Significance
The vast majority of familial Alzheimer’s disease (AD) cases are linked to mutations in presenilin-1 (PS1) in an autosomal dominant manner. PS1 is the catalytic component of γ-secretase, which cleaves amyloid precursor protein into Aβ peptides. It remains unclear whether the causal role of PS1 mutations in AD development is effected through γ-secretase, and if yes, whether the dominant negative effect of PS1 mutant allele is effected through the proteolytic activity of γ-secretase. In this study, we provide compelling evidence to prove a dominant negative effect by the loss-of-function γ-secretase mutants on the production of Aβ42/40 by WT γ-secretase through hetero-oligomerization. These data, together with our prior knowledge on the function of γ-secretase, have important ramifications on the two key questions listed here.
Keywords: dominant negative effect, γ-secretase, presenilin 1, oligomerization, Alzheimer’s disease
Abstract
γ-secretase is an intramembrane protease complex consisting of nicastrin, presenilin-1/2, APH-1a/b, and Pen-2. Hydrolysis of the 99-residue transmembrane fragment of amyloid precursor protein (APP-C99) by γ-secretase produces β-amyloid (Aβ) peptides. Pathogenic mutations in PSEN1 and PSEN2, which encode the catalytic subunit presenilin-1/2 of γ-secretase, lead to familial Alzheimer’s disease in an autosomal dominant manner. However, the underlying mechanism of how the mutant PSEN gene may affect the function of the WT allele remains to be elucidated. Here we report that each of the loss-of-function γ-secretase variants that carries a PSEN1 mutation suppresses the protease activity of the WT γ-secretase on Aβ production. Each of these γ-secretase variants forms a stable oligomer with the WT γ-secretase in vitro in the presence of the detergent CHAPSO {3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate}, but not digitonin. Importantly, robust protease activity of γ-secretase is detectable in the presence of CHAPSO, but not digitonin. These experimental observations suggest a dominant negative effect of the γ-secretase, in which the protease activity of WT γ-secretase is suppressed by the loss-of-function γ-secretase variants through hetero-oligomerization. The relevance of this finding to the genesis of Alzheimer’s disease is critically evaluated.
The γ-secretase, comprising nicastrin, presenilin, APH-1, and Pen-2 (1–3), cleaves the substrate 99-residue transmembrane fragment of amyloid precursor protein (APP-C99) into Aβ peptides of varying lengths, ranging from Aβ37 to Aβ45 (4–6). The longer Aβ peptides, exemplified by Aβ42, form amyloid plaque in the brain and are thought to be detrimental to the neuronal system (7–11). Formation of amyloid plaque is a hallmark of Alzheimer’s disease (AD) (12). Aberrant cleavage of APP-C99 by γ-secretase results in an elevated molar ratio of Aβ42 over Aβ40 (13), which presumably acts as a trigger of AD (14). About 1% of all patients develop early-onset familial AD with autosomal dominant inheritance (15). Mutations from the catalytic component of γ-secretase, presenilin-1 (PS1) or presenilin-2 (PS2), contribute to most of the early-onset familial AD cases. The molecular mechanism of dominant inheritance has been a subject of intense investigation and debate.
In one hypothesis, mutant PS1 is thought to exert a dominant negative effect on the WT allele in trans through functional interaction (16). This hypothesis potentially explains the puzzling observation that AD-derived PSEN1 mutations are predominantly missense in nature, with only a few deletions and insertions, but never nonsense or frameshift. The nonsense or frameshift mutations, but usually not missense mutations, result in gross alteration of the PS1 primary sequence, and consequently the 3D structure. Such an alteration may render the resulting PS1 mutant protein incapable of functionally interacting with the WT PS1. The dominant negative hypothesis may also explain the finding that heterozygous mouse with one WT PSEN1 allele and one missense mutant allele, but not PSEN1+/−, develops AD (17). Because PS1 is mainly known as the catalytic component of γ-secretase, the dominant negative effect of mutant PS1 may be reflected by the corresponding mutant γ-secretases. Consistent with this analysis, the vast majority of the AD-derived PS1 mutations lead to decreased cleavage of APP-C99 by the corresponding γ-secretases (18).
The transdominant negative hypothesis requires γ-secretase to interact with each other (16). Relying on immunoprecipitation of cellular proteins, earlier studies strongly suggest γ-secretase may form a dimer (19–21) or a higher-order protein complex (22–26). Subsequent biochemical investigations of recombinant γ-secretases, however, unambiguously showed γ-secretase to be a 230-kDa complex, with one copy each of its four components (27–29). In contrast to the proposed dominant negative effect, the WT allele of PSEN1 was reported to compensate for the decreased protease activity of some pathogenic PS1 mutants (30). These seemingly conflicting claims require reconciliation, perhaps through a revisit of the dominant negative effect and a careful comparison of the experimental conditions.
In this study, we demonstrate a clear dominant negative effect by the γ-secretase variants that have lost the proteolytic activity toward APP-C99. These γ-secretase mutants suppress the proteolytic activity of WT γ-secretase through a direct, stable interaction between mutant and WT γ-secretases. This interaction was found to strictly depend on the choice of detergents. Under conditions in which γ-secretase no longer interacts with each other, there is a complete loss of the protease activity. These findings have important ramifications on our understanding of the functional mechanism of γ-secretase and its relationship to AD development.
Results
Enzyme and Substrate Concentrations in the Cleavage Assay.
In this manuscript, loss of function strictly refers to the loss of the proteolytic activity of γ-secretase toward the substrate APP-C99. More specifically, because the production of Aβ40 and Aβ42 is closely correlated with the proteolytic activity of γ-secretase (31, 32), it is used as an exclusive indicator throughout this study. We sought to investigate the potential dominant negative effect of AD-derived γ-secretase mutants on the proteolytic activity of WT γ-secretase. Both WT and mutant γ-secretases used in this study were individually overexpressed in HEK293 cells and biochemically purified to homogeneity (33). Dominant negative effect may be achieved by two mutually nonexclusive means: sequestration of substrate by the loss-of-function γ-secretase mutants and hetero-oligomerization between WT and mutant γ-secretases (34, 35). Discovery and confirmation of the second possibility requires sufficient molar excess of the substrate over the γ-secretase mutant.
