Colon Cancer Patients at Different Stages of Disease.
IFNγ is a critical cytokine for immunity against viral and bacterial infections, as well as tumor surveillance (17, 18). Samples of blood from the peripheral circulation were collected from patients with colon cancer at various disease stages. Peripheral blood mononuclear cells (PBMCs) were isolated, including 70–90% lymphocytes (i.e., T cells, B cells, and NK cells) (19). The PBMCs were stimulated with phorbol-12-myristate acetate (PMA) and ionomycin (20), RSK2 mRNA levels were analyzed using human RSK2 primers, and IFNγ levels were detected in supernatant fractions using a human IFNγ ELISA kit. The results showed lower levels of RSK2 mRNA expression in the patients with colon cancer compared with normal control subjects (Fig. S1A). Furthermore, IFNγ levels decreased spontaneously, corresponding with advanced disease stage (Fig. 1A). Serum cytokine levels were also assayed in colon cancer patients and normal, healthy controls, and no significant differences were observed (Fig. S1 B–G). GM09621 and GM03317 are human lymphoblast cell lines that express RSK2 WT (RSK2+) and RSK2 mutant (RSK2−) genes, respectively (7). When stimulated with PMA and ionomycin, RSK2− lymphoblasts showed decreased IFNγ mRNA levels compared with RSK2+ lymphoblasts (Fig. 1B).
Fig. 1.
IFNγ levels in the PBMCs of patients with colon cancer of different stages. (A) PBMCs were stimulated, and IFNγ secretion was detected using a human IFNγ ELISA kit. (B) RSK2− lymphoblasts showed lower IFNγ mRNA levels compared with RSK2+. GM09621 (RSK2+) and GM03317 (RSK2−) were treated with PMA and ionomycin or untreated. Gene expression of Ifnγ was analyzed by PCR. Gapdh served as an internal control. (C) Analysis of IFNγ populations in the spleens and lymph nodes of WT and RSK2 KO mice. The average fluorescent intensity of IFNγ expression was analyzed by flow cytometry.
RSK2 KO mice were generated for studying brain function and body size. These mice exhibit characteristics consistent with the mental retardation and reduced growth characteristics observed in persons with CLS, who lack functional RSK2 proteins (21), and thus provide a useful model for studying RSK2 function. The spleen and lymph nodes are known to be important for proper functioning of the immune system, acting as filters for foreign particles and cancer cells. Primary cells isolated from mouse spleen and lymph nodes were analyzed by flow cytometry. The results showed that CD4+, CD8+, and NK cell populations and their rates of proliferation were not significantly different between WT and RSK2 KO mice (Fig. S2); however, the IFNγ+-stained cell population in these organs was dramatically decreased in the RSK2 KO mice (Fig. 1C).
The Rate of Colon Cancer Liver Metastasis Is Significantly Increased in RSK2 KO Mice.
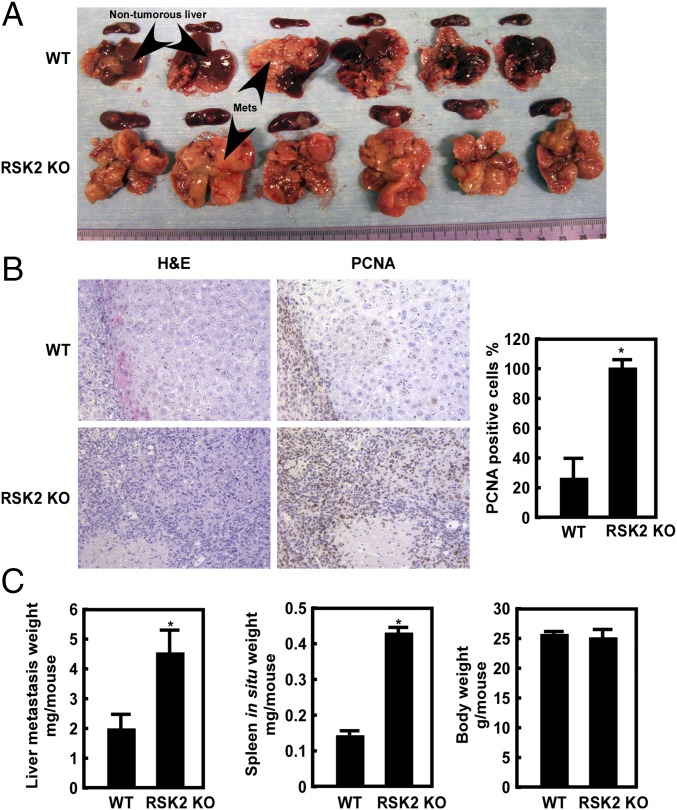
IFNγ is a critical component of immunity to metastatic carcinomas (22), and our results indicate that RSK2 deficiency is associated with decreased IFNγ secretion (Fig. 1 B and C). This observation led us to investigate whether RSK2 depletion could promote cancer metastatic growth. To do so, we implanted CT26 colon carcinoma cells, a mouse cancer cell line widely used in metastasis studies, into the spleens of WT and RSK2 KO mice for 10–14 d (23). On necropsy, we found that RSK2 KO mouse livers were fully occupied with cancer cells compared with WT mice (Fig. 2A). Histological examination of H&E-stained liver tissues revealed that very large areas of the liver contained metastases (Fig. 2B, Left).
