Abstract
The regulation of uterine and peripheral blood natural killer (NK) cells has been associated with problems related to reproductive immunology such as recurrent pregnancy loss (RPL), implantation failure or preeclampsia. NKp46, one of the natural cytotoxicity receptors (NCRs), is a unique marker that functions in NK cell cytotoxicity and cytokine production. Expression of NKp46 on NK cells is lower in women with recurrent pregnancy loss and pregnancy‐induced hypertension. Moreover, expression of NKp46 on peritoneal fluid NK cells is lower in women with pelvic endometriosis. Therefore, evaluation of NKp46 on peripheral blood NK cells may provide a means of screening for reproductive abnormalities. Recently, a new type of NK cell, the NK22 cell, has been reported. This cell may be a regulator not only of the mucosal barrier but also of reproduction. For women with RPL showing abnormal uterine and/or peripheral blood NK cells, both intravenous immunoglobulin treatment and intralipid treatment have been reported. The effects of these treatments are still controversial, and further studies are needed in order to clarify their true impact. The present review examines variations in the expression of NCRs on NK cells, the participation of NK22 cells in reproduction, and the possible use of intravenous immunoglobulin or intralipid treatment for women with recurrent pregnancy loss and NK cell abnormality.
Keywords: Intralipid, Intravenous immunoglobulin, Natural cytotoxicity receptor, NK cell, Recurrent pregnancy loss
Introduction
Natural killer (NK) cells play a very important role in human pregnancy. They bear a specific surface marker, CD56, and comprise 5–10 % of peripheral blood cells, 30–50 % of uterine endometrial cells, and 70 % of decidual lymphocytes. NK cells can be divided into CD56dim cells and CD56bright cells according to the intensity of their CD56 fluorescence. CD56bright cells account for 10 % of NK cells and are located mainly in the uterine endometrium and the decidua. Their main function is cytokine production. On the other hand, CD56dim cells account for 90 % of NK cells, representing the main population of circulating (peripheral blood) NK cells and showing high cytotoxicity. Moreover, NK cells express various kinds of activating and inhibitory receptors, and NK cell cytotoxicity is determined by the balance of these activating and inhibitory receptors.
The regulation of uterine and circulating peripheral blood NK cells is associated with various problems related to reproductive immunology, such as recurrent pregnancy loss (RPL), implantation failure, and preeclampsia. As NK cells exist in the endometrium and decidua [1], it is not unlikely that endometrial or decidual NK cells play a role in the establishment or maintenance of pregnancy. Researchers have been investigating various roles of uterine endometrial or decidual and peripheral NK cells [2, 3]. As mentioned above, NK cells exist in the uterine endometrium and decidua. At the implantation site, the chorion consists of syncytiotrophoblasts and cytotrophoblasts. These cells do not express classical class I human leukocyte antigen (HLA)‐A and HLA‐B or class II (HLA‐DP, HLA‐DQ or HLA‐DR) alloantigens. NK cells preferentially kill targets with lower expression of major histocompatibility complex (MHC) class I proteins, because fewer inhibitory receptors engage ligands. As a consequence, syncytiotrophoblasts are not free from peripheral blood NK cell cytotoxicity. Therefore, both decidual (endometrial) and peripheral blood NK cells may be important for successful pregnancy.
Recently, the predictive value of preconceptional peripheral blood NK cell activity has been evaluated, and Katano et al. [4] have reported that measurement of peripheral blood NK cells is not useful for evaluation of recurrent pregnancy loss. The prognostic value of endometrial and peripheral blood NK cells has also been reviewed [5]. The authors of that review concluded that a higher percentage of peripheral blood pre‐pregnancy NK cells and a higher number of uterine pre‐pregnancy NK cells are not associated with subsequent pregnancy outcome in women with infertility or RPL. However, they considered that the value of measuring NK cell activity or number as a prognostic indicator of pregnancy success was still undetermined. On the other hand, various reports have documented the usefulness of measuring pre‐pregnancy peripheral blood or endometrial NK cells as an indicator of reproductive success [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18]. NK cell cytotoxicity at the time of embryo transfer is significantly higher in women who miscarry [9], and the count of pre‐pregnancy peripheral CD56+ cells is higher in women with RPL [6, 7, 8]. The count of pre‐pregnancy endometrial CD16+/CD56dim NK cells is also significantly higher in women who miscarry [9] or those with RPL [18]. In women with reproductive failure, pre‐pregnancy cytokine production by NK cells shows a shift from type 2 to type 1 [11], and expression of NK cell activating and inhibitory receptors also alters [10, 12, 13, 14, 17].
