Abstract
In recent years, postponement of marriage and childbearing in women of reproductive age has led to an increase in the incidence of age‐related infertility. The reproductive aging process in women is assumed to occur due to a decrease in both the quantity and quality of the oocytes, with the ultimate result being a decline in fecundity. This age‐related decline in fecundity is strongly dependent on oocyte quality, which is critical for fertilization and subsequent embryo development. Aged oocytes display increased chromosomal abnormality and dysfunction of cellular organelles, both of which factor into oocyte quality. In particular, mitochondrial dysfunction has been suggested as a major contributor to the reduction in oocyte quality as well as to chromosomal abnormalities in aged oocytes and embryos. Participation of oxidative stress in the oocyte aging process has been proposed because oxidative stress has the capacity to induce mitochondrial dysfunction and directly damage many intracellular components of the oocytes such as lipids, protein, and DNA. In an attempt to improve mitochondrial function in aged oocytes, several therapeutic strategies have been investigated using both animal models and assisted reproductive technology. Here, we review the biological mechanisms and present status of therapeutic strategies in the female reproductive aging field and indicate possible future therapeutic strategies.
Keywords: Endoplasmic reticulum, Mitochondria, Oocyte aging, Oxidative stress, Reproductive aging
Introduction
It is widely known that aging reduces fecundity and reproductive capacity in women [1, 2, 3]. The incidence of aging‐related female infertility has increased in industrialized countries, including Japan [4, 5, 6]. Increasing women's higher education and social advancement are common in these countries. These changes in women's lifestyle result in changes in female marriage and reproductive behavior. In this situation, women tend to postpone marriage and childbirth. The mean age at which women marry has risen from 25.9 years in 1990 to 29.2 years in 2012. In addition, the mean age at which women deliver their first child has risen from 27.0 years in 1990 to 30.1 years in 2011 in Japan (Ministry of Health, Labour and Welfare, Japan. http://www.Mhlw.go.jp). Marriage at an older age has led to reduction in female fecundity. Surprisingly, although female fertility peaks at around 25 years of age, the decrease in female fecundity starts after 30 years of age [2, 7]. For example, women aged 35–39 years have 31 % lower fecundity than women aged 20–24 years [8].
Numerous studies involving assisted reproductive technology (ART) clarify that advanced maternal age has a remarkable negative impact on ART outcomes [9, 10, 11]. Despite recent progress in ART, the treatment outcomes of ART have not improved because of increases in the treatment cycles of advanced reproductive aged (generally, 35 years and older) women in Japan. Large‐scale data obtained from patient records in the Japanese national ART registry show an increase in the treatment cycles of patients aged ≥40 years. These data show that the percentage of treatment cycles administered to patients aged ≥40 years was 31.2, 32.1, 33.4, and 35.7 %, respectively, from 2007 to 2010 [5]. Decreases in pregnancy rate per embryo transfer and increases in miscarriage rate were significantly correlated with maternal age and resulted in very low live birth rates in advanced‐age women. A similar trend showing that the percentage of live births decreased progressively with maternal age has been reported in the United States [12, 13]. The percentage of live births per treatment cycle was 41.5 % in women younger than 35 years, 31.9 % in women aged 35–37 years, 22.1 % in women aged 38–40 years, 12.4 % in women aged 41–42 years, 5 % in women aged 43 or 44 years, and 1 % for women aged >44 years [14]. In contrast, as shown in Fig. 1, the aging‐related decline in ART results was negated in patients who used oocytes obtained from healthy young donors. Although the reproductive aging process is assumed to decrease both the quantity and quality of the oocytes held within the follicles present in the ovarian cortex (i.e., ovarian reserve) [15] (Fig. 2), these data strongly suggest that aging‐related decline in fecundity is strongly dependent on oocyte aging rather than a decrease in ovarian reservation and changes in both endocrine function and endometrial receptivity.
Figure 1.
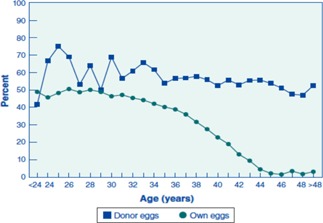
Percentage of transfers that resulted in live births for ART cycles using fresh embryos from the patient's own eggs and ART cycles using fresh embryos from donor eggs, according to the age of the women, in 2011. Reprinted with permission from the U.S. Centers for Disease Control and Prevention (http://www.cdc.gov/art/ART2011/PDFs/ART_2011_National_Summary_Report.pdf)
Figure 2.

The reproductive aging process is assumed to result in decreases in the quantity (solid line) and increases in poor‐quality oocytes held within the follicles present in the ovarian cortex. Modified with permission from Ref. [15]
It is well established that oocyte quality is responsible for fertilization and subsequent embryonic development potential [16, 17]. Although low oocyte quality is not clearly defined, an increased rate of chromosomal aberrations in oocytes [18, 19] and cellular organelle dysfunction [20, 21] are regarded as determinants of oocyte quality. Thus, the concept that aged oocytes represent low‐quality oocytes is widely accepted. Major implications of oocyte aging are low fertilization rate, poor embryonic development, increased likelihood of spontaneous miscarriage, and abnormalities in offspring [16, 22]. However, although the deterioration of oocyte quality along with oocyte aging is well established, the mechanisms of oocyte aging are not fully understood. Aneuploidy observed in aged oocytes can result in miscarriages and birth defects. A recent study in mice shows that aging‐related aneuploidy is caused by the deterioration of chromosomal cohesion that occurs during the extended prophase I (meiosis I) arrest [19]. In addition, the dysfunction of cellular organelles, especially the endoplasmic reticulum (ER) and mitochondria, is essential for the deterioration of oocyte quality observed in aged oocytes.
In the present review, we have summarized (i) aging‐related changes in ER and mitochondrial function that are responsible for oocyte quality, (ii) the contribution of oxidative stress to oocyte aging, and (iii) current and future therapeutic strategies for countering oocyte aging.
