Abstract
Processes of conceptus implantation and placentation, unique to mammalian reproduction, have been extensively studied. It was once thought that processes of these events varied greatly, notably between invasive and noninvasive modes of implantation and/or placentation. Regardless of the mode of implantation, however, physiological and biochemical processes in conceptus implantation to the maternal endometrium including the kinds of gene expression and their products are now considered not to differ so much. Recent progress has identified that in addition to the hormones, cytokines, proteases and cell adhesion molecules classically characterized, epithelial–mesenchymal transition, molecules related to lymphocyte homing, the expression of endogenous retroviruses and possibly exosomes are all required for the progression of conceptus implantation to placentation. In this review, therefore, new findings related to these events are integrated into the context of conceptus implantation to the maternal endometrium.
Keywords: EMT, ERV, Exosome, Gene expression, Implantation
Introduction
The uterine structures in mammalian species as we know them today are the product of a long and complex evolutionary process. A novel characteristic of mammalian reproduction is that fertilization and embryonic development proceed within the oviduct and uterus, respectively [1]. These structures must then provide an adequate environment for fertilization and embryonic growth; however, this process presents new challenges, most immediately the attachment of two epithelial layers between uterine epithelium and outer embryonic/conceptus membrane cells, trophectoderm (TE) cells. This interaction is also immunogenically complex because the conceptus carries paternal genes allogeneic to the maternal immune system. As these events proceed, the outer layer of TE cells plays a major role in the attachment and invasion to the uterine endometrium, and in the formation of placenta. All placentas with both conceptus and maternal cell structures assume the responsibility of supporting and nourishing the conceptus. However, extensive variation in TE cell types and placental structures exists across different mammalian species. In this review, new information on conceptus implantation, processes and its gene regulation, to the maternal endometrium and events proceeding to placental formation will be integrated.
Processes of implantation
It is generally accepted that there are five phases of conceptus implantation to the maternal endometrium, preceding placentation [2]. Phase 1, migration/hatching; the blastocyst/conceptus migrates and sheds from zona pellucida (ZP) in the uterus. During this phase, the blastocyst/conceptus enters and migrates within the uterus and hatching allows the expansion of the blastocyst to spherical shape, or it may migrate and change in its shape from spherical to tubular and filamentous form, as in domestic animals. Phase 2, pre‐contact; the blastocyst/conceptus reorients to be apposed to appropriate regions of the uterine lining. During this phase, the blastocyst/conceptus migrates or elongates without definitive contact between the TE cells and endometrial epithelium. In domestic animals, this is the period when the process of maternal recognition of pregnancy is initiated for the prevention of corpus luteum (CL) demise, resulting from biochemical communication between the developing conceptus and mother. Phase 3, attachment; TE cells of the blastocyst/conceptus attach to the uterine epithelium. During this phase, the outer TE cells of blastocyst/conceptus establish definitive contact with the uterine epithelium. Phase 4, adhesion; TE cells attach firmly to the uterine epithelium. In some cases, superficial glandular epithelium, during which mononucleate TE cells differentiate into binucleate and/or multinucleate syncytiotrophoblast cells. Phase 5, invasion; the blastocyst/conceptus invades the uterine endometrium. This phase is when many mammalian species begin to diverge greatly in their development as invasive TE cells cause the formation of decidualized endometrium, whereas noninvasive does not. For the first four phases, however, implantation processes among mammalian species appear fairly similar in cell–cell interactions and gene expression associated with these events [2].
Maternal recognition of pregnancy
In mammalian species, the continued secretion of a steroid hormone, progesterone (P4), by functional CL is a prerequisite for the establishment and continuation of pregnancy. It has been well established that P4 is involved directly and/or indirectly in various gene expressions in utero, which regulate numerous uterine functions through endometrial secretions, alteration of blood flow at implantation sites and promotion of physiological and/or immune environments suitable for normal embryonic development. Despite similar requirements, the biochemical as well as molecular mechanisms by which CL is maintained for continued P4 production differ from species to species. In higher primates including humans, CL is maintained by a luteotrophic factor, chorionic gonadotropin (CG), produced by the TE cells as they face and invade the uterine epithelium during implantation [3]. In rodents, CL is prolonged through the release of copulation‐induced pituitary prolactin surges [4]. Whichever the molecules associated with the maintenance of CL life span, they must be produced long before CL regression begins, and the period during which CL is protected from a luteolytic signal is known as the period of maternal recognition of pregnancy [5]. During such period, trophoblast and uterine endometrium under the influence of P4 must communicate with each other, resulting in the establishment of the proper uterine environment necessary for conceptus survival, implantation, and subsequent placental formation. It should be noted, however, that as the administration of human CG (hCG) does not prevent CL regression in non‐pregnant women, hCG may not be the only factor maintaining P4 production [6]. However, to date, no factors other than hCG have been definitively identified for CL maintenance in humans.
