Significance
Hedgehog pathway-inhibitor drugs effectively treat basal cell carcinoma, a common skin cancer. However, many patients taking such drugs report severe taste disturbances that impair their quality of life. To understand the biology behind these adverse effects, we studied the consequences of Hedgehog pathway inhibition on taste organs and neural sensation in mice. Taste bud progenitor-cell proliferation and differentiation were altered, resulting in taste bud loss. Nerve responses to lingual taste stimuli were also eliminated, while responses to touch and cold stimuli remained. After stopping Hedgehog pathway inhibition, taste buds and sensory responses recovered. This study advances our understanding of Hedgehog signaling in taste homeostasis and the reported taste recovery after clinical treatments with Hedgehog pathway-inhibiting drugs.
Keywords: taste receptor cell, fungiform papilla, circumvallate papilla, taste regeneration, chorda tympani nerve
Abstract
Striking taste disturbances are reported in cancer patients treated with Hedgehog (HH)-pathway inhibitor drugs, including sonidegib (LDE225), which block the HH pathway effector Smoothened (SMO). We tested the potential for molecular, cellular, and functional recovery in mice from the severe disruption of taste-organ biology and taste sensation that follows HH/SMO signaling inhibition. Sonidegib treatment led to rapid loss of taste buds (TB) in both fungiform and circumvallate papillae, including disruption of TB progenitor-cell proliferation and differentiation. Effects were selective, sparing nontaste papillae. To confirm that taste-organ effects of sonidegib treatment result from HH/SMO signaling inhibition, we studied mice with conditional global or epithelium-specific Smo deletions and observed similar effects. During sonidegib treatment, chorda tympani nerve responses to lingual chemical stimulation were maintained at 10 d but were eliminated after 16 d, associated with nearly complete TB loss. Notably, responses to tactile or cold stimulus modalities were retained. Further, innervation, which was maintained in the papilla core throughout treatment, was not sufficient to sustain TB during HH/SMO inhibition. Importantly, treatment cessation led to rapid and complete restoration of taste responses within 14 d associated with morphologic recovery in about 55% of TB. However, although taste nerve responses were sustained, TB were not restored in all fungiform papillae even with prolonged recovery for several months. This study establishes a physiologic, selective requirement for HH/SMO signaling in taste homeostasis that includes potential for sensory restoration and can explain the temporal recovery after taste dysgeusia in patients treated with HH/SMO inhibitors.
Cancer patients treated with Hedgehog (HH) pathway inhibition (HPI) drugs experience severe taste disturbances (1–5). The Food and Drug Administration-approved HPI drug sonidegib (LDE225) blocks HH signaling at the Smoothened (SMO) receptor (Fig. 1A) (6). We had reported that oral gavage with sonidegib in mice for 16 or 28 d results in progressive fungiform papilla (FP) taste-organ disruption, taste bud (TB) loss, and elimination of taste nerve responses to chemical stimuli (7). Now we present data for the initial time course of HPI effects on taste organs and cell types and on sensation; study of Smo deletion; and the presence of the HH ligand in the nerve fibers of taste organs. Importantly, the potential for and nature of recovery from HPI effects in taste organs and taste neurophysiology are demonstrated.
Fig. 1.
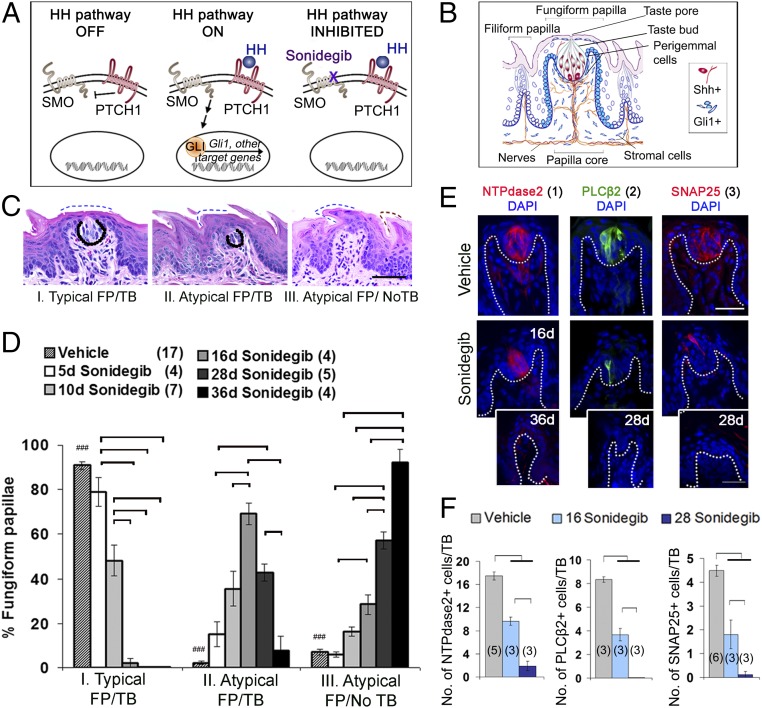
Sonidegib alters FP and TB morphology and reduces all TB cell types. (A) The HH pathway in OFF and ON signaling and INHIBITED at SMO by sonidegib. (B) Schematic: FP and filiform papillae with HH signaling components. (C) H&E staining for FP/TB categories. Dotted lines demarcate TB or regions of TB remnants. Blue dashed surface lines indicate epithelial keratinization in FP; a red dashed line indicates epithelial keratinization in filiform papilla in type III atypical FP/no TB. (Scale bar: 50 μm.) (D) Percentage of FP/TB categories: typical FP/TB (category I), atypical FP/TB (category II), and atypical FP/no TB (category III), after vehicle or sonidegib treatment. Bars are mean ± SEM. Numbers of tongues per group are in parentheses. Brackets indicate significant differences for treatment durations (two-way ANOVA and Tukey’s HSD post hoc tests); ###P < 0.001 for vehicle vs. sonidegib treatments. Complete F and P values are given in Fig. S1A. (E) Antibody detection of TB cell types: NTPdase2 (type 1), PLCβ2 (type 2), SNAP25 (type 3) after vehicle or sonidegib treatment. Cell nuclei are identified with DAPI (blue). (Insets) TB cell types 2 and 3 were eliminated after 28 d of sonidegib treatment. Type 1 cells (NTPdase2) were eliminated after 36 d. (Scale bar: 50 μm.) (F) Number of TB cell types after 16 or 28 d of sonidegib treatment compared with vehicle treatment. Data are mean ± SEM. Numbers of tongues are in parentheses in bars. Brackets indicate significant differences (one-way ANOVA with Tukey’s HSD post hoc tests). Complete F and P values are given in Fig. S1C.
Lingual taste organs are composed of (i) the TB, about 80–100 modified epithelial/neuroepithelial cells of several types; (ii) the sensory innervation to the TB and surrounding epithelium; and (iii) the papilla residence, a stratified epithelium over a core of stromal cells and connective tissue elements (8). Precise controls within each compartment are essential to integrate the cell processes that support taste organs and sensation. Here we investigated homeostatic lingual taste organ regulation by HH/SMO signaling in FP and circumvallate (CV) papillae and tested the hypothesis that morphologic and functional recovery would occur following cessation of HH/SMO inhibition by sonidegib treatment.
Taste cells turn over continuously in adults (9, 10), but TB are eliminated when essential signaling pathways, including HH, are disrupted (11–13). However, the taste cells are resilient in specific contexts, as illustrated by degeneration after nerve cut in adult mammals and regeneration as nerves grow back into the taste papilla epithelium (14). The taste organ therefore is ideally suited to explore fundamental questions about the biology of tissue homeostasis and renewal, the mechanisms that sustain sensation in constantly renewing organs comprised of heterogeneous cell types, and the potential restoration of organs and function after TB elimination resulting from deregulation of essential controls.
Although the HH pathway is a major regulator in several organ systems (15), there is sparse fundamental knowledge about the function and timing of HH signaling in taste organs, especially the mechanisms of cell regulation, the regulation across anterior and posterior tongue tissues, and the effects on taste sensation per se. Whereas transcriptional-level regulation of HH/GLI signaling has been studied by genetic manipulation of Gli2 (11), the consequences of HH signal disruption at the cell surface remain largely unexplored, although most pharmacologic HH inhibitors act at this level (16).
