Significance
Relative processing is a ubiquitous feature of neural and cognitive function. In perception, a prominent example of relative processing is adaptation, in which both sensory neural responses and resulting percepts depend on the history of past stimuli. Neurons in decision-related brain areas also adapt, representing value information relative to previous rewards, but whether adaptive value coding affects behavior is unknown. Here, we show adaptation in the subjective valuation process of human subjects, with values consistently dependent on the recent history of presented values. This adaptive valuation can be explained by divisive normalization, a canonical neural computation widely observed in sensory processing, offering a unifying biological mechanism for temporal context effects in perception and decision making.
Keywords: adaptation, context dependence, decision making, divisive normalization, reward
Abstract
The notion of subjective value is central to choice theories in ecology, economics, and psychology, serving as an integrated decision variable by which options are compared. Subjective value is often assumed to be an absolute quantity, determined in a static manner by the properties of an individual option. Recent neurobiological studies, however, have shown that neural value coding dynamically adapts to the statistics of the recent reward environment, introducing an intrinsic temporal context dependence into the neural representation of value. Whether valuation exhibits this kind of dynamic adaptation at the behavioral level is unknown. Here, we show that the valuation process in human subjects adapts to the history of previous values, with current valuations varying inversely with the average value of recently observed items. The dynamics of this adaptive valuation are captured by divisive normalization, linking these temporal context effects to spatial context effects in decision making as well as spatial and temporal context effects in perception. These findings suggest that adaptation is a universal feature of neural information processing and offer a unifying explanation for contextual phenomena in fields ranging from visual psychophysics to economic choice.
Relative rather than absolute evaluation is a fundamental feature of cognition. In sensory processing, the perception of stimulus properties depends markedly on the context in which that stimulus appears. This contextual influence is driven by both spatial context (the surrounding sensory environment) and temporal context (stimuli in the recent past). For example, the perceived brightness of a gray square depends on whether it appears on a light or a dark background, and whether the viewer was previously in a bright or a dark environment. Across multiple sensory modalities, psychophysical context effects can be explained by corresponding contextual modulation in sensory neural responses (1, 2). The prevalence of contextual modulation in perception suggests that relative evaluation, in which a stimulus is represented relative to its context, is a fundamental feature of neural coding, contributing to functions such as predictive coding, Bayesian inference, and the efficient representation of information (3–6).
Consistent with a general role in neural processing, context effects extend beyond perception to higher order cognitive functions—in particular, option evaluation and decision making. Value is a central notion in decision theory, integrating all relevant information into a single decision variable to guide choice. In traditional theories of rational choice (7, 8), value is assumed to be a static quantity, determined solely by the properties of an individual option. Under such an absolute valuation, preference between two given options should be independent of contextual factors such as other available alternatives or the previous history of rewards. Context-independent choice is a foundation of traditional normative theories that describe how rational choosers should behave. However, in contrast to the predictions of normative theory, empirical choice behavior in diverse species varies with the decision context (9–11). Such context-dependent preferences suggest that biological choosers employ a relative rather than absolute valuation process, but the neural origins of these context effects are not well understood.
However, emerging evidence suggests that context-dependent perception and decision making may share common underlying neural mechanisms. In sensory processing, many perceptual effects of spatial context can be explained by the contextual modulation of sensory neural responses. For example, surround suppression in visual cortical neurons is thought to mediate the effect of background stimuli on perceptual sensitivity and salience (12). In decision making, analogous behavioral effects of spatial context have been widely observed in both animal and human choosers (13–17), where the architecture of the choice set can markedly influence observed decisions. For example, the relative preference between two options can be altered by the addition of a third alternative, despite the additional option never being chosen. Recent neurophysiological evidence has linked spatial context effects in decision making to relative value representation in neural circuits, suggesting a biological basis for context-dependent deviations from rational benchmarks (18–20). Importantly, both sensory (21, 22) and value-related (18, 19, 23) spatial context effects can be explained by divisive normalization, a canonical neural computation that incorporates contextual information via divisive scaling.
In contrast to spatial context effects, the effects of temporal context on choice behavior are less well documented, and the responsible neural mechanisms are unknown. In sensory processing, a large body of work has examined the relationship among temporal context, neural representation, and behavior (1, 24, 25). This work has shown that the history of previously presented stimuli induces perceptual adaptation to both simple (e.g., luminance) and complex (e.g., face identity) stimulus features, driven by adaptive changes in sensory neuron response properties. For example, in the waterfall illusion, the viewing of a moving visual stimulus induces perceived motion in the opposite direction in a subsequent stationary stimulus, an effect linked to neural adaptation in motion-sensitive brain areas (26). Adaptation effects extend to different stimulus features, hierarchical levels, and modalities in sensory processing, suggesting that adaptation is a characteristic feature of how the brain represents behaviorally relevant information. In decision making, although less prevalent than widely documented spatial context phenomena, temporal context effects in which choices depend on previous decisions have been described (27, 28). Furthermore, prominent theories of decision making highlight the importance of expectations, which can be substantially shaped by previous experience (29–31); these expectations can drive adaptation in even complex behavior, such as responses to social norm violations (32).
Given the extensive nature of adaptation, an attractive explanation for temporal context dependence in decision making is adaptation in the cognitive representation of value. Such adaptation is consistent with a large extant literature on successive incentive contrast effects, where motivational behaviors like running speed and consumption are dependent on both current and past reward environments (33). Recent findings show that value-coding neurons also adapt to the recent history of rewards (34–36), raising the possibility that adaptation in reward coding underlies temporal context-dependent decision behavior. However, corresponding changes in behavior have not been examined in existing value adaptation experiments, and the influence of reward history on subsequently perceived value is unknown.