In the ruminant ungulates including cows, sheep and goats [5], interferon tau (IFNT), a major cytokine produced by TE cells during the peri‐implantation period, is the anti‐luteolytic factor essential for the prolongation of CL life span [7, 8, 9, 10, 11]. IFNT exhibits structural and functional similarities to those of type I IFNs such as IFNA and IFNB [12]. These include antiviral and anti‐proliferative activities, but IFNT shows much less cytotoxic activity than do IFNA or IFNB [13, 14, 15, 16, 17]. Type I IFNs are known to bind to a common receptor complex with two polypeptide subunits (IFNAR1 and IFNAR2) [18], both of which are present in ovine uterine epithelial cells [19]. It has been thought that the luminal epithelium of the uterine endometrium is the primary target for IFNT [11, 20]. Identification of the receptor or receptor subunits suggests that IFNT can reach the stroma, and even the uterine myometrium [21, 22, 23]. Indications are that IFNT likely reaches the circulating immune cells and the ovaries as well [24]. Upon binding to the receptor, type I IFNs activate the Janus kinase‐signal transducer and activator of transcription‐interferon regulatory factor (JAK‐STAT‐IRF) signaling pathway [25, 26], causing the activation of a group of interferon‐stimulated genes (ISGs) [27, 28]. In addition to ISGs, wingless‐type MMTV integration site family (WNTs) and LGALS gene expression [29, 30], IFNT induces several chemokines in endometrial tissues including chemokine ligand 10 (CXCL10) and CXCL9 [31, 32]. Endometrial CXCL10 in turn attracts immune cells, particularly NK cells, to the caruncular regions of the endometrium [33], and by acting through the CXCL10 receptor, CXCR3, this chemokine regulates TE cell migration and integrin expressions [32]. Together with P4, IFNT regulates endometrial gene expression necessary for the establishment of the proper uterine environment during the implantation period [28]. These changes result in conceptus migration, apposition and initial attachment to the uterine epithelial cells in mammals including ruminant species [33, 34] (Fig. 1).
Figure 1.
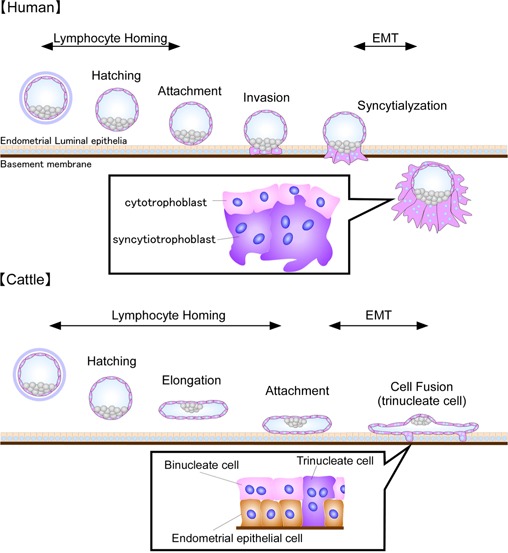
Processes of conceptus implantation to the maternal endometrium; hatching, attachment, invasion, and syncytialization or cell fusion. During the implantation period, molecules related to lymphocyte homing and EMT are used in both human and bovine species. Upper human implantation. Cell structures of cytotrophoblast and syncytiotrophoblast are shown in the black box. Lower Bovine (cattle) implantation. Unique to the ruminant ungulates, the trophoblast after hatching elongates (conceptus) and the elongated conceptus attachment to the maternal luminal epithelium occurs, but the TE cell invasion is kept minimal. During conceptus elongation, binucleate cells form in the TE cell layer, which take on nuclei from the epithelial cells of maternal endometrium, resulting in the formation of trinucleate cells
Use of lymphocyte homing molecules in conceptus attachment