Fig. 2.
The rate of colon cancer liver metastasis is significantly increased in RSK2 KO mice. (A) Representative photographs of spleen and liver metastases (Mets) of WT and RSK2 KO mice. (B) Immunohistochemical analysis of tumor tissues. Tumor tissues were stained with H&E or a PCNA antibody. The number of PCNA+-stained cells was significantly higher in the RSK2 KO group compared with the WT group. *P < 0.05. (C) Average liver metastasis weight of RSK2 KO mice was significantly higher than that of WT at 11 d after tumor implantation (Left; *P < 0.05, t test). Average spleen weight of RSK2 KO mice was significantly higher than that of WT spleen at 11 d after tumor implantation (Center; *P < 0.05, t test). The average body weight of WT and RSK2 KO mice was not different (Right).
Tumor cell proliferation was examined by immunostaining tissue sections using an antibody against proliferating cell nuclear antigen (PCNA), a proliferation marker. Staining results showed that the average level of PCNA in the liver metastases of RSK2 KO mice was more than five times higher than that of WT mice (Fig. 2B, Right). In addition, the average tumor weight in the liver was more than twofold greater in the RSK2 KO mice compared with the WT mice at 11 d after implantation (Fig. 2C, Left), indicating that RSK2 deficiency promotes liver metastatic growth in mice. Moreover, the average weight of the spleen was more than threefold greater in the RSK2 KO mice (Fig. 2C, Center), but average body weight did not differ between the RSK2 KO and WT mice (Fig. 2C, Right). Consistent with our ex vivo data, the liver metastasis results demonstrate that the absence of RSK2 promotes both tumor growth and metastasis in mice.
IFNγ Levels Are Down-Regulated in RSK2 KO Mice.
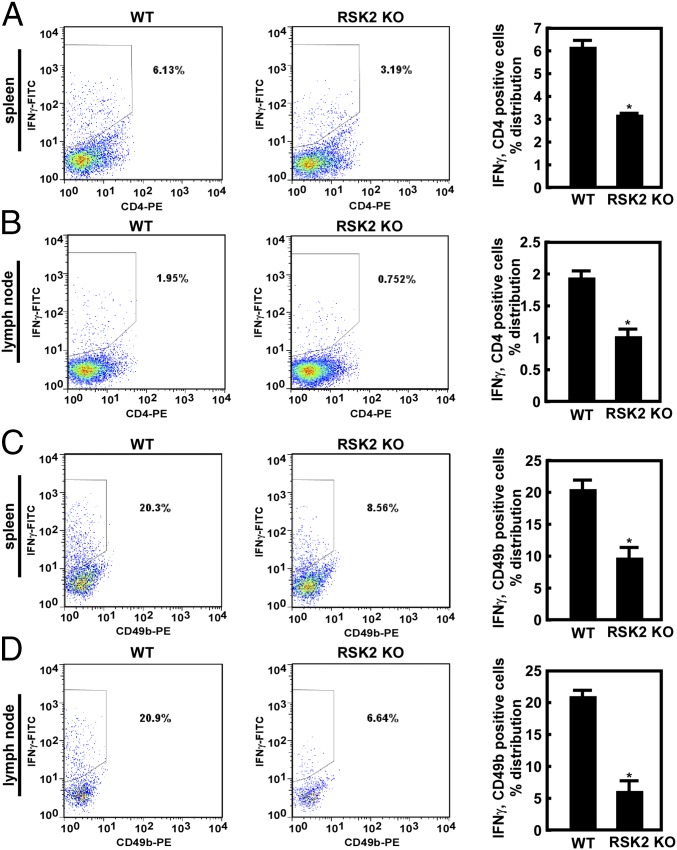
Previous studies have identified CD4+, CD8+, and NK cells as critical components of immunity against metastatic tumor growth (24, 25). IFNγ is produced by CD4+ and CD8+ T cells and plays important roles in inhibiting and killing tumor cells, thereby impeding tumor growth (17). Liver cells were collected from RSK2 KO and WT mice and analyzed by flow cytometry. CD4+ and CD8+ cell populations were significantly higher in the RSK2 KO mice compared with WT controls (Fig. S3A). In addition, because antitumor-specific effector cells are developed within the draining lymph nodes (26), we collected lymph nodes and spleens from mice with liver metastases. Primary cells were isolated and stained with IFNγ/CD4, IFNγ/CD49b (i.e., NK cell marker), or IFNγ/CD8. The data show significantly lower IFNγ expression in CD4+, NK, and CD8+ cells in RSK2 KO mice compared with WT mice even under CT26 colon tumor cell-challenging conditions (Fig. 3 and Fig. S3B).