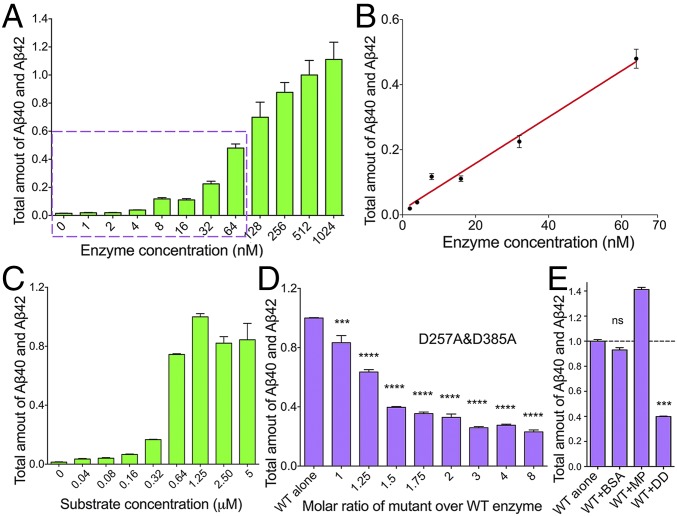
First, we determined the working concentrations of γ-secretase at which the proteolytic cleavage assays would be performed. Because γ-secretase exhibits a generally low level of proteolytic activity toward the substrate APP-C99, an incubation time of 3 h was used for all γ-secretase cleavage assays reported in this study. With a fixed substrate concentration of 5 µM, a wide range of WT γ-secretase concentrations from 1 nM to 1.02 μM was examined (Fig. 1A). Within the concentration range of 2–64 nM, the combined production of Aβ40 and Aβ42 is approximately linearly proportional to the amount of WT γ-secretase (Fig. 1B). To ensure sufficient excess of the substrate, we chose 8 or 16 nM for the WT γ-secretase for all subsequent experiments.
Fig. 1.
Catalytically inactive γ-secretase inhibits the catalytic activity of WT γ-secretase toward the production of Aβ40 and Aβ42. (A) Production of Aβ40 and Aβ42 peptides by a wide range of concentrations of the WT γ-secretase. The total amount of Aβ40 and Aβ42 production is plotted against the concentrations of WT γ-secretase, which vary from 1 nM to 1.02 μM. The concentration of the substrate APP-C99 is 5 μM. The total amount of Aβ40 and Aβ42 produced by 512 nM WT γ-secretase is normalized as 1. Each experiment was repeated three times, with the SD shown. Protein concentration was measured by the Bradford method. (B) The production of Aβ40 and Aβ42 is linearly proportional to the γ-secretase concentration in the range of 2–64 nM. (C) Production of Aβ40 and Aβ42 peptides by a wide range of concentrations of the substrate APP-C99. The concentration of WT γ-secretase is 8 nM. The substrate concentration varies from 40 nM to 5 μM. The combined production of Aβ40 and Aβ42 in the presence of 1.25 μM APP-C99 is normalized as 1. (D) The catalytic mutant γ-secretase (PS1-D257A, D385A) suppresses the production of Aβ40 and Aβ42 by WT γ-secretase in a concentration-dependent manner. The concentrations of WT γ-secretase and APP-C99 are 8 nM and 5 μM, respectively. The total amount of Aβ40 and Aβ42 produced by WT γ-secretase alone is normalized as 1. One-way ANOVA was applied to evaluate the statistical significance compared with WT γ-secretase. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. (E) The catalytic mutant γ-secretase specifically inhibits the proteolytic activity of WT γ-secretase compared with the control proteins BSA and a membrane protein. ns, not significant.
Next, with a fixed concentration of 8 nM for the WT γ-secretase, we further examined the requirement of substrate concentration. Within the substrate concentration range of 40–640 nM, the combined production of Aβ40 and Aβ42 steadily increases with increasing APP-C99 concentrations, indicating that the substrate is not yet in sufficient excess over the enzyme (Fig. 1C). At a substrate concentration of 1.25 µM or above, the production of Aβ40 and Aβ42 remains largely the same, suggesting the substrate is no longer the limiting factor. We chose 5 µM for the substrate APP-C99 for all subsequent experiments.
Dominant Negative Effect by Loss-of-Function γ-Secretase Mutants.
With both catalytic aspartate residues of PS1 mutated to alanine (Ala), the γ-secretase with PS1-D257A/D385A (γ-secretase-DD) represents a loss-of-function mutant (Fig. S1A). We incubated varying amounts of the catalytic mutant γ-secretase-DD with 8 nM WT enzyme and examined the combined production of Aβ40 and Aβ42. Strikingly, the proteolytic activity of the WT γ-secretase decreases significantly with increasing concentrations of the catalytic mutant (Fig. 1D), with the production of Aβ40 and Aβ42 both decreasing (Fig. S1 B and C). The ratio of Aβ42 over Aβ40 remains largely the same (Fig. S1D). The decreased cleavage activity was specifically caused by the mutant γ-secretase-DD, because neither a control membrane protein nor BSA was able to suppress the proteolytic activity of the WT γ-secretase (Fig. 1E). The decreased activity of WT γ-secretase cannot be explained by potential substrate sequestration by the mutant γ-secretase, because the concentration of the substrate APP-C99 was 80-fold higher than the highest concentration of the mutant γ-secretase used. This analysis strongly suggests a dominant negative effect for the catalytic mutant γ-secretase-DD over the proteolytic activity of WT γ-secretase.
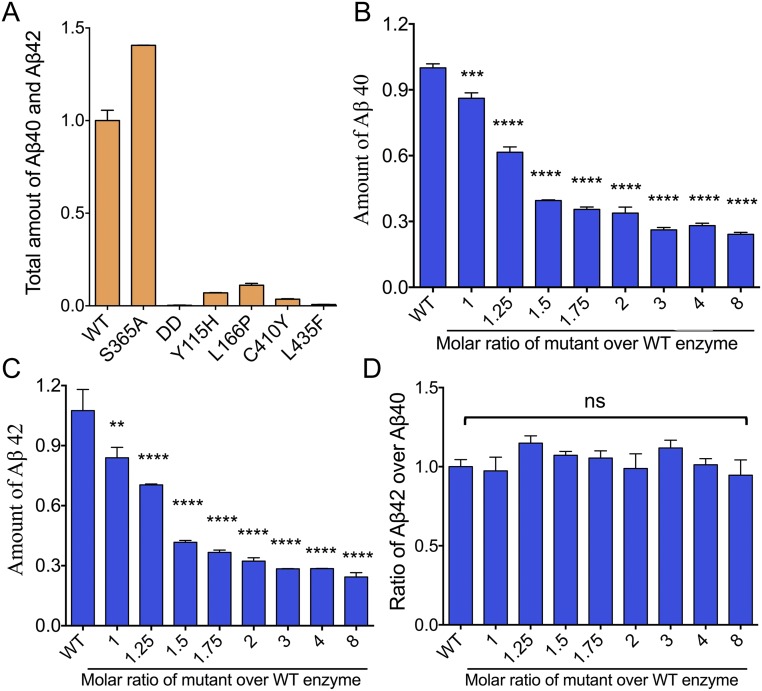
Fig. S1.
The dominant negative effect of the catalytically inactive γ-secretase-DD (PS1-D257A, D385A) over the WT γ-secretase in terms of the production of Aβ40 and Aβ42. (A) The proteolytic activities of WT γ-secretase and six representative mutants (18). Shown here are the combined production of Aβ40 and Aβ42. The proteolytic activity of WT γ-secretase is normalized as 1. (B) The dominant negative effect of the catalytic mutant γ-secretase-DD over WT γ-secretase in terms of the production of Aβ40. (C) The dominant negative effect of the catalytic mutant γ-secretase-DD over WT γ-secretase in terms of the production of Aβ42. (D) The ratio of Aβ42 over Aβ40 remains largely the same. The Aβ42/Aβ40 ratio of WT γ-secretase alone is normalized as 1. **P < 0.01; ***P < 0.001; ****P < 0.0001. ns, not significant.