It is well documented that cell–cell interactions and integrin (ITG)‐mediated signaling between the conceptus and endometrium are critical for successful implantation in humans and murine species [35, 36]. Specifically, the extracellular domain of ITGs acts as a receptor for extra‐cellular matrix components (ECMs) such as fibronectin, vitronectin, laminin, collagen‐type IV, and osteopontin (SPP) [37, 38]. In goats, sheep, and cattle, constituents of uterine histotroph such as IP‐10 (CXCL10), galactoside‐binding soluble 15 (LGALS 15), and insulin‐like growth factor binding protein (IGFBP)‐1 have been characterized to activate ITGs through their RGD domain during the period of TE cell attachment to the uterine epithelium [35, 36, 37]. In the bovine species, the expression of ITGs has been characterized at the uteroplacental interface during the periods of TE attachment [38, 39] and placentation [40]. During the stages of bovine TE binucleate cell migration and fusion with the uterine epithelial cells, five α subunits (ITGA2B, ITGA3, ITGA5, ITGA8 and ITGAV) and two β subunits (ITGB1 and ITGB3) have been characterized [40]. In the previous investigation [41], we found that integrin subunits α (ITGAV, ITGA5) and β (ITGB1, ITGB3 and ITGB5) are constitutively expressed in bovine peri‐attachment TE cells, whereas the expression of ITGA4 and ITGA8 is induced after attachment of TE cells to uterine epithelial cells is initiated.
Vascular cell adhesion molecule (VCAM‐1), a trans‐membrane glycoprotein member of the immunoglobulin gene superfamily [42], is well known as a cell adhesion mediator during the process of lymphocyte homing [43], angiogenesis [44] and allantoic membrane fusion to the chorion [45]. In VCAM‐1 gene ablation study [45], the allantois fails to fuse with the chorion, resulting in abnormal placental development and embryonic losses at 9.5–11.5 days of gestation, although a minority of VCAM‐1‐deficient mice with abnormal distribution of allantoic mesoderm over the chorionic surface survives. In humans, VCAM‐1 is present on the endometrial side, specifically localized on decidual stromal cells in the areas where migrating TE cells are present, but not on vascular endothelial cells in decidua parietalis. Endometrial expression of VCAM‐1 at the peri‐implantation stage of patients with unexplained infertility was significantly lower than in control patients [46], suggesting that the expression of VCAM‐1 might be essential for the preparation of the endometrium for invasive mode of implantation. In the study of early pregnancy in sheep, VCAM‐1 is first found in endothelial cells on days 17–19 in both caruncular and intercaruncular areas of the endometrium, and becomes strongly induced in endothelial cells on days 26–27 [47].
VCAM‐1, induced by various cytokines in different tissues or organs in mice [48], functions through integrin α4β1 (ITGA4/ITGB1), also known as very late antigen‐4 (VLA4) [49]. Homozygous loss of ITGB1 expression in mice was lethal during early post‐implantation development, resulting in inner cell mass failure [50]. It was also identified that homozygous ITGA4 null knockout mice fail to complete fusion of the allantois with the chorionic membrane during placentation period [51], the cellular event similar to that of VCAM‐1 gene ablation. In our previous investigation on bovine conceptuses, ITGA4 mRNA was found at elevated expression levels on day 22, 2–3 days after the initiation of trophoblast attachment to the endometrial epithelium [41, 52]. We also found that changes in TE cells’ gene expression including ITGs were seen when bovine TE (CT‐1) cells were cocultured with endometrial epithelial cells (EECs), which was further enhanced with the addition of uterine flushings from pregnant animals [52, 53]. These results suggest that components of uterine flushings/histotroph including ECMs and/or various cytokines, as well as cell–cell interactions are important in the progression of conceptus attachment to the uterine epithelium in the bovine and other mammalian species.