Fig. 3.
IFNγ is down-regulated in RSK2 KO mice. (A and B) Flow cytometry analysis of the percentage of IFNγ/CD4+ cells in primary cells isolated from spleens (A) or lymph nodes (B) of WT and RSK2 KO mice. (C and D) Flow cytometry analysis of the percentage of IFNγ/CD49b+ cells from primary cells isolated from spleens (C) or lymph nodes (D) of WT and RSK2 KO mice (*P < 0.05, t test).
As reported previously (27), IL-2 is one of the major cytokines regulated by RSK2. Analysis of IL-2 mRNA expression in primary cells from the spleen and lymph nodes showed less IL-2 expression in the RSK2 KO mice compared with WT mice (Fig. S3C).
Taken together, the foregoing results suggest that RSK2 KO mice may have an immune-suppressed environment in vivo that accelerates colon cancer metastasis and growth (28) (Fig. 2). We explored this idea in additional experiments.
RSK2 Binds and Phosphorylates T-Bet.
RSK2 is a serine/threonine kinase that phosphorylates numerous substrates involved in mediating multiple cellular functions. The role of RSK2 in the immune system has not been well studied, however. IFNγ cannot be directly regulated by kinases; however, T-bet, a member of the T-box family of transcription factors, can directly activate Ifnγ gene transcription (11). In addition, as reported previously, T-bet protein posttranslational modification may play an important role in its function (12).
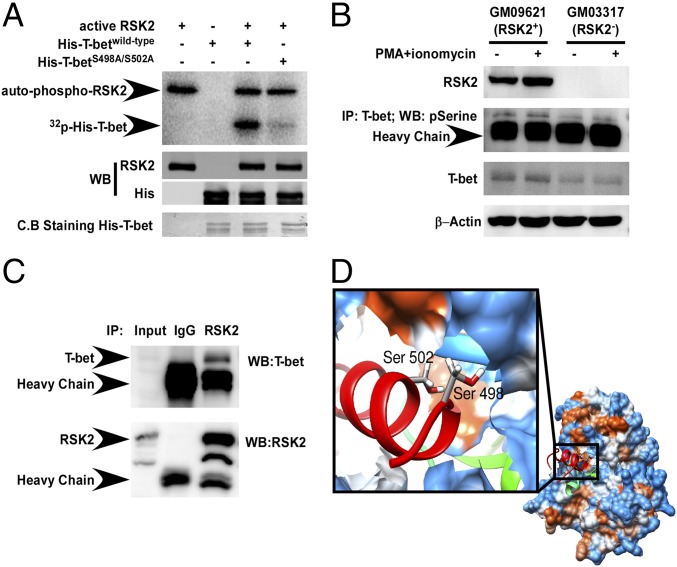
To examine whether RSK2 works as an upstream kinase for T-bet, we carried out LTQ Orbitrap hybrid MS analysis to identify T-bet sites potentially phosphorylated by RSK2. The results indicate that RSK2 phosphorylated serines 498 and 502 on T-bet (Table S1). Furthermore, an in vitro kinase assay proved that full-length T-bet was strongly phosphorylated by RSK2 (Fig. 4A, Upper, lane 3). However, mutation of T-bet serines 498 and 502 to alanine (T-betS498A/S502A) almost totally abolished the phosphorylation by RSK2 (Fig. 4A, Upper, lane 4).
Fig. 4.
RSK2 binds with and phosphorylates T-bet. (A) RSK2 phosphorylates T-bet at serines 498 and 502. His–T-betwild-type and a mutant His–T-betS498A/S502A fusion proteins were subjected to an in vitro kinase assay by active RSK2. Results were visualized by autoradiography. (B) T-bet showed a higher phosphorylation level in RSK2+ cells. RSK2+ and RSK2− were untreated or treated with PMA and ionomycin. After immunoprecipitation with a T-bet antibody, phosphorylated T-bet was detected using a p-serine antibody. Panels represent individual blots. (C) Confirmation of T-bet and RSK2 binding. Endogenous RSK2 was pulled down with an RSK2 antibody, and coimmunoprecipitated T-bet was detected at the same time in Jurkat cells. Panels represent individual blots. (D) Binding model of the N-terminal kinase domain of RSK2 with a T-bet fragment (residues 490–510). RSK2 is shown as a surface representation, and the T-bet fragment is shown as a carton representation in red. The enlarged view shows the two phosphorylation sites of T-bet, serines 498 and 502, represented in a stick model.