Natural cytotoxicity receptors and reproduction
Natural cytotoxicity receptors
Natural cytotoxicity receptors (NCRs) are unique surface markers of NK cells, playing a role in NK cell cytotoxicity and cytokine production. NCRs, which include NKp30, NKp44, and NKp46, are expressed exclusively on NK cells, NKp46 and NKp30 being constitutively expressed, whereas NKp44 expression is induced after NK cells become activated. We have previously reported that three‐quarters of peripheral blood NK cells are NKp46+, whereas half are NKp30+ NK cells [10].
NCRs are the major receptors involved in NK cell cytotoxicity and play a role in the recognition and lysis of tumor cells by NK cells. NKp46 and NKp44, but not NKp30, recognize viral proteins such as the hemagglutinin of the influenza virus, or the hemagglutinin‐neuraminidase of the parainfluenza virus [19, 20]. The endogenous cellular ligands recognized by NCRs have still not been characterized. Recently, NCR ligands were found to be expressed by murine lymphoma and myeloma cell lines [21] and in human primary nevi and melanomas [22, 23]. The NKp30 and NKp46 receptors are expressed on the surface of activated and non‐activated NK cells, whereas NKp44 is expressed only on the surface of activated NK cells. In addition, NKp30 and NKp46 play a role in NK cell cytotoxic activity and cytokine production. Recently, we have reported the role of the natural cytotoxicity receptor, NKp46, in cytokine production by NK cells using peripheral blood and endometrium from infertile women in vitro [24]. NKp46 expression was associated with cytokine‐producing NK cells of both the CD56bright and CD56dim types. We concluded that expression of NKp46 is involved in cytokine production by CD56+ NK cells in the peripheral blood and uterine endometrium.
It has been reported that NKp46 is a ubiquitous marker for NK cells [25]. However, our studies have revealed that about 60 % of peripheral blood NK cells and uterine endometrial CD56dim cells express NKp46, and that 90 % or more of CD56bright cells do so [10, 14]. Moreover, NKp46 shows lower expression in women with various forms of reproductive failure such as RPL, implantation failure, preeclampsia, and pelvic endometriosis. Further studies are needed to clarify the participation of NCRs, especially NKp46, expressed on endometrial, peripheral blood, and peritoneal fluid NK cells, in reproduction.
Natural cytotoxicity receptors and recurrent pregnancy loss or implantation failure
In the uterus, it has been reported that endometrial NK cells have a unique receptor repertoire, being positive for NKp46, and negative or weakly positive for NKp30 and NKp44, in both the proliferative and secretory phases [26]. However, our data showed that endometrial NK cells are positive not only for NKp46 but also for NKp30 and NKp44 in the secretory phase [14, 15]. Our study showed that 80 % of endometrial NK cells are NKp46+, 25 % are NKp30+, and about 10 % of peripheral blood NK cells are NKp44+. In decidual NK cells from spontaneous abortion, Zhang et al. [27] have reported higher expression of NKp44 and NKp46, and we have reported higher expression of NKp44 and NKp30 [15]. As NCR+ NK cells have higher cytotoxicity, they may play a role in reproductive failures. On the other hand, in women with RPL and implantation failure, we have reported lower expression of NKp46 on circulating peripheral blood NK cells [10], and an abnormal correlation between NCR expression and cytokine production by peripheral blood NK cells [13]. Briefly, there was a significant positive correlation between the percentage of CD56bright/NKp46+ cells and the percentage of tumor necrosis factor (TNF)‐α‐ and interferon (IFN)‐γ‐producing NK cells in normal women, whereas women with RPL showed a significant negative correlation. The presence of aberrant NCRs or interruption of the signal transduction process after NCR activation may disturb the relationship between NCRs and cytokine production in NK cells. In addition, excessive pro‐inflammatory cytokine production by NK cells may occur through disruption of NCR expression or signal transduction processes.