SMO is the core signal transduction component of HH signaling (Fig. 1A) (17, 18). In HH signaling, pathway activity is repressed in the absence of ligand via the transmembrane receptor PTCH, which represses the pivotal HH signaling protein SMO (19–21). HH binding to PTCH1 blocks its inhibition of SMO; downstream signaling is then initiated, resulting in the modulation of GLI transcription factors (GLI1, GLI2, GLI3) and leading to the transcription of target genes, including Gli1 and Ptch1 (Fig. 1A). In the tongue, HH pathway components are in distinct compartments of adult taste papillae and TB (Fig. 1B), including actively proliferating TB progenitor cells (8, 13). The secreted SHH ligand in epithelium is produced principally within TB cells (13, 22), whereas the HH targets, Ptch1 and Gli1, are expressed within perigemmal cells around the TB, in basal cells of the stratified epithelium that lines the papilla core, and in stromal or connective tissue cells of the papilla core (Fig. 1B) (11, 13). Paracrine signaling from SHH in TB cells to surrounding epithelial and connective tissue cells has been proposed (13, 23).
Sonidegib and other HPI drugs block the HH pathway at SMO and are effective in treating patients with advanced BCC (3, 16, 24–26). However, these patients experience dysgeusia and ageusia that compromise treatment adherence and quality of life (4, 5, 27, 28). There are reports that patients have some recovery 2–3 mo after stopping treatment (5), but the nature and extent of this recovery are unclear. Regenerative potential after HPI drug treatment has not been explored in animal models, so the basic biology of patient recovery is not known.
Here we used the cancer drug sonidegib in mice, in parallel with conditional Smo deletion targeting the whole body or epithelium, to test the main site of inhibitory effects and discern the mechanisms for HH/SMO inhibition in FP and CV taste organs and in sensory responses from the chorda tympani nerve that innervates TB in the FP. Further, we assessed taste organs and nerve responses for periods of several months after cessation of HPI drug treatment to determine whether recovery is possible. We demonstrate coordinated cell proliferation and differentiation regulated by HH/SMO signaling in taste papillae and TB, selective regulation of oral sensory modalities of taste, touch, and temperature, and the recovery of taste organs and sensation. Our data provide insight into the regenerative biology and clinical consequences in patients treated with sonidegib who experience dysgeusia.
Results
Treatment with HPI Drug Sonidegib Alters FP Taste-Organ Morphology Within 10 D.
Before testing recovery from HPI drug treatment, it was important first to determine the temporal aspects of HH/SMO signaling inhibition in mice gavaged with sonidegib for 5–36 d. We quantified effects by characterizing FP and TB morphology as category I (typical FP/TB), II (atypical FP/TB), or III (atypical FP/no TB) (Fig. 1C). Compared with vehicle-treated mice, there were no differences in FP/TB categories after 5 d of sonidegib gavage (Fig. 1D). However, after just 10 d of sonidegib treatment the proportion of category I FP (typical FP/TB) FP was reduced by half, and category II FP (atypical FP/TB) had increased several fold to 40% of all FP, compared with ∼2% in vehicle treatment. After 16 d of sonidegib gavage only ∼1% of all FP were category I (typical FP/TB) FP; category II (atypical FP/TB) FP comprised almost 70%, and category III (atypical FP/no TB) FP accounted for ∼30% of all FP (Fig. 1D). With 28 d of sonidegib gavage, category I (typical FP/TB) were essentially eliminated, replicating our prior results (7); notably, after 36 d, virtually all FP were category III, atypical FP/no TB (Fig. 1D). Therefore, in a detailed time course, HPI drug duration of 10–16 d eliminated typical FP and TB, whereas 5 d was not effective, and in progressive effects by 36 d only atypical papillae that lack TB (category III) remained on the tongue. (Statistics for data in Fig. 1D are given in Fig. S1A.)
Importantly, we quantified all FP numbers in a standard 600-µm region of vehicle- and sonidegib-treated tongues and found no reduction in papilla density across treatments (Fig. S1B). That is, FP were not eradicated, and we were not categorizing fewer FP at long treatment durations. Rather, these duration-specific results suggest that HPI drug effects are related to the disruption of physiologic cell renewal in taste organs.
To further understand the progression of TB loss in FP, we used immunostaining to test whether all TB cell types were susceptible to sonidegib treatment or whether specific taste cell types (type 1: NTPDase2+; type 2: PLCβ2; type 3: SNAP25+) (29) were lost in a particular sequence or time course or were retained. Whereas cell types 2 and 3 were essentially eliminated in TB after 28 d, type 1 cells were not completely lost until 36 d of sonidegib gavage (Fig. 1 E and F). The type 1 cells are most numerous in the TB and have a long turnover time, up to 24 d (29–31), which could account for their longer retention after HPI treatment. However, turnover for no TB cell type was resistant to the HPI drug, suggesting effects on progenitor(s) for all TB cells. (Complete statistics for data in Fig. 1F are in Fig. S1C.)
Cell proliferation and cell death.
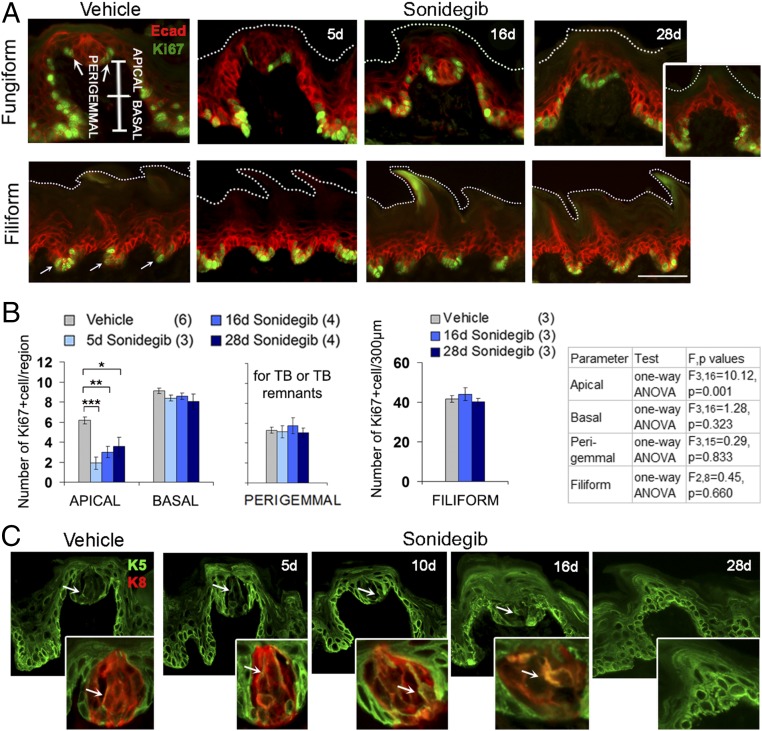
To test whether HPI altered turnover in lingual epithelium, we studied cell proliferation within FP and the nontaste filiform papillae. In FP we designated three regions, basal, apical, and perigemmal zones of FP basal cells (Fig. 2A, vehicle), and quantified Ki67+ cells for proliferation. Cells in all these regions include TB progenitors (13, 32). In basal cells of the apical FP wall there were substantial reductions in Ki67+ cells as early as 3–5 d of HPI treatment (Fig. 2 A and B), that is even before the FP/TB organs had noticeable morphological disruption (Fig. 1D). This early decrease in the number of Ki67+ TB progenitors in the FP was sustained after 16 and 28 d of drug treatment (Fig. 2 A and B). Alterations in proliferating cell numbers were not observed in basal or perigemmal regions of FP epithelial cells (Fig. 2 A and B). Note that perigemmal counts were made only within category I and category II FP, which have TB or TB remnants, because there were no TB in type III FP (Fig. 2A, 28 d and Fig. 2B). Notably, there was no reduction in Ki67+ cells in the basal cells of filiform papillae (Fig. 2 A and B).
Fig. 2.
Sonidegib treatment reduces apical epithelial cell proliferation in FP and leads to the accumulation of suprabasal K5+ cells. (A) Immunofluorescent antibody detection of E-cadherin (Ecad, red) and Ki67 (green) in basal epithelial cells of FP and filiform papillae after vehicle or 5 d, 16 d, or 28 d sonidegib treatments. The image for FP, vehicle, shows three regions for quantifying Ki67+ cells (apical, basal, and perigemmal). (Inset) Twenty-eight days sonidegib illustrates the absence of Ki67+ perigemmal cells in some category III FP (atypical FP/no TB). The vehicle image for filiform papillae illustrates Ki67+ cells, clustered at the base of rete ridges (arrows). White dotted lines indicate the surface of the epithelium. (Scale bar: 50 μm, also applies to C.) (Magnification: Inset, 0.9×.) (B) Number of Ki67+ cells in FP regions in vehicle- and sonidegib-treated mice. Counts in the perigemmal region were done only when TB or TB remnants remained in the papilla, i.e., in category I (typical FP/TB) or category II (atypical FP/TB) FP. In filiform papillae Ki67+ proliferating cells were quantified in a 300-μm length of tongue. Numbers of tongues are in parentheses. Statistical analysis was performed with one-way ANOVA with Tukey’s HSD post hoc tests (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). F and P values are shown in the table at the right of the graphs. (C) Antibody detection in FP of K5 (green, arrows point to K5+ cells in TB or TB remnants) in TB cells after vehicle or 5, 10, 16, or 28 d sonidegib treatment. (Insets) K5 coexpression with a few K8+ TB cells (red) indicated by arrows. (Magnification: Insets, 3.5×.)