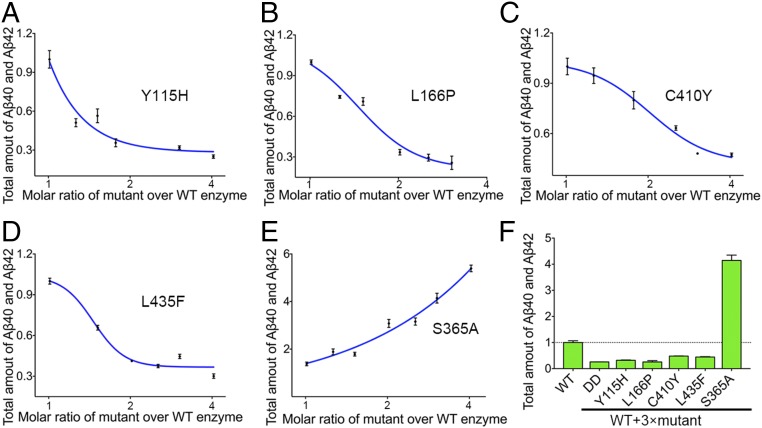
The vast majority of AD-derived PS1 mutations give rise to loss-of-function γ-secretase mutants (18). To investigate whether these γ-secretase mutants are dominant negative over the WT enzyme, we chose four such representative PS1 missense mutations: Y115H, L166P, C410Y, and L435F. Each of these four mutations was reconstituted into a distinct γ-secretase mutant. In contrast to the WT γ-secretase, these four loss-of-function mutants exhibit very low levels of the proteolytic activity (18) (Fig. S1A). With a fixed concentration of 16 nM for the WT γ-secretase, the potential dominant negative effect for each of the four mutants was examined (Fig. 2 A–D). In all cases, the total production of Aβ40 and Aβ42 by the WT γ-secretase decreases with increasing concentrations of the mutant enzyme. Thus, all four loss-of-function mutants exhibit dominant negative effect in the in vitro cleavage assays.
Fig. 2.
The loss-of-function γ-secretase mutants, each containing an AD-derived mutation in PS1, inhibit the production of Aβ40 and Aβ42 by WT γ-secretase. (A) Dominant negative effect of the γ-secretase mutant (PS1-Y115H) on WT γ-secretase. The concentration of WT γ-secretase is 16 nM in A–E. The total amount of Aβ40 and Aβ42 produced by WT γ-secretase alone is normalized as 1 in all panels. Each experiment was repeated three times, with the SD shown. (B) Dominant negative effect of the γ-secretase mutant (PS1-L166P) on WT γ-secretase. (C) Dominant negative effect of the γ-secretase mutant (PS1-C410Y) on WT γ-secretase. (D) Dominant negative effect of the γ-secretase mutant (PS1-L435F) on WT γ-secretase. (E) The γ-secretase mutant (PS1-S365A), which has a proteolytic activity comparable to that of the WT γ-secretase, exhibits no dominant negative effect. In fact, the proteolytic activity increases with increasing amounts of the mutant γ-secretase. (F) A summary of the dominant negative effect by five γ-secretase mutants. The concentrations of WT and mutant γ-secretases are 16 and 48 nM, respectively, in each case.
A number of the AD-derived PS1 mutations have little effect on the proteolytic activity of the corresponding γ-secretase mutants. To investigate whether these γ-secretase mutants have any effect on the WT enzyme, we examined one such representative PS1 mutation S365A (Fig. S1). In contrast to the loss-of-function γ-secretase mutants, increasing concentrations of the mutant γ-secretase-S365A led to proportionally increased production of Aβ40 and Aβ42 (Fig. 2E). Therefore, for all PS1 mutations examined, only the loss-of-function γ-secretase mutants exhibit a dominant negative effect on the proteolytic activity of the WT γ-secretase (Fig. 2F).
Physical Basis of the Dominant Negative Effect.
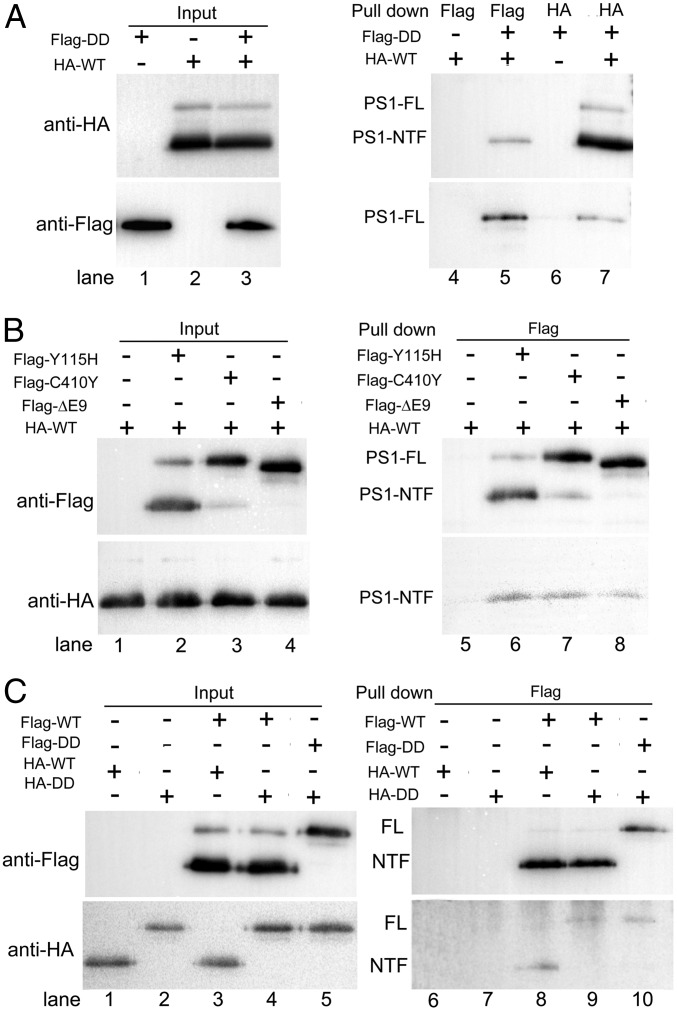
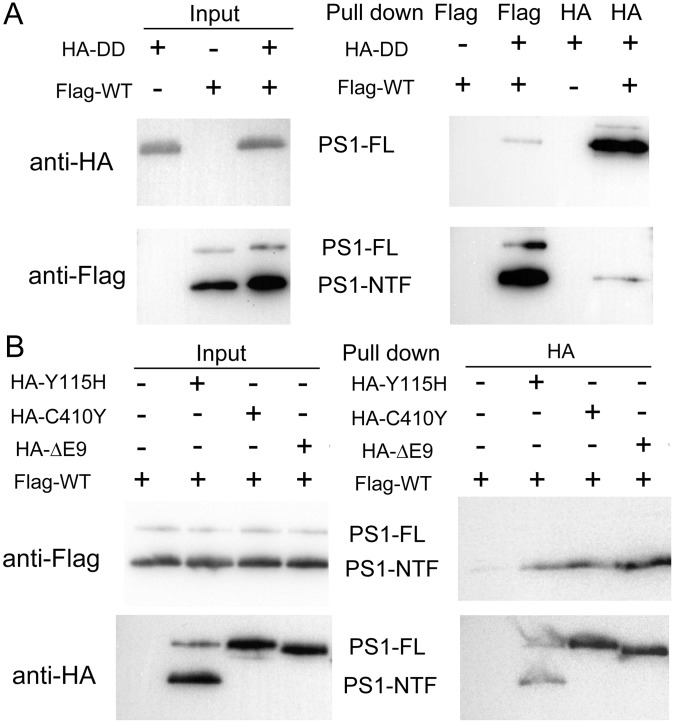
The dominant negative effect of the loss-of-function γ-secretase mutants on the proteolytic activity of the WT enzyme strongly suggests physical association. To examine this scenario, we individually overexpressed and biochemically purified a number of γ-secretase variants. First, we investigated potential interactions between HA-tagged WT γ-secretase and Flag-tagged catalytic mutant γ-secretase-DD, using an in vitro pull-down assay (Fig. 3A). The HA or Flag tag is placed at the N terminus of PS1 and can be easily detected by a monoclonal antibody raised against the tag (Fig. 3A, lanes 1–3). Using the anti-Flag antibody to perform the pull-down step, the HA-tagged WT γ-secretase was detected in the pellet only in the presence of the Flag-tagged γ-secretase-DD (lane 5), but not in its absence (lane 4). Conversely, using the anti-HA antibody to pull-down, the Flag-tagged γ-secretase-DD was detected in the pellet only in the presence of the HA-tagged WT γ-secretase (lane 7), but not in its absence (lane 6). These results demonstrate a direct interaction between the HA-tagged WT γ-secretase and the Flag-tagged γ-secretase-DD. Swapping of the HA and Flag tags had no effect on this conclusion (Fig. S2A).