Biological mechanisms of oocyte aging
Effects of oocyte aging on the ER
At fertilization, the sperm evokes drastic changes in intracellular Ca2+ ([Ca2+]i) in mammalian oocytes: a single, relatively long‐lasting (~5 min) rise in [Ca2+]i is seen, followed by short repetitive transients of [Ca2+]i lasting several hours until pronucleus (PN) formation [23, 24]. These changes in [Ca2+]i at fertilization are called Ca2+ oscillations. The Ca2+ oscillations appear to be necessary for early events following fertilization (e.g., resumption of cell cycle, cortical granule exocytosis, recruitment of maternal mRNAs) [24, 25]. In addition, several studies have shown that the oscillatory pattern of [Ca2+]i is essential for normal embryonic development [26, 27]. For example, manipulation of [Ca2+]i by using electroporation to induce parthenogenic activation of oocytes shows that the amplitude, number, and frequency of transient Ca2+ fluctuations influence oocyte activation, developmental performance, morphology [26], and gene expression [27]. Overexpression of egg phospholipase C (PLC) β1 by microinjection of a Plcb1 cRNA significantly perturbed the duration and frequency of Ca2+ transients and disrupted the characteristic shape of the first transient at fertilization [28]. These changes in Ca2+ oscillations induce significant delays in PN formation [28]. Taken together, these findings strongly suggest that Ca2+ oscillations following fertilization are an important factor that determines normal development of embryos.
The effect of maternal aging on Ca2+ oscillations has not been fully studied because of the lack of an appropriate maternal‐aging animal model. It is known that maternal‐aged oocytes and postovulatory‐aged oocytes show common phenomena [22], such as low fertilization rates, poor embryonic development, chromosomal abnormalities, and increased miscarriage rates. Thus, instead of maternal‐aged oocytes, postovulatory‐aged oocytes that do not fertilize after ovulation for a prolonged time in the oviduct are often used as models of poor‐quality oocytes to investigate mechanisms of oocyte aging [29, 30]. However, it should be noted that the oocyte of postovulatory aging is clearly distinguished from that of maternal aging that exists in the ovaries of females who show a progressive decline in fecundity towards menopause. We have demonstrated that postovulatory‐aged mouse oocytes (insemination at 20 h after hCG injection) show significant alterations in the pattern of Ca2+ oscillations at fertilization [31]. Compared with freshly ovulated oocytes (insemination at 14 h after hCG injection), aged oocytes show significantly higher frequencies and lower amplitudes of individual Ca2+ elevations. Furthermore, during exposure to high extracellular [Ca2+]o, aged oocytes show sustained elevation of [Ca2+]i and disruption of normal patterns of Ca2+ oscillations. Unique experiments using the chelator Nitr‐5, which can load Ca2+ into the cytoplasm, show that 80 % of intracellular Ca2+ is restored into the ER, and that this restoration is significantly reduced in aged oocytes. We have also reported that the size of Ca2+ storage in the ER in aged oocytes is decreased. Therefore, these aging‐related changes in Ca2+ oscillations arise from alterations in Ca2+ handling in aged oocytes, particularly those in the ER. These data also indicate that aged oocytes may have decreased intracellular ATP because ER Ca2+ storage is maintained by smooth ER Ca2+‐ATPase (SERCA), the activity of which depends on intracellular ATP levels. On the other hand, inositol 1,4,5‐triphosphate (InsP3)‐induced Ca2+ release (IICR) from ER Ca2+ channels via the introduction of sperm‐specific PLCζ is an important mechanism by which Ca2+ oscillations are generated and maintained. We [32] and another group [33] have reported that Ca2+ release from IICR‐sensitive Ca2+ stores is significantly decreased, and Ca2+ store size is significantly reduced, in aged oocytes. These data strongly suggest that aged oocytes have a diminished capacity for storing intracellular Ca2+ and regulating the normal pattern of Ca2+ oscillations. In addition, mRNA and protein levels of the apoptotic regulatory protein Bal‐2 show a prominent decrease in aged oocytes [21, 30, 34]. Under this condition, abnormal Ca2+ transients induce embryo fragmentation and cell death.
In summary, abnormal Ca2+ oscillations elicited by ER dysfunction observed in aged oocytes may adversely affect normal fertilization and subsequent embryonic development.
Effects of oocyte aging on mitochondria
In all cells, mitochondria play a central role in energy supply and apoptotic regulation. The main function of mitochondria is production of ATP (phosphorylation of ADP) by oxidative phosphorylation in the electron transfer system. Most of the energy required for various cellular activities is supplied by mitochondria directly or indirectly in the form of ATP. Many stressors, such as oxidative stress and DNA damage, induce apoptosis through anti‐apoptotic proteins, that is, the Bcl‐2 family proteins, and the apoptosis‐inducing protein p53. These proteins change the membrane potential of mitochondria, resulting in leakage of cytochrome c from the respiratory chain of mitochondria into the cytosol, and induce apoptosis. Therefore, the concept that mitochondrial function reflects oocyte quality is widely accepted [18, 20, 35, 36]. It has been suggested that mitochondrial dysfunction is a major factor in chromosomal anomalies during the first and second meiotic divisions in oocytes and mitosis in embryos and influences the developmental competence of oocytes and pre‐implantation embryos [18, 37, 38]. Many studies using animal models and humans have shown that oocyte aging induces loss of mitochondrial function. The fluorescent dye JC‐1 (5,5′,6,6′‐tetrachloro‐1,1′,3,3′‐tetraethylbenzimidazolylcarbocyanine iodide), which is used to analyze inner mitochondrial membrane potential, has provided reliable estimates of mitochondrial activity [39]. Data from experiments using JC‐1 in mouse and human oocytes have clarified the relationship between mitochondria activity and developmental competence for oocytes and embryos [39].
It is well known that aged oocytes have decreased intracellular ATP content in many mammalian species, including humans [40], rats, hamsters [41], pigs [42], and mice [41, 43]. Intracellular ATP content may be the most relevant factor in determining mitochondrial function, fertilization‐related events, and embryonic development. In studies on human embryogenesis, a close relationship between intracellular ATP content in pre‐embryos and the developmental potency of the early cleavage stage of embryos has been demonstrated [40]. Furthermore, the intracellular ATP content of cleavage‐stage human embryos (8–10 cells) might be related to development speed because embryos that developed more slowly tended to have a low intracellular ATP content [44]. Thus, higher ATP levels have been correlated with embryo quality and lead to an increase in successful pregnancies. In a post‐ovulatory aging model, we have demonstrated that Ca2+ oscillations at fertilization stimulate ATP production and read just intracellular ATP levels in fresh mouse oocytes and that aged oocytes fail to adequately read just their intracellular ATP levels [43]. Interestingly, not only sperm‐induced Ca2+ oscillations but also strontium‐ and thimerosal‐inducible Ca2+ oscillations stimulate intracellular ATP production. This phenomenon strongly suggests that Ca2+ elevation is essential for intracellular ATP production (probably via Ca2+‐sensitive dehydrogenases in mitochondria).