Epithelial and mesenchymal transition
The outer layer of TE cells possesses epithelial characteristics, including apicobasal cell polarity, lateral junctions with neighboring cells and basal contact with the basement membrane proteins [54, 55, 56]. Despite the fact that the apical plasma membranes of simple epithelia normally lack adhesive properties, TE cells still manage to adhere to the uterine epithelium through its apical domains as part of the pre‐implantation process. Thus, the adhesion between TE cells and uterine epithelial cells has long been considered a cell biological paradox [57]. With the exception of rodents, in which the conceptus enters a receptive uterus and attaches immediately to the uterine epithelium, primates and most domestic animals have a prereceptive phase during which the conceptus does not physically interact with the uterine epithelium. In the bovine species, attachment between TE cells and endometrial epithelium is first seen on day 20 of gestation, and subsequent stable adhesion occurs between days 20 and 22 [58].
Another surprising finding was that changes in gene expression associated with the epithelial–mesenchymal transition (EMT) occurred not before attachment, but rather on day 22, 2–3 days after the initiation of conceptus attachment to the uterine epithelium [41]. Positive signals for both the epithelial marker cytokeratin and the mesenchymal marker vimentin were seen in the elongated TE on day 22. Increased transcripts of N‐cadherin, vimentin, matrix metalloproteinase 2 (MMP2), and MMP9 were also found on day 22, concurrent with E‐cadherin mRNA and protein down‐regulation [41]. These observations indicate that after the conceptus‐endometrium attachment, EMT‐related transcripts as well as cytokeratin are present in the bovine TE, and suggest that in addition to extracellular matrix expression, partial EMT is required for proper adhesion of elongated conceptus to the maternal endometrium.
In that study, we also identified that transcription factor SNAI2, ZEB1, ZEB2, TWIST1, TWIST2, and KLF8 transcripts were up‐regulated concurrent with cytokeratin expression in the TE cells [41]. It has been characterized that SNAIL, ZEB, and KLF8 factors bind to and repress E‐cadherin promoter activity [59, 60], whereas TWIST1 and TWIST2 repress E‐cadherin transcription indirectly [61]. In addition, SNAIL and ZEB factors are known to induce the expression of MMPs that can degrade the basement membrane, thereby favoring invasion [62]. Although the bovine conceptus does not penetrate into the endometrium, the confirmation that MMP2 and MMP9 transcripts are up‐regulated not only suggests that they play a role in noninvasive trophoblasts, but also confirms the similarity between invasive and noninvasive modes of implantation.
Placenta: structural diversity
Fertilized eggs differentiate into an inner cell mass (ICM) and an outer TE in the early development of the mammalian trophoblast. ICM develops into the embryo as well as the amnion, yolk sac and allantois whereas the TE forms chorionic membrane and later becomes a major part of the conceptus placenta. To receive nutrients and gases from the mother in utero, the conceptus forms the placenta; however, its cell types as well as structures vary considerably among mammalian species. Various cell types that form a barrier between the fetal and maternal blood in epitheliochorial placentation (pigs and horse) are: (1) the endothelium of the maternal capillary, (2) uterine endometrium (stroma and/or decidua), (3) the epithelial layer of the uterine endometrium, (4) the layer or layers of TE cells that make up the chorionic epithelium, (5) fetal connective tissues, and (6) the endothelium of the fetal capillary [63, 64, 65, 66]. In hemochorial placentation (rodents and primates), as maternal blood directly reaches the TE cells, only three layers exist between maternal‐fetal circulations. In any form of placentation, maternal nutrients and gases must traverse these cell layers to reach the fetus, and waste materials must be expelled back to the maternal circulatory system.
In an epitheliochorial placenta, the uterine epithelium is in direct contact with the chorionic TE cells. This type of placentation is found in several orders including even‐toed ungulates, whales and dolphins and lower primates. In an endotheliochorial placenta, the endothelium of the maternal capillaries is located close to the TE cells, resulting from stromal thinning and a loss of uterine epithelium. This type of placenta is seen in carnivores, but is also found in the distantly related elephants [67]. In hemochorial placentation, by contrast, maternal blood is directly in touch with the trophoblast, functioning without the capillary endothelium. This type of placentation is seen in many rodents and in higher primates including humans. In this type of placentation, the multinucleate TE, syncytiotrophoblast, serves the function of efficient nutrient and gas transfer. However, molecular mechanisms by which this cell type forms have not been definitively elucidated.