To examine the phosphorylation levels ex vivo, we stimulated GM03317 (RSK2+) and GM03317 (RSK2−) cells with PMA and ionomycin. We found a significantly higher T-bet phosphorylation level in GM09621 (RSK2+) cells compared with GM03317 (RSK2−) cells (Fig. 4B). To study the interaction of T-bet and RSK2, we pulled down the endogenous RSK2 with an RSK2 antibody and also detected endogenous T-bet in a coimmunoprecipitation assay (Fig. 4C). These results provide further confirmation that RSK2 binds T-bet.
Our laboratory previously solved the crystal structure of the N-terminal domain of RSK2 (29), which is critical for downstream activation (6). Using a computational homology method, we constructed a T-bet fragment (residues 490–510) that includes serines 498 and 502. After docking with the RSK2 N terminal and conducting molecular dynamics, the binding model showed that the RSK2 ATP-binding cavity specifically recognizes the phosphorylation sites, serines 498 and 502 (Fig. 4D). Based on these overall results, we conclude that T-bet is a phosphorylation target of RSK2.
RSK2 Promotes T-Bet-Mediated IFNγ mRNA Expression.
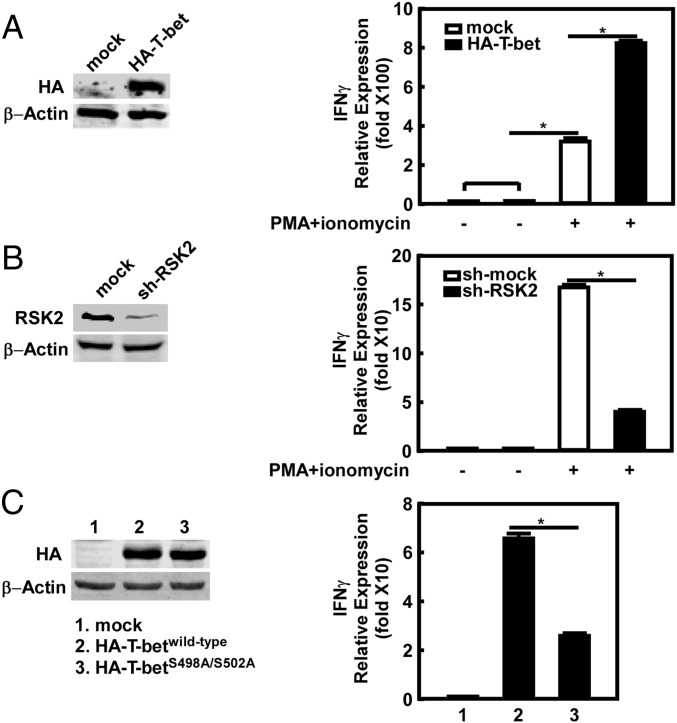
T-bet binds the Ifnγ promoter region and regulates IFNγ expression (13, 30). Using Jurkat cells, a human T lymphocyte cell line, we found that ectopic overexpression of T-bet could significantly promote IFNγ mRNA expression (Fig. 5A). We also found that lentivirus expression of sh-RSK2 in these cells blocked RSK2 protein expression (Fig. 5B, Left), and IFNγ mRNA expression was suppressed compared with sh-mock–transfected cells (Fig. 5B, Right). Finally, to elucidate the function of T-bet S498/S502 phosphorylation, we overexpressed T-betwild-type or mutant T-betS498A/S502A (Fig. 5C, Left). The results indicated that T-betwild-type could be phosphorylated by RSK2, whereas the mutant form of T-bet could not be phosphorylated (Fig. 4A, lanes 3 and 4). As expected, the mutant T-betS498A/S502A-promotion of IFNγ expression was dramatically decreased compared with that of WT T-bet (Fig. 5C, Right). These data further confirm that RSK2 phosphorylation of T-bet is required for IFNγ expression ex vivo.
Fig. 5.
RSK2 promotes T-bet–mediated IFNγ mRNA expression. (A) T-bet overexpression promotes IFNγ mRNA expression. The T-bet expression level was detected by Western blot analysis; β-actin served as a loading control. (B) Knockdown of RSK2 expression inhibits the expression of IFNγ mRNA. The RSK2 expression level was detected by Western blot analysis; β-actin served as a loading control. (C) Phosphorylation of T-betS498/S502 is required for the expression of IFNγ mRNA. Ectopic expression of T-betwild-type or mutant T-betS498A/S502A was detected by Western blot analysis; β-actin served as a loading control. *P < 0.05.
RSK2 Promotes IFNγ Transcription Activity.