Oocytes have extremely large numbers of mitochondria and mitochondrial DNA (mtDNA) (approximately 2 × 105 copies/cell) compared with other somatic cells (several thousand copies/cell) [45]. mtDNA point mutations in human oocytes are associated with maternal age [46] and are correlated with IVF outcome [47, 48]. Similarly, unfertilized human oocytes from advanced‐age women are more likely to contain deleted mtDNA than oocytes from young women [49]. Additionally, in both the mouse and hamster, mtDNA numbers are significantly lower in old oocytes than in young oocytes [41]. These data strongly suggest that mutations, deletions, and the number of mtDNA are good indicators of mitochondrial dysfunction that causes oocyte aging. Importantly, mitochondrial function is closely associated with maintenance of genomic stability because ATP is necessary for motor protein activity during spindle formation and for regulation of enzymes involved in chromosome segregation [37]. Mitochondrial dysfunction is a major factor involved in predisposition to chromosomal nondisjunction during the first and second meiotic divisions in oocytes and mitotic errors in embryos [19]. The incidence of maternal age‐related trisomy (e.g., Down's syndrome) could be explained by mitochondrial dysfunction.
In summary, aging‐related mitochondrial dysfunction is mainly responsible for low‐quality oocytes characterized by reduced ATP levels and increases in chromosomal abnormalities. Poor developmental competence of embryos and increased miscarriage rates observed in advanced‐age women are attributable to mitochondrial dysfunction.
Contribution of oxidative stress to oocyte aging
The participation of oxidative stress in aging or aging‐related diseases is widely accepted. Mitochondria produce many reactive oxygen species (ROS) via oxidative phosphorylation during ATP production. When the ROS level exceeds the antioxidant defense capacity, such as that provided by superoxide dismutase (SOD), oxidative stress is induced. Oxidative stress damages many cellular components, including mitochondria, lipids, proteins, enzymes, and DNA, and is thought to be involved in aging and aging‐related diseases. In particular, mitochondria are the most important target of oxidative stress because they are pivotal in controlling cell survival and death. For example, disruption of mitochondrial functions induced by cumulative oxidative stress is associated with neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease [50].
In oocyte aging, oxidative stress may play a central role in deterioration of oocyte quality [22, 51]. In both post‐ovulatory‐aging and maternal‐aging models, aging reduces the developmental competency and fertilization rates of the oocytes and is closely related to elevated ROS levels [30, 52, 53]. In the post‐ovulatory‐aging model, aged mouse oocytes display significantly higher levels of lipid peroxidation in the oolemma than fresh mouse oocytes [52]. Moreover, exposure of fresh mouse oocytes to oxidative stress changes the pattern of Ca2+ oscillations to become more similar to those of aged oocytes, and leads to reduced fertilization rates and poor embryo development [52]. However, there is no direct evidence that shows participation of oxidative stress in the aging process of human oocytes. As indirect evidence, oxidative stress evaluated by the 8‐oxo‐2′‐deoxyguanosine content of granulosa cells obtained during ART cycles has shown a negative correlation with fertilization rate and embryo quality [54]. Aging‐related changes in the antioxidant defense system have also been reported. When the gene expression profiles of 5–6‐week‐old (young) mouse oocytes and 42–45‐week‐old (aged) mouse oocytes were compared, a group of genes that provide protection against oxidative stress were found to be downregulated in the aged oocytes [55]. A unique aging mouse model that induced repeated ovulation shows poor embryonic development and decreases in some antioxidant enzymes in the ovary. Expression of SOD1 and SOD2, but not SOD3 and catalase, decreased with repeated ovulation [56]. Although the development of zygotes to the morula stage was not affected, the rate of development to the blastocyst stage decreased with repeated ovulation. These reports strongly suggest that impairment of the antioxidant defense system is also important for the process of oocyte aging.
In summary, equilibration of the production and consumption of oxidants fails in aged oocytes, leading to the induction of oxidative stress. Mitochondrial dysfunction induced by oxidative stress may play a central role in the deterioration of quality seen in aged oocytes. Figure 3 shows a schematic summary of the molecular mechanisms of poor embryonic development in aged oocytes.
Figure 3.
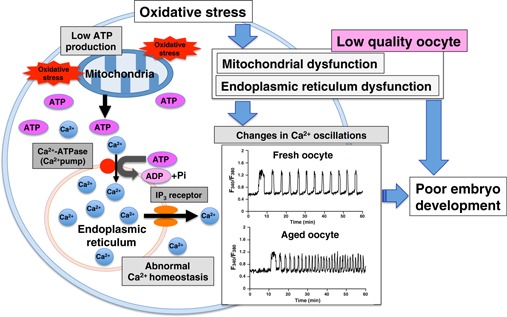
Schematic illustration of putative mechanisms of poor embryonic development observed in aged oocytes. Oocyte aging results in increased mitochondrial oxidative stress that induces mitochondrial dysfunction followed by low ATP production. A decrease in intracellular ATP levels reduces the capacity for Ca2+ restoration by Ca2+‐ATPase (Ca2+ pump) in the endoplasmic reticulum (ER) membrane. Simultaneously, decrease in Ca2+ release via the inositol 1,4,5‐triphosphate (InsP3) receptor on the ER and reduction in ER Ca2+ stores occur during oocyte aging. The abnormal Ca2+ homeostasis in aged oocytes induces changes in Ca2+ oscillation patterns characterized by both high frequency and low amplitude of Ca2+ transients. Low ATP levels involved in mitochondrial dysfunction could result in poor embryonic development. Furthermore, the abnormal pattern of Ca2+ oscillations may contribute to oocyte aging. These mechanisms have mainly been studied using post‐ovulatory‐age oocytes (from Refs. [31, 32, 43, 53])
Therapeutic strategies for oocyte aging
Infertility in advanced‐age patients that is caused by aging‐related deterioration of oocyte quality is intractable and effective therapies are currently lacking. Oxidative stress is thought to be greatly responsible for oocyte aging processes, including mitochondrial dysfunction and increases in chromosomal aberrations. Many different therapeutic strategies have been tried in animal models to improve oocyte quality, with particular emphasis on demonstrating the efficacy of antioxidants and improvement of mitochondrial function.
Supplementation with antioxidants
Antioxidants are thought to improve low‐quality oocytes by reducing oxidative stress. Recently, limited evidence has suggested that antioxidants improve fertility in animal models and ART cycles. In one mouse model, oral administration of vitamins C and E counteracted the aging‐related negative effects on ovarian reserve (number of ovarian oocytes and number of ovulated oocytes) and oocyte quality (chromosomal aberration in metaphase II oocytes and morphological apoptotic oocytes) [57]. It is important to note that oral administration of antioxidants to mice starting either at weaning (early onset administration) or at the age of 32 weeks (late‐onset administration) can counteract the aging‐related negative impact on ovarian reserve and oocyte quality. Despite these positive effects of oral antioxidant administration, caution is warranted in human applications. Human doses of vitamin C (104–198 g) and E (6–12 g) equivalent to those used in the mouse studies greatly exceed the maximum permissible doses for a 60‐kg woman.