Endogenous retroviruses and pregnancy
Numerous analyses on mammalian genomic DNAs revealed that endogenous viral elements (EVEs) make up at least 45 and 40 % of human and mouse genomes, respectively (Human and mouse genomic sequencing consortium, Nature 2001, 2002). Among EVEs, endogenous retroviruses (ERVs) and long terminal repeat (LTR) retrotransposons make up 8 and 10 % of human and murine genomes, respectively. ERVs, as parts of an organism's genome, can potentially exhibit functions, although their nucleotide structures largely consist of deletions and/or mutations. In general, the genome of retroviruses contain gag, pro, pol, and env genes, and 5′‐ and 3′ LTRs, some of which are still active in their transcription and could code for proteins. Extensive studies of mammalian ERVs have provided insight into their env proteins, which enable ERV infection to the host cells through specific receptors and induce cell–cell fusion. In humans, 18 ERV‐env nucleotide structures have been identified as having actual protein expression, three of which possess fusogenic activity [68, 69].
Evidence has accumulated that ERVs are now realized as factors implicated in development and differentiation of TE cells in mammalian species such as humans, rodents, dogs/cats, rabbits, sheep, cattle, tenrecs and opossums [70, 71, 72, 73, 74, 75, 76, 77, 78]. During the course of evolution, all vertebrates have been exposed to multiple waves of cross‐species infection by exogenous retroviruses, some of which infected germ cells and have been inherited in an integrated, proviral form [79]. Despite this prevalence in the mammalian genome, these were once considered non‐functional junk DNAs. However, it is now realized that ERVs play biological roles in protection against retroviral infection [80] and in placental development [81, 82]. Recently, it was found that high levels of transcripts found in ES cells, most of which are expressed in two‐cell stage embryos, are induced by ERVs’ LTRs, suggesting the possibility that the foreign sequences have helped to drive cell‐fate regulation of early embryos in placental mammals [83].
Similar to malignant cells, TE cells possess the ability to invade non‐TE cells. If unchecked, therefore, TE cells have the potential to damage or destroy uterine structures, and this aggression must be regulated for the protection of uterine endometrium. When the cell cycles of TE cells are restricted, these cells go through endoreduplication, resulting in the formation of giant trophoblast cells in murine species. In other mammalian species, TE cells become multinucleate cells through cell fusion. These multinucleate cells do not go through cell cycles, and thereby their invasiveness is held under control [84]. For example, syncytin 1 and 2 are products of the two human ERV envelope (env) genes, and are involved in the fusion of trophoblast cells, resulting in multinucleated syncytiotrophoblast formation [70, 71]. It was determined that syncytin 2 entered the primate lineage more than 40 million years ago (MYA) while syncytin‐1 entered the lineage 25–40 MYA [71]. Under physiological conditions, syncytin 1 possesses stronger fusogenic activity than that of syncytin 2. In addition, syncytin 2, not syncytin 1, has immunosuppressive activity.
From murine genome analysis, two env genes, syncytin A and B, with fusogenic activity in vitro were found and are homologous to human syncytin‐1 and ‐2, respectively [70, 85]. There are three layers in mice trophoblasts, and syncytin A is found in the second layer, syncytiotrophoblast layer‐I (ST‐I) whereas syncytin B is localized in the third layer, syncytiotrophoblast layer‐II (ST‐II) [72]. Ablation of syncytin A results in the failure of ST‐1 formation, and these embryos die between days 11.5 and 13.5 of pregnancy. Because syncytin A exhibits fusogenic activity in Green monkey Vero and human 293T cells, it is also thought to be involved in trophoblast cell fusion. Similar to human syncytins, GCM1 functions as a transcription factor bound to the upstream region of syncytin A gene, regulating its gene transcription [86]. This is in agreement with the observation that GCM1 gene ablation precludes the development of labyrinth zone in mice placenta [87]. On the other hand, syncytin B gene ablation does not result in embryonic death, although ST‐II layer formation is insufficient and the number of pups born is smaller than that for the control mice possessing syncytin B function. In in vitro assay, syncytin B exhibits fusogenic activity only in canine MDBK cells; however, it possesses strong immunosuppressive activity [88]. These findings for syncytin B, found in murine trophoblasts, closely resemble those for syncytin 2 in human cytotrophoblasts.