We did not observe differences in the extent or distribution of cell death markers [TUNEL assay, Cleaved caspase 3 (CC3) antibody] in FP or filiform papillae of vehicle- or sonidegib-treated tongues (Fig. S2 A and B). That is, accelerated or altered cell death did not contribute to TB cell loss in HPI.
Cell differentiation.
When TB were eliminated from the FP after sonidegib gavage, as seen in category III FP (atypical FP/no TB), the papilla apex acquired a particular conical shape (Figs. 1C and 2A, 28 d, Inset). To learn whether TB progenitor cells were within this conical FP region, we used immunostaining for the basal cell marker keratin 5 (K5). K5+/K14+ cells are among the HH-responding progenitors for TB cells in FP (13, 32), and K5+/K14+ cells in CV differentiate into mature K8+ TB cells (33). In vehicle-treated mice a few cells within the TB were dual-labeled for the TB cell marker K8 (34) and K5 (Fig. 2C). After sonidegib treatment for 5, 10, and 16 d there was a reduction in K8+ TB cells and an increase in K5+ cells in the perigemmal region; a few cells within the TB were dual-labeled for K8 and K5 (Fig. 2C, Insets). However, at 28 d in category III FP (atypical FP/no TB), where there were no TB or TB remnants, the FP had K5+ cells in suprabasal layers throughout the conical apex (Fig. 2C, 28 d sonidegib). Thus, K5+ cells occupy tissue territory where TB had been located.
We also assessed the expression of K14+ and p63+ cells in the FP apex and observed both K14+ and p63+ cells in suprabasal cells of the apex after 28 d of sonidegib treatment (Fig. S2C), as with K5 cells. Ki67+ cells were sometimes found in the FP apex but generally were not suprabasal (Fig. S2D). As the K5+/K14+/p63+ cells in the tongue differentiate to TB, their expressions are lost (32). After sonidegib treatment, at the apex of category III FP (atypical FP/no TB), the suprabasal expression of K5+/K14+/p63+ points toward a differentiation defect from a normal progression of K5+/K14+/p63 expression to K8+ cells.
Smo Deletion Mimics HPI Drug Effects on FP Taste Organs.
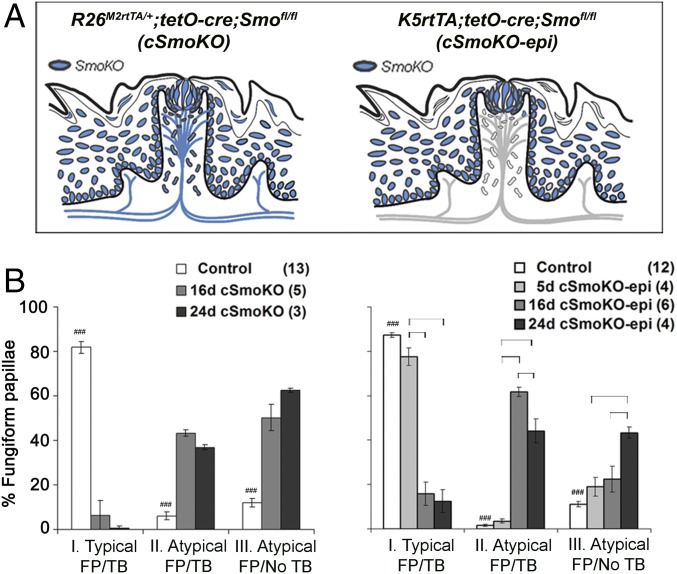
To establish that the effects observed in sonidegib-treated mice reflected the blockade of SMO, the HH signaling effector targeted by the drug, we generated mice to conditionally (doxycycline-regulated) delete Smo globally (R26M2rtTA/+;tetO-cre;Smofl/fl, referred to as “cSmoKO” mice) or in the epithelium (K5rtTA;tetO-cre;Smofl/fl, referred to as “cSmoKO-epi” mice), diagrammed in Fig. 3A. In cSmoKO mice, the category I FP (typical FP/TB) were reduced to less than 10% of all FP after 16 d of Smo deletion (Fig. 3B). With epithelial deletion of Smo, in cSmoKO-epi mice, there were no effects at 5 d after gene deletion, but after 16 d only 15% of FP were category I (typical FP/TB) (Fig. 3B). After 24 d, more than 40% of all FP were category III (atypical FP/no TB), which is comparable to the 16-d effects in cSmoKO mice. Therefore the major target cell population on which sonidegib acts to alter FP and TB is likely to be epithelial. Statistical analyses for the data in Fig. 3B are in Fig. S3A. The FP density did not alter with time in either Smo deletion model (Fig. S3B).
Fig. 3.
Smo deletion alters FP morphology and reduces TB. (A) Diagrams showing the conditional deletion of Smo from all tissues (cSmoKO) or from epithelial cells and progeny (cSmoKO-epi). The gray/shaded region on the cSmoKO-epi diagram indicates normal Smo expression. (B) Percentage of category I (typical FP/TB), category II (atypical FP/TB), and category III (atypical FP/no TB) FP in cSmoKO or cSmoKO-epi mice. Bars are mean ± SEM. Numbers of tongues are in parentheses. Brackets indicate significant differences (two-way ANOVA with Tukey’s HSD post hoc tests); ###P ≤ 0.001 for control vs. cSmoKO or cSmoKO-epi. Complete F and P values are given in Fig. S3A.
Overall the data from two cSmoKO models are similar in time course, extent, and effects after sonidegib treatment. Further, when comparing sonidegib and Smo-deletion experiments a similar phenotype was seen in category III FP (atypical FP/no TB) (Fig. S3C, H&E). That is, a heavily cornified, conical papilla apex was observed with a discernible cell collection where the K8+ taste cells had been located. As with sonidegib treatment, in the category III papilla after Smo deletion, K5+ cells occupied the conical papilla apex (Fig. S3C, K5/K8). With HPI, therefore, K5+ cells replace differentiated cells of the apical FP that typically include TB cells and accumulate as suprabasal, stratified epithelial cells.
After Smo Deletion, SHH+ and K8+ Cells Are Reduced in FP, and HH-Responding Cells Are Eliminated from the Epithelium but Remain in the Connective Tissue Core.
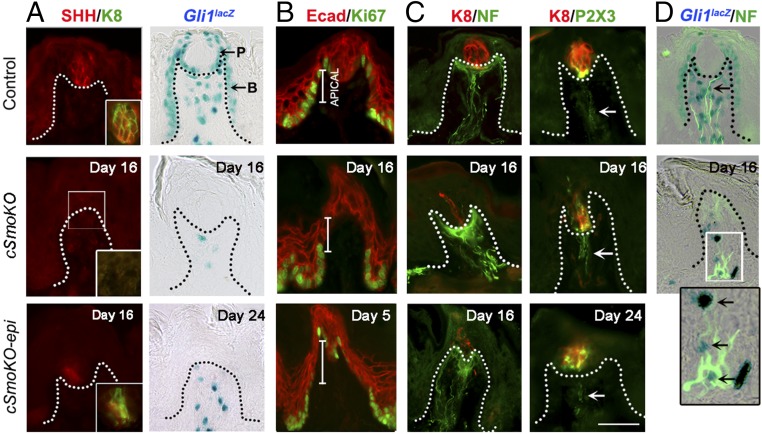
Associated with the loss of TB cells (using K8 as a taste-cell marker), the SHH+ TB cells also were reduced in cSmoKO and cSmoKO-epi mice (Fig. 4A, SHH/K8). Furthermore, Gli1lacZ-positive HH-responding cells were not retained in the FP epithelium, in either the perigemmal cells or basal cells of the FP epithelial wall (Fig. 4A, Gli1lacZ). In the papilla core, however, HH-responding cells remained but were reduced in number after global Smo deletion compared with controls. The remaining HH-responding cells in the FP core of cSmoKO mice might relate to noncanonical signaling; alternatively, the efficiency of Smo deletion in stromal cells might be less than in epithelial cells.
Fig. 4.