Melatonin is secreted from the pineal gland and acts as a regulator of circadian rhythms in some biological functions. Melatonin also protects cells, including oocytes, from oxidative stress by acting as a free radical scavenger and by stimulating antioxidant enzymes [58]. Because the melatonin concentration in human preovulatory follicular fluid is threefold higher than that in serum [59], melatonin may improve oocyte quality and the reproductive outcome of ART. Therefore, melatonin supplementation during controlled ovarian hyperstimulation (COH) for women undergoing ART has been attempted [60, 61]. Among the patients who failed to become pregnant with a low fertilization rate (≤50 %) in the previous in vitro fertilization‐embryo transfer (IVF‐ET) cycle, melatonin supplementation (3 mg/day) markedly improved fertilization rates compared to those for previous IVF‐ET cycles and resulted in high pregnancy rates [60]. However, meta‐analysis of randomized controlled trials with melatonin supplementation during COH for women undergoing ART could not conclusively demonstrate beneficial clinical outcomes, including those related to the number of oocytes retrieved, clinical pregnancy rates, and miscarriage rates [62]. Melatonin supplementation may improve the clinical outcomes of ART only in a subset of infertile patients. Cochrane's systematic review also concluded that oral antioxidants, including combinations of N‐acetyl‐cysteine, melatonin, l‐arginine, vitamin E, myo‐inositol, vitamin C, vitamin D‐calcium, and omega‐3‐polyunsaturated fatty acids, are not associated with increased live birth rates (OR 1.25, 95 % CI 0.19–8.26, p = 0.82) or clinical pregnancy rates (OR 1.30, 95 % CI 0.92–1.85, p = 0.14) compared to those seen with placebo or no treatment [63].
Resveratrol is a polyphenolic compound found in red wine and yeast, and is an activator of mammalian orthologs of sirtuin 1 (SIRT1) in vitro, thereby mimicking some of the molecular and functional effects of dietary restriction [64, 65]. The protective effects of resveratrol against aging‐related diseases such as cardiovascular disease, ischemic injury, diabetes, and carcinogenesis are well established (reviewed in [66]). The molecular mechanisms of resveratrol's anti‐aging effects underlie its mitochondrial protective effects [65, 67], which have been attributed to the induction of the mitochondrial antioxidant system and activation of SIRT1 [68]. The human cell line MRC‐5 cultured in resveratrol‐containing medium for 2 weeks showed significant increases in Mn superoxide dismutase (MnSOD) [69]. In addition, MnSOD protein levels and citrate synthesis activity significantly increased after only 2 h of resveratrol treatment. Furthermore, in mouse oocytes, resveratrol can reverse the adverse effects of methylglyoxal (MG), a reactive dicarbonyl compound that induces oxidative stress [70]. MG leads to increases in DNA double‐strand breaks, excess reactive oxygen, aberrant mitochondrial distribution, and high levels of lipid peroxidation, and resveratrol can block these cytotoxic changes. SIRT1 is a deacetylase that is activated by a variety of stressors and targets transcriptional regulators, including the following: p53; nuclear factor‐kappa B (NF‐κB); heat‐shock factor 1 (HSF1); forkhead transcription factor (FOXO) 1, 3, and 4; and peroxisome proliferator‐activated receptor‐γ coactivator 1 (PGC‐1α) (reviewed in [71]). PGC‐1α acts as the “master regulator” of mitochondrial biogenesis [65, 72], thermogenesis, and gluconeogenesis [71]. Thus, SIRT1 plays key roles in the regulation of cell cycle, apoptosis, and metabolic processes and modulation of life span [71]. Recently, the protective effect of resveratrol against the reduction in fertility seen with reproductive aging has been investigated [73]. Resveratrol was administered with drinking water to young female mice for 6 or 12 months, and their fertility and ovarian function were compared with those of control mice [73]. The mice administered resveratrol for 12 months retained reproductive functions, exhibiting larger litter sizes and larger follicular oocyte pools. Furthermore, telomerase activity, telomerase length, and expression of age‐related genes in the ovaries of mice administered resveratrol for 12 months were similar to those of young mice. Administration of resveratrol for 12 months also improved oocyte quality, as evaluated by improvement in chromosomal alignment on metaphase plates and decreased meiotic spindle abnormalities. Taken together, these results suggest that resveratrol could improve aging‐related deterioration of ovarian function and oocyte quality by induction of the mitochondrial antioxidant system and activation of SIRT1.
At present, there is insufficient evidence to conclude that antioxidants improve aging‐related deterioration of oocyte quality and reproductive outcomes in advanced‐age patients. Resveratrol is a promising candidate therapeutic that should be tested in clinical trials to more fully characterize its effects on oocyte quality and reproductive benefits.
Calorie restriction
Nutrient‐sensing pathways play fundamental roles in the aging process. Calorie restriction (CR, typically ranging from 10 to 50 %) increases longevity in organisms as diverse as yeast and humans [74], and has been reported to prevent various aging‐related diseases, including metabolic diseases, neurodegenerative diseases, cardiovascular diseases, and cancer (reviewed in [71, 74]). The mechanisms by which CR increases longevity are becoming increasingly clear. In mammals, CR reduces the activity of various signal transduction pathways either directly or indirectly through reduced levels of insulin/insulin‐like growth factor‐1 (IGF‐1) and reduced activity of mammalian target of rapamycin (mTOR) [74]. Insulin/IGF‐1 and mTOR both control cell growth and energy intake, and upstream activation of these pathways accelerates aging. Another important pathway involves modulation of key metabolic sensors, the SIRTs [75], especially SIRT1, which is the most extensively studied member of the SIRT family [65, 71]. SIRT1 is closely coupled to AMP‐activated protein kinase (AMPK) in a mutually enforcing mechanism that adjusts cellular physiology in response to limited energy sources [76]. Downstream of SIRT1 and AMPK, the deacetylation of two transcriptional regulators, PGC‐1α [77] and FOXO [78], regulates mitochondrial function and stress resistance [79]. Taken together, these observations suggest that these mechanisms underlie the ability of CR to mediate increases in longevity.