Unlike in primates and rodents, syncytiotrophoblasts are not formed in TE cells of ruminant ungulates. However, bovine TE cells form binucleate cells (BNCs) as well as trinucleate cells (TNCs). While it has not been definitively determined whether BNCs result from cell fusion or endoreduplication, it is clear that TNCs are products of fusion between binucleate cells and uterine epithelial cells [89, 90, 91, 92]. In addition, TE cells of ruminants are not invasive, and thus do not penetrate deep into uterine stroma or spiral arteries; however, BNCs from bovine placenta possess one ERV, BERV‐K1 [91], with fusogenic activity [92]. It should be noted that multi‐nucleate and syncytiotrophoblast cell formation results from homologous cell fusions, however, TNC formation results from heterologous cell fusion between TE cells and maternal endometrial epithelia. TNCs are only located in the endometrium [93], suggesting that this cell fusion may strengthen the adhesion between conceptus and uterine endometrium at the placentomes. Similar to syncytiotrophoblasts of primates, these cells are in closest proximity to maternal immune cells, possibly suggesting that TNCs may play a role in the protection of the allogeneic embryo during the course of pregnancy.
Recently, a bovine ERV, BERV‐K1, with strong fusogenic activity was considered to be the main factor involved in TNC formation and was therefore named Fematrin‐1 [92]. It was also reported that syncytin‐Rum1 has been integrated into ruminant genomes, including cattle and sheep, and was possibly involved in fetomaternal cell–cell fusion in both species [77]. It should be noted however that Fematrin‐1 integrated into the bovine genome, but not in the sheep genome. Current hypothesis supports that syncytin‐Rum1 was integrated into ruminant genomes 20 MYA while Fematrin‐1 was integrated into the bovine genome 11 MYA [94]. As for bERVE‐A, its mRNA is found only in binucleate cells throughout the gestation period, and this gene contains syncytin 1‐like SU domain and ASCT2 binding domain; however, it does not possess fusogenic activity due to a loss of the fusion peptide [76]. Furthermore, the integration of BERV‐P was recently found in ruminants [95]. Nucleotide structures of BERV‐P closely resemble that of syncytin found in dogs/cats, syncytin‐Car1 [75]; however, BERV‐P does not possess fusogenic activity. Although several ERVs are found in ruminants, syncytin‐Rum1 and Fematrin‐1 are unique in possessing fusogenic activity. Integration of syncytin‐Rum1, followed by Fematrin‐1 into the bovine genome suggests that a two‐step process could be required for viral particle integration into the genome. First, unless viral activity is lost, viral genomes may not be endogenized into the genome. Second, if in fact, a part of integrated viral genes could be used by the host animals, it is possible that the host may utilize them for more efficient cellular and/or genomic functions (Fig. 2).
Figure 2.
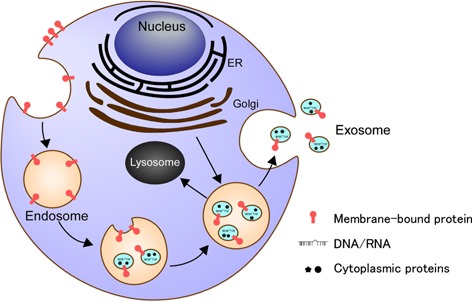
Exosome production. At endosomes of various cell types, cytosolic proteins and nucleic acids are packaged into exosomes. Exosome‐filled cytosolic structures are either fused with plasma membrane to release their contents in the extracellular spaces or send their contents to lysosomes for degradation. Released exosomes are often used for intercellular communications, including cell–cell fusion
Exosomes promote TE cell fusion?