Natural cytotoxicity receptors in pregnant women
It has been reported that the expression of NKp46 was significantly lower in peripheral blood from women with a history of RPL compared with controls [15]. Interestingly, this difference was observed at 12 and 20 weeks of gestation, but disappeared after that. These data may mean that even though their pregnancies are ongoing, women with a history of RPL may experience dysfunction of cytokine production by NK cells. Moreover, it has been reported that pregnant women with preeclampsia have a significantly lower percentage of CD56+/NKp46+ cells and CD56bright/NKp46+ cells than pregnant women without preeclampsia [28, 29, 30]. Interestingly, expression of CD56+/NKp46+ cells in pregnant women with preeclampsia was lower 3–4 months before the onset of preeclampsia and remained at this level until delivery. This finding strongly suggests that NKp46 might be a potentially useful marker for prediction of preeclampsia, as is the case for other factors such as sFlt1 and PlGF [31, 32].
Natural cytotoxicity receptors in women with pelvic endometriosis
It has been reported that the percentages of CD56+/NKp46+ cells and CD56dim/NKp46+ cells in peritoneal fluid of infertile women with R‐ASRM stage III to IV pelvic endometriosis are significantly lower than those in women without endometriosis [33]. As CD56dim NK cells are considered to be cytotoxic, it seems likely that CD56dim/NKp46+ cells are also cytotoxic. Differential expression of NKp46 on peritoneal fluid NK cells in women with severe endometriosis may allow the proliferation of endometriotic cells.
NK22 cells and reproduction
A new type of NKp46+ NK cell, which produces interleukin (IL)‐22, has been reported [34, 35, 36]. These cells, known as NCR22 or NK22 cells, can be distinguished from conventional NK cells, and it is considered that their IL‐22 production might mediate mucosal immune defense in respiratory organs, intestine, skin, and liver. In these organs, NK22 cells play a role in the prevention of infection and protection of the mucosa, and abnormality of NK22 cell function causes asthma, ulcerative colitis, psoriasis or atopic dermatitis [37]. In the liver, NK22 cells play a role in the proliferation of hepatocytes, and NK22 abnormality is associated with hepatocellular carcinoma. It has also been reported that IL‐22‐producing NK cells are present in the uterine mucosa [38]. However, their function in reproduction is still unclear.
Recently, a relationship between the expression of NKp46 and the cytokine production by NK cells was reported [39]. It was shown that reduced expression of NKp46 led to lower production of IFNγ. Moreover, cells showing lower expression of NKp46 produced lower amounts not only of IFNγ but also of IL‐13 in comparison with cells showing normal NKp46 expression. We have demonstrated a direct relationship between expression of NKp46 on NK cells and corresponding cytokine production using peripheral blood and endometrium from infertile women in vitro [24]. Generally, NKp46+ NK cells can be divided into NKp46bright cells and NKp46dim cells. NKp46bright NK cells in peripheral blood and the uterine endometrium show significantly higher IFNγ production than NKp46dim cells. Moreover, among peripheral blood and uterine NKp46+ NK cells, production of IFNγ is highest for NKp46bright/CD56bright NK cells. What is the key factor connecting the expression of NCR (NKp46) with cytokine production by NK cells?
Recently, it has been reported that a reduced level of IL‐22/IL‐22 receptor α1 (IL‐22R1) in villi might be involved in the occurrence of spontaneous miscarriage [40]. The expression of IL‐22R1 in villi of women with unexplained spontaneous miscarriage is lower than that of women in the early stage of normal pregnancy. Wang et al. [40] concluded that IL‐22 secreted by decidual NK cells might promote the survival of trophoblasts and participate in the maintenance of pregnancy by binding to IL‐22R1. Therefore, IL‐22‐producing NK cells may be important for reproduction.