Conventionally, CR has a negative impact on female fertility because CR sometimes induces secondary amenorrhea associated with body weight loss. However, current data from animal models indicate that moderate CR may have beneficial effects on female fecundity [80, 81, 82]. In an aged‐mouse‐model study, the effects of moderate CR on ovarian function and oocyte quality, including aneuploidy and mitochondrial function, were examined [80]. Twelve‐month‐old (aged) female mice maintained at 40 % CR from 3.5 to 11 months (CR‐aged mice) did not show aging‐related declines in ovulated oocyte numbers compared to ad libitum‐fed age‐matched controls. This result suggests that CR may prevent the decline in ovarian reserves normally associated with aging. Furthermore, oocytes obtained from CR‐aged mice did not show aging‐related increases in aneuploidy, chromosomal misalignment on metaphase plates, and meiotic spindle abnormalities. Similarly, mitochondrial dysfunction (abnormal mitochondrial aggregation, impaired ATP production) was not observed in these oocytes. Interestingly, PGC‐1α‐deficient aged mice as well as CR‐aged mice showed increases in ovulated oocyte numbers and decreases in both chromosomal aberrations and abnormal mitochondrial aggregation. These results suggest that (a) the nutrient‐sensing pathways participate in the oocyte aging process as well as other aging‐related diseases (b) moderate CR prevents mitochondrial dysfunction with aging, and (c) mitochondrial function may be important for maintaining normal chromosomal structure. In contrast, a high‐fat diet (HFD) has a negative impact on oocyte quality and embryonic development [83]. Female mice fed an HFD (36 % fat and 20 % protein) were compared with female mice fed a control diet (13 % fat and 25 % protein). Embryos from HFD mice demonstrated a significantly higher frequency of degradation and delayed developmental progression. Furthermore, fetuses and placentas from HFD mice were significantly smaller than those fed the control diet. In addition, oocyte quality in HFD mice significantly increased not only in terms of spindle and chromosome alignment defects but also in terms of mitochondrial abnormalities. Western‐blot analysis of germinal vesicle oocytes from control vs. HFD mice revealed increased expression of PGC‐1α and Drp‐1 (dynamin‐related protein 1, a large GTPase that mediates mitochondrial fission in mammalian cells). These data strongly suggest that an HFD, via nutrient‐sensing pathways, has adverse effects on oocyte quality and embryonic development.
In summary, modification of the nutrient‐sensing pathways induced by moderate CR may have beneficial effects on mitochondrial function and chromosomal aberration associated with oocyte aging. Although additional studies are necessary to clarify the downstream mechanisms that mediate these beneficial effects, modification of the nutrient‐sensing pathways induced by moderate CR is a potential therapeutic strategy for advanced‐age patients.
Mitochondrial transfer
From the standpoint that mitochondrial function determines oocyte quality and is responsible for embryonic development and reproductive outcome, trials involving a fertility procedure termed ooplasmic transfer were attempted in the late 1990s [84]. The basis of ooplasmic transfer is improvement of poor oocyte quality by ooplasm transplantation from donors, containing healthy mitochondria and other beneficial components, into recipients who had repeatedly failed to become pregnant following ART due to poor embryonic development. These attempts at ooplasmic transfer resulted in about 30 live births and the success rate of this procedure was reported as “remarkable” and “higher than expected”. However, although ooplasmic transfer may have been effective for these patients, who had repeatedly failed ART outcomes due to the poor quality of their own oocytes, the safety of this procedure was not fully confirmed. The same group that had attempted the ooplasmic transfer reported contamination from donor mtDNA in the healthy children who were born from the recipient oocytes of the ooplasmic transfer [85, 86]. Thus, ooplasmic transfer resulted in mitochondrial heteroplasmy, which is contamination from two different maternal mtDNAs, among the offspring. Although the long‐term effects of mitochondrial heteroplasmy on health are not fully understood, ethical and legal issues regarding ooplasmic transfer should be considered. The US. Food and Drug Administration views ooplasmic transfer as genetic manipulation of human germ cells and prohibited its use as an ART in 2001 [87]. Despite the issue of mitochondrial heteroplasmy, ooplasmic transfer could improve oocyte quality by enhancement of mitochondrial functions such as ATP production, and could result in improvements in reproductive outcomes.
To resolve mitochondrial heteroplasmy, the use of autologous mitochondria from somatic cells has been proposed instead of the use of donor ooplasm. Some reports have shown that microinjection of mitochondria derived from somatic cells into oocytes does indeed affect oocyte quality. Although oocytes from female FVB mice have inherently high rates of apoptosis in vitro, microinjection of a small number of mitochondria derived from somatic (granulosa) cells into these oocytes prevented apoptosis [88]. The same report showed that the incidence of a common mtDNA deletion in unfertilized human oocytes was higher than that in fertilized oocytes. These results suggest that transplantation of exogenous mitochondria may compensate for the mitochondrial function of poor‐quality oocytes and improve oocyte competency. Autologous mitochondrial transfer may become a promising new fertility treatment for patients who have repeatedly unsuccessful ART outcomes, especially for advanced‐age patients. In order to establish autologous mitochondrial transfer, autologous mitochondria appropriately suited for oocytes are required. Although the generation of human oogonial stem cells (OSCs), which are natural precursor of human oocytes, from ovaries of reproductive‐age women has been established [89], human OSCs may be a potential source of patient‐matched autologous mitochondria [90]. AUGMENT (Autologous Germline Mitochondrial Energy Transfer)‐IVF has been proposed to enhance mitochondrial function and improve human IVF outcomes [91]. Microinjection of mitochondria or cytoplasm from the patient's own OSCs into the same patient's oocytes at the time of intracytoplasmic sperm injection provides sufficient ATP for successful fertilization and embryonic development. The advantage of this method is that sufficient amounts of mitochondria with characteristics (e.g., bioenergetic potential) extremely similar to those of oocyte mitochondria can be obtained. The methodology of AUGMET could clear the ethical and biological issues surrounding ooplasmic transfer, but the safety and efficacy of AUGMENT need to be confirmed in animal models and preclinical studies before performing clinical trials.
In summary, autologous mitochondrial transfer may enhance mitochondrial function and boost the ATP production of aged oocytes, resulting in successful fertilization and embryonic development and hence better pregnancy outcomes. AUGMENT‐IVF may become a powerful ART for advanced‐age women in the relatively near future.
Conclusions
We have reviewed the mechanisms of oocyte aging underlying female reproductive aging and the proposed therapeutic strategies for this intractable aging‐related infertility (Table 1). Because advanced‐age women characteristically have deteriorated mitochondrial function and relative low ATP levels in their oocytes, improvement of mitochondrial function is a fundamental strategy for aging‐related infertility. We believe that combination of supplementation with antioxidants and mitochondrial nutrients such as resveratrol along with CR is currently the best available treatment option. In addition, autologous mitochondrial transfer represented by AUGMENT may provide a new strategy when its efficacy and safety are confirmed.