Smo deletion leads to reduced TB, HH ligand, HH-responding cells and reduced proliferation in FP, while innervation is retained. (A) Immunofluorescent antibody detection of SHH (red) and K8 (green) in TB cells; X-Gal staining (blue) of Gli1lacZ-positive cells from control, cSmoKO, and cSmoKO-epi mice. (Insets) Merged images for SHH and K8. No TB cells remain in the cSmoKO image (white box, Inset). (B) Antibody detection of E-cadherin (Ecad, red) and Ki67 (green) in control and Smo deletions. Ki67+ cells were reduced in apical FP walls shown by white bars. (C) Antibody detection of K8+ (red) TB cells and nerves (NF, green or P2X3, green) in cSmoKO or cSmoKO-epi mice. Arrows indicate P2X3 fibers in the FP core. (Scale bar: 50 μm, all images.) (D) Antibody detection of nerves. NF (green) with X-Gal staining (blue) of Gli1lacZ in control and 16-d cSmoKO mice. (Inset) Enlarged image of the boxed region shows the association of Gli1lacZ-positive, HH-responding cells with nerves (NF+) in FP stroma (arrows). Dotted lines in in A, C, and D indicate the basal lamina. (Magnification: D, Inset, 5×.)
As HH signaling activity was eliminated in the epithelium, there was an apparent decrease in Ki67+ proliferating cells in the basal cell layer of the apical papilla wall but not in the basal region (Fig. 4B, Ecad/Ki67). This demonstrates a disruption in the supply of a subset of TB cell progenitors.
After Smo Deletion, Nerves Remain Within the FP Connective Tissue Core.
Whereas TB cells were absent after Smo deletion in both cSmoKO and cSmoKO-epi mice, innervation remained within the FP core from the lingual nerve that typically innervates the FP walls and perigemmal regions (neurofilament, NF+, nerve fibers) and from the chorda tympani nerve that typically innervates TB cells (P2X3+ taste nerve fibers) (Fig. 4C, K8/NF and K8/P2X3). In addition, X-Gal staining for Gli1lacZ-positive cells plus NF immunofluorescence demonstrates a close physical association of nerves and HH-responding cells in the FP connective tissue core in control and cSmoKO mice (Fig. 4D), suggesting potential interactions between the papilla innervation and stromal cells. Furthermore, the results demonstrate that nerve fibers sustained within the FP are not able to maintain TB in the context of HPI effects in lingual epithelium.
HPI Leads to Decreased TB in CV on Posterior Tongue.
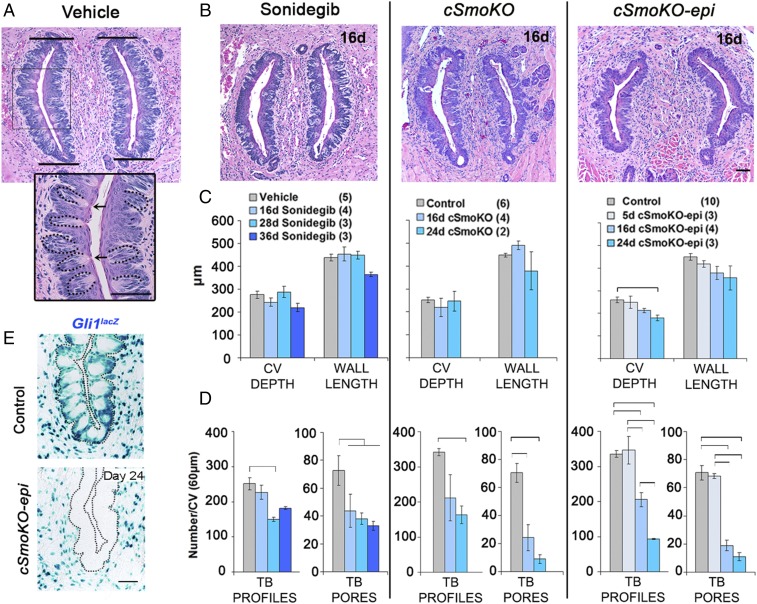
We found that sonidegib gavage and Smo gene deletion each substantially reduced TB and altered morphology in ectoderm-derived FP of the anterior tongue. To test whether HH/SMO inhibition could also affect TB and morphology of the CV (an organ with endodermal derivation), we extended studies to include a major posterior-tongue taste organ. We used H&E staining to quantify CV size and TB numbers. TB were identified as cell collections with a taste pore (Fig. 5A, Inset, arrows) or as collections of cells with or without a taste pore, termed “TB profiles” (Fig. 5A, Inset, dotted lines encompass TB profiles). CV depth (all sections in which the papilla appeared, multiplied by section thickness) and the length of papilla walls (Fig. 5A, bars denote wall length measure) were assessed for size determinations. Across sonidegib-treated and Smo-deletion mice, there was not an apparent gross alteration in CV form (Fig. 5B). Nor were depth or wall length different, except in cSmoKO-epi mice, in which CV depth was reduced over time and there was a trend for wall length to be shorter (Fig. 5C). (Statistical analyses for data in Fig. 5C are presented in Fig. S4A.)
Fig. 5.
Pharmacologic and genetic HH pathway blockade decreases TB in CV papillae. (A) H&E staining for CV/TB morphology in vehicle-treated mice. Bars delimit the CV walls, for measuring wall length. (Inset) An enlarged image of the boxed region illustrating TB profiles (dotted lines) and TB pores (arrows). (Scale bars: 50 μm.) (B) H&E staining for CV/TB morphology after 16 d of HPI by sonidegib or whole-body (cSmoKO) or epithelial Smo (cSmoKO-epi) deletion. (Scale bar: 50 μm for all images in A and B.) (C and D) Graphs for depth and wall length (C) and TB profiles and TB pores (D). Brackets in D denote significant differences (one-way ANOVA with Tukey’s HSD post hoc tests). Bars are mean ± SEM. F and P values are given in Fig. S4 A and B. (E) X-Gal staining (blue) for Gli1lacZ illustrates HH-responding cells in epithelium and stroma in control and 24-d cSmoKO-epi mice. Dotted lines outline the epithelium. (Scale bar: 50 μm for both images.)
Importantly, in pharmacologic and genetic HPI models, numbers of TB profiles were reduced, and numbers of TB with pores decreased by 25–75% after 16 d (Fig. 5D). Notably, the effects were more pronounced in Smo-deletion mice than after sonidegib gavage. In the CV of cSmoKO-epi mice we examined early-time-point effects and found that the number of TB with pores was not reduced after 5 d treatment, but there was a significant decrease after 16 d (Fig. 5D). This is similar to effects in FP/TB. (Detailed statistics for Fig. 5D are given in Fig. S4B.)
Overall, the TB loss is substantial and demonstrates that HPI affects the CV as well as FP taste organs and, therefore, can alter two major sets of TB on the anterior and posterior tongue. Although not all of the more than 100 TB in the CV were totally eliminated, as they were in the FP, which have a single TB each, the effects are substantial. In assessing the efficacy of HPI in cSmoKO-epi mice, we further observed that Gli1lacZ-positive HH-responding cells were eliminated in the CV epithelium but not from the stroma (Fig. 5E and Fig. S4C). Similar to effects in the FP, HH-responding cells were reduced in the stroma in cSmoKO mice (Fig. S4C). Furthermore, even with loss of TB cells, innervation to the CV was retained in physical association with HH-responding cells (Fig. S4C).
Recovery of FP and TB Cells and Molecular Phenotypes When Sonidegib Treatment Is Discontinued.
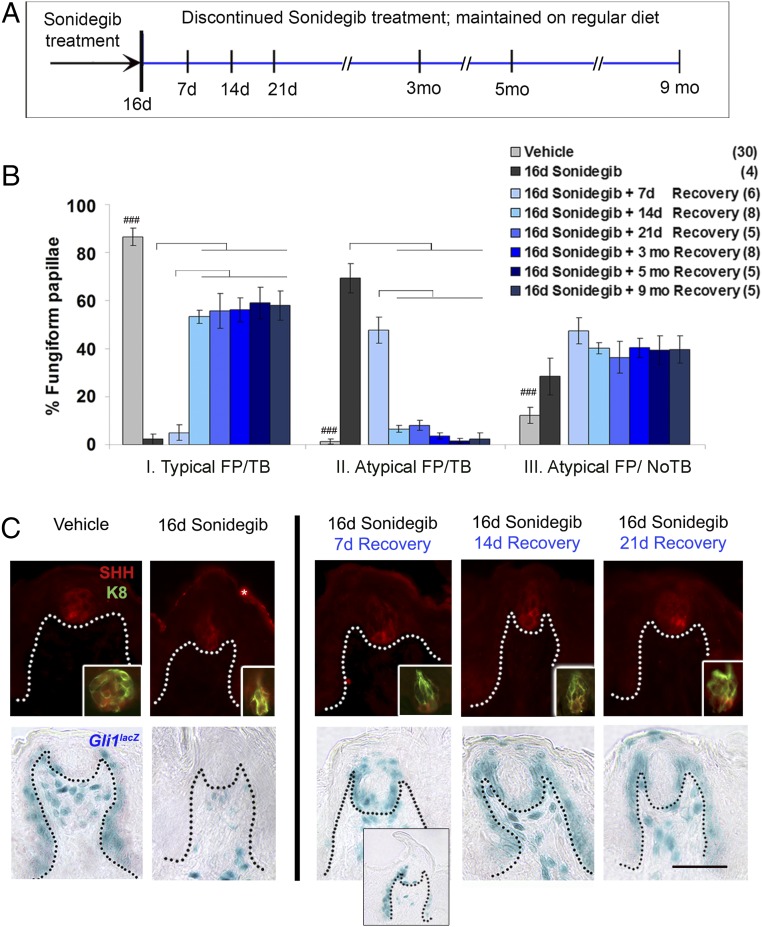
To test potential FP taste-organ restoration after HPI, we gavaged mice with sonidegib for 16 d and then discontinued treatment and maintained mice for recovery periods of 7, 14, or 21 d and for 3, 5, or 9 mo (Fig. 6A). A recovery period of 7 d was not sufficient to restore papillae to category I morphology (typical FP/TB); rather most papillae were atypical, in category II or III (Fig. 6B). Remarkably, however, 14 d were sufficient to restore about 50% of all papillae to category I morphology, and only 5% or fewer of all FP were category II FP (atypical FP/TB). About 40% of all papillae were category III (atypical FP/no TB).