We have evaluated the physiological role of NK22 cells in women with and without unexplained RPL using samples of pre‐pregnancy peripheral blood and midsecretory uterine endometrium [41]. The relative proportions of NK22 cells in both peripheral blood and uterine endometrium were significantly higher in women with RPL than in women without RPL. Moreover, a higher proportion of IL‐22‐producing NK cells showed lower production of IFNγ and TNFα in both peripheral blood and endometrium. For other cytokines such as IL‐4, IL‐10 and transforming growth factor (TGF)‐β, there was no relationship between the NK cells producing them and NK22 cells. In addition, there was a significantly negative correlation between NK22 cells and NKp46+ NK cells. These findings shed some light on the relationships existing between NKp46+ NK cells, production of cytokines (IFNγ and TNFα) by NK cells, and NK22 cells [41]. We speculated that in normal pregnancy, NK22 cells could produce IL‐22 whereas conventional NK cells produce IFNγ and TNFα. On the other hand, in women with reproductive failure, there is lower expression of NKp46 and higher production of IFNγ and TNFα. Then, the proportion of NK22 cells increases, and these cells may regulate the production of cytokines by NK cells. Thus, NK22 cells may be a type of regulatory cell (Fig. 1).
Figure 1.
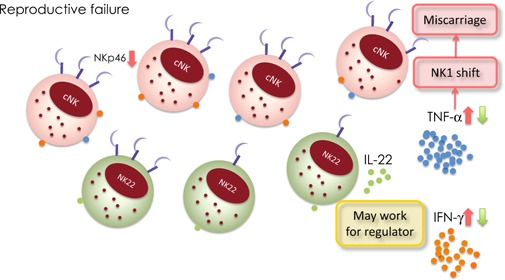
Natural cytotoxicity receptors and NK22 cells in reproductive failure. NK22 cells can produce IL‐22 whereas conventional NK cells produce IFNγ and TNFα. On the other hand, in women with reproductive failure, there is lower expression of NKp46 and higher production of IFNγ and TNFα. Then, the proportion of NK22 cells increases, and these cells may regulate the production of cytokines by NK cells. Thus, NK22 cells may be a type of regulatory cell
IVIG treatment for women with reproductive failure and abnormal NK cells
NK cells play an important role in maternal tolerance to the fetus and maintenance of pregnancy. Therefore, it seems to be important to modify the NK cells of women with reproductive failure and NK cell abnormalities such as high cytotoxicity, abnormal surface antigen expression, and/or abnormal cytokine production. Intravenous immunoglobulin (IVIG) therapy has been applied for the treatment of RPL, although its effects are still controversial. According to a previous review of IVIG treatment for women with RPL [42], IVIG was not effective for women with unexplained RPL. In another review by Polanski et al. [43] of reproductive outcomes in women with elevated levels of NK cells undergoing assisted reproductive technology (ART), some data indicated that IVIG treatment seemed to confer some benefit. However, Polanski et al. concluded that, in terms of quality and quantity, the existing data still do not support the use of IVIG in women undergoing ART who are found to have elevated numbers or activity of NK cells. The most reliable methods for testing of NK cells and for determining their normal levels remain to be unequivocally defined.
With regard to the mechanism of action of IVIG therapy for high NK cell cytotoxicity, Type 1 cytokines such as TNFα and IFNγ induce higher NK cell cytotoxicity, and IVIG exerts either a direct antibody effect or works through immunomodulation. Thus, IVIG inhibits antibody production by B cells, macrophage‐inhibitory Fcγ receptors, and NK cell activity. Accordingly, IVIG treatment for high NK cell cytotoxicity may help to maintain pregnancy and prevent spontaneous abortion. Heilmann et al. [44] have reported the effects of IVIG treatment in women with recurrent implantation failure. They measured the percentages of the peripheral blood NK cell subpopulations (CD16 and CD56) before embryo transfer, and concluded that for women with high levels of CD16+/CD56+ NK cells, additional application of IVIG improves the outcome of pregnancy. Moreover, Shimada et al. [45] reported the effect of IVIG treatment in women with recurrent pregnancy loss. They measured the percentage of inhibitory CD94 on peripheral blood NK cells before and after IVIG treatment, and showed the higher percentage of inhibitory CD94+ NK cells after IVIG treatment. They concluded that one of the mechanisms of IVIG treatment might be enhancement of CD94 expression and subsequent suppression of NK cell cytotoxicity.