Fig. 3.
γ-secretase molecules interact with each other in the presence of the detergent CHAPSO. (A) WT γ-secretase interacts with the catalytic mutant γ-secretase (PS1-D257A, D385A). All pull-down experiments described in this figure were performed using purified recombinant proteins. In the experiments described in A and B, the WT and mutant γ-secretases are differentially tagged and incubated together. Immunoprecipitation using one specific antibody was followed by Western blots using another specific antibody. (B) WT γ-secretase interacts with each of the three mutant γ-secretase proteins (PS1-Y115H, C410Y, and ΔE9). (C) WT or catalytic mutant γ-secretase forms oligomers. In these experiments, the WT (or catalytic mutant) γ-secretase proteins are differentially tagged. The pull-down experiments were performed to assess the WT–WT or mutant–mutant γ-secretase interactions, with WT–mutant interactions as the control. A similar pull-down efficiency is observed in all combinations.
Fig. S2.
γ-secretase molecules interact with each other in the presence of the detergent CHAPSO. (A) WT γ-secretase interacts with the catalytic mutant γ-secretase-DD (PS1-D257A, D385A). All pull-down experiments described in this figure were performed using purified recombinant proteins. The WT and mutant γ-secretases are differentially tagged and incubated together. Immunoprecipitation using one specific antibody was followed by Western blots using another specific antibody. Compared with the experiments described in Fig. 3, the tags were swapped for the WT and mutant γ-secretases. (B) WT γ-secretase interacts with each of the three mutant γ-secretase proteins (PS1-Y115H, C410Y, and ΔE9).
Next, we individually examined the potential interactions of the HA-tagged WT γ-secretase with three Flag-tagged γ-secretase mutants: PS1-Y115H, PS1-C410Y, and PS1-ΔE9 (Fig. 3B). Similar amounts of the γ-secretase variants were used in these assays (lanes 1–4). The results are unambiguous: the HA-tagged WT γ-secretase was detectable only in the presence of the Flag-tagged γ-secretase mutants (lanes 6–8), but not in their absence (lane 5). Swapping of the tags on the WT and mutant γ-secretases still allowed the mutual interactions (Fig. S2B). The observed interactions should not be restricted to those between the WT and mutant γ-secretases. The Flag-tagged WT γ-secretase and the Flag-tagged γ-secretase-DD were able to pull-down the HA-tagged WT γ-secretase and the HA-tagged γ-secretase-DD, respectively (Fig. 3C, lanes 8 and 10).
Effect of Detergents on γ-Secretase Oligomerization.
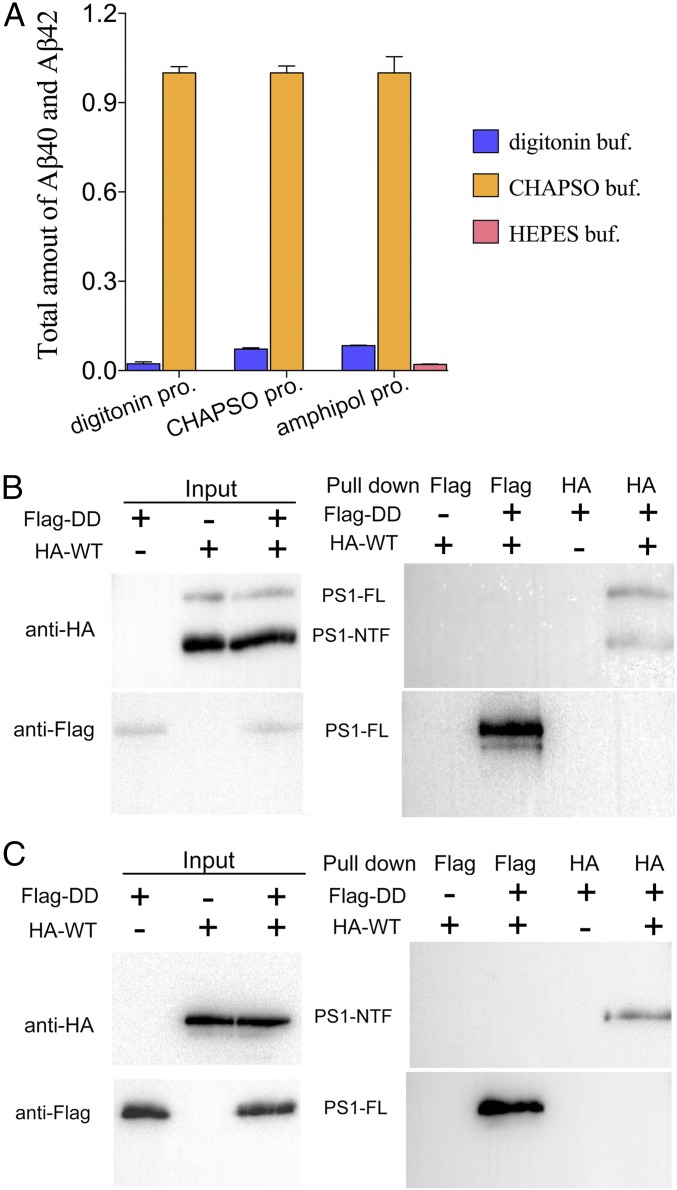
Our results clearly demonstrate that purified, recombinant γ-secretase oligomerizes in vitro, which explains the dominant negative effect of the loss-of-function γ-secretase mutants over the WT enzyme. We suspected that the proteolytic activity toward the substrate APP-C99 may strictly depend on the oligomerization of γ-secretase. Notably, both the proteolytic activity assays and the pull-down experiments were performed in the presence of the detergent CHAPSO {3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate}. In contrast to CHAPSO, other detergents such as digitonin are known to cripple the proteolytic activity of γ-secretase extracted from cells (22). Regardless of the choice of the extraction detergent for the WT γ-secretase, inclusion of CHAPSO in the assay buffer allowed robust proteolytic activity (Fig. 4A, orange bars). The use of digitonin (Fig. 4A, blue bars) or the inclusion of amphipol, but not detergent (Fig. 4A, pink bars), in the assay buffer led to reduction of the proteolytic activity by at least 90%.