Here, we test the hypothesis that the valuation process in human subjects exhibits history-dependent adaptation. Using an incentive-compatible auction procedure designed to elicit true valuations, we show that the subjective value of a given option depends on the previous value environment in a specific manner, with current item values varying inversely with the average value of recently observed items. Consistent with normalization-driven adaptation, this relative valuation process introduces a dynamic dependence on the history of past values. These findings suggest that adaptation is a key feature of the valuation process and that—analogous to contextual effects in sensory processing—spatial and temporal forms of contextual modulation in decision making share a common computational mechanism.
Results
Bid Adaptation Task.
To examine the influence of temporal context on the valuation process, we quantified valuation behavior in human subjects (n = 43, 24 female, mean age 23.43 y) using a bid adaptation task (Fig. 1). This task comprised alternating blocks of two types of trials: bid trials and rating trials (Fig. 1A). In each bid trial, subjects viewed a single snack food item and reported their maximum willingness to pay for that item ($0–$6). To elicit true subject valuations, realization of a randomly selected bid trial was conducted at the end of the experiment via an incentive-compatible auction procedure (37) (Becker–DeGroot–Marschak auction). In each rating trial, subjects viewed a single snack food item and reported the pleasantness of that item on a visual analog scale. Together, these trial types allowed us to examine subject valuations (in bid trials) as a function of recently experienced value history (in rating trials).
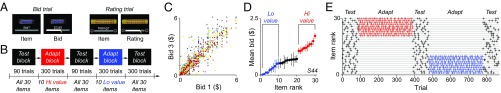
Fig. 1.
Value adaptation task. (A) Bid trial and rating trial designs. In bid trials, subjects reported their willingness to pay for a snack food item using a slider bar. In rating trials, subjects reported the pleasantness of a presented item on a visual analog scale. (B) Experimental session block design. Blocks of bid trials (Test block) and rating trials (Adapt block) alternated across a session. Test blocks presented all 30 items, and Adapt blocks presented a subset of 10 low (Lo) or 10 high (Hi) value items, enabling a quantification of how valuation behavior changed following adaptation to either low- or high-value exposure. The sequence of Adapt block conditions (Lo-Hi or Hi-Lo) was randomized across subjects. (C) Repeat bid stability. First versus third bids for individual items are plotted for all subjects. Consistent with items having intrinsic subjective valuations, repeat bids were significantly correlated across the population (r = 0.89, P = 5.40 × 10−46) as well as in each individual subject (P < 0.05). (D) Example bid distribution and item classification. Data show sorted mean bids for all 30 items (error bars, SEM). The 10 lowest- and 10 highest-value items were designated Lo-value (blue) and Hi-value (red) items, respectively. (E) Example item rank structure. Crosses show the value rank of items presented in Test blocks (black), Hi-value Adapt blocks (red), and Lo-value Adapt blocks (blue).
Experimental sessions were organized as alternating blocks of bid and rating trials to test and adapt valuation behavior, respectively (Fig. 1B). Each Test block comprised 90 bid trials, with three repetitions for each of 30 items in pseudorandom order. Consistent with subjects reporting item-specific valuations, repeat bids for individual items were strongly correlated in each subject (mean correlation between first and third bids: r = 0.89; P = 5.40 × 10−46, t test; Fig. 1C). Following the first Test block, items were rank-ordered by their mean bid price, and the 10 lowest-valued (Lo value) and 10 highest-valued (Hi value) items were identified (Fig. 1D). Subjects then underwent an Adapt block, comprising 300 rating trials with only Lo value (Hi value) items, followed by another Test block where bids for all 30 goods were again elicited. Finally, subjects underwent a second Adapt block with only Hi value (Lo value) items and a final Test block. To control for presentation order, subjects were randomly assigned to the sequence of Adapt block values (Lo-Hi vs. Hi-Lo). This task structure quantifies valuations across the entire set of 30 items following exposure to either low- or high-value items (Fig. 1E).
Postadaptation Bid Changes.
We hypothesized that adaptation in value coding would produce systematic changes in valuation behavior, observable as willingness to pay measures that depend on previously viewed item values. In sensory processing, adaptation paradigms elicit characteristic history-dependent changes in perception that are classically repulsive in nature (24). For example, prolonged exposure to a high-contrast visual stimulus reduces the perceived contrast of subsequent test stimuli, and prolonged exposure to a moving visual stimulus induces a perceived motion in the opposite direction in subsequent stationary stimuli. We hypothesized that if similar adaptation occurs in neural value coding, subject bids will differ as a function of the preceding Adapt block condition: bids will decrease following Hi Adapt blocks and increase following Lo Adapt blocks.