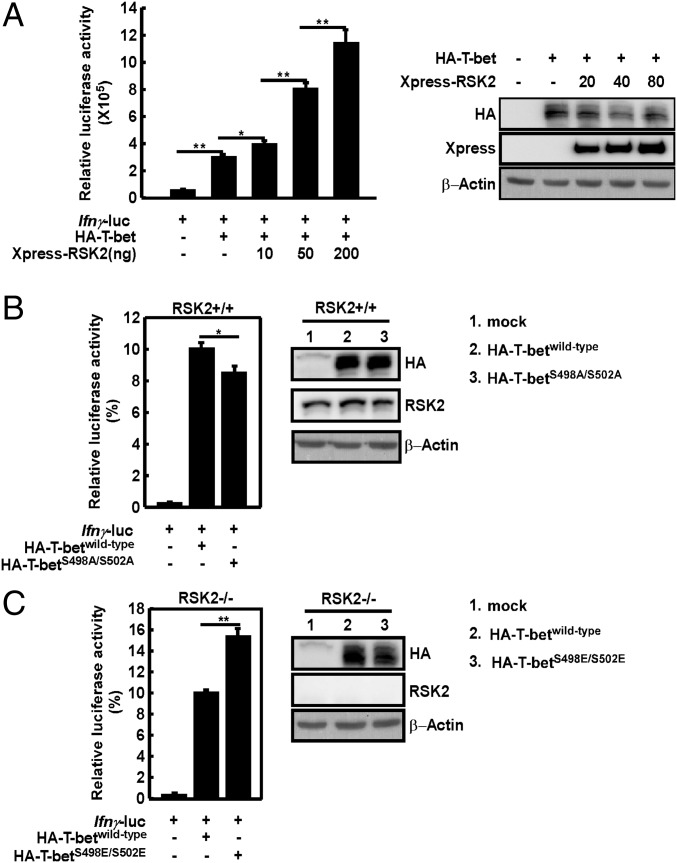
T-bet is a transcription factor regulating Ifnγ gene transcription (13, 30). A reporter gene assay was conducted using the Ifnγ-luc reporter plasmid carrying the luciferase gene containing the T-bet promoter region. After cotransfection with a fixed amount of T-bet but with increasing amounts of RSK2, T-bet transcription activity was significantly increased dose-dependently by RSK2 in RSK2−/− murine embryonic fibroblasts (MEFs) (Fig. 6A). To examine the function of the T-bet phosphorylation sites, T-betwild-type and mutant T-betS498A/S502A or T-bet expressing serines 498 and 502 mutated to glutamine (T-betS498E/S502E, which mimics a constitutively phosphorylated status of T-bet), were each cotransfected with the Ifnγ-luc reporter plasmid. As expected, in RSK2+/+ MEF cells, the T-betwild-type showed higher transcriptional activity compared with cells expressing the T-betS498A/S502A mutant form (Fig. 6B). Furthermore, RSK2−/− MEFs expressing the active mutant T-betS498E/S502E showed greater IFNγ transcriptional activity compared with cells expressing the T-betwild-type (Fig. 6C). These data indicate that T-bet promotion of IFNγ transcription activity relies on its phosphorylation and activation by RSK2.
Fig. 6.
RSK2 promotes IFNγ transcription activity. (A) The Ifnγ-luc reporter plasmid and pCMV-HA-T-bet plasmid were cotransfected with pcDNA4-mock (a control vector) or different doses of pcDNA-Xpress-RSK2 into RSK2−/− MEF cells. The luciferase activity was measured and normalized against Renilla luciferase activity (Left), and T-bet and RSK2 expression levels were detected by Western blot analysis (Right). The Ifnγ-luc reporter plasmid and pCMV-HA-T-betwild-type, pCMV-HA-T-betS498A/S502A, or pCMV-HA-T-betS498E/S502E plasmid were cotransfected with pCMV-HA-mock (a control vector) into RSK+/+ cells (B) or RSK2−/− cells (C). The luciferase activity was measured and normalized against Renilla luciferase activity (Left), and T-bet expression level was detected by Western blot analysis (Right). *P < 0.05. Panels represent individual blots.
Phosphorylation of T-Bet at Serines 498 and 502 Attenuates Colon Cancer Metastasis in RSK2 KO Mice.
To further investigate the role of T-betS498/S502 phosphorylation by RSK2 in vivo, we conducted a bone marrow cell transplantation assay. Female RSK2 KO mice served as recipient mice, and male RSK2 KO mice were donors. After isolation of bone marrow cells, mock, T-betwild-type, or T-betS498E/S502E (i.e., mimicking phosphorylated status) was overexpressed in these bone marrow cells using an electropulse method. Simultaneously, female RSK2 KO mice were sublethally irradiated using an X-ray generator to fully abolish bone marrow function, and then received i.v. transplantation with transfected bone marrow cells within 3 h after irradiation. Peripheral lymphohematopoietic reconstitution of all cell lineages should be normal by day 21 after bone marrow transplantation (31).