Table 1.
Effects of each therapy against oocyte aging
| Model | Mechanism | Effects | Refs. | |
|---|---|---|---|---|
| Vitamin C, E | Mouse | Antioxidant action | Increase in ovulated oocyte Improvement of oocyte quality | [57] |
| Melatonin | Human a | Antioxidant action | Improvement of fertilization rate in ART cycle Improvement of pregnancy rate in ART cycle | [60] |
| Resveratrol | Mouse | SIRT1 activation | Increase in follicular oocyte pool Improvement in chromosomal alignment on metaphase plates Decrease in meiotic spindle abnormalities | [73] |
| Calorie restriction | Mouse | Modification of insulin/IGF‐1, mTOR, and SIRT1 pathways | Increase in ovulated oocyte numbers Decrease in chromosomal aberrations Decrease in abnormal mitochondrial aggregation | [80] |
| Mitochondrial transfer b | Human | Supplementation of mitochondrial function | Improvement of fertilization rate in ART cycle Improvement of pregnancy rate in ART cycle | [91] |
aAmong the patients who failed to become pregnant with a low fertilization rate (≤50 %) in the previous ART cycle
bAUGMENT (Autologous Germline Mitochondrial Energy Transfer)‐IVF has been proposed, but the effect of this therapy has not been validated as yet
Conflict of interest
Hideki Igarashi, Toshifumi Takahasi, and Satoru Nagase declare that they have no conflict of interest.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study.
Animal rights
This article does not contain any studies with animal subjects performed by any of the authors.
References
- 1. Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update, 2002, 8, 141–154 10.1093/humupd/8.2.141 [DOI] [PubMed] [Google Scholar]
- 2. Broekmans FJ, Knauff EA, Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab, 2007, 18, 58–65 10.1016/j.tem.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 3. Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Steirteghem A, Cohen J, Crosignani PG, Devroey P et al. Fertility and ageing. Hum Reprod Update, 2005, 11, 261–276 10.1093/humupd/dmi006 [DOI] [PubMed] [Google Scholar]
- 4. Dougall K, Beyene Y, Nachtigall RD. Age shock: misperceptions of the impact of age on fertility before and after IVF in women who conceived after age 40. Hum Reprod, 2013, 28, 350–356 10.1093/humrep/des409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeshima K, Saito H, Nakaza A, Kuwahara A, Ishihara O, Irahara M, Hirahara H, Yoshimura Y, Sakumoto T. Efficacy, safety, and trends in assisted reproductive technology in Japan—analysis of four‐year data from the national registry system. J Assist Reprod Genet, 2014, 31, 477–484396946710.1007/s10815‐014‐0181‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, Mouzon J, Castilla JA, Erb K, Korsak V, Nyboe Andersen A European IVFMCftESoHR, Embryology . Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod, 2013, 28, 2318–2331 10.1093/humrep/det278 [DOI] [PubMed] [Google Scholar]
- 7. Noord‐Zaadstra BM, Looman CW, Alsbach H, Habbema JD, Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ, 1991, 302, 1361–1365167005510.1136/bmj.302.6789.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menken J, Trussell J, Larsen U Age and infertility. Science, 1986, 233, 1389–1394 [DOI] [PubMed] [Google Scholar]
- 9. Toner JP, Grainger DA, Frazier LM. Clinical outcomes among recipients of donated eggs: an analysis of the U.S. National Experience, 1996‐1998. Fertil Steril, 2002, 78, 1038–1045 10.1016/S0015‐0282(02)03371‐X [DOI] [PubMed] [Google Scholar]
- 10. Spandorfer SD, Bendikson K, Dragisic K, Schattman G, Davis OK, Rosenwaks Z. Outcome of in vitro fertilization in women 45 years and older who use autologous oocytes. Fertil Steril, 2007, 87, 74–76 10.1016/j.fertnstert.2006.05.081 [DOI] [PubMed] [Google Scholar]
- 11. Gleicher N, Weghofer A, Barad D. Too old for IVF: are we discriminating against older women?. J Assist Reprod Genet, 2007, 24, 639–644345499710.1007/s10815‐007‐9182‐1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sunderam S, Kissin DM, Crawford S, Anderson JE, Folger SG, Jamieson DJ, Barfield WD. Assisted reproductive technology surveillance—United States, 2010. MMWR Surveill Summ, 2013, 62, 1–24 [PubMed] [Google Scholar]
- 13. Sunderam S, Kissin DM, Flowers L, Anderson JE, Folger SG, Jamieson DJ, Barfield WD. Assisted reproductive technology surveillance—United States, 2009. MMWR Surveill Summ, 2012, 61, 1–23 [PubMed] [Google Scholar]
- 14. American College of . O, Gynecologists Committee on Gynecologic P, Practice C. Female age‐related fertility decline. Committee Opinion No. 589. Fertil Steril, 2014, 101, 633–634 10.1016/j.fertnstert.2013.12.032 [DOI] [PubMed] [Google Scholar]
- 15.Klinkert ER. Clinical significance and management of poor response in IVF. Thesis Utrecht University. 2005.