Fig. 4.
The interactions among γ-secretase molecules are strictly dependent on the choice of detergents and correlate with the proteolytic activity. (A) WT γ-secretase exhibits robust proteolytic activity in the reaction buffer containing CHAPSO, but not digitonin or amphipol A8-35. WT γ-secretase was purified in three different detergents: CHAPSO, digitonin, and amphipol A8-35. Then the proteolytic activity assays were performed in three different buffers. Regardless of the original detergent used in purification, WT γ-secretase is highly active, as long as the reaction buffer contains the detergent CHAPSO. Each experiment was repeated three times, with the SD shown. (B) γ-secretase containing catalytic mutations and WT γ-secretase cannot pull down each other in digitonin buffer. (C) γ-secretase containing catalytic mutations and WT γ-secretase in amphipol A8-35 cannot pull down each other.
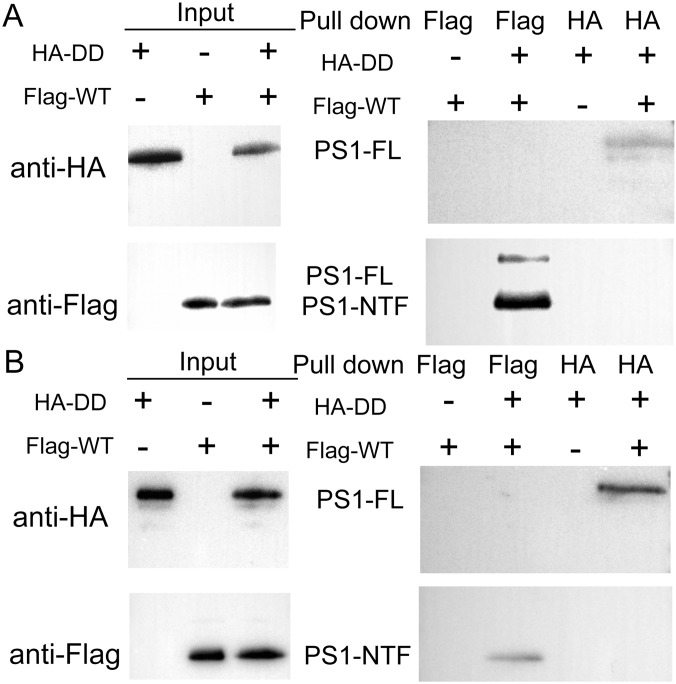
If the oligomerization of γ-secretase is required for its proteolytic activity, then the crippled activity in digitonin might be caused by disruption of γ-secretase oligomerization. To investigate this scenario, we re-examined the interactions between the HA-tagged WT γ-secretase and the Flag-tagged γ-secretase-DD in the presence of digitonin, using the pull-down assay (Fig. 4B). In contrast to the results obtained under CHAPSO, no physical association was detected between these two γ-secretase variants, using the anti-Flag or the anti-HA antibody. Compared with that in CHAPSO, the proteolytic activity of γ-secretase is severely compromised in the presence of amphipol (Fig. 4A, pink bars). Consistently, the HA-tagged WT γ-secretase no longer interacted with the Flag-tagged γ-secretase-DD in the presence of amphipol (Fig. 4C). Swapping of the HA and Flag tags on the WT and mutant γ-secretases had no effect on this conclusion (Fig. S3).
Fig. S3.
The interactions between WT and catalytic mutant γ-secretases are strictly dependent on the choice of detergents. (A) The WT and catalytic mutant γ-secretases cannot pull down each other in the digitonin buffer. Compared with the experiments described in Fig. 4, the tags were swapped for the WT and mutant γ-secretases. (B) The WT and catalytic mutant γ-secretases cannot pull down each other in the reaction buffer containing amphipol A8-35.
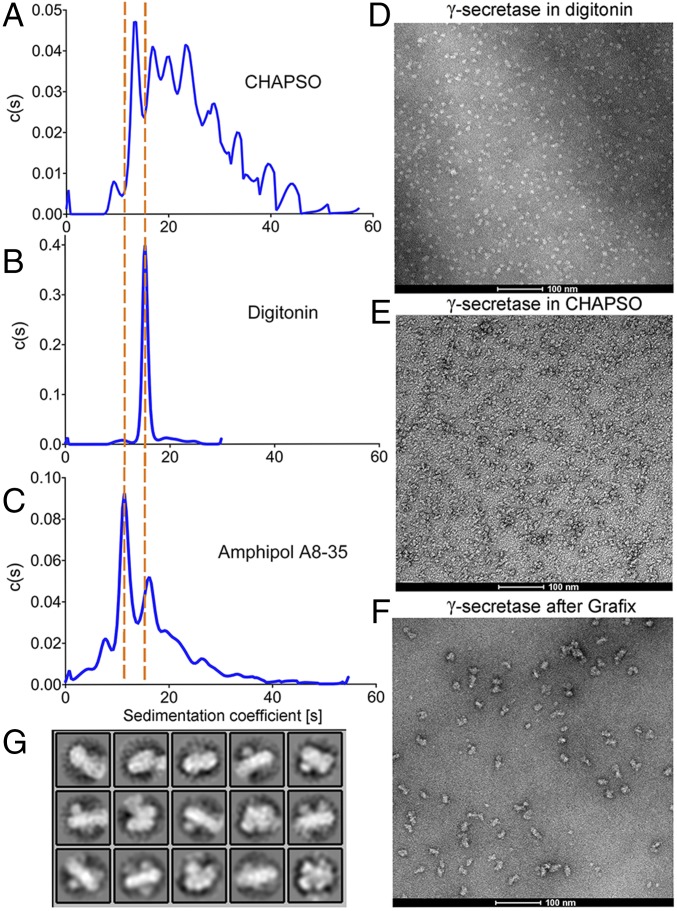
We used analytical ultracentrifugation to investigate the oligomerization status of γ-secretase in the presence of detergents. Consistent with the results of the in vitro pull-down assays, WT γ-secretase is highly poly-disperse in the presence of CHAPSO, appearing in a number of peaks, each with a distinct sedimentation coefficient (Fig. 5A). In contrast, WT γ-secretase is monodisperse in the presence of digitonin (Fig. 5B) and exhibits a low level of poly-dispersity in the presence of amphipol A8-35 (Fig. 5C). The extent of γ-secretase oligomerization under the three detergents correlates well with the proteolytic activity of γ-secretase.