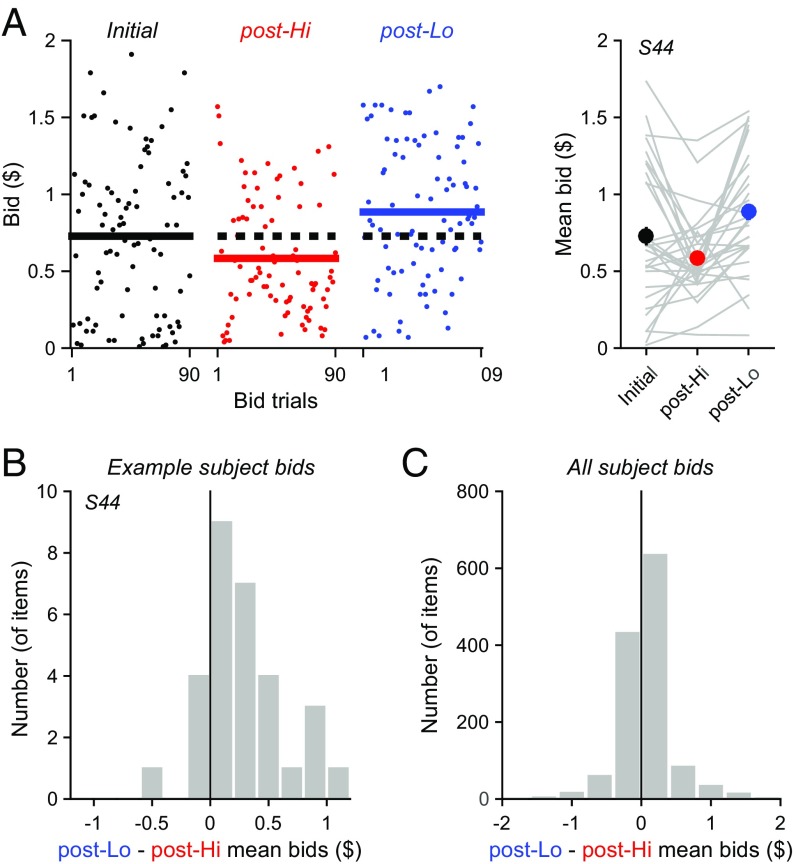
Examination of individual subject bids reveals that the average item bids differed depending on the identity of the preceding adaptation block (Fig. 2). As shown for an example subject (Fig. 2A), bids following the Hi Adapt block were lower than bids for the very same items following a Lo Adapt block. We quantified this adaptation effect by examining the average difference between post-Lo and post-Hi bids across all 30 items. Bid differences were significantly positive in the example subject (mean bid difference = $0.30; P = 5.46 × 10−5, paired t test; Fig. 2B) and across the entire population (mean bid difference = $0.05; P = 6.55 × 10−6, paired t test; Fig. 2C). These bid differences in both the example subject and population were also significant when analyzed with permutation tests that do not require specific distributional assumptions (P < 0.0001; SI Results and Fig. S1). Thus, consistent with a repulsive effect of recent values, subject valuations are lower after high-value environments and higher after low-value ones.
Fig. 2.
Postadaptation bids depend on value history. (A) Example of observed bids across time. (Left) Individual bids in three Test blocks (black, initial block; red, post-Hi block; blue, post-Lo block). Solid lines show the average bid across all items in each Test block; for comparison, dotted black line denotes average bid in the initial test block. (Right) Mean bids by block (error bars, SEM). Gray lines connect mean bids for an individual item across blocks. (B) Example distribution of bid differences, calculated for each snack food item as the difference between mean bids in the post-Lo and post-Hi Test blocks. As evident in the shifted distribution, bid differences were positive in this example subject, indicating significantly higher bids in post-Lo versus post-Hi blocks (P = 5.46 × 10−5, paired t test). (C) Distribution of population bid differences. Across all items in all subjects, post-Lo bids significantly exceeded post-Hi bids (P = 6.55 × 10−6, paired t test).
Quantifying Bid Deviations.
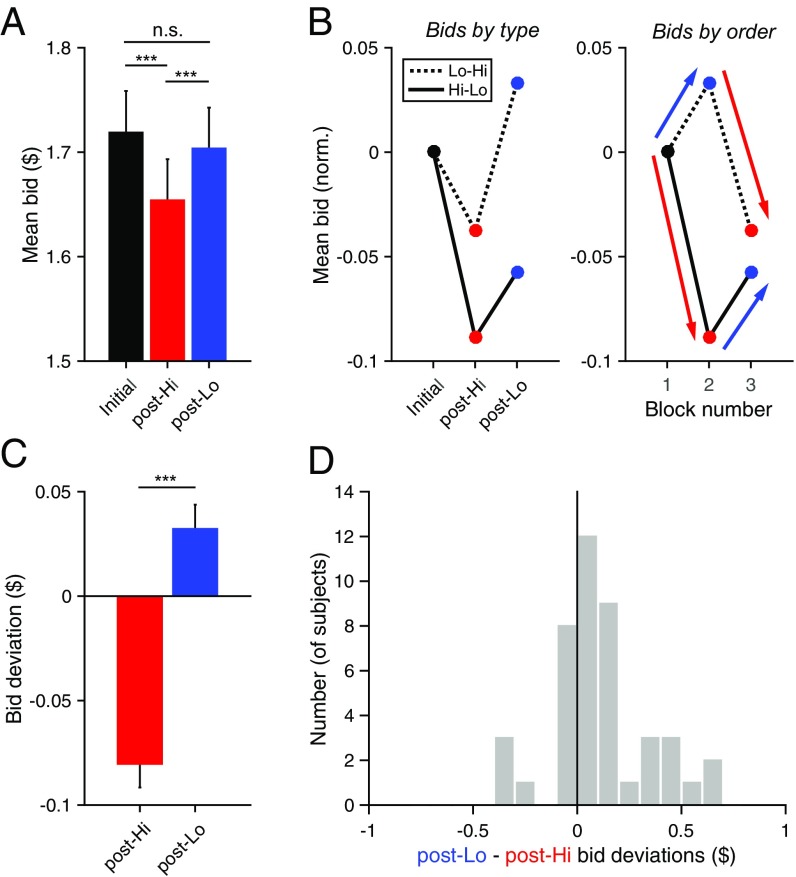
We next examined the dynamic nature of value adaptation by quantifying how bids changed relative to previous bids. We hypothesized that if adaptation is an intrinsic and ongoing feature of the valuation process, bidding behavior would be better characterized by changes in bids between blocks than bids alone. Consistent with this hypothesis, subject bid data were strongly dependent on the sequence of presented Adapt block conditions (Fig. 3 A and B). When examining bids alone (Fig. 3A), post-Hi bids across all subjects were significantly lower than bids in the initial Test block (P = 3.00 × 10−7, paired t test), but post-Lo bids were not significantly higher than initial bids (P = 0.26, paired t test). This finding differs from the expected effect of adaptation, which predicts an increase in valuation following exposure to low-value items. However, examination of bid data segregated by adaptation sequence showed consistent effects of both high- and low-value adaptation relative to preceding bids (Fig. 3B). Specifically, bids following Lo adaptation were higher than previous bids (blue arrows; Fig. 3B, Right) and bids following Hi adaptation were lower (red arrows; Fig. 3B, Right). Therefore, post-Hi and post-Lo changes were similar in subjects undergoing either adaptation sequence (Hi-Lo or Lo-Hi), suggesting that bids continually changed over the course of experimental sessions and that bid changes are the most informative measure of value adaptation effects.