It is reported that high pre‐conceptional peripheral blood NK cytotoxicity has been associated with abortions in the next pregnancy in women with RPL [6, 46, 47]. Successful pregnancy cases in women with RPL were associated with lower levels of peripheral blood NK cell activity [48, 49]. Is IVIG treatment effective for RPL women with high NK cell cytotoxicity? To resolve this question, we carried out an in vitro study to evaluate NK cell cytotoxicity using K562 cells treated with IVIG. Addition of IVIG was found to decrease NK cell cytotoxicity in a dose‐dependent manner (Fig. 2). IVIG may thus be applicable for women with RPL and high NK cell cytotoxicity, and further studies are warranted to investigate this possibility. A new clinical study is now underway in Japan to evaluate NK cell cytotoxicity in women with unexplained RPL who have suffered spontaneous abortion four times or more. It is expected that the results will shed further light on the feasibility of IVIG for this population of women.
Figure 2.
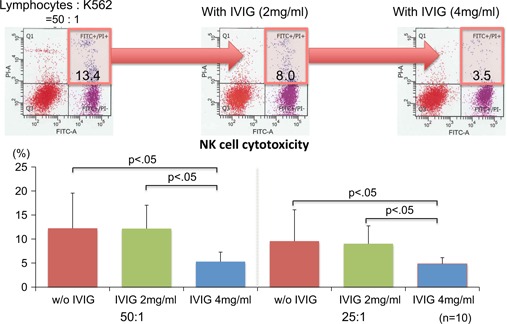
Change in NK cell cytotoxicity on addition of IVIG using K562 cells. On addition of IVIG, NK cell cytotoxicity decreased in a dose‐dependent manner
Intralipid treatment for women with reproductive failure and NK cell abnormality as an alternative to IVIG
Recently, successful pregnancy after injection of intralipid, a form of fat emulsion, has been reported [50]. The active ingredient of intralipid is purified soybean oil, together with purified egg phospholipids. In general, intralipid has been administered to patients with malnutrition to supply energy and essential fatty acids. However, it remains unclear whether intralipid has immunoregulatory properties [51]. The effect of intralipid in pregnancy has yet to be fully elucidated, particularly for women with RPL. Some reports have suggested that intralipid may have possible immune effects [52, 53, 54]. Its active ingredient, soybean oil, is capable of suppressing pro‐inflammatory mediators (Th1 cells) [52]. Roussev et al. [53, 54] have reported suppression of NK cell cytotoxicity after addition of intralipid, and indicated that this effect is almost the same as that of IVIG. After addition of intralipid, a low level of NK cell cytotoxicity is maintained. Moreover, the live birth rates following IVIG treatment and intralipid treatment are the same [55]. These findings agree with our own observations using K562 cells as mentioned above. Unexpectedly, however, intralipid did not decrease NK cell cytotoxicity, suggesting that the effect of intralipid on NK cells may be an indirect one. Moreover, there are no reports to date on the expression of NCR such as NKp46 or the percentage of NK22 cells by addition of IVIG or intralipid in women with reproductive failure. The effect of intralipid and IVIG should be investigated further to see if it is applicable for the treatment of RPL or implantation failure.
Conclusions
Abnormal expression and/or function of NK cells such as abnormality of NK cell subpopulations (CD16/CD56), abnormalities of NK cell surface markers such as NCR (especially NKp46), and abnormal cytokine production by NK cells (IFNγ, TNFα, and IL‐22) may all be risk factors for RPL. IVIG treatment may be useful for women with RPL due to NK cell abnormality.
It is still controversial whether abnormal function or abnormal expression of NK cells causes RPL or infertility. The cause of RPL still remains unexplained in up to 60 % of diagnosed patients. However, many patients with unexplained RPL do have abnormal NK cell function. Further analysis is needed to clarify the roles of NK cells in women with reproductive failure, and new forms of treatment for them should be developed.
Disclosures
Conflict of interest
Atsushi Fukui, Mai Kamoi, Ayano Funamizu, Kohei Fuchinoue, Hitomi Chiba, Megumi Yokota, Rie Fukuhara and Hideki Mizunuma declare that they have no conflict of interest.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study.
Animal rights
This article does not contain any studies with animal subjects performed by any of the authors.