Fig. 5.
Distinct oligomerization states of γ-secretase in different detergents and γ-secretase form a suspicious dimer after GraFix. (A–C) Representatives of the analytical ultracentrifugation results of γ-secretase in different detergents as indicated. (D) The negative staining image of γ-secretase in digitonin. (E) Negative staining image of γ-secretase in CHAPSO. (F) Negative staining image of GraFixed γ-secretase. (G) Representative negative staining 2D classification of GraFixed γ-secretase.
We further examined the oligomerization status of γ-secretase under detergents, using electron microscopy (EM). Consistent with the results of analytical ultracentrifugation, WT γ-secretase appeared to be mostly monomeric in the presence of digitonin by negative staining EM (Fig. 5D). In sharp contrast, WT γ-secretase appeared to form oligomers of several different sizes in the presence of CHAPSO (Fig. 5E). To stabilize the oligomers, we applied a low-concentration range of the crosslinking reagent glutaraldehyde to γ-secretase, using the Grafix protocol (36), and collected EM micrographs (Fig. 5F). 2D classification of the EM particles reveals tantalizing features of γ-secretase (Fig. 5G), which strongly suggest oligomerization.
Discussion
In this manuscript, we present compelling evidence to support the dominant negative effect of the loss-of-function γ-secretase mutants on the proteolytic activity of the WT γ-secretase. The physical basis for the dominant negative effect appears to be the oligomerization of γ-secretase. Both the proteolytic activity measurements and the pull-down assays were performed in vitro, using highly purified, recombinant γ-secretase. Such an experimental design, which contrasts with previous cell-based studies, allows clear delineation of the experimental logic and clean interpretation of the results. Nonetheless, the observed dominant negative effect in vitro is yet to be thoroughly examined in cells. Preliminary experiments appear to confirm the same dominant negative effect in cells (Fig. S4), which confirms the conclusion of an earlier study (16). Consistently, PS1 homodimers have been previously detected by fluorescent lifetime imaging microscopy in live mammalian cells (37). Intriguingly, the substrate APP-C99 has been reported to dimerize in previous studies (38–40).
Fig. S4.
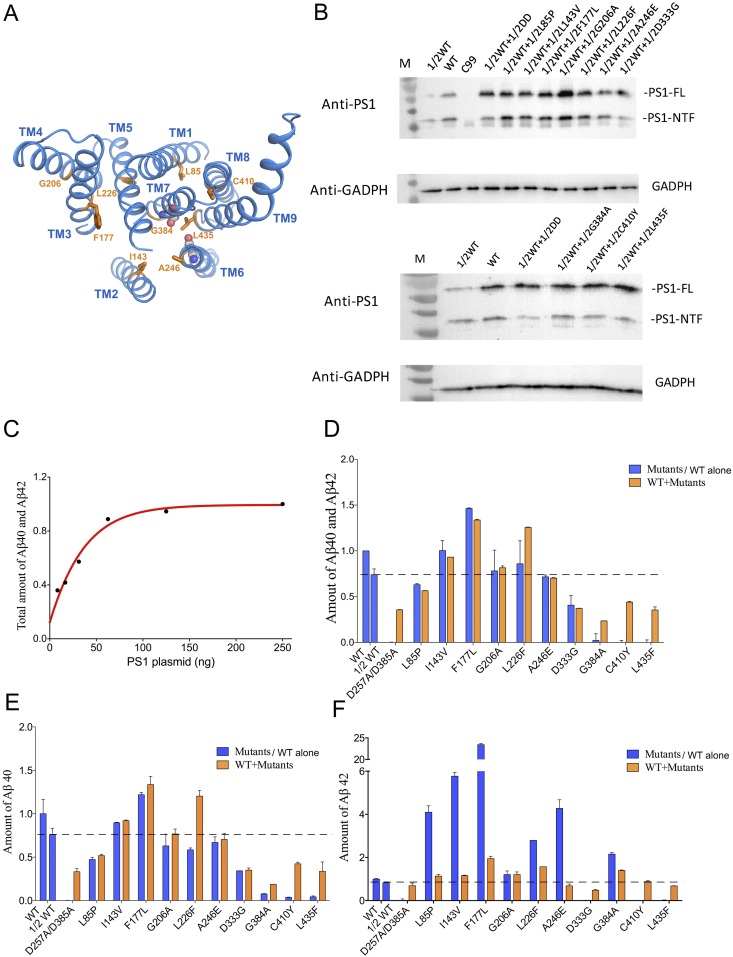
Results of the cell-based cleavage assays are consistent with a dominant negative effect by mutant γ-secretases. (A) Location of the amino acids targeted for mutations in the PS1 structure. Except for Asp333 in the disordered loop between TM6 and TM7, the other nine amino acids targeted for mutations are distributed throughout all nine TMs of PS1. (B) Determination of the amount of PS1-expressing plasmid for transfection into 1 mL HeLa cells. The combined production of Aβ40 and Aβ42 was monitored. Within the range of 7.8–62.5 ng of the transfected PS1 plasmid, the total amount of Aβ40 and Aβ42 is roughly linearly proportional to the amount of transfected PS1-expressing plasmid under the condition of fixed C99-expressing plasmid (500 ng). (C) The expression levels of the WT and mutant PS1 proteins in HeLa cells. Plasmids that overexpress the WT and mutant PS1 proteins were transfected into PSEN1/PSEN2 double-knockout HeLa cells. Western blots were performed on these cells using an anti-PS1 monoclonal antibody. The total amount of PS1 is similar for these different transfections. The label “1/2” indicates one-half of the full amount (62.5 ng) of that particular plasmid for transfection into 1 mL HeLa cells. For example, “1/2 WT + 1/2 DD” means 31.25 ng of the plasmid for WT PS1 plus 31.25 ng of the plasmid for the catalytic mutant PS1-DD. (D) The combined production of Aβ40 and Aβ42 was detected from medium after transfection of plasmids carrying PS1(62.5 ng as full amount) and the substrate APP-C99 (500 ng). Coexpression of 1/2 loss-of-function PS1 mutants with 1/2 WT PS1 results in compromised activities compared with 1/2 WT alone, indicating a dominant negative effect. This effect applies to all four PS1 mutants: D257A/D385A, G384A, C410Y, and L435F. (E) The production of Aβ40 in the cell-based assay. (F) The production of Aβ42 in the cell-based assay.
Theoretically, the dominant negative effect should allow elimination of the proteolytic activity of the WT γ-secretase at sufficiently high concentrations of the loss-of-function mutants. Curiously, however, compared with that of the WT γ-secretase alone, the proteolytic activity of the WT γ-secretase in the presence of high concentrations of the loss-of-function mutants appears to level off at the 20–30% level (Figs. 1C and 2). One potential explanation is that the proteolytic activity of the WT γ-secretase in the hetero-oligomer with the loss-of-function mutants is reduced to a fixed level (20–30%), rather than complete elimination. In this case, the physical basis remains to be elucidated.