Fig. 3.
Bid deviations quantify effect of adaptation. (A) Mean bids by Test block condition (***P < 0.001). (B) Mean normalized bids segregated by adaptation sequence. To facilitate comparison, bids are normalized by subtracting mean initial Test block bid value. Solid and dashed lines show data from subjects presented with Hi-Lo and Lo-Hi adaptation sequences, respectively. (Left) Data as a function of Test block condition. (Right) Data as a function of sequential block number. As shown by the arrows, bid changes relative to previous bids were similar following Lo-value (blue) and Hi-value (red) adaptation. (C) Mean bid deviations across all subjects. For each bid, bid deviation was calculated as the difference between the bid and the average bid for that item in the previous Test block (error bars, SEM). Post-Hi deviations are significantly negative (P = 1.91 × 10−12, t test), and post-Lo deviations are significantly positive (P = 5.4 × 10−3, t test). (D) Distribution of subject bid deviation differences. Across subjects, post-Lo deviations significantly exceed post-Hi deviations (P = 3.3 × 10−3, paired t test).
To quantify adaptation-induced bid changes, we computed the deviation of each bid for a specific item from the average bid for that particular good during the preceding Test block (Materials and Methods). This metric accounts for changes in valuation that have occurred over the course of a behavioral session; because the observed mean change in bids (relative to preceding bids) is the same regardless of presentation order (Fig. 3B), bid deviations provide a sequence-independent measure of adaptation effects. Quantified in this manner (Fig. 3C), average bid deviations across all subjects were significantly negative after high-value adaptation (P = 1.91 × 10−12, t test) and positive after low-value adaptation (P = 5.4 × 10−3, t test), with a significant difference in deviations between adaptation conditions (P = 4.49 × 10−10, paired t test). This difference in bid deviations (post-Lo – post-Hi) at the subject level was significantly positive across the population (P = 3.3 × 10−3, paired t test; Fig. 3D). Finally, bid deviation differences were still significant (P = 2.5 × 10−3, paired t test) when we restricted the analysis to items that were never presented in rating trials (Fig. 1D, item rank 11–20), suggesting that the adaptation process affects the general valuation process rather than the values of adapted items alone. These bid deviation results show a consistent effect of recent history on willingness to pay measures, with low-value contexts increasing, and high-value contexts decreasing, subsequent bids for identical snack food items.
Bid Deviation Dynamics and the Role of Normalization.
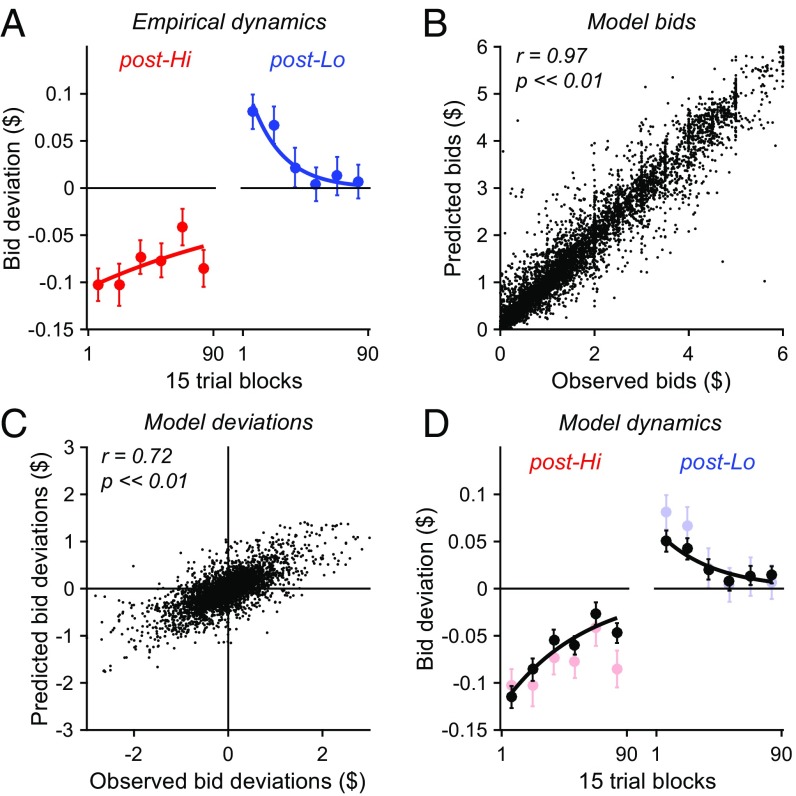
In addition to blockwise adaptation effects, we examined whether bid deviations exhibit intrablock dynamics. In sensory processing, blockwise adaptation effects arise from a dynamic adaptation process incorporating a weighted average of recent stimuli. If value adaptation incorporates a similar dependence on past rewards, we hypothesized that bid deviations will change across a Test block. Bids early in a block are preceded primarily by rating trials with extreme value items, and should exhibit the strongest adaptation; bids late in a block are preceded by other bid trials, and should exhibit the weakest adaptation. To investigate bid dynamics, we examined average bid deviations as a function of trial number in both postadaptation bidding blocks (Fig. 4A). In both blocks, bids deviations are largest in the initial trials immediately following the Adapt blocks and diminish over successive bid trials. When fit with simple exponential decay functions (see Materials and Methods), bid deviation magnitudes decreased across the duration of the Test block for both post-Hi (decay constant λ = −0.104; P = 0.046, bootstrap) and post-Lo (λ = −0.552; P = 3.0 × 10−4, bootstrap) bids. Examination of rating trial data suggests that similar adaptive changes may affect pleasantness ratings (SI Results and Fig. S2); however, the current experiment was not designed to test adaptation in rating trials, and the relationship between these changes and value adaptation will require further research.
