The studies by Vidaurre et al. (1) and Vatansever et al. (2) in PNAS provide contrasting, yet complementary insights into the role that regions of transmodal cortex, including those in the default mode network (DMN) (3) and the fronto-parietal network (FPN) (4), play in cognition.
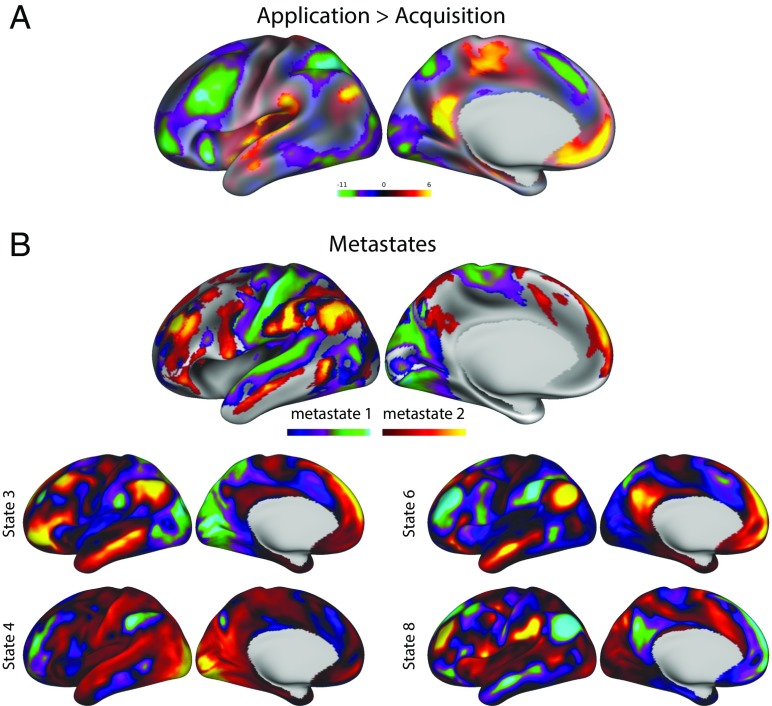
Vatansever et al. (2) used task-based fMRI to illustrate how the DMN and FPN work collectively to guide complex behavior. In their study, participants performed the Wisconsin Card Sorting Task (WCST) (5) while brain activity was measured using fMRI. In this task, participants sort shapes based on features (color, shape, or number). The feature is not revealed to the participant at the outset of a block of trials, and must instead be learned based on feedback presented after each trial. The feedback is used to identify the current feature rule, which is then applied on subsequent trials. Periodically, the rule changes and feedback is used to update the current goal representation. Vatansever et al. (2) demonstrate that the FPN is active after a rule change—the “acquisition phase”—suggesting its involvement in encoding the contingencies upon which the sorting decision is based. In later sections of the block—the “application phase”—when the individual understands the rule, activity within the FPN is reduced, and activity within the DMN increases (Fig. 1A). Therefore, the activity within the DMN corresponds to periods when contingencies determining cognitive decisions are established. Additionally, and importantly, Vatansever et al. (2) show that patterns of increased DMN connectivity are also linked to better response latency during correct trials. Together, these results corroborate recent studies showing that the DMN supports external task processing when behavior depends on preexisting representations guiding cognition (6–9).
Fig. 1.
Brain states demonstrating the active role of the DMN and FPN in cognition. (A) Contrast of application versus acquisition phases during performance of the WCST (2). (B) Metastates described using a HMM applied to resting-state fMRI data (1), as well as two example states from metastate 1 (Bottom Left) and metastate 2 (Bottom Right).
Using resting-state fMRI data from the Human Connectome Project (10), Vidaurre et al. (1) apply a hidden Markov model (HMM) (11) that exploits the spatiotemporal patterns in activity to infer a number of different brain states. Vidaurre et al. (1) show that the time spent in each state, known as “fractional occupancy,” remains consistent across different scanning sessions and is heritable. The ability to use HMMs to identify states based on intrinsic dynamics captured by fMRI constitutes an advance in our ability to characterize the nature of ongoing brain activity, providing an intuitive method for understanding how neurocognitive processing can be understood as a succession of neurocognitive states (12, 13).