Although γ-secretase forms an oligomer, the nature of the oligomer remains to be investigated. Notably, under our experimental conditions, only a very small fraction of the Flag-tagged mutant γ-secretase-DD was pulled out by the HA-tagged WT γ-secretase (Fig. 3A). Similarly, the efficiency of hetero-oligomeric interaction remains poor in all cases of the pull-down experiments, suggesting a relatively weak interaction among the γ-secretase molecules. In the case of a low binding affinity, formation of the γ-secretase oligomer should be more favorable at higher concentrations, which predicts a nonlinear increase of the proteolytic activity with increasing γ-secretase concentrations.
A key finding of our study is that detergents modulate the interactions among γ-secretase molecules. In contrast to CHAPSO, the detergent digitonin and the surfactant amphipol negatively affect the interactions. In addition, the detergent dodecyl β-D-maltoside was found to mediate the dissociation of the γ-secretase complex, resulting in inactive subcomplexes (41). In light of our current study, the published, seemingly conflicting observations on the basal state of γ-secretase can be fully reconciled. The finding of γ-secretase as a homo-dimer (19–21) or a higher-order protein complex (22–26) was made through immunoprecipitation of cellular proteins under the detergent CHAPSO, which in our current studies has been proven essential for maintenance of the oligomeric state of γ-secretase. In contrast, the observation of γ-secretase as a 230-kDa monomeric complex (27–29) was obtained using recombinant γ-secretase under the detergent digitonin, which disrupts formation of the γ-secretase oligomer. Supporting this analysis, the higher-order complexes of γ-secretase (670 or 900 kD) appeared to exhibit higher proteolytic activities (22–26). The compensation of some pathogenic PSEN1 mutations by the WT allele (30) can also be explained by the remaining proteolytic activity of the WT PS1 in the presence of excess loss-of-function mutant PS1 proteins (Figs. 1 and 2).
Our experimental data reveal two important findings: γ-secretases interact with each other under select detergents, and the proteolytic activity of γ-secretase depends on this interaction. These findings give rise to the observed dominant negative effect of the loss-of-function γ-secretases (with corresponding PS1 mutations). We wish to stress that our experimental data provide no supporting evidence for a potential role of γ-secretase in the development of AD. In fact, a number of the γ-secretase variants with pathogenic PS1 mutations, exemplified by S365A, have WT-level proteolytic activity in terms of Aβ42 and Aβ40 production (18). The development of AD in the patients with these PS1 mutations cannot be explained by the WT-level proteolytic activity of these γ-secretase variants in vitro. Nonetheless, the dominant negative effect of these PS1 mutations in patients with AD still applies, suggesting such an effect may not be recapitulated by the catalytic function of PS1 in γ-secretase. We speculate that, for the vast majority of patients with AD, the dominant negative effect of PS1 is perhaps effected through other mechanisms that are independent of γ-secretase.
Importantly, the observed dominant negative effect can only be efficiently achieved by considerably higher concentrations of the mutant γ-secretase over the WT enzyme (Figs. 1D and 2). Under an equimolar ratio of 1:1 between WT and mutant γ-secretases, the proteolytic activity was only slightly reduced (Fig. 1D), which is again consistent with the compensation by WT γ-secretase, as previously suggested (30). To gain a better understanding of the moderate dominant negative effect, the expression level of the WT and mutant PSEN1 alleles, as well as the oligomerization state of γ-secretases, should be carefully examined under in vivo circumstances, especially in patients’ brains. In our study, the production of Aβ40 and Aβ42 is used as an exclusive indicator of the proteolytic activity. It remains to be investigated whether such a mechanism applies to other Aβ species such as Aβ43, which is thought to play a crucial role in amyloidogenesis and AD pathology (42, 43). Nevertheless, the dominant negative effect indicated by production of Aβ40 and Aβ42 had been previously suggested for the production of the intracellular domain of APP (16). Cleavage of other type I substrates by γ-secretase may also follow the same mechanism (16).
Materials and Methods
Protein Purification.
γ-secretase for enzymatic assay was expressed and purified as described (33). For pull-down assay, the proteins were purified through strep tag on PEN2 by Strep-Tactin Superflow resin (IBA). Either flag tag or HA tag was fused to the N terminus of PS1. In the case of analytical ultracentrifugation, the detergents used for γ-secretase were exchanged via gel filtration in Superose-6 column (GE Healthcare).
Activity Assays.
A different amount of purified WT or mutant γ-secretase was mixed with APP-C99 substrate in 0.5% CHAPSO, 25 mM Hepes at pH 7.0, 150 mM NaCl, 0.1% phosphatidylcholine, and 0.025% phosphatidylethanolamine and incubated in 37 °C for 3 h. Aβ42 and Aβ40 production was detected by the AlphaLISA assay (PerkinElmer), as described (18). Protein concentration was determined by the Bradford method.
Cell-Based Activity Assays.
PSEN-1/PSEN-2 double-knockout HeLa cell line was generated using CRISPR/Cas9 technology coupled with a CMV/PuroR reporter system, as reported (44). To determine a suitable amount of PS1 plasmid for transfection, a different amount of plasmid-containing PS1 and 500 ng plasmid carrying substrate APP-C99 was cotransfected into 70% confluent HeLa cells in a 12-well plate, using Neofect, and 62.5 ng PS1 plasmid was finally used as total amount to transfect for each reaction. Transfected cells were cultured for 60 h before the cell medium was harvested. 2 μL medium was detected by the AlphaLISA assay, as described (18).
Pull-Down Assays.
Around 10 μg differently tagged γ-secretase was preincubated at room temperature for about 15 min. The γ-secretases were then mixed and incubated with anti-Flag M2 or anti-HA affinity resin for 2 h at 4 °C. The resin was washed by using the corresponding buffer with ∼30-fold volume to eliminate the nonspecifically bound protein. γ-secretase was eluted with Flag peptide or HA peptide and analyzed by Western blot.
Analytical Ultracentrifugation Analysis.
The peak fractions of WT γ-secretase purified by different detergents were collected and concentrated to 1 mg⋅mL−1, as measured by OD280. The samples were applied to analytical ultracentrifugation analysis, using a Beckman XL-I analytical ultracentrifuge (Beckman Coulter) equipped with an eight-cell An-50 Ti rotor. When centrifuge γ-secretase with 35,000 rpm (89, 180 g-98, 784 g from cell center to cell bottom) for 5 h at 20 °C, the ultraviolet (UV) absorbance was recorded to determine the distribution of protein complex. The data of sedimentation velocity was analyzed using Sedfit (45).
GraFix of γ-Secretase.
Next, 1 mg⋅mL−1 γ-secretase purified by CHAPSO was prepared in low-salt buffer (50 mM Hepes at pH 7.4, 50 mM NaCl, 0.5% CHAPSO), and 200 µL protein sample was added to a gradient consisting of 10–30% (vol/vol) glycerol and 0–0.05% glutaraldehyde in the low-salt buffer. Ultracentrifugation was performed for 20 h at 33,000 rpm(82, 274 g-186, 575 g along the gradient) in a SW41 Ti rotor (Beckman). The fractionated sample was quenched by 50 mM Tris at pH 7.4 and applied to negative stain preparation.