Fig. 6.
Cessation of sonidegib treatment results in the recovery of FP/TB morphology, SHH ligand, and HH-responding cells within 14 d and continuing up to 9 mo. (A) Time line for studying recovery from the effects of 16 d sonidegib treatment. (B) Percentage of category I (typical FP/TB), category II (atypical FP/TB), and category III (atypical FP/no TB) FP after 16 d of sonidegib treatment followed by recovery periods of 7, 14, or 21 d and 3, 5, or 9 mo. Examples of FP/TB morphology are shown in Fig. S5A. Bars are mean ± SEM. Numbers in parentheses are number of mice analyzed. Brackets denote significant differences for treatment durations (two-way ANOVA with Tukey’s HSD post hoc tests); ###P ≤ 0.001 for vehicle vs. sonidegib treatments. Complete F and P values are given in Fig. S6A. (C) Antibody detection of SHH (red) and K8 (green, Inset) for TB cells and X-Gal staining (blue) for Gli1lacZ after 16 d of sonidegib treatment and recovery for 7, 14, or 21 d. The Gli1lacZ Inset at 7 d recovery is included to illustrate the variability in the recovery phenotype at 7 d. Dotted lines demarcate the basal lamina. (Scale bar: 50 μm.) (Magnification: C, Lower, Inset, 0.6×.)
We used prolonged recovery times to determine whether an increased proportion of FP was restored to category I after several months. Notably, the proportions of typical FP/TB ( category I) and atypical FP/no TB (category III) did not change from 14 d through 9 mo of recovery (Fig. 6B). This suggests that a proportion of FP is not resilient and cannot recover after HPI drug treatment, whereas another FP subset recovers and maintains TB after sonidegib treatment is discontinued. Details of FP/TB morphology during treatment and the recovery transition period from 7 to 14 d after stopping sonidegib treatment, and after 9 mo of recovery are seen with H&E staining in Fig. S5A. The category III (atypical FP/no TB) phenotype persists throughout treatment and recovery. This category, which loses all TB cells, apparently cannot recover from HPI effects, whereas category II FP (atypical FP/TB), which retain TB cell remnants, presumably can recover.
Statistical analysis for FP/TB categories across recovery in Fig. 6B is presented in Fig. S6A, and additional data are included to demonstrate that FP numbers per tissue region do not differ across sonidegib treatment and recovery periods (Fig. S6B).
To test whether all taste cell types in the FP/TB recover after discontinuing sonidegib treatment or whether some types were resistant to recovery, we used immunostaining and quantified cell types. As noted, all cell types were eliminated after the HPI drug (Fig. 1D), but when treatment was discontinued the three cell types were restored, with similar time courses, after 14 d in category I (typical FP/TB) and category II (atypical FP/TB) FP to the values seen in vehicle-treated FP (Fig. S6C). Category III FP (atypical FP/no TB), were not quantified because no TB were present.
During recovery from HPI treatment, which occurs between 7 and 14 d after stopping sonidegib gavage, there was an increase in SHH+ and K8+ cells in the reconstituted taste buds (Fig. 6C, SHH/K8), associated with recovery of Gli1lacZ-positive cells in the perigemmal region and FP epithelial walls (Fig. 6C, Gli1lacZ). A gradual restoration of Gli1lacZ-positive cells was apparent in the varied expression patterns seen at 7 d recovery, when HH-responding cells were observed in perigemmal regions only or partially patterned in perigemmal cells and papilla walls (Fig. 6C, 7 d recovery and Inset). Overall, the HH ligand and responding cells returned to FP/TB in concert at 14 d after drug cessation.
Recovery of TB in the CV.
TB in the posterior tongue CV papillae were decreased after 16 d of sonidegib treatment (Fig. 5). When sonidegib gavage was discontinued, there was no restoration of TB with pores after 7 d (Fig. S5B), but there was a trend toward increased numbers at 14 d. By 3–9 mo of recovery the numbers of TB with pores were not different from the numbers in vehicle-treated mice. Therefore, there was recovery from HPI effects in the CV as well as in the FP.
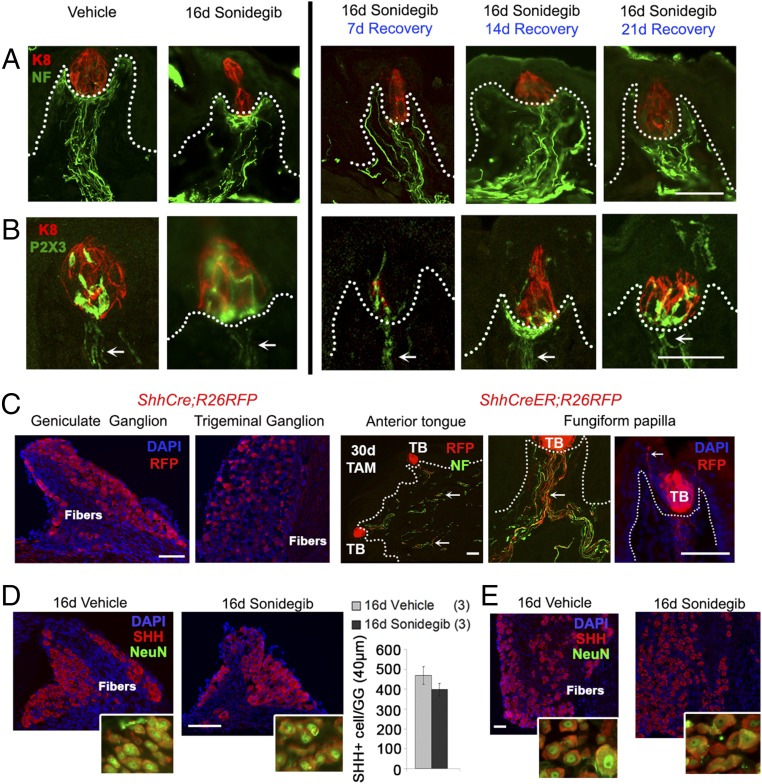
Innervation in the FP.
Innervation, shown with NF+ and TB-specific P2X3+ fibers, was retained within the FP core during sonidegib treatment and also was obvious and robust during recovery from HPI (Fig. 7 A and B). The nerve fibers were observed even in category III papillae (atypical FP/no TB) that have no taste buds (Fig. S7A) and in FP after prolonged HPI for 36 d (Fig. S7B). There were no detectable differences in the extent of innervation in papillae treated with vehicle or the HPI drug (Fig. S7C). However, nerves alone could not maintain or restore TB in FP with epithelial effects of HH/SMO inhibition.
Fig. 7.
Retained innervation during and after sonidegib treatment and identification of Shh expression in GG and TG and in anterior tongue and FP nerve fibers. (A and B). Immunofluorescent antibody detection of TB cells (K8, red) and nerves (NF, green, in A; or P2X3, green, in B). Even in category III (atypical FP/no TB) FP, with no K8+ cells retained during sonidegib exposure or recovery periods, the innervation was intact, as illustrated in Fig. S7A. Dotted lines indicate the basal lamina. Arrows in B point to P2X3 fibers. (Scale bars: 50 μm.) (C) RFP (red) expression in constitutive ShhCre;R26RFP mice and after tamoxifen (TAM) administration in ShhCreER;R26RFP mice. In both GG and TG, all cell bodies expressed Shh. Anterior tongue TB include Shh+ cells and their progeny. Arrows denote Shh+ nerve fibers in the anterior tongue, FP core, and surrounding the TB reaching into the apical epithelium. Dotted lines demarcate the basal lamina. (Scale bars: 50 μm.) (D) Immunofluorescent antibody detection of SHH (red) demonstrates SHH+ cells in GG in vehicle- or sonidegib-treated mice. Insets show merged and magnified images for SHH and NeuN (green). (Scale bar: 50 μm.) The graph indicates that number of SHH+ cells in four noncontiguous 10-μm sections of GG did not change after 16 d sonidegib treatment compared with vehicle (t test; t = 1.32, P = 0.26). Bars represent mean ± SEM. Numbers in parentheses are the number of mice per group. (Magnification: Insets, 2×.) (E) Immunofluorescent antibody detection of SHH (red) demonstrates SHH+ cells in TG. Insets show merged and magnified images for SHH and NeuN (green). (Scale bar: 50 μm.) (Magnification: Insets, 3×.)