One observation emerging from the analysis of Vidaurre et al. (1) is that specific states are hierarchically organized, forming temporal groupings referred to as “metastates.” These reflect a dissociation between states anchored by regions of the cortex concerned with constrained neural processing (such as the sensorimotor systems), from those anchored by transmodal regions of the cortex, hypothesized to serve more abstract functions (14, 15) (Fig. 1B). Vidaurre et al. (1) show that participants spending more time in the transmodal metastate perform better on measures of intelligence, executive control, and processing speed. Crucially, the metastates have a stronger association to behavior than the individual states, indicating that they are functionally relevant above and beyond their constituent elements.
Both studies provide accounts of the transmodal cortex anchored in both neural and psychological measurements (16), and thus constrain accounts of the role that transmodal cortex, and in particular the DMN, plays in cognition. Historically, the DMN was argued to be task-negative, supporting forms of cognition irrelevant to external goals (17), because its activity emerged in simple nondemanding tasks (3), as well as states, such as mind-wandering (18) and attentional lapses (19). However, the links with cognition revealed in these two studies are inconsistent with this account of the DMN.
Vidaurre et al. (1) show that spending time in states anchored in transmodal cortex, including those dominated by the DMN (see state 6 in Fig. 1B), predict better executive control and higher intelligence (Fig. 1). Thus, even though brain activity analyzed by Vidaurre et al. (1) takes place at rest, the functional implications of their analysis extend to forms of cognition measured in the context of a task (such as intelligence). A similar conclusion emerges from the study by Vatansever et al. (2). The progression to a state of rule-based behavior in the WCST corresponds to a shift in brain activity from an initial focus in fronto-parietal regions to those in the DMN (Fig. 1A). Critically, participants performed the task better in periods when the DMN was more active, and connectivity within this network supported better task performance. Together, both studies highlight the need to move beyond a task-negative account of the DMN in cognition.
Both studies are broadly consistent with an overarching view of the DMN as important when cognition is guided by representations from memory (20). Vatansever et al. (2) found that the DMN is active after participants have acquired the rule in the WCST, corresponding to periods when memory input is most relevant for task performance. Although determining the specific functional processes of the metastates identified by Vidaurre et al. (1) is challenging since they occur at rest, we do note recent studies have linked patterns of integration at rest within both the DMN and FPN as important for different types of spontaneous thoughts (21–25). It is possible, therefore, that the fractional occupancy of the transmodal metastate may reflect types of spontaneous thought that derive their content from memory (26). The hypothesis that the DMN is relevant when cognition requires information from memory can account for many conditions that activate this system. For example, the DMN is active during periods of future thinking (27), semantic decisions that depend on strong conceptual associations (28), mind-wandering (29), moral reasoning (30) and, perhaps most tellingly, during spatial or numerical decisions made based on memory rather than perceptual input (6, 8). These all reflect situations when cognitive operations cannot flourish based on environmental input alone, and suggest the DMN may reflect the process through which existing representations in memory guide cognition (8). The ability to guide cognition using preexisting representations allows more complex cognition to emerge in an efficient manner, explaining why the DMN is linked to complex forms of cognition that can seem effortless and automatic.
Finally, these studies highlight the importance of time in understanding the functions of transmodal cortex. The analysis by Vatansever et al. (2) demonstrates that transitions between the acquisition and application phases of the WCST are instantiated by a transition from higher FPN activity to a state of higher DMN activity. Since the information encoded during acquisition is used to guide behavior in the application phase, these opposing states reflect cognitive operations that are nested within the broader goal of performing the task. In the analysis by Vidaurre et al. (1), a similar
The studies by Vidaurre et al. and Vatansever et al. in PNAS provide contrasting, yet complementary insights into the role that regions of the transmodal cortex, including those in the default mode network (DMN) and the fronto-parietal network (FPN), play in cognition.
hierarchical pattern emerges, although at a much coarser level. Within the transmodal metastate, states 6 and 8 (Fig. 1B) correspond to patterns of opposition between the DMN and other networks within transmodal cortex. The authors also make complimentary predictions for behavior, suggesting that even though these states may be anticorrelated at specific moments in time, they could be linked to overarching cognitive states or processes. Thus, a common theme emerging from both studies (1, 2) is that shifting patterns of dominance between the FPN and DMN may reflect the organization of cognition across a broad time frame. Perhaps the most important implication of this temporal perspective is that it allows the anticorrelation between the DMN and FPN to be reevaluated. Although both Vidaurre et al. (1) and Vatansever et al. (2) capture the anticorrelated nature of these two networks in their analyses, their results are consistent with the possibility that patterns of dominance between these two networks may reflect aspects of cognition that, at least on certain occasions, can collaborate in the service of a temporally extended goal. Future research, motivated by the methodological and conceptual advances made in these two studies, may help to further understand how the interactions between these large-scale networks of transmodal cortex contribute to cognition that extends over time.