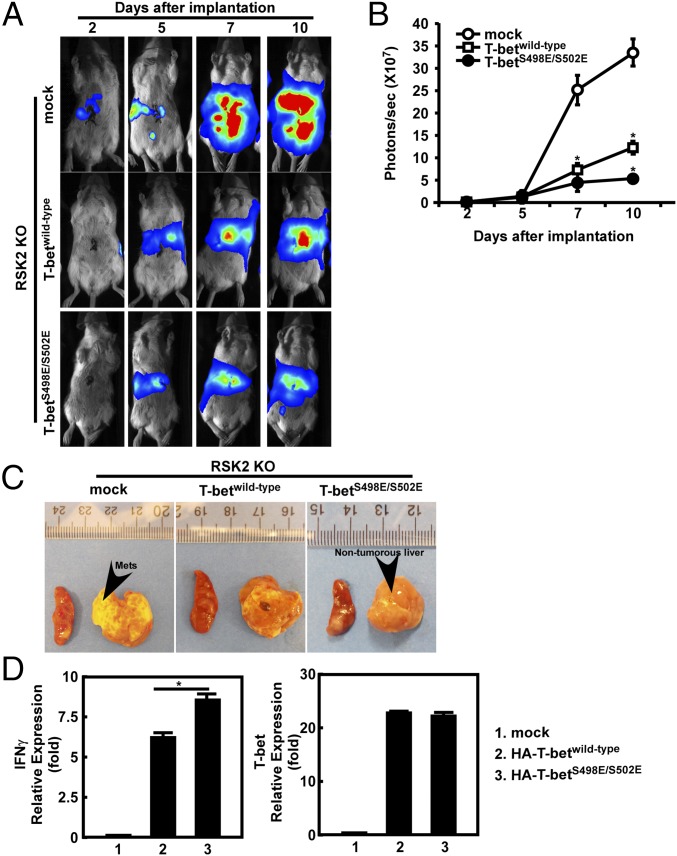
Peripheral blood was withdrawn from mice for identification of successful reconstitution of recipient mice. The data show that the sry (male-specific) gene (32) was detected in all female recipient mouse blood samples (Fig. S4A). We used these RSK2 KO mice with bone marrow cells overexpressing mock, T-betwild-type, or T-betS498E/S502E as our study model and implanted CT26-luciferase mouse colorectal cancer cells into the spleen of each mouse. As in a previous study of liver metastasis (33), the implanted CT26-luciferase cells quickly multiplied and occupied the mouse liver (by spleen injection) or lung (by tail vein injection) (34) in vivo. In vivo Xenogen imaging (35), which measures the bioluminescence of CT26 cells, revealed that starting on day 7 after tumor implantation, significantly more CT26 cells were detected in RSK2 KO mock-transfected mice than in RSK2 KO T-betwild-type– or T-betS498E/S502E–transfected mice (Fig. 7 A and B and Fig. S4 B and C). In addition, RSK2 KO T-betS498E/S502E–transfected mice had significantly less CT26 liver and lung metastasis compared with RSK2 KO T-betwild-type–transfected mice (Fig. 7 A–C and Fig. S4 B–E).
Fig. 7.
Phosphorylation of T-betS498/S502 attenuates colon cancer liver metastasis in RSK2 KO mice. RSK2 KO mice overexpressing mock, T-betwild-type, or T-betS498E/S502E were established by bone marrow transplant assay. CT26 cells (1 × 106) tagged with firefly luciferase were injected into the spleens of these mice, and bioluminescence of CT26 cells was visualized using in vivo Xenogen imaging at different days after tumor implantation. (A) Representative images from each group (n = 5) are shown. (B) Data were analyzed using Bruker MI SE software. *P < 0.05, ANOVA. (C) Representative photographs of spleen and liver metastases (Mets) of mice. (D) Gene expression of Ifnγ and T-bet was analyzed by PCR with specific primers, and gapdh was used as an internal control to verify equal amounts of cDNA. *P < 0.05.
Finally, we isolated immune cells from the thymus of mice from each group and found that the mRNA level of Ifnγ was significantly up-regulated in both T-betwild-type– and T-betS498E/S502E–transfected groups. Interestingly, IFNγ levels were higher in the T-betS498E/S502E–transfected mice than in the T-betwild-type–transfected mice (Fig. 7D). These data indicate that the inappropriate phosphorylation of T-bet accelerates colorectal tumor metastasis and growth because of an impaired immune response due to a deficiency of RSK2.
Discussion
Failing immunity is known to contribute to cancer development and progression (36); thus, enhancing and maintaining T-cell activation might be an effective approach in cancer treatment (37). All immunosuppressive treatments can potentially impair the immune system defense capacity, leading to an increased incidence of cancers (38). Although RSK family proteins have been extensively studied for their involvement in multiple cellular functions, little is known of their role(s) in the immune system in vivo. As reported previously, RSK2 is catalytically activated by T-cell receptor (TCR) stimulation, and plays an essential role in T-cell activation. T, B, and NK cells from RSK2 KO mice develop normally (27), and similar observations were made in our in-house RSK2 KO mouse breeding colony. When primary cells were isolated from spleens and lymph nodes and stained to detect the CD4, CD8, or NK cell marker, no significant differences were found between WT and RSK2 KO groups (Fig. S2). Both T and B cells can recognize a diverse array of potential tumor antigens, and also can detect small antigenic differences between normal and transformed cells (36). Our RSK2 KO mice showed a significantly increased rate of liver metastasis with a colon cancer cell challenge (Fig. 2A).