References
- 1. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med, 2001, 345, 1400–1408 10.1056/NEJMra000763 [DOI] [PubMed] [Google Scholar]
- 2. Moffett A, Regan L, Braude P. Natural killer cells, miscarriage, and infertility. BMJ, 2004, 329, 1283–128553445110.1136/bmj.329.7477.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhalla A et al. Comparison of the expression of human leukocyte antigen (HLA)‐G and HLA‐E in women with normal pregnancy and those with recurrent miscarriage. Reproduction, 2006, 131, 583–589 10.1530/rep.1.00892 [DOI] [PubMed] [Google Scholar]
- 4. Katano K et al. Peripheral natural killer cell activity as a predictor of recurrent pregnancy loss: a large cohort study. Fertil Steril, 2013, 100, 1629–1634 10.1016/j.fertnstert.2013.07.1996 [DOI] [PubMed] [Google Scholar]
- 5. Tang AW, Alfirevic Z, Quenby S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod, 2011, 26, 1971–1980 10.1093/humrep/der164 [DOI] [PubMed] [Google Scholar]
- 6. Kwak‐Kim J, Gilman‐Sachs A. Clinical implication of natural killer cells and reproduction. Am J Reprod Immunol, 2008, 59, 388–400 10.1111/j.1600‐0897.2008.00596.x [DOI] [PubMed] [Google Scholar]
- 7. Kwak JY et al. Up‐regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol, 1995, 34, 93–99 10.1111/j.1600‐0897.1995.tb00924.x [DOI] [PubMed] [Google Scholar]
- 8. Coulam CB et al. Systemic CD56+ cells can predict pregnancy outcome. Am J Reprod Immunol, 1995, 33, 40–46 10.1111/j.1600‐0897.1995.tb01136.x [DOI] [PubMed] [Google Scholar]
- 9. Fukui A et al. Natural killer cell subpopulations and cytotoxicity for infertile patients undergoing in vitro fertilization. Am J Reprod Immunol, 1999, 41, 413–422 10.1111/j.1600‐0897.1999.tb00456.x [DOI] [PubMed] [Google Scholar]
- 10. Fukui A et al. Expression of natural cytotoxicity receptors and a2V‐ATPase on peripheral blood NK cell subsets in women with recurrent spontaneous abortions and implantation failures. Am J Reprod Immunol, 2006, 56, 312–320 10.1111/j.1600‐0897.2006.00431.x [DOI] [PubMed] [Google Scholar]
- 11. Fukui A et al. Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures. Fertil Steril, 2008, 89, 157–165 10.1016/j.fertnstert.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 12. Fukui A et al. Expression of natural cytotoxicity receptors and intracellular cytokine production of natural killer cell subsets in women with implantation failures. J Fertil Implant, 2009, 26, 341–347 [Google Scholar]
- 13. Fukui A et al. Correlation between natural cytotoxicity receptors and intracellular cytokine expression of peripheral blood NK cells in women with recurrent pregnancy losses and implantation failures. Am J Reprod Immunol, 2009, 62, 371–380 10.1111/j.1600‐0897.2009.00750.x [DOI] [PubMed] [Google Scholar]
- 14. Fukui A et al. Expression of natural cytotoxicity receptors on midsecretory endometrial or decidual natural killer cells. J Fertil Implant, 2010, 27, 369–374 [Google Scholar]
- 15. Fukui A et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol, 2011, 90, 105–110 10.1016/j.jri.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 16. Chernyshov VP et al. Elevated NK cell cytotoxicity, CD158a expression in NK cells and activated T lymphocytes in peripheral blood of women with IVF failures. Am J Reprod Immunol, 2010, 64, 58–67 [DOI] [PubMed] [Google Scholar]
- 17. Junovich G et al. Endometrial CD16(+) and CD16(−) NK cell count in fertility and unexplained infertility. Am J Reprod Immunol, 2013, 70, 182–189 10.1111/aji.12132 [DOI] [PubMed] [Google Scholar]
- 18. Lachapelle MH et al. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol, 1996, 156, 4027–4034 [PubMed] [Google Scholar]
- 19. Arnon TI et al. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol, 2001, 31, 2680–2689 10.1002/1521‐4141(200109)31:9<2680::AID‐IMMU2680>3.0.CO;2‐A [DOI] [PubMed] [Google Scholar]