Supplementary Material
Acknowledgments
J.S. was supported by the European Research Council (WANDERINGMINDS 646927).
Footnotes
References
- 1.Vidaurre D, Smith SM, Woolrich MW. Brain network dynamics are hierarchically organized in time. Proc Natl Acad Sci USA. 2017;114:12827–12832. doi: 10.1073/pnas.1705120114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vatansever D, Menon DK, Stamatakis EA. Default mode contributions to automated information processing. Proc Natl Acad Sci USA. 2017;114:12821–12826. doi: 10.1073/pnas.1710521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole MW, et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milner B. Effects of different brain lesions on card sorting: The role of the frontal lobes. Arch Neurol. 1963;9:90–100. [Google Scholar]
- 6.Smallwood J, et al. Escaping the here and now: Evidence for a role of the default mode network in perceptually decoupled thought. Neuroimage. 2013;69:120–125. doi: 10.1016/j.neuroimage.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Spreng RN, et al. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 2014;34:14108–14114. doi: 10.1523/JNEUROSCI.2815-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konishi M, McLaren DG, Engen H, Smallwood J. Shaped by the past: The default mode network supports cognition that is independent of immediate perceptual input. PLoS One. 2015;10:e0132209. doi: 10.1371/journal.pone.0132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA. Default mode network connectivity during task execution. Neuroimage. 2015;122:96–104. doi: 10.1016/j.neuroimage.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Van Essen DC, et al. WU-Minn HCP Consortium The WU-Minn human connectome project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabiner LR. A tutorial on hidden Markov models and selected applications in speech recognition. Proc IEEE. 1989;77:257–286. [Google Scholar]
- 12.Smallwood J. Distinguishing how from why the mind wanders: A process-occurrence framework for self-generated mental activity. Psychol Bull. 2013;139:519–535. doi: 10.1037/a0030010. [DOI] [PubMed] [Google Scholar]
- 13.Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: A dynamic framework. Nat Rev Neurosci. 2016;17:718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam M-M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 15.Buckner RL, Krienen FM. The evolution of distributed association networks in the human brain. Trends Cogn Sci. 2013;17:648–665. doi: 10.1016/j.tics.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D. Neuroscience needs behavior: Correcting a reductionist bias. Neuron. 2017;93:480–490. doi: 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 20.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smallwood J, et al. Representing representation: Integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS One. 2016;11:e0152272. doi: 10.1371/journal.pone.0152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vatansever D, et al. Varieties of semantic cognition revealed through simultaneous decomposition of intrinsic brain connectivity and behaviour. Neuroimage. 2017;158:1–11. doi: 10.1016/j.neuroimage.2017.06.067. [DOI] [PubMed] [Google Scholar]
- 23.Golchert J, et al. Individual variation in intentionality in the mind-wandering state is reflected in the integration of the default-mode, fronto-parietal, and limbic networks. Neuroimage. 2017;146:226–235. doi: 10.1016/j.neuroimage.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Mooneyham BW, et al. States of mind: Characterizing the neural bases of focus and mind-wandering through dynamic functional connectivity. J Cogn Neurosci. 2017;29:495–506. doi: 10.1162/jocn_a_01066. [DOI] [PubMed] [Google Scholar]
- 25.Raij TT, Riekki TJJ. Dorsomedial prefontal cortex supports spontaneous thinking per se. Hum Brain Mapp. 2017;38:3277–3288. doi: 10.1002/hbm.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poerio GL, et al. The role of the default mode network in component processes underlying the wandering mind. Soc Cogn Affect Neurosci. 2017;12:1047–1062. doi: 10.1093/scan/nsx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schacter DL, et al. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davey J, et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. Neuroimage. 2016;137:165–177. doi: 10.1016/j.neuroimage.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiong W, et al. The salience network causally influences default mode network activity during moral reasoning. Brain. 2013;136:1929–1941. doi: 10.1093/brain/awt066. [DOI] [PMC free article] [PubMed] [Google Scholar]