All of the foregoing effects seem to be due to the inappropriate phosphorylation of T-bet, which impairs the immune response (Fig. 7 and Fig. S4); however, how RSK2 enhances antitumor CD8+ T-cell responses directly or indirectly through CD4+ T cells or another mechanism requires further study. Clinically, heterogeneous loss-of-function mutations in the hRSK2 gene (RPS6KA3) cause CLS, and ∼70–80% of diagnosed patients have no family history (9). An RSK2-null mouse has been created as a model for CLS. Current clinical studies of CLS are focused mainly on patients with serious developmental retardation, and the extent of immune deficiencies remains unclear.
T-bet directly activates the Ifnγ gene by binding to the IFNγ promoter and to multiple distal regulatory elements located upstream and downstream of the Ifnγ gene (39). T-bet expression has been correlated with increasing IFNγ expression (40). Similar results were also obtained in our study (Figs. 5A and 6A). A high percentage of T cells produce IFNγ after TCR stimulation, and T-bet deficiency results in reduced IFNγ production (41). Selective expression of T-bet accounts for TH1 cell development and for the TH1 cell-specific expression of IFNγ (11). T-bet expression is rapidly induced by TCR and IL-12R signaling, and is required for the early production of IFNγ by antigen-specific CD8+ T cells (42). T-cell kinase-mediated phosphorylation of T-bet at Tyr525 promotes the interaction of T-bet with GATA3, which interferes with the binding of GATA-3 to its target DNA (43). T-bet phosphorylation at Thr302 is crucial for the interaction of T-bet with NFAT1, and loss of this interaction abrogates the ability of T-bet to suppress NFAT1-dependent cytokine expression (44). T-bet phosphorylation at Ser508 by casein kinase I and glycogen synthase kinase 3 (GSK3) promotes the interaction of T-bet with RelA, which impairs RelA binding to the Il2 promoter and the subsequent transcriptional activation of the Il2 gene (45). Mutation of lysine 313 (K313) decreases ubiquitination-mediated T-bet degradation and completely abrogates T-bet functions involving DNA binding and transcriptional activation of IFNγ (44). Based on recent studies, we hypothesized that T-bet activity might be regulated by posttranslational modification, specifically phosphorylation. We found that T-bet is phosphorylated by RSK2 at serines 498 and 502, and plays an important role in regulating IFNγ mRNA (Figs. 5C and 7D) and transcription level (Fig. 6 B and C).
Overall, our results show that RSK2 deficiency can result in dramatically decreased IFNγ secretion through inappropriate phosphorylation of T-bet. This can lead to immune suppression, which accelerates colon cancer metastasis and growth. The clinical relevance of these findings requires additional study. In addition, analysis of the cancer incidence and immune function in CLS patients could provide valuable information.
Materials and Methods
The in vivo Xenogen imaging of mice was performed using the Xtreme Imaging system (CareStream Health), and bioluminescence was quantified using Bruker MI software. The materials and methods used in this study are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Rebecca Morris and Kelly Johnson for assisting with the bone marrow transplant assay, Drs. Dan Li, Xuejiao Liu, Haitao Li, Young Jin Jeon, and Do Young Lim and Todd Schuster for supporting experiments; and Dr. Tia Rai and Nicki Brickman for assistance with manuscript submission. This work was supported by The Hormel Foundation; National Institutes of Health (Grants CA166001, CA172457, CA196639, and CA187027, to Zigang Dong), and the National Science Foundation of Henan Province, China (Grant 162300410337).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710756114/-/DCSupplemental.