Three different mechanisms of cell–cell communication have been recognized: (a) by the use of soluble factors such as hormones, cytokines, chemokines, bioactive ions and lipids in an autocrine, paracrine or endocrine manner, (b) through direct adhesion contacts between cells such as receptor‐ligand signaling and trogocytosis, and (c) by shuttling of information through intercellular nanotubes [96, 97]. Recently, a fourth way of intercellular communication through release and uptake of extracellular membrane‐bound microvesicles (EMV) has been identified. This type of intercellular exchange relies on the transport of membranally bound molecules, containing cytosolic proteins and nucleic acids such as mRNA and microRNA (miRNA), and delivers information in the near vicinity and/or at a distance [98]. EMVs have been found in all living organisms, including plants, and produced by both prokaryotic and eukaryotic cells. Evidence has been accumulated that EMVs comprise a heterogeneous group of vesicles, and are present in blood and all kind of bodily fluids, including plasma and serum, ocular effluent and aqueous humor, cerebrospinal fluid, saliva, breast milk, synovial fluid, nasal and bronchial secretions, bile, urine, amniotic fluid, semen, and from pleural and peritoneal effusions in pathological conditions [98]. Although an official definition has not been finalized yet [99], the current definition of “exosome” among EMVs is as follows: (a) secreted membrane‐bound nanovesicles of endosomal origin, (b) size from 30 to 100 nm or approximately 150 nm, (c) cup‐shaped form, (d) tetraspanin‐, cholesterol‐ and sphingophospholipid‐rich detergent‐resistant membrane, and (e) buoyant density of 1.13–1.19 g/ml on sucrose gradient [100]. In addition, the specific pathway of biogenesis of exosomes separates them from all other known EMVs. Exosomes are produced in the late endosomal compartment by inward budding of the limiting membrane of multi‐vesicular bodies (MVBs) [101]. The membrane invagination process fills the MVBs with intraluminal microvesicles (ILVs). Cytosolic proteins and nucleic acids are packaged and carried inside exosomes. Exosome‐filled MVBs are either fused with the plasma membrane to release their contents in the extracellular space or send their contents to lysosomes for degradation [98].
In humans, syncytin‐1 triggers a cell fusion process, resulting in the formation of syncytiotrophoblasts. To initiate the cell fusion processes, the fusion‐active domain of the protein must bring the phospholipid bilayer of two cells into close proximity [102], allowing the next steps of the fusion processes to proceed. These steps involve the protein‐free areas on the cell surface, allowing lipid mixing between the two cell membranes. This lipid interdigitation proceeds favorably in cell surface areas enriched in fusogenic lipids such as phosphatidic acid in the presence of calcium. Calcium is known to bind to the phosphate moiety of the phosphatidic acid molecule and disrupts the thermodynamic barrier made by the water molecules bound to the membrane phospholipids, from which lipid interdigitation is initiated. Exosomes are enriched in phosphatidic acid [103], and also contain the endosomal phospholipid Bis(Monoacylglycero)Phosphate (BMP) [104] which is fusogenic [103]. It has been thought that fusion between exosomes and another membrane might occur more readily at acidic pH since BMP is fusogenic at endosomal pH [103, 105]. However, the presence of syncytin in the exosome membrane could enable BMP to function even at neutral pH. More importantly, exposure of phosphatidylserine on the outer layer of cell membranes has been reported to favor fusion [106], because exosomes expose this phospholipid on their external membranes [107]. These recent observations favor that together with syncytin‐1, exosomes could well be involved in the promotion of intercellular fusion, resulting in the initiation of syncytiotrophoblast formation.
Conclusions
The placentas as we see them today are considered to be a fairly recent development in mammals, and the conceptus side consists of mononucleate TE cells and fused TEs, syncytiotrophoblasts. Until recently, processes of conceptus implantation to the maternal endometrium had been studied from the standpoint of attachment and invasion through extracellular matrices, cell adhesion molecules, cytokines, and/or proteinases and their inhibitor expression. Recent progress suggests that implantation processes should be analyzed as a whole as well as in specific events; however, each event and/or their gene expression must be studied on a continuum sequence of events. In particular, any implantation study must include ERV genes and their specific expression as well as other components and/or players such as exosomes and EMT related molecules. ERV and exosome research in reproduction represents fairly new directions and with various ERV genes and other components yet to be found, our current understanding of implantation and placental formation may be far from finalized. We must then treat these processes as a work still in progress, and, therefore, prepare for much work ahead in the elucidation of molecular mechanisms associated with implantation and placentation, all of which result in reproductive advantages in mammalian evolution.
Acknowledgments
This was supported by the Program for Promotion of Basic research Activities for Innovative Bioscience (BRAIN) and by Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry. The authors would like to thank Mr. Robert Moriarty for his thorough evaluation of the manuscript.