Fig. 4.
Bid deviation dynamics are explained by a value normalization model. (A) Bid deviation dynamics. Points plot average bid deviations in successive 15 trial windows across post-Hi (red) and post-Lo (blue) blocks (error bars, SEM). Lines plot exponential curves fit to the deviation dynamics. (B) Normalization model bids. Data points show predicted versus observed bids for all postadaptation Test trials. Model-predicted bids are significantly correlated with observed bids (r = 0.97, P << 0.01). (C) Normalization model bid deviations. Data points show predicted versus observed bid deviations. Model-predicted bid deviations are significantly correlated with observed bid deviations (r = 0.72, P << 0.01). (D) Normalization model bid dynamics. Black, model-predicted bid deviations. Data plotted as in A, with observed bid deviations for comparison.
To test whether this dynamic bidding behavior is consistent with known mechanisms of adaptation, we fit bid data with a divisive normalization model of adaptive valuation. Normalization models employ a divisive representation, in which the output of a neuron reflects the feedforward input to the neuron divided by the summed activity of a larger pool of neurons; to explain adaptation, this divisive factor incorporates information about stimuli presented in the recent past. In sensory processing, history-dependent divisive normalization explains neural and perceptual aspects of a variety of sensory adaptation phenomena (22, 38–40). In decision making, recurrent circuit implementations of value normalization can introduce an intrinsic dependence on past value information (23, 41). To examine if a normalization process can explain the observed bid adaptation effects, we fit individual subject bid data with a simple normalization model of adaptive value coding:
where the predicted bid pbidt on trial t depends on the value Vt of the item presented on that trial, the values of recently presented items (up to N previous trials), and the parameters K and α. Individual good values were calculated as the average bid for that item across all experimental Test bids. Because the timescale of bid deviation dynamics following adaptation suggests a slow integration over many trials, the following results employed a model with value information from the previous 60 trials; however, qualitatively similar modeling results were obtained with a range of different previous history windows.
We found that the normalization model captured multiple features of observed bidding behavior, including postadaptation bid changes, bid deviations, and intrablock bid dynamics. Model-predicted bids were significantly correlated with observed bids in individual subjects (P < 0.05 in 43/43 subjects, mean r = 0.94) and across the entire population (r = 0.97, P << 0.01; Fig. 4B). However, because differences in individual item values capture much of the variance in item bids, a stronger test of the normalization model is whether it captures adaptation-related changes in bidding behavior. Such changes depend on the history of previous items and cannot be predicted solely from the value of an item in a given trial. We first examined whether predicted bids differed depending on the preceding Adapt block. As in the observed data, model-predicted bids were significantly lower in post-Hi bid trials compared with post-Lo bid trials (P = 9.98 × 10−26, paired t test). We next examined model-predicted bid deviations, quantified in the same manner as observed bid deviations but using predicted rather than observed bids. Model-predicted bid deviations mirrored the pattern of observed data, with significantly negative post-Hi deviations (mean deviation = −0.067, P = 9.26 × 10−17, t test) and significantly positive post-Lo deviations (mean deviation = 0.027, P = 3.06 × 10−4, t test). Furthermore, predicted and observed deviations were significantly correlated across all bid trials (r = 0.72, P << 0.01; Fig. 4C). Finally, we examined the intrablock dynamics of predicted bid deviations. Like the observed bid deviations, predicted deviations in both post-Hi and post-Lo bid trials were largest immediately following Adapt blocks (Fig. 4D). When fit with exponential decay functions, predicted bid deviation magnitudes decreased across the duration of the Test block for both post-Hi (λ = −0.238; P << 0.01, bootstrap) and post-Lo (λ = −0.346; P = 4.0 × 10−4, bootstrap) bids. Notably, the normalization model captured experimental asymmetry between high- and low-value adaptation: observed post-Hi deviations exhibited larger magnitudes and a slower decay than post-Lo deviations, an effect mirrored in deviations predicted by the model (potential causes of asymmetry addressed in SI Results and Fig. S3). Together, these results suggest that a simple normalization model captures both static and dynamic features of adaptive valuation in human subjects.
Discussion
Our findings show that the valuation process in human subjects adapts dynamically to the history of recent values. Consistent with repulsive forms of sensory adaptation, a recent history of low-value increases, and high-value decreases, how human choosers value subsequent options. Critically, this adaptive change is not limited to items presented during adaptation blocks but extends to all subsequent test items, suggesting that it reflects a change in the general valuation process rather than an effect of repeated exposure. Furthermore, the observed changes are more consistent with adaptation to value than to low-level stimulus characteristics: value was a function of individual subject preference rather than stimulus features, low- and high-value adaptation induced opposite bid effects despite no apparent difference between adapter images, and the same item produced low- or high-value adaptation depending on its value to an individual subject. However, to fully establish the generalizability of value adaptation, further research will have to examine whether evaluating options is required to induce adaptation and whether adaptation occurs for other domains of value (e.g., monetary lotteries instead of goods).