Negative Staining Electron Microscopy.
Samples of WT or GraFixed γ-secretase (4 µL) were applied to glow-discharged continuous carbon-coated grids (Zhongjingkeyi Technology) and stained by 2% uranyl acetate. The grids were imaged on a FEI Tecnai F20 microscope at 200 kV. Approximately 140 images were collected for the GraFixed sample. About 7,000 particles were automatically picked to perform 2D classification with RELION (46).
Supplementary Material
Acknowledgments
We thank Y. Zhou and W. Wei (Peking University) for providing the PSEN-1/PSEN-2 double-knockout HeLa cell line. We also thank the protein purification and identification platform at Tsinghua University Branch of China National Center for Protein Sciences. This work was supported by funds from the Ministry of Science and Technology (2014ZX09507003006 to Y.S.) and the National Natural Science Foundation of China (31130002 and 31321062 to Y.S.).
Footnotes
Conflict of interest statement: Y.S. and Y.-M.L. were co-authors on a 2015 paper.
See Commentary on page 12635.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713605114/-/DCSupplemental.
References
- 1.Kimberly WT, et al. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci USA. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takasugi N, et al. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 3.De Strooper B. Aph-1, Pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 4.Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992;255:728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- 5.Estus S, et al. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992;255:726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- 6.Takami M, et al. Gamma-secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheuner D, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki N, et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 9.Citron M, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 10.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 11.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alzheimer A. About a peculiar disease of the cerebral cortex. Centralblatt für Nervenheilkunde Psychiatrie. 1907;30:177–179. [Google Scholar]
- 13.Borchelt DR, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 14.De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 15.Campion D, et al. Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65:664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilig EA, Gutti U, Tai T, Shen J, Kelleher RJ., 3rd Trans-dominant negative effects of pathogenic PSEN1 mutations on γ-secretase activity and Aβ production. J Neurosci. 2013;33:11606–11617. doi: 10.1523/JNEUROSCI.0954-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen J, et al. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Zhou R, Yang G, Shi Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc Natl Acad Sci USA. 2017;114:E476–E485. doi: 10.1073/pnas.1618657114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeter EH, et al. A presenilin dimer at the core of the gamma-secretase enzyme: Insights from parallel analysis of Notch 1 and APP proteolysis. Proc Natl Acad Sci USA. 2003;100:13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cervantes S, Saura CA, Pomares E, Gonzàlez-Duarte R, Marfany G. Functional implications of the presenilin dimerization: Reconstitution of gamma-secretase activity by assembly of a catalytic site at the dimer interface of two catalytically inactive presenilins. J Biol Chem. 2004;279:36519–36529. doi: 10.1074/jbc.M404832200. [DOI] [PubMed] [Google Scholar]
- 21.Hébert SS, Godin C, Lévesque G. Oligomerization of human presenilin-1 fragments. FEBS Lett. 2003;550:30–34. doi: 10.1016/s0014-5793(03)00813-5. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, et al. The presenilin proteins are components of multiple membrane-bound complexes that have different biological activities. J Biol Chem. 2004;279:31329–31336. doi: 10.1074/jbc.M401548200. [DOI] [PubMed] [Google Scholar]
- 23.Evin G, et al. Transition-state analogue gamma-secretase inhibitors stabilize a 900 kDa presenilin/nicastrin complex. Biochemistry. 2005;44:4332–4341. doi: 10.1021/bi0481702. [DOI] [PubMed] [Google Scholar]
- 24.Li YM, et al. Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state. Proc Natl Acad Sci USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edbauer D, Winkler E, Haass C, Steiner H. Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc Natl Acad Sci USA. 2002;99:8666–8671. doi: 10.1073/pnas.132277899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmery MR, et al. Partial purification and characterization of gamma-secretase from post-mortem human brain. J Biol Chem. 2003;278:24277–24284. doi: 10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, et al. Active gamma-secretase complexes contain only one of each component. J Biol Chem. 2007;282:33985–33993. doi: 10.1074/jbc.M705248200. [DOI] [PubMed] [Google Scholar]
- 28.Osenkowski P, et al. Cryoelectron microscopy structure of purified gamma-secretase at 12 A resolution. J Mol Biol. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai XC, et al. An atomic structure of human γ-secretase. Nature. 2015;525:212–217. doi: 10.1038/nature14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szaruga M, et al. Qualitative changes in human γ-secretase underlie familial Alzheimer’s disease. J Exp Med. 2015;212:2003–2013. doi: 10.1084/jem.20150892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 32.Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 33.Lu P, et al. Three-dimensional structure of human γ-secretase. Nature. 2014;512:166–170. doi: 10.1038/nature13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- 35.Wilkie AO. The molecular basis of genetic dominance. J Med Genet. 1994;31:89–98. doi: 10.1136/jmg.31.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kastner B, et al. GraFix: Sample preparation for single-particle electron cryomicroscopy. Nat Methods. 2008;5:53–55. doi: 10.1038/nmeth1139. [DOI] [PubMed] [Google Scholar]
- 37.Herl L, et al. Detection of presenilin-1 homodimer formation in intact cells using fluorescent lifetime imaging microscopy. Biochem Biophys Res Commun. 2006;340:668–674. doi: 10.1016/j.bbrc.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 38.Nadezhdin KD, Bocharova OV, Bocharov EV, Arseniev AS. Dimeric structure of transmembrane domain of amyloid precursor protein in micellar environment. FEBS Lett. 2012;586:1687–1692. doi: 10.1016/j.febslet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 39.Gorman PM, et al. Dimerization of the transmembrane domain of amyloid precursor proteins and familial Alzheimer’s disease mutants. BMC Neurosci. 2008;9:17. doi: 10.1186/1471-2202-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, et al. Familial Alzheimer’s mutations within APPTM increase Aβ42 production by enhancing accessibility of ε-cleavage site. Nat Commun. 2014;5:3037. doi: 10.1038/ncomms4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraering PC, et al. Detergent-dependent dissociation of active gamma-secretase reveals an interaction between Pen-2 and PS1-NTF and offers a model for subunit organization within the complex. Biochemistry. 2004;43:323–333. doi: 10.1021/bi035748j. [DOI] [PubMed] [Google Scholar]
- 42.Saito T, et al. Potent amyloidogenicity and pathogenicity of Aβ43. Nat Neurosci. 2011;14:1023–1032. doi: 10.1038/nn.2858. [DOI] [PubMed] [Google Scholar]
- 43.Veugelen S, Saito T, Saido TC, Chávez-Gutiérrez L, De Strooper B. Familial Alzheimer’s disease mutations in presenilin generate amyloidogenic Aβ peptide seeds. Neuron. 2016;90:410–416. doi: 10.1016/j.neuron.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Zhang H, Wei W. Simultaneous generation of multi-gene knockouts in human cells. FEBS Lett. 2016;590:4343–4353. doi: 10.1002/1873-3468.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheres SH. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]