We found that HH-responding cells and nerve fibers remain within the FP connective core after HPI and continue in recovery periods (Figs. 6C and 7 A and B). Nerve fibers and neurons in the dorsal root ganglion are a source of SHH in signaling to Gli1-expressing cells in the hair follicle (35). To determine whether fibers in the FP and their soma express SHH, we studied geniculate ganglion (GG) and trigeminal ganglion (TG) neurons in Shh reporter mice. SHH expression has been reported in TG neurons (36), and we confirmed that finding (Fig. 7C). Importantly, we further observed Shh expression within GG neurons and Shh+ fibers within the tongue and FP and projecting into TB (Fig. 7C). To test whether SHH+ neurons were altered after HH pathway inhibition, we used immunoreactions for SHH in ganglia after vehicle or sonidegib treatment and quantified SHH+ neurons with NeuN coexpression in the GG. No differences were detected (Fig. 7D). Although not quantified, similar results were seen in the TG (Fig. 7E). This indicates that a GG ganglion source of SHH ligand remains during pathway inhibition, although the lingual epithelial SHH associated with TB cells is eliminated. The GG- and TG-derived SHH sources could potentially maintain HH signaling in papilla stroma via secretion from nerves.
Responses to Chemical Stimuli from the Chorda Tympani Nerve Recover After Sonidegib Treatment Is Stopped, and Tactile and Cold Responses Are Maintained Throughout.
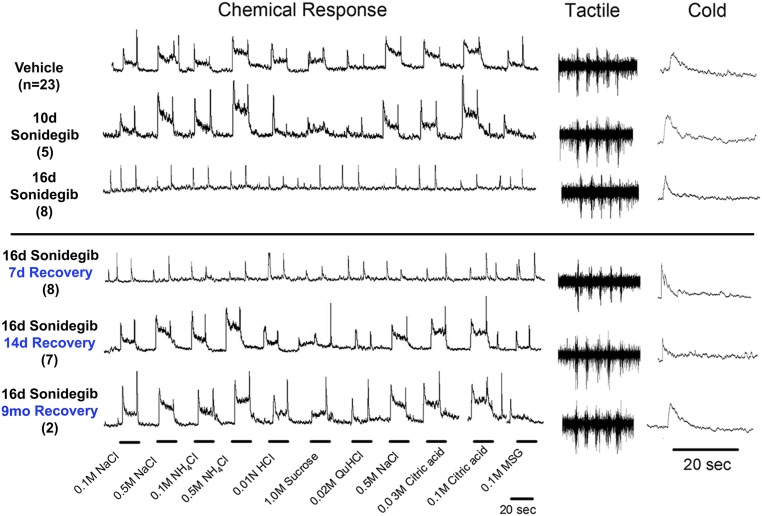
To determine early sensory effects after HPI and to test whether taste sensation is restored in concert with taste-organ recovery, we recorded from the chorda tympani nerve that innervates TB in FP and stimulated the tongue with chemicals including salts, acids, sucrose, and quinine to elicit taste responses. Compared with vehicle treatment, after 10 d of sonidegib treatment the taste responses were fully maintained (Fig. 8). Therefore, although 10 d of HPI drug treatment effectively reduced the proportion of category I (typical FP/TB) FP to about 60% of the proportion in vehicle treatment (Fig. 1D), neurophysiological responses were not altered. However, after 16 d of sonidegib, responses to chemicals were essentially eliminated, replicating our prior data for 16-d treatment (7), and category I (typical FP/TB) papillae were reduced to less than 3% of all FP (Fig. 1D).
Fig. 8.
Chorda tympani nerve responses to lingual stimulation with chemical, tactile, and cold stimuli after HPI with sonidegib and recovery. Chemical response: Integrated electrophysiological whole-nerve recordings in response to chemical stimuli applied to the tongue for 20 s followed by a distilled water rinse for 30 s. The height at the steady-state integrated recording for an individual stimulus above baseline was measured as response. Tactile response: Whole-nerve, not integrated, responses to tactile stimuli, which consisted of five strokes with a wooden rod, on the anterior tongue. Cold response: Integrated recordings to water at 4 °C applied for 20 s.
We further tested whether neural responses could be restored after HPI that had effectively eliminated intact FP taste organs. When sonidegib treatment was stopped after 16 d, a recovery period of 7 d was not sufficient to restore chorda tympani nerve responses to lingual taste stimuli (Fig. 8). Nor is this recovery period sufficient for restoring category I (typical FP/TB) papillae to the tongue in large proportion (Fig. 6B). In contrast, after 14 d recovery and continuing through 9 mo, chorda tympani nerve responses to taste stimuli were fully restored. However, during this period the category I (typical FP/TB) papillae were ∼55% and category III (atypical FP/no TB) taste organs were ∼40% of all papillae (Fig. 6B). Therefore, normal chorda tympani nerves responses can be obtained with only about 55% of intact FP taste organs recovered. (Quantified analysis for data in Fig. 8 is presented in Fig. S8A.)
Modality-specific effects.
Importantly, we also stimulated the anterior tongue with tactile (stroking with a wooden rod) and cold (water at 4 °C) stimuli. Although HPI for 16 d can eliminate chorda tympani nerve taste responses, responses to tactile and cold modalities were retained (Fig. 8), replicating our prior data (7). This particularly noteworthy result demonstrates that HH differentially regulates oral sensory modalities and suggests that the receptor organs for touch and cold are not within the TB cells per se.
The tactile and cold responses were sustained throughout all the recovery periods, from 7 d through 9 mo (Fig. 8). We have observed Shh+ nerve fibers in ShhCre reporter mice that extend in apical FP outside the bounds of TB cells (Fig. 7C) and propose that these might represent fibers that respond to touch and/or cold FP stimulation or fibers that connect to epithelial receptors for tactile and temperature.
Response/concentration series and chloride salt stimuli.
We challenged the taste organs and neurophysiological properties of the chorda tympani nerve with ability to generate graded responses to a series of NaCl (from 0.05–1.0 M) during HPI and with recovery. After sonidegib treatment for 16 d, nerve responses were essentially not discernible, except as very small responses to 0.5 and 1.0 M NaCl (Fig. S9A). A 7-d recovery period after sonidegib treatment was not sufficient for restoration of responses. However, after 14 d graded responses to lingual stimulation with increasing concentrations of NaCl were apparent (Fig. S9A). The timing of recovery, between 7 and 14 d, matches that for responses to a broad range of taste stimuli (Fig. 8). Further, response/concentration functions demonstrated typical curves after 14 d of recovery and through 9 mo (Fig. S9B).
We also used a series of 0.5 M chloride salts, which have distinctive “tastes” and receptor mechanisms, after HPI and with recovery. After sonidegib treatment the chorda tympani nerve did not respond to stimulation with Mg2+, Ca2+, K+, NH4+, or Na+ chloride salts (Fig. S8B). However, recovery in responses to values comparable to vehicle treatment was apparent after sonidegib had been discontinued for 14 d.
Therefore, chorda tympani nerve responses to a range of chemical taste qualities, a concentration series of NaCl, and high-concentration disparate chloride salts were eliminated by HPI but recovered by 2 wk after treatment was discontinued. On the other hand, responses to lingual stimulation with touch or temperature modalities were not affected by treatment with the HPI drug sonidegib.
Discussion
We tested the potential for restoration of taste homeostasis after HH signaling deregulation and show that taste organs and sensory responses can recover after severe loss from HH/SMO inhibition with the cancer drug sonidegib. Indeed, after elimination of TB and of chorda tympani nerve responses to taste stimuli, there is a remarkable restoration of TB in taste organs and full recovery of taste nerve responses. TB elimination based on the deregulation of essential HH signaling controls for cell proliferation and differentiation and loss of all TB cell types is rapid, within 2 wk. Whereas nerves remain during HH/SMO inhibition and recovery, they are not able to sustain or restore TB in the context of selective taste papilla epithelial effects. However, when sonidegib treatment is stopped, the taste organs are restored, and neural taste sensation returns to typical response patterns within 2 wk. Although restoration of a full complement of TB numbers is not achieved, the recovery of chorda tympani nerve responses can be supported by about 55% of all FP taste buds. Redundancy in the taste periphery has long been noted (37, 38) as being crucial for life-essential sensations but has not previously recognized as important in recovery from HPI drug effects. We suggest that the reversible taste disruption in HPI-treated patients is, therefore, a specific, directed effect reflecting the physiologic requirement for HH/SMO signaling in taste-organ homeostasis.