A history-dependent change in subjective value may underlie a number of previously described behavioral phenomena. In the psychology literature, successive incentive contrast effects describe how the response to current conditions depends on whether they are better or worse than the recent past. For example, in rats trained to approach a reward, approach speed for a given size reward is faster or slower if the rat had previously been trained with smaller or larger rewards, respectively (42, 43). Analogous positive and negative effects have been demonstrated in diverse species, including insects, birds, and humans (44–46). Such contrast effects have largely been observed in either consummatory (e.g., licking) or instrumental (e.g., running speed) behaviors, raising the question of whether these effects are driven by changes in the valuation process itself or broader psychological processes like generalization, inhibition, and response to novelty (33). Our findings show that subjective values themselves adapt to reward history, offering a direct mechanism for the history-dependent behaviors evident in successive contrast effects.
In addition to successive contrast effects, adaptive valuation provides a potential mechanism for history-dependent effects in value-guided choice behavior. In the behavioral economics literature, prospect theory describes how choosers differentially treat losses and gains in decisions under uncertainty; a key component of the theory is a reference point to which outcomes are compared to determine their valence (29). Although the original theory left the origin of the reference point unspecified, subsequent work argues that it represents an expectation of future outcomes as determined from recent experience (30, 31). Consistent with the influence of reward statistics on risk preferences (47, 48), value adaptation introduces an intrinsic expectation of future outcomes based on past values that provides a mechanism for context-dependent choice under uncertainty (11). More broadly, value adaptation may play a role in examples of history dependence identified in real-world decision making, such as commuting behavior and housing choice (49, 50).
How might neural systems incorporate previous value information into an adaptive value code? Here, we show that a simple normalization model utilizing a running average of previous values explains both average bid changes and their posttransition dynamics, providing a conceptual bridge between adaptive value coding and well-studied mechanisms of adaptation in sensory processing. Although most research on sensory normalization has focused on spatial context, several studies have proposed that normalization explains adaptation in sensory neural responses (22, 38–40). A common element in these approaches is that adaptation effects are driven by a change in the normalization denominator that modifies the degree of divisive scaling. A simple model of this adaptation in sensory physiology postulates that the denominator incorporates an expectation term, using past stimulus information to adjust sensory responses to likely future stimuli. Consistent with this idea, we found that bid adaptation was explained with a normalization model incorporating an expectation term calculated as the average value of items over a finite number of previous trials. Although we used average value as a proxy for reward expectation, neural circuits may determine this quantity in a different manner, such as an exponential weighting kernel of past rewards; an exponential dependence on past activity arises naturally in dynamic models of recurrent normalization circuits (23, 41) and may be a more biologically plausible mechanism for integrating past values. The precise relationship between the behavioral adaptation effects observed here and neural adaptation in decision-related brain areas (34–36) is unknown, and experimental paradigms that elicit context dependence in both behavior and neural activity is a key target for future research.
The relationship between history-dependent valuation and divisive normalization provides a computational link between value adaptation and general contextual processing in both decision making and perception. Originally proposed to explain nonlinear neural responses in primary visual cortex, the normalization computation has been identified in multiple species, sensory modalities, and levels of processing (21). This ubiquity suggests that normalization may serve as a canonical form of computation employed by neural systems. In sensory processing, normalization can explain both temporal (e.g., retinal light adaptation) and spatial (e.g., surround suppression) forms of contextual modulation. Recent studies show that normalization extends beyond early sensory processing to higher order cognitive functions, including attention, multisensory integration, and decision making (18, 19, 23, 51, 52). In decision making, normalization describes how value coding activity in the parietal cortex depends on the structure of the choice set, an example of spatial context dependence; notably, this relative value coding predicts context-dependent choice behavior that is also characterized by divisive normalization. Our current results show that value adaptation can also be explained by a history-dependent form of normalization, emphasizing the generality of this computation across temporal and spatial effects and sensory and decision-related processing.
These findings provide further evidence that valuation is a relative process, relying on canonical comparative mechanisms rather than static evaluation to determine the subjective worth of potential outcomes. Although context-dependent preferences are largely framed in terms of spatial context, value adaptation suggests that temporal context may play a similarly important role in shaping the decision process. In traditional rational choice frameworks, such context dependence is often viewed as suboptimal deviations from normative choice behavior. However, adaptation is thought to be crucial for the efficient representation of time-varying information, raising the possibility that value adaptation confers distinct benefits to a decision maker in a dynamic world.
Materials and Methods
Participants.
Forty-three adults (24 female, ages 18–45 y) participated in the experiment after giving informed consent. All procedures involving human subjects were approved by the University Committee on Activities Involving Human Subjects of New York University.
Experimental Task.
The experimental task comprised three Test blocks separated by two intervening Adapt blocks. Each Test block comprised 90 bid trials, in which subjects reported their maximum willingness to pay for individual snack food items. In each bid trial, an image of an individual item was presented on a computer screen above a slider bar. The dollar amount represented by the current slider bar position was indicated above the bar ($0–$6, in $0.01 increments). Subjects adjusted the cursor to their intended bid amount and confirmed their bid with a single mouse click. The slider bar was reset to empty at the beginning of each trial. Each of 30 items was presented three times each, in randomized order. Before beginning the task, subjects were endowed with $6 for use in these auctions. Realization of a randomly selected bid trial at the end of the session was conducted as a Becker–Degroot–Marschak auction, a procedure designed to elicit an individual’s exact subjective valuation for an item (37).