- 16. Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update, 2009, 15, 573–585 10.1093/humupd/dmp014 [DOI] [PubMed] [Google Scholar]
- 17. Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion, 2011, 11, 797–813 10.1016/j.mito.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 18. Eichenlaub‐Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online, 2004, 8, 45–58 10.1016/S1472‐6483(10)60497‐X [DOI] [PubMed] [Google Scholar]
- 19. Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell, 2012, 11, 1121–1124349112310.1111/j.1474‐9726.2012.00866.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet, 2011, 28, 773–783316968210.1007/s10815‐011‐9588‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eichenlaub‐Ritter U, Wieczorek M, Luke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion, 2011, 11, 783–796 10.1016/j.mito.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 22. Lord T, Aitken RJ. Oxidative stress and ageing of the post‐ovulatory oocyte. Reproduction, 2013, 146, R217–R227 10.1530/REP‐13‐0111 [DOI] [PubMed] [Google Scholar]
- 23. Miyazaki S. Thirty years of calcium signals at fertilization. Semin Cell Dev Biol, 2006, 17, 233–243 10.1016/j.semcdb.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 24. Miyazaki S. Intracellular calcium oscillations in mammalian eggs at fertilization. J Physiol, 2007, 584, 713–714227700510.1113/jphysiol.2007.144238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol, 2006, 17, 324–332 10.1016/j.semcdb.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 26. Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development, 2001, 128, 917–928 [DOI] [PubMed] [Google Scholar]
- 27. Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2 + oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol, 2006, 300, 534–544 10.1016/j.ydbio.2006.08.041 [DOI] [PubMed] [Google Scholar]
- 28. Igarashi H, Knott JG, Schultz RM, Williams CJ. Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev Biol, 2007, 312, 321–330217053310.1016/j.ydbio.2007.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi T, Igarashi H, Amita M, Hara S, Matsuo K, Kurachi H. Molecular mechanism of poor embryo development in postovulatory aged oocytes: mini review. J Obstet Gynaecol Res, 2013, 39, 1431–1439 [DOI] [PubMed] [Google Scholar]
- 30. Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction, 2002, 124, 745–754 10.1530/rep.0.1240745 [DOI] [PubMed] [Google Scholar]
- 31. Igarashi H, Takahashi E, Hiroi M, Doi K. Aging‐related changes in calcium oscillations in fertilized mouse oocytes. Mol Reprod Dev, 1997, 48, 383–390 10.1002/(SICI)1098‐2795(199711)48:3<383::AID‐MRD12>3.0.CO;2‐X [DOI] [PubMed] [Google Scholar]
- 32. Takahashi T, Saito H, Hiroi M, Doi K. Effects of aging on inositol 1,4,5‐triphosphate‐induced Ca(2+) release in unfertilized mouse oocytes. Mol Reprod Dev, 2000, 55, 299–306 10.1002/(SICI)1098‐2795(200003)55:3<299::AID‐MRD8>3.0.CO;2‐G [DOI] [PubMed] [Google Scholar]
- 33. Jones KT, Whittingham DG. A comparison of sperm‐ and IP3‐induced Ca2 + release in activated and aging mouse oocytes. Dev Biol, 1996, 178, 229–237 10.1006/dbio.1996.0214 [DOI] [PubMed] [Google Scholar]
- 34. Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod, 2002, 66, 1828–1837 10.1095/biolreprod66.6.1828 [DOI] [PubMed] [Google Scholar]
- 35. Blerkom J. Mitochondria in early mammalian development. Semin Cell Dev Biol, 2009, 20, 354–364 10.1016/j.semcdb.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 36. Eichenlaub‐Ritter U. Oocyte ageing and its cellular basis. Int J Dev Biol, 2012, 56, 841–852 10.1387/ijdb.120141ue [DOI] [PubMed] [Google Scholar]
- 37. Schon EA, Kim SH, Ferreira JC, Magalhaes P, Grace M, Warburton D, Gross SJ. Chromosomal non‐disjunction in human oocytes: is there a mitochondrial connection?. Hum Reprod, 2000, 15 (Suppl 2) 160–172 10.1093/humrep/15.suppl_2.160 [DOI] [PubMed] [Google Scholar]
- 38. Desler C, Munch‐Petersen B, Stevnsner T, Matsui S, Kulawiec M, Singh KK, Rasmussen LJ. Mitochondria as determinant of nucleotide pools and chromosomal stability. Mutat Res, 2007, 625, 112–124 10.1016/j.mrfmmm.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 39. Acton BM. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod, 2004, 10, 23–32 10.1093/molehr/gah004 [DOI] [PubMed] [Google Scholar]
- 40. Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in vitro fertilization and embryo transfer. Hum Reprod, 1995, 10, 415–424 [DOI] [PubMed] [Google Scholar]
- 41. Simsek‐Duran F, Li F, Ford W, Swanson RJ, Jones HW Jr, Castora FJ. Age‐associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE, 2013, 8, e64955366921510.1371/journal.pone.0064955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS ONE, 2014, 9, e94488399160510.1371/journal.pone.0094488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Igarashi H, Takahashi T, Takahashi E, Tezuka N, Nakahara K, Takahashi K, Kurachi H. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod, 2005, 72, 1256–1261 10.1095/biolreprod.104.034926 [DOI] [PubMed] [Google Scholar]
- 44. Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization. ATP content and competence. Hum Reprod, 2000, 15, 2621–2633 10.1093/humrep/15.12.2621 [DOI] [PubMed] [Google Scholar]
- 45. May‐Panloup P, Chretien MF, Malthiery Y, Reynier P. Mitochondrial DNA in the oocyte and the developing embryo. Curr Top Dev Biol, 2007, 77, 51–83 10.1016/S0070‐2153(06)77003‐X [DOI] [PubMed] [Google Scholar]
- 46. Barritt JA, Cohen J, Brenner CA. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod Biomed Online, 2000, 1, 96–100 10.1016/S1472‐6483(10)61946‐3 [DOI] [PubMed] [Google Scholar]
- 47. Yesodi V, Yaron Y, Lessing JB, Amit A, Ben‐Yosef D. The mitochondrial DNA mutation (deltamtDNA5286) in human oocytes: correlation with age and IVF outcome. J Assist Reprod Genet, 2002, 19, 60–66346822910.1023/A:1014439529813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reynier P, May‐Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, Barriere P, Malthiery Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod, 2001, 7, 425–429 10.1093/molehr/7.5.425 [DOI] [PubMed] [Google Scholar]
- 49. Keefe DL, Niven‐Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril, 1995, 64, 577–583 [PubMed] [Google Scholar]
- 50. Ramalingam M, Kim SJ. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J Neural Transm, 2012, 119, 891–910 10.1007/s00702‐011‐0758‐7 [DOI] [PubMed] [Google Scholar]
- 51. Tarin JJ. Potential effects of age‐associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod, 1996, 2, 717–724 10.1093/molehr/2.10.717 [DOI] [PubMed] [Google Scholar]
- 52. Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev, 2003, 66, 143–152 10.1002/mrd.10341 [DOI] [PubMed] [Google Scholar]
- 53. Thouas GA, Trounson AO, Jones GM. Effect of female age on mouse oocyte developmental competence following mitochondrial injury. Biol Reprod, 2005, 73, 366–373 10.1095/biolreprod.105.040956 [DOI] [PubMed] [Google Scholar]