Whereas HH signaling is required for TB maintenance and restoration, it is not clear why some papillae can recover but others cannot after HPI is stopped. We suggest that as long as some TB cells remain, and some HH-responding cells are in the epithelium (in category II, atypical FP/TB papillae), there is potential for TB reconstitution. However, if TB are eradicated along with putative TB stem cells, then there is no TB restoration even up to 9 mo after stopping treatment. This places TB stem cells within the TB. In fact, basal cells of the TB have been indicated as stem/progenitor cells (13, 22, 30). In addition, after pathway inhibition we observed the loss of HH-responding cells in FP walls, known regions of TB progenitors (13, 32); thus, another proposed progenitor/stem cell population is eliminated. We believe that there is more than one source of TB stem/progenitor cells and that these are regulated by HH signaling (8, 11).
HH Pathway Regulation of Proliferation and Differentiation.
With complementary pharmacologic and genetic approaches to inhibit SMO-dependent HH signaling, we found consistent phenotypes across models. There were rapid effects on TB loss in both ectoderm-derived anterior tongue FP taste organs and endoderm-derived posterior tongue CV taste organs, but there were no discernible effects on the nontaste filiform papillae. Thus, HH/SMO signaling is an essential and selective regulator of gustatory epithelia and TB that turn over constantly in dynamic papilla organs. Cell cycles of the three TB cell types range from 3 to 30+ days (9, 10, 31), with an average TB cell life span of 10 d (9). TB elimination began after 10 d and derived from the loss of all three taste-cell types in FP and a reduction in proliferation among TB cell progenitors in the apical wall of FP. HH/SMO signaling regulation of all TB cell progenitors is indicated.
Progenitor differentiation to taste cells also is affected in HPI. As TB cells are eliminated, the apical integrity of FP is compromised by the acquisition of a heavily cornified, conical cap over epithelial cell clusters that are devoid of orientation and stain intensely with hematoxylin. We identified these cells as K5+ and K14+, characteristic of lingual and FP basal epithelial cells that include progenitors for TB (13, 32). Typically, in stratified epithelia the K5+ cells include transit-amplifying cells that leave the basal cell layer and differentiate to suprabasal cells that are not proliferative and express different keratins (39). In lingual papillae these differentiated cells include those of the TB, which are K8+ cells. With prolonged HPI and attendant TB loss, the epithelial SHH ligand production is correspondingly eliminated. HH-responding cells are reduced or eliminated. We propose that in the absence of HH signaling, the K5+ and K14+ cells do not differentiate to K8+ cells but instead occupy the apical FP epithelial layers where the TB had been located. Alternatively, K8+ cells could revert to K5+ expression in a transdifferentiation process seen in other tissues (40). Overall, K5+ cell differentiation is disrupted when HPI deregulates taste organs. Notably this notion is consistent with a genetic overexpression of SHH, where a role for HH signaling in regulating TB differentiation has also been proposed (41).
The acquisition of a conical apex in the FP that accompanies the loss of TB cells is characteristic of reduced HH pathway activity, as previously reported (7, 8) and as observed here after sonidegib administration or Smo deletion. This reproducible phenotype with HH signaling suppression or inhibition is reminiscent of an acquired filiform papilla-like form observed in FP after prolonged periods of denervation (42). A key feature in all these experimental models is the loss of TB accompanied by the loss of HH-responding epithelial cells. This indicates that HH signaling is a principal regulator not only of TB maintenance but also of FP as distinct from filiform papilla morphology.
Functional Effects of HPI on Lingual Sensation.
The rapid effects of HPI on taste organs were associated with loss of neurophysiological taste responses. Here we found that the altered neural taste responses to stimuli representing all taste qualities relate directly to the loss of all taste-cell types. Further, effects were observed even though the TB were challenged with stimulation across high concentrations of varied chloride salts that might have elicited nonspecific effects.
Interestingly, however, typical taste nerve responses were still obtained after 10 d of drug HPI, although only about 50% of FP were category I (typical FP/TB) at this time point. Whereas chorda tympani nerve taste responses were absent after 16 d of sonidegib gavage, it is notable that nerve responses to lingual tactile or cold stimuli were not altered. Previously we suggested that the receptors for responses to tactile and cold stimuli must not be TB cells per se, because these have been eliminated, and we indicated that mechanoreceptor or thermal receptor endings might be adjacent to TB (7). Now we have shown nerve endings next to TB that are Shh+. Studies of receptive fields of GG neurons (soma of chorda tympani nerve fibers) have demonstrated FP that respond only to tactile or temperature stimuli and not taste (43). This suggests that there are GG/nerve fiber subsets, possibly with end organs that are specifically somatosensory. HH signaling presumably has different roles in maintaining epithelial sense organ specializations vs. receptor nerve endings.
FP Innervation and HH Signaling.
Whereas TB are dependent on an intact innervation (14), the retention of nerves within FP in the face of TB elimination indicates that nerves alone are not sufficient to maintain TB in an epithelium that is devoid of SHH ligand and HH-responding cells. Similarly, nerves in the CV labeled with NF immunostaining are retained in the papilla core but apparently are not able to sustain the full complement of TB. Notably these results substantiate our data after the suppression of HH/GLI signaling (11).
Here we have shown physical association between papilla innervation and Gli1lacZ-positive HH-responding cells during genetic HPI in both FP and CV stroma. This affords an opportunity for nerve interactions with HH-responding stromal cells in the papilla, not previously proposed. SHH secreted from dorsal root ganglion nerve fibers activates signaling via Gli1+ HH-responding cells in touch domes (44) and maintains homeostasis in the hair follicle (35). Because we have observed SHH protein expression in neurons of the GG and TG that include the soma for nerves innervating FP and have observed Shh+ expression in fibers within the FP, we propose interactions between SHH+ nerve fibers and Gli1lacZ-positive stromal cells in maintaining the FP structure. Furthermore, Shh+ fibers contact basal lamina components of the FP and remaining epithelial cells; these could participate in signaling interactions within a taste-organ niche (8).
SHH likely has varied roles in taste-organ development and maintenance. SHH is a morphogen in developing FP placodes (45) but might also serve as an axon-guidance molecule or chemoattractant via noncanonical signaling (46, 47) to direct growing nerves specifically into the developing papillae (48). In the adult taste organ, HH signaling is required for epithelial cell proliferation and differentiation in FP and TB homeostasis. We suggest that SHH, retrogradely secreted from nerve fibers in the FP core, signals to HH-responding cells and tissue elements in the connective tissue.
CV Papillae and HPI.
In our current experiments, we did not record from the glossopharyngeal nerve that innervates taste buds in the CV. Because we noted TB loss in the CV after 16 d of HPI drug treatment and the recovery of about 80% of TB after 14 d, we predict that any loss of posterior tongue sensation would recover also. In mice treated with the HPI drug vismodegib for 15 wk (49), statistically significant but biologically small effects were seen on TB cell numbers in the CV, whereas we found a substantial loss of TB (up to about half of control values) after 4 wk of sonidegib gavage. The only other reports of altered HH signaling on TB in the CV are from HH/GLI suppression in mice, where TB were substantially reduced/lost (11). Here we observed effects in TB of both the FP and CV, that is, in two major lingual taste regions. Although there were no reported behavioral effects in two bottle preference/avoidance tests for sucrose or denatonium benzoate in vismodegib-treated mice (49), we predict behavioral effects after HH/SMO signaling inhibition.
Implications for Adverse Taste-Disturbance Effects in Patients Treated with HPI Drugs.
This work is at the confluence of HH/SMO signaling as a principal regulator of taste organs and taste sensation, deregulated HH signaling in basal cell carcinoma (BCC), and BCC treatment with drugs that inhibit HH signaling with associated severe taste disruption (4, 5, 26, 28, 50) that can alter patient quality of life (27). The demonstrated duration-dependent effects on TB in the FP and CV and the profound loss of chorda tympani nerve responses to all taste stimuli are the biological bases for severe taste disturbances in patients. We found substantial effects in anterior and posterior tongue TB in both the FP and CV, which have different innervation and chemical response profiles (51). Effects in patients taking HPI drugs therefore are likely to be wide ranging across taste chemical stimuli and oral regions, and the reported ageusia in >20% of patients (5) is not unexpected. Further, based on our neurophysiological data, it is not likely that even very strong chemicals would elicit responses in patients during treatment with HPI drugs. However, our modality-specific results support consideration of overall lingual sensation, and patient diets focused on the texture and temperature characteristics of nutrients could temper the loss of taste sensation in overall flavor of meals.