Following the initial Test block, items were ranked by their mean bids and the 10 lowest-valued (Lo value) and 10 highest-valued (Hi value) items were identified for use in Adapt blocks. Each Adapt block comprised 300 rating trials, in which subjects viewed a picture of a snack food item and reported the pleasantness of the item via a visual analog scale (in 9/43 subjects, Adapt blocks were 260 trials; no qualitative differences were observed and data from all subjects were combined for the main analyses). Subjects made ratings by adjusting the cursor position on the slider bar with a computer mouse and performing a mouse click; happy and sad face icons on either end of the bar indicated positive and negative valence. Icon positions were flipped on every other trial to avoid potential motor adaptation effects. Each Adapt block comprised 30 trials of each of the 10 items in one of the extreme value categories (Lo value or Hi value), presented in randomized order. Immediately after completion of each adaptation block, subjects repeated the bidding procedure on all 30 items three times each in a subsequent Test block. The presentation order of low- and high-adaptation blocks was randomized across subjects.
Bid Data Analysis.
To quantify the effects of adaptation with nonstationary valuations, we examined how individual postadaptation bids for specific items changed relative to previous bids for that item. The bid deviation corresponding to a bid for item i at time t was calculated as
where is the bid in question and is the average bid for item i in the immediately preceding Test block. Bid deviations were determined for each bid in the two postadaptation Adapt blocks. To examine the dynamics of bid deviations across Test blocks, bid deviations were averaged across all subjects in 15 trial windows and fit with an exponential decay function:
where the parameters B0 and λ represent the initial value and decay constant, respectively. Separate exponential decay functions were fit to post-Lo value and post-Hi value Test block data. Significance of exponential function parameters was determined by bootstrap resampling (10,000 iterations).
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health NIH Award R01MH104251.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715293114/-/DCSupplemental.
References
- 1.Schwartz O, Hsu A, Dayan P. Space and time in visual context. Nat Rev Neurosci. 2007;8:522–535. doi: 10.1038/nrn2155. [DOI] [PubMed] [Google Scholar]
- 2.Phillips WA, Clark A, Silverstein SM. On the functions, mechanisms, and malfunctions of intracortical contextual modulation. Neurosci Biobehav Rev. 2015;52:1–20. doi: 10.1016/j.neubiorev.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Rao RP, Ballard DH. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- 4.Lochmann T, Ernst UA, Denève S. Perceptual inference predicts contextual modulations of sensory responses. J Neurosci. 2012;32:4179–4195. doi: 10.1523/JNEUROSCI.0817-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nat Neurosci. 2001;4:819–825. doi: 10.1038/90526. [DOI] [PubMed] [Google Scholar]
- 6.Vinje WE, Gallant JL. Natural stimulation of the nonclassical receptive field increases information transmission efficiency in V1. J Neurosci. 2002;22:2904–2915. doi: 10.1523/JNEUROSCI.22-07-02904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephens DW, Krebs JR. Foraging Theory. Princeton Univ Press; Princeton, NJ: 1986. [Google Scholar]
- 8.Von Neumann J, Morgenstern O. Theory of Games and Economic Behavior. Princeton Univ Press; Princeton, NJ: 1944. [Google Scholar]
- 9.Louie K, Glimcher PW. Efficient coding and the neural representation of value. Ann N Y Acad Sci. 2012;1251:13–32. doi: 10.1111/j.1749-6632.2012.06496.x. [DOI] [PubMed] [Google Scholar]
- 10.Rangel A, Clithero JA. Value normalization in decision making: Theory and evidence. Curr Opin Neurobiol. 2012;22:970–981. doi: 10.1016/j.conb.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tymula A, Plassmann H. Context-dependency in valuation. Curr Opin Neurobiol. 2016;40:59–65. doi: 10.1016/j.conb.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Seriès P, Lorenceau J, Frégnac Y. The “silent” surround of V1 receptive fields: Theory and experiments. J Physiol Paris. 2003;97:453–474. doi: 10.1016/j.jphysparis.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Huber J, Payne JW, Puto C. Adding asymmetrically dominated alternatives: Violations of regularity and the similarity hypothesis. J Consum Res. 1982;9:90–98. [Google Scholar]
- 14.Tversky A, Simonson I. Context-dependent preferences. Manage Sci. 1993;39:1179–1189. [Google Scholar]
- 15.Shafir S, Waite TA, Smith BH. Context-dependent violations of rational choice in honeybees (Apis mellifera) and gray jays (Perisoreus canadensis) Behav Ecol Sociobiol. 2002;51:180–187. [Google Scholar]
- 16.Bateson M, Healy SD, Hurly TA. Context-dependent foraging decisions in rufous hummingbirds. Proc Biol Sci. 2003;270:1271–1276. doi: 10.1098/rspb.2003.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart N, Chater N, Stott HP, Reimers S. Prospect relativity: How choice options influence decision under risk. J Exp Psychol Gen. 2003;132:23–46. doi: 10.1037/0096-3445.132.1.23. [DOI] [PubMed] [Google Scholar]