- 54. Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight‐hydroxy‐2′‐deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization‐embryo transfer program. Fertil Steril, 2002, 77, 1184–1190 10.1016/S0015‐0282(02)03103‐5 [DOI] [PubMed] [Google Scholar]
- 55. Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age‐associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet, 2004, 13, 2263–2278 10.1093/hmg/ddh241 [DOI] [PubMed] [Google Scholar]
- 56. Miyamoto K, Sato EF, Kasahara E, Jikumaru M, Hiramoto K, Tabata H, Katsuragi M, Odo S, Utsumi K, Inoue M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic Biol Med, 2010, 49, 674–681 10.1016/j.freeradbiomed.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 57. Tarin JJ, Perez‐Albala S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev, 2002, 61, 385–397 10.1002/mrd.10041 [DOI] [PubMed] [Google Scholar]
- 58. Carlomagno G, Nordio M, Chiu TT, Unfer V. Contribution of myo‐inositol and melatonin to human reproduction. Eur J Obstet Gynecol Reprod Biol, 2011, 159, 267–272 10.1016/j.ejogrb.2011.07.038 [DOI] [PubMed] [Google Scholar]
- 59. Brzezinski A, Seibel MM, Lynch HJ, Deng MH, Wurtman RJ. Melatonin in human preovulatory follicular fluid. J Clin Endocrinol Metab, 1987, 64, 865–867 10.1210/jcem‐64‐4‐865 [DOI] [PubMed] [Google Scholar]
- 60. Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J, 2013, 60, 1–13 10.1507/endocrj.EJ12‐0263 [DOI] [PubMed] [Google Scholar]
- 61. Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo‐inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol, 2011, 27, 857–861 10.3109/09513590.2011.564687 [DOI] [PubMed] [Google Scholar]
- 62. Seko LM, Moroni RM, Leitao VM, Teixeira DM, Nastri CO, Martins WP. Melatonin supplementation during controlled ovarian stimulation for women undergoing assisted reproductive technology: systematic review and meta‐analysis of randomized controlled trials. Fertil Steril, 2014, 101 (154–61) e4 [DOI] [PubMed] [Google Scholar]
- 63.Showell MG, Brown J, Clarke J, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev 2013; 8:Cd007807 [DOI] [PubMed]
- 64. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature, 2003, 425, 191–196 10.1038/nature01960 [DOI] [PubMed] [Google Scholar]
- 65. Ungvari Z, Sonntag WE, Cabo R, Baur JA, Csiszar A. Mitochondrial protection by resveratrol. Exerc Sport Sci Rev, 2011, 39, 128–132312340810.1097/JES.0b013e3182141f80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov, 2006, 5, 493–506 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- 67. Price NL, Gomes AP, Ling AJ, Duarte FV, Martin‐Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab, 2012, 15, 675–690354564410.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takeo S, Sato D, Kimura K, Monji Y, Kuwayama T, Kawahara‐Miki R, Iwata H. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J Reprod Dev, 2014, 60, 92–99399939910.1262/jrd.2013‐102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: specific and progressive induction of MnSOD. Biochem Biophys Res Commun, 2008, 367, 406–412 10.1016/j.bbrc.2007.12.138 [DOI] [PubMed] [Google Scholar]
- 70. Liu Y, He XQ, Huang X, Ding L, Xu L, Shen YT, Zhang F, Zhu MB, Xu BH, Qi ZQ, Wang HL. Resveratrol protects mouse oocytes from methylglyoxal‐induced oxidative damage. PLoS ONE, 2013, 8, e77960380679210.1371/journal.pone.0077960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guarente L, Franklin H. Epstein Lecture: sirtuins, aging, and medicine. N Engl J Med, 2011, 364, 2235–2244 10.1056/NEJMra1100831 [DOI] [PubMed] [Google Scholar]
- 72. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC‐1 family of transcription coactivators. Cell Metab, 2005, 1, 361–370 10.1016/j.cmet.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 73. Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Resveratrol protects against age‐associated infertility in mice. Hum Reprod, 2013, 28, 707–717 10.1093/humrep/des437 [DOI] [PubMed] [Google Scholar]
- 74. Fontana L, Partridge L, Longo VD. Extending healthy life span‐from yeast to humans. Science, 2010, 328, 321–326360735410.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Park S, Mori R, Shimokawa I. Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol Cells, 2013, 35, 474–480388787210.1007/s10059‐013‐0130‐x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Boutant M, Canto C. SIRT1 metabolic actions: integrating recent advances from mouse models. Mol Metab, 2014, 3, 5–18392991310.1016/j.molmet.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu C, Lin JD. PGC‐1 coactivators in the control of energy metabolism. Acta Biochim Biophys Sin (Shanghai), 2011, 43, 248–257 10.1093/abbs/gmr007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Daitoku H, Sakamaki J, Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein–protein interactions. Biochim Biophys Acta, 2011, 1813, 1954–1960 10.1016/j.bbamcr.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 79. Corton JC, Brown‐Borg HM. Peroxisome proliferator‐activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci, 2005, 60, 1494–1509 10.1093/gerona/60.12.1494 [DOI] [PubMed] [Google Scholar]
- 80. Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging‐associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA, 2011, 108, 12319–12324314569710.1073/pnas.1018793108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xiang Y, Xu J, Li L, Lin X, Chen X, Zhang X, Fu Y, Luo L. Calorie restriction increases primordial follicle reserve in mature female chemotherapy‐treated rats. Gene, 2012, 493, 77–82 10.1016/j.gene.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 82. Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell, 2008, 7, 622–629299091310.1111/j.1474‐9726.2008.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS ONE, 2012, 7, e49217349576910.1371/journal.pone.0049217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cohen J, Scott R, Alikani M, Schimmel T, Munne S, Levron J, Wu L, Brenner C, Warner C, Willadsen S. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod, 1998, 4, 269–280 10.1093/molehr/4.3.269 [DOI] [PubMed] [Google Scholar]
- 85. Brenner CA, Barritt JA, Willadsen S, Cohen J. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil Steril, 2000, 74, 573–578 10.1016/S0015‐0282(00)00681‐6 [DOI] [PubMed] [Google Scholar]
- 86. Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod, 2001, 16, 513–516 10.1093/humrep/16.3.513 [DOI] [PubMed] [Google Scholar]
- 87. Templeton A. Ooplasmic transfer–proceed with care. N Engl J Med, 2002, 346, 773–775 10.1056/NEJM200203073461013 [DOI] [PubMed] [Google Scholar]
- 88. Perez GI, Trbovich AM, Gosden RG, Tilly JL. Mitochondria and the death of oocytes. Nature, 2000, 403, 500–501 10.1038/35000651 [DOI] [PubMed] [Google Scholar]
- 89. Woods DC, White YA, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view toward the future. Reprod Sci, 2013, 20, 7–15367624010.1177/1933719112462632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc, 2013, 8, 966–988 10.1038/nprot.2013.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab, 2013, 17, 838–850375609610.1016/j.cmet.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]