- 20. Mandelboim O et al. Recognition of haemagglutinins on virus‐infected cells by NKp46 activates lysis by human NK cells. Nature, 2001, 409, 1055–1060 10.1038/35059110 [DOI] [PubMed] [Google Scholar]
- 21. Halfteck GG et al. Enhanced in vivo growth of lymphoma tumors in the absence of the NK‐activating receptor NKp46/NCR1. J Immunol, 2009, 182, 2221–2230 10.4049/jimmunol.0801878 [DOI] [PubMed] [Google Scholar]
- 22. Lakshmikanth T et al. NCRs and DNAM‐1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest, 2009, 119, 1251–1263267386610.1172/JCI36022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cagnano E et al. Expression of ligands to NKp46 in benign and malignant melanocytes. J Invest Dermatol, 2008, 128, 972–979 10.1038/sj.jid.5701111 [DOI] [PubMed] [Google Scholar]
- 24. Yokota M et al. Role of NKp46 expression in cytokine production by CD56‐positive NK cells in the peripheral blood and the uterine endometrium. Am J Reprod Immunol, 2013, 69, 202–211 10.1111/aji.12062 [DOI] [PubMed] [Google Scholar]
- 25. Sivori S et al. p46, a novel natural killer cell‐specific surface molecule that mediates cell activation. J Exp Med, 1997, 186, 1129–1136221171210.1084/jem.186.7.1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manaster I et al. Endometrial NK cells are special immature cells that await pregnancy. J Immunol, 2008, 181, 1869–1876 10.4049/jimmunol.181.3.1869 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y et al. Expressions of natural cytotoxicity receptors and NKG2D on decidual natural killer cells in patients having spontaneous abortions. Fertil Steril, 2008, 90, 1931–1937 10.1016/j.fertnstert.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 28. Fukui A. NK cells and its role in reproduction. Am J Reprod Immunol, 2010, 64, 1 10.1111/j.1600‐0897.2010.00951.x 20331586 [Google Scholar]
- 29. Fukui A. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy losses, implantation failures or preeclampsia. J Reprod Immunol, 2010, 86, 14 10.1016/j.jri.2010.08.015 [DOI] [PubMed] [Google Scholar]
- 30. Fukui A et al. Changes of NK cells in preeclampsia. Am J Reprod Immunol, 2012, 67, 278–286 10.1111/j.1600‐0897.2012.01120.x [DOI] [PubMed] [Google Scholar]
- 31. Moore Simas TA. Angiogenic factors for the prediction of preeclampsia in high‐risk women. Am J Obstet Gynecol, 2007, 197 (244) e1–e8 [DOI] [PubMed] [Google Scholar]
- 32. Poon LC et al. First‐trimester prediction of hypertensive disorders in pregnancy. Hypertension, 2009, 53, 812–818 10.1161/HYPERTENSIONAHA.108.127977 [DOI] [PubMed] [Google Scholar]
- 33. Funamizu A et al. Expression of natural cytotoxicity receptors on peritoneal fluid natural killer cell and cytokine production by peritoneal fluid natural killer cell in women with endometriosis. Am J Reprod Immunol, 2014, 71, 359–367 10.1111/aji.12206 [DOI] [PubMed] [Google Scholar]
- 34. Cella M et al. A human natural killer cell subset provides an innate source of IL‐22 for mucosal immunity. Nature, 2009, 457, 722–725377268710.1038/nature07537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Colonna M. Interleukin‐22‐producing natural killer cells and lymphoid tissue inducer‐like cells in mucosal immunity. Immunity, 2009, 31, 15–23 10.1016/j.immuni.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 36. Veiga‐Fernandes H, Kioussis D, Coles M. Natural killer receptors: the burden of a name. J Exp Med, 2010, 207, 269–272282261110.1084/jem.20100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang X, Zheng SG. Interleukin‐22: a likely target for treatment of autoimmune diseases. Autoimmun Rev, 2014, 13, 615–620396695410.1016/j.autrev.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brosnahan MM et al. IL‐22 is expressed by the invasive trophoblast of the equine (Equus caballus) chorionic girdle. J Immunol, 2012, 188, 4181–4187374683710.4049/jimmunol.1103509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghadially H et al. NKp46 regulates allergic responses. Eur J Immunol, 2013, 43, 3006–3016386765910.1002/eji.201343388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y et al. IL‐22 secreted by decidual stromal cells and NK cells promotes the survival of human trophoblasts. Int J Clin Exp Pathol, 2013, 6, 1781–17903759484 [PMC free article] [PubMed] [Google Scholar]