- 18.Louie K, Grattan LE, Glimcher PW. Reward value-based gain control: Divisive normalization in parietal cortex. J Neurosci. 2011;31:10627–10639. doi: 10.1523/JNEUROSCI.1237-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie K, Khaw MW, Glimcher PW. Normalization is a general neural mechanism for context-dependent decision making. Proc Natl Acad Sci USA. 2013;110:6139–6144. doi: 10.1073/pnas.1217854110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soltani A, De Martino B, Camerer C. A range-normalization model of context-dependent choice: A new model and evidence. PLOS Comput Biol. 2012;8:e1002607. doi: 10.1371/journal.pcbi.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2011;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci. 1992;9:181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- 23.Louie K, LoFaro T, Webb R, Glimcher PW. Dynamic divisive normalization predicts time-varying value coding in decision-related circuits. J Neurosci. 2014;34:16046–16057. doi: 10.1523/JNEUROSCI.2851-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn A. Visual adaptation: Physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- 25.Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol. 2007;17:423–429. doi: 10.1016/j.conb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huk AC, Ress D, Heeger DJ. Neuronal basis of the motion aftereffect reconsidered. Neuron. 2001;32:161–172. doi: 10.1016/s0896-6273(01)00452-4. [DOI] [PubMed] [Google Scholar]
- 27.Simonson I, Tversky A. Choice in context: Tradeoff contrast and extremeness aversion. J Mark Res. 1992;29:281–295. [Google Scholar]
- 28.Waite TA. Background context and decision making in hoarding gray jays. Behav Ecol. 2001;12:318–324. [Google Scholar]
- 29.Kahneman D, Tversky A. Prospect theory: Analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- 30.Koszegi B, Rabin M. A model of reference-dependent preferences. Q J Econ. 2006;121:1133–1165. [Google Scholar]
- 31.Koszegi B, Rabin M. Reference-dependent risk attitudes. Am Econ Rev. 2007;97:1047–1073. [Google Scholar]
- 32.Xiang T, Lohrenz T, Montague PR. Computational substrates of norms and their violations during social exchange. J Neurosci. 2013;33:1099–1108. doi: 10.1523/JNEUROSCI.1642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty CF. Incentive contrast: A review of behavioral changes following shifts in reward. Anim Learn Behav. 1982;10:409–440. [Google Scholar]
- 34.Padoa-Schioppa C. Range-adapting representation of economic value in the orbitofrontal cortex. J Neurosci. 2009;29:14004–14014. doi: 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi S, Pinto de Carvalho O, Schultz W. Adaptation of reward sensitivity in orbitofrontal neurons. J Neurosci. 2010;30:534–544. doi: 10.1523/JNEUROSCI.4009-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox KM, Kable JW. BOLD subjective value signals exhibit robust range adaptation. J Neurosci. 2014;34:16533–16543. doi: 10.1523/JNEUROSCI.3927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker GM, DeGroot MH, Marschak J. Measuring utility by a single-response sequential method. Behav Sci. 1964;9:226–232. doi: 10.1002/bs.3830090304. [DOI] [PubMed] [Google Scholar]
- 38.Wainwright MJ, Schwartz O, Simoncelli EP. Natural image statistics and divisive normalization: Modeling nonlinearity and adaptation in cortical neurons. In: Rao RPN, Olshausen BA, Lewicki MS, editors. Probabilistic Models of the Brain: Perception and Neural Function. MIT Press; Cambridge, MA: 2002. pp. 203–222. [Google Scholar]
- 39.Wissig SC, Kohn A. The influence of surround suppression on adaptation effects in primary visual cortex. J Neurophysiol. 2012;107:3370–3384. doi: 10.1152/jn.00739.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaliukhovich DA, Vogels R. Divisive normalization predicts adaptation-induced response changes in macaque inferior temporal cortex. J Neurosci. 2016;36:6116–6128. doi: 10.1523/JNEUROSCI.2011-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LoFaro T, Louie K, Webb R, Glimcher P. The temporal dynamics of cortical normalization models of decision-making. Lett Biomath. 2014;1:209–220. [Google Scholar]
- 42.Crespi LP. Quantitative variation of incentive and performance in the white rat. Am J Psychol. 1942;55:467–517. [Google Scholar]
- 43.Zeaman D. Response latency as a function of the amount of reinforcement. J Exp Psychol. 1949;39:466–483. doi: 10.1037/h0060477. [DOI] [PubMed] [Google Scholar]
- 44.Couvillon PA, Bitterman ME. The overlearning-extinction effect and successive negative contrast in honeybees (Apis mellifera) J Comp Psychol. 1984;98:100–109. [PubMed] [Google Scholar]
- 45.Freidin E, Cuello MI, Kacelnik A. Successive negative contrast in a bird: Starlings’ behaviour after unpredictable negative changes in food quality. Anim Behav. 2009;77:857–865. [Google Scholar]
- 46.Kobre KR, Lipsitt LP. A negative contrast effect in newborns. J Exp Child Psychol. 1972;14:81–91. doi: 10.1016/0022-0965(72)90033-1. [DOI] [PubMed] [Google Scholar]
- 47.Rigoli F, Rutledge RB, Dayan P, Dolan RJ. The influence of contextual reward statistics on risk preference. Neuroimage. 2016;128:74–84. doi: 10.1016/j.neuroimage.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rigoli F, Friston KJ, Dolan RJ. Neural processes mediating contextual influences on human choice behaviour. Nat Commun. 2016;7:12416. doi: 10.1038/ncomms12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonsohn U. New Yorkers commute more everywhere: Contrast effects in the field. Rev Econ Stat. 2006;88:1–9. [Google Scholar]
- 50.Simonsohn U, Loewenstein G. Mistake #37: The effect of previously encountered prices on current housing demand. Econ J (Lond) 2006;116:175–199. [Google Scholar]
- 51.Ohshiro T, Angelaki DE, DeAngelis GC. A normalization model of multisensory integration. Nat Neurosci. 2011;14:775–782. doi: 10.1038/nn.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.