Notably, a majority of taste organs and TB regain cell integrity, and taste nerve responses rapidly return to normal patterns in the post-HPI drug recovery period in mice. Therefore, the reported recovery from taste disturbances after treatment is discontinued in patients (5) is related directly to the recovery of taste organs and sensory responses. Importantly, management of reported decreased food and fluid intake in patients should address flavor perception. We are investigating responses to gustatory and olfactory stimuli in patients treated with HPI drugs to learn about possible contrasting effects in taste and smell.
Materials and Methods
Animals.
Animal use and care procedures were performed according to the guidelines of the National Institutes of Health and approved protocols of the University of Michigan Institutional Animal Care and Use Committee. C57BL/6 mice were treated for 3–36 d by daily oral gavage with sonidegib [NVP-LDE225 diphosphate salt; Chemietek catalog no. CT-LDE225] dissolved in vehicle, PEG 400/5% dextrose in water (75:25 vol/vol), at a dose of 20 mg/kg, or with vehicle alone. Dose determination was after Kumari et al. (7). For recovery experiments, mice were treated with sonidegib or vehicle for 16 d; then the drug was discontinued, and animals were maintained for 7, 14, or 21 d or for 3, 5, or 9 mo in standard housing. Mouse genotypes and sources are given in SI Materials and Methods.
Tissue Analyses.
Tongues were collected and prepared for tissue analysis, or recordings were made from the chorda tympani nerve and then tongues were dissected. Tissue processing, immunohistochemistry and neurophysiological protocols, and data and quantification analyses are described in SI Materials and Methods.
Statistics.
Animal numbers and statistical tests are included in the graphs and figure legends. Details are supplied in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ariell M. Joiner in the B.L.A. laboratory and Libo Li in the C.M.M. laboratory for technical assistance with tissue collection, analyses, and immunostaining, and the staff in the University of Michigan Dentistry Histology Core for technical support. This work was supported by NIH funding from the National Institute on Deafness and Other Communication Disorders Multi Principal Investigator Grant R01 DC014428 (to B.L.A., R.M.B., A.A.D., and C.M.M.); National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01 AR045973 (to A.A.D.); University of Michigan Comprehensive Cancer Center Core Grant P30 CA046592 (to A.A.D.); and a University of Michigan Center for Organogenesis Postdoctoral Fellowship (to A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712881114/-/DCSupplemental.
References
- 1.Migden MR, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716–728. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 2.Rodon J, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20:1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 3.Sekulic A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang JY, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fife K, et al. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017;13:175–184. doi: 10.2217/fon-2016-0296. [DOI] [PubMed] [Google Scholar]
- 6.Pan S, et al. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett. 2010;1:130–134. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumari A, et al. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 2015;113:1034–1040. doi: 10.1152/jn.00822.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistretta CM, Kumari A. Tongue and taste organ biology and function: Homeostasis maintained by hedgehog signaling. Annu Rev Physiol. 2017;79:335–356. doi: 10.1146/annurev-physiol-022516-034202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 2006;141:2129–2138. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 11.Ermilov AN, et al. Maintenance of taste organs is strictly dependent on epithelial hedgehog/GLI signaling. PLoS Genet. 2016;12:e1006442. doi: 10.1371/journal.pgen.1006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard D, Xu M, Liu F, Millar SE, Barlow LA. β-catenin signaling biases multipotent lingual epithelial progenitors to differentiate and acquire specific taste cell fates. PLoS Genet. 2015;11:e1005208. doi: 10.1371/journal.pgen.1005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu HX, et al. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev Biol. 2013;382:82–97. doi: 10.1016/j.ydbio.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guagliardo NA, Hill DL. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J Comp Neurol. 2007;504:206–216. doi: 10.1002/cne.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee RTH, Zhao Z, Ingham PW. Hedgehog signalling. Development. 2016;143:367–372. doi: 10.1242/dev.120154. [DOI] [PubMed] [Google Scholar]
- 16.Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: Review of smoothened and GLI inhibitors. Cancers (Basel) 2016;8:E22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone DM, et al. The tumour-suppressor gene patched encodes a candidate receptor for sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 18.van den Heuvel M, Ingham PW. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature. 1996;382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- 19.Briscoe J, Thérond PP. The mechanisms of hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 20.Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 21.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 22.Miura H, Scott JK, Harada S, Barlow LA. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev Dyn. 2014;243:1286–1297. doi: 10.1002/dvdy.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 2006;69:209–225. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- 24.Jain S, Song R, Xie J. Sonidegib: Mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017;10:1645–1653. doi: 10.2147/OTT.S130910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silapunt S, Chen L, Migden MR. Hedgehog pathway inhibition in advanced basal cell carcinoma: Latest evidence and clinical usefulness. Ther Adv Med Oncol. 2016;8:375–382. doi: 10.1177/1758834016653605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen AA, Aldahan AS, Hughes OB, Shah VV, Strasswimmer J. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: A systematic review and pooled analysis of interventional studies. JAMA Dermatol. 2016;152:816–824. doi: 10.1001/jamadermatol.2016.0780. [DOI] [PubMed] [Google Scholar]
- 27.Lacouture ME, et al. Characterization and management of hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist. 2016;21:1218–1229. doi: 10.1634/theoncologist.2016-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang JY, et al. Inhibition of the hedgehog pathway in patients with basal-cell nevus syndrome: Final results from the multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:1720–1731. doi: 10.1016/S1470-2045(16)30566-6. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan JM, Borecki AA, Oleskevich S. Stem and progenitor cell compartments within adult mouse taste buds. Eur J Neurosci. 2010;31:1549–1560. doi: 10.1111/j.1460-9568.2010.07184.x. [DOI] [PubMed] [Google Scholar]
- 31.Perea-Martinez I, Nagai T, Chaudhari N. Functional cell types in taste buds have distinct longevities. PLoS One. 2013;8:e53399. doi: 10.1371/journal.pone.0053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asano-Miyoshi M, Hamamichi R, Emori Y. Cytokeratin 14 is expressed in immature cells in rat taste buds. J Mol Histol. 2008;39:193–199. doi: 10.1007/s10735-007-9151-0. [DOI] [PubMed] [Google Scholar]
- 34.Knapp L, Lawton A, Oakley B, Wong L, Zhang C. Keratins as markers of differentiated taste cells of the rat. Differentiation. 1995;58:341–349. doi: 10.1046/j.1432-0436.1995.5850341.x. [DOI] [PubMed] [Google Scholar]
- 35.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–173. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartoshuk LM. Genetic and pathological taste variation: What can we learn from animal models and human disease? Ciba Found Symp. 1993;179:251–262, discussion 262–267. doi: 10.1002/9780470514511.ch16. [DOI] [PubMed] [Google Scholar]
- 38.Spector AC, Smith JC. 2004. Neurobiology of Food and Fluid Intake, Handbook of behavioral neurobiology, eds Stricker E, Woods SC (Kluwer Academic/Plenum Publishers, New York), Vol 14, pp 63–87.
- 39.Fuchs E. Skin stem cells: Rising to the surface. J Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lafkas D, et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature. 2015;528:127–131. doi: 10.1038/nature15715. [DOI] [PubMed] [Google Scholar]
- 41.Castillo D, et al. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development. 2014;141:2993–3002. doi: 10.1242/dev.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oakley B, Wu LH, Lawton A, deSibour C. Neural control of ectopic filiform spines in adult tongue. Neuroscience. 1990;36:831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- 43.Yokota Y, Kumari A, Mistretta CM, Bradley RM. 2016 Thermal and tactile responses of the rat chorda tympani nerve. Neuroscience Meeting Planner. Available at http://www.abstractsonline.com/pp8/index.html#!/4071/presentation/8790. Accessed October 27, 2017.
- 44.Peterson SC, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mistretta CM, Liu HX, Gaffield W, MacCallum DK. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: Fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol. 2003;254:1–18. doi: 10.1016/s0012-1606(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 46.Yam PT, Charron F. Signaling mechanisms of non-conventional axon guidance cues: The Shh, BMP and Wnt morphogens. Curr Opin Neurobiol. 2013;23:965–973. doi: 10.1016/j.conb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 48.Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: Nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Cong WN, Yoon JS, Egan JM. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2015;4:245–252. doi: 10.1002/cam4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dummer R, et al. The 12-month analysis from basal cell carcinoma outcomes with LDE225 treatment (BOLT): A phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol. 2016;75:113–125.e5. doi: 10.1016/j.jaad.2016.02.1226. [DOI] [PubMed] [Google Scholar]
- 51.Colbert CL, Garcea M, Spector AC. Effects of selective lingual gustatory deafferentation on suprathreshold taste intensity discrimination of NaCl in rats. Behav Neurosci. 2004;118:1409–1417. doi: 10.1037/0735-7044.118.6.1409. [DOI] [PubMed] [Google Scholar]
- 52.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 53.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 54.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.