- 41.Kamoi M, et al. NK22 cells in the uterine mid‐secretory endometrium and peripheral blood of women with recurrent pregnancy loss and unexplained infertility. Am J Reprod Immunol. 2015. doi:10.1111/aji.12356. [DOI] [PubMed]
- 42. Ata B et al. A systematic review of intravenous immunoglobulin for treatment of unexplained recurrent miscarriage. Fertil Steril, 2011, 95 (1080–5) e1–e2 [DOI] [PubMed] [Google Scholar]
- 43. Polanski LT et al. Interventions to improve reproductive outcomes in women with elevated natural killer cells undergoing assisted reproduction techniques: a systematic review of literature. Hum Reprod, 2014, 29, 65–75 10.1093/humrep/det414 [DOI] [PubMed] [Google Scholar]
- 44. Heilmann L, Schorsch M, Hahn T. CD3‐CD56+CD16+ natural killer cells and improvement of pregnancy outcome in IVF/ICSI failure after additional IVIG‐treatment. Am J Reprod Immunol, 2010, 63, 263–265 10.1111/j.1600‐0897.2009.00790.x [DOI] [PubMed] [Google Scholar]
- 45. Shimada S et al. A high dose of intravenous immunoglobulin increases CD94 expression on natural killer cells in women with recurrent spontaneous abortion. Am J Reprod Immunol, 2009, 62, 301–307 10.1111/j.1600‐0897.2009.00739.x [DOI] [PubMed] [Google Scholar]
- 46. Aoki K et al. Preconceptional natural‐killer‐cell activity as a predictor of miscarriage. Lancet, 1995, 345, 1340–1342 10.1016/S0140‐6736(95)92539‐2 [DOI] [PubMed] [Google Scholar]
- 47. Hadinedoushan H, Mirahmadian M, Aflatounian A. Increased natural killer cell cytotoxicity and IL‐2 production in recurrent spontaneous abortion. Am J Reprod Immunol, 2007, 58, 409–414 10.1111/j.1600‐0897.2007.00524.x [DOI] [PubMed] [Google Scholar]
- 48. Perricone R et al. High levels of peripheral blood NK cells in women suffering from recurrent spontaneous abortion are reverted from high‐dose intravenous immunoglobulins. Am J Reprod Immunol, 2006, 55, 232–239 10.1111/j.1600‐0897.2005.00356.x [DOI] [PubMed] [Google Scholar]
- 49. Katano K et al. Clinical trial of immunostimulation with a biological response modifier in unexplained recurrent spontaneous abortion patients. J Clin Immunol, 1997, 17, 472–477 10.1023/A:1027319710387 [DOI] [PubMed] [Google Scholar]
- 50. Ndukwe G. Recurrent embryo implantation failure after in vitro fertilisation: improved outcome following intralipid infusion in women with elevated T Helper 1 response. Hum Fertil (Camb), 2011, 14, 131–146 10.3109/14647273.2011.575667 [Google Scholar]
- 51. Shreeve N, Sadek K. Intralipid therapy for recurrent implantation failure: new hope or false dawn?. J Reprod Immunol, 2012, 93, 38–40 10.1016/j.jri.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 52. Granato D et al. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. JPEN J Parenter Enteral Nutr, 2000, 24, 113–118 10.1177/0148607100024002113 [DOI] [PubMed] [Google Scholar]
- 53. Roussev RG et al. Duration of intralipid's suppressive effect on NK cell's functional activity. Am J Reprod Immunol, 2008, 60, 258–263 10.1111/j.1600‐0897.2008.00621.x [DOI] [PubMed] [Google Scholar]
- 54. Roussev RG, Ng SC, Coulam CB. Natural killer cell functional activity suppression by intravenous immunoglobulin, intralipid and soluble human leukocyte antigen‐G. Am J Reprod Immunol, 2007, 57, 262–269 10.1111/j.1600‐0897.2007.00473.x [DOI] [PubMed] [Google Scholar]
- 55. Coulam CB, Acacio B. Does immunotherapy for treatment of reproductive failure enhance live births?. Am J Reprod Immunol, 2012, 67, 296–304 10.1111/j.1600‐0897.2012.01111.x [DOI] [PubMed] [Google Scholar]