References
- 1.Mehlen P, Puisieux A. Metastasis: A question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 2.Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–4075. doi: 10.3748/wjg.v17.i36.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess KR, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 4.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, et al. Computational and biochemical discovery of RSK2 as a novel target for epigallocatechin gallate (EGCG) PLoS One. 2015;10:e0130049. doi: 10.1371/journal.pone.0130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YY, et al. A regulatory mechanism for RSK2 NH(2)-terminal kinase activity. Cancer Res. 2009;69:4398–4406. doi: 10.1158/0008-5472.CAN-08-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Ataxia telangiectasia mutated proteins, MAPKs, and RSK2 are involved in the phosphorylation of STAT3. J Biol Chem. 2003;278:12650–12659. doi: 10.1074/jbc.M210368200. [DOI] [PubMed] [Google Scholar]
- 8.Jacquot S, Zeniou M, Touraine R, Hanauer A. X-linked Coffin-Lowry syndrome (CLS, MIM 303600, RPS6KA3 gene, protein product known under various names: pp90(rsk2), RSK2, ISPK, MAPKAP1) Eur J Hum Genet. 2002;10:2–5. doi: 10.1038/sj.ejhg.5200738. [DOI] [PubMed] [Google Scholar]
- 9.Pereira PM, Schneider A, Pannetier S, Heron D, Hanauer A. Coffin-Lowry syndrome. Eur J Hum Genet. 2010;18:627–633. doi: 10.1038/ejhg.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trivier E, et al. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature. 1996;384:567–570. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- 11.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 12.Lazarevic V, Glimcher LH, Lord GM. T-bet: A bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci USA. 2003;100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 15.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 16.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 18.Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15:407–422. doi: 10.1038/sj.cr.7290309. [DOI] [PubMed] [Google Scholar]
- 19.Du X, et al. Genomic profiles for human peripheral blood T cells, B cells, natural killer cells, monocytes, and polymorphonuclear cells: Comparisons to ischemic stroke, migraine, and Tourette syndrome. Genomics. 2006;87:693–703. doi: 10.1016/j.ygeno.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Ai W, Li H, Song N, Li L, Chen H. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int J Environ Res Public Health. 2013;10:3834–3842. doi: 10.3390/ijerph10093834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dufresne SD, et al. Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice. Mol Cell Biol. 2001;21:81–87. doi: 10.1128/MCB.21.1.81-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulaski BA, Smyth MJ, Ostrand-Rosenberg S. Interferon-gamma-dependent phagocytic cells are a critical component of innate immunity against metastatic mammary carcinoma. Cancer Res. 2002;62:4406–4412. [PubMed] [Google Scholar]
- 23.Gorden DL, et al. Resident stromal cell-derived MMP-9 promotes the growth of colorectal metastases in the liver microenvironment. Int J Cancer. 2007;121:495–500. doi: 10.1002/ijc.22594. [DOI] [PubMed] [Google Scholar]
- 24.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6:123–133. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, et al. Antitumor activity of T cells generated from lymph nodes draining the SEA-expressing murine B16 melanoma and secondarily activated with dendritic cells. Int J Biol Sci. 2009;5:135–146. doi: 10.7150/ijbs.5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin JX, Spolski R, Leonard WJ. Critical role for Rsk2 in T-lymphocyte activation. Blood. 2008;111:525–533. doi: 10.1182/blood-2007-02-072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakuta S, Tagawa Y, Shibata S, Nanno M, Iwakura Y. Inhibition of B16 melanoma experimental metastasis by interferon-gamma through direct inhibition of cell proliferation and activation of antitumour host mechanisms. Immunology. 2002;105:92–100. doi: 10.1046/j.0019-2805.2001.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malakhova M, et al. Structural diversity of the active N-terminal kinase domain of p90 ribosomal S6 kinase 2. PLoS One. 2009;4:e8044. doi: 10.1371/journal.pone.0008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh S, Hwang ES. The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res. 2014;2014:589672. doi: 10.1155/2014/589672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duran-Struuck R, Dysko RC. Principles of bone marrow transplantation (BMT): Providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci. 2009;48:11–22. [PMC free article] [PubMed] [Google Scholar]
- 32.Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. Biotechniques. 2005;38:702–706. doi: 10.2144/05385BM05. [DOI] [PubMed] [Google Scholar]
- 33.Ohana G, et al. Inhibition of primary colon carcinoma growth and liver metastasis by the A3 adenosine receptor agonist CF101. Br J Cancer. 2003;89:1552–1558. doi: 10.1038/sj.bjc.6601315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng YL, Liao HF, Li AF, Chang JC, Chiou RY. Oral administration of resveratrol in suppression of pulmonary metastasis of BALB/c mice challenged with CT26 colorectal adenocarcinoma cells. Mol Nutr Food Res. 2010;54:259–267. doi: 10.1002/mnfr.200900049. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, et al. IQGAP1 suppresses TβRII-mediated myofibroblastic activation and metastatic growth in liver. J Clin Invest. 2013;123:1138–1156. doi: 10.1172/JCI63836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghirelli C, Hagemann T. Targeting immunosuppression for cancer therapy. J Clin Invest. 2013;123:2355–2357. doi: 10.1172/JCI69999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerlini G, Romagnoli P, Pimpinelli N. Skin cancer and immunosuppression. Crit Rev Oncol Hematol. 2005;56:127–136. doi: 10.1016/j.critrevonc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Hatton RD, et al. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity. 2006;25:717–729. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Klose CS, et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, et al. Epigenetic and transcriptional programs lead to default IFN-gamma production by gammadelta T cells. J Immunol. 2007;178:2730–2736. doi: 10.4049/jimmunol.178.5.2730. [DOI] [PubMed] [Google Scholar]
- 42.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 43.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 44.Jang EJ, Park HR, Hong JH, Hwang ES. Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet. J Immunol. 2013;190:5764–5770. doi: 10.4049/jimmunol.1203403. [DOI] [PubMed] [Google Scholar]
- 45.Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med. 2005;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.