References
- 1. Amoroso EC. The evolution of viviparity. J R Soc Med., 1968, 61, 1188–1200 [PMC free article] [PubMed] [Google Scholar]
- 2. Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction, 2009, 138, 195–209 10.1530/REP‐09‐0158 [DOI] [PubMed] [Google Scholar]
- 3. Hearn JP, Webley GE, Gidley‐Baird AA. Chorionic gonadotrophin and embryo‐maternal recognition during the peri‐implantation period in primates. J Reprod Fertil, 1991, 92, 497–509 10.1530/jrf.0.0920497 [DOI] [PubMed] [Google Scholar]
- 4. Soares MJ, Faria TN, Roby KF, Deb S. Pregnancy and the prolactin family of hormones: coordination of anterior pituitary, uterine, and placental expression. Endocr Rev, 1991, 12, 402–423 10.1210/edrv‐12‐4‐402 [DOI] [PubMed] [Google Scholar]
- 5.Short RV. Implantation and the maternal recognition of pregnancy. In: Wolstenhome GEW, O'Connor M, editors. “Foetal Anatomy”, Ciba Foundation Symposium. London: J&A Churchill; 1969. p. 2–26.
- 6. Quagliarello J, Goldsmith L, Steinetz B, Lustig DS, Weiss G. Induction of relaxin secretion in nonpregnant women by human chorionic gonadotropin. J Clin Endocrinol Metab, 1980, 51, 74–77 10.1210/jcem‐51‐1‐74 [DOI] [PubMed] [Google Scholar]
- 7. Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J Reprod Fertil, 1982, 65, 141–150 10.1530/jrf.0.0650141 [DOI] [PubMed] [Google Scholar]
- 8. Imakawa K, Anthony RV, Kazami M, Marotti KR, Polites HG, Roberts RM. Interferon‐like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature, 1987, 330, 377–379 10.1038/330377a0 [DOI] [PubMed] [Google Scholar]
- 9. Stewert HJ, McCann SHE, Barker PJ, Lee KE, Lamming GE, Flint APF. Interferon sequence homology and receptor binding activity of ovine trophoblast antiluteolytic protein. J Endocrinol, 1987, 115, R13–R15 10.1677/joe.0.115R013 [DOI] [PubMed] [Google Scholar]
- 10. Charpigny G, Reinard P, Huet JC, Guillomot M, Charlier M, Pernollet JC, Martal J. High homology between a trophoblastic protein (trophoblastin) isolated from ovine embryo and α‐interferon. FEBS Lett, 1988, 228, 12–16 10.1016/0014‐5793(88)80574‐X [DOI] [PubMed] [Google Scholar]
- 11. Roberts RM, Cross JC, Leaman DW. Interferons as hormones of pregnancy. Endocr Rev, 1992, 13, 432–452 [DOI] [PubMed] [Google Scholar]
- 12. Imakawa K, Hansen TR, Malathy PV, Anthony RV, Polites HG, Marotti KR, Roberts RM. Molecular cloning and characterization of complementary deoxyribonucleic acids corresponding to bovine trophoblast‐1: a comparison with ovine trophoblast protein‐1 and bovine interferon‐alpha II. Mol Endocrinol, 1989, 3, 127–139 10.1210/mend‐3‐1‐127 [DOI] [PubMed] [Google Scholar]
- 13. Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annual Rev Biochem., 1987, 56, 727–777 10.1146/annurev.bi.56.070187.003455 [DOI] [PubMed] [Google Scholar]
- 14. Niwano Y, Hansen TR, Kazemi M, Malathy PV, Johnson HD, Roberts RM, Imakawa K. Suppression of T‐lymphocyte blastogenesis by ovine trophoblast protein‐1 and human interferon‐alpha may be independent of interleukin‐2 production. Am J Reprod Immunol, 1989, 20, 21–26 10.1111/j.1600‐0897.1989.tb00632.x [DOI] [PubMed] [Google Scholar]
- 15. Roberts RM, Imakawa K, Niwano Y, Kazemi M, Malathy PV, Hansen TR, Glass AA, Kronenberg LH. Interferon production by the preimplantation sheep embryo. J Interferon Res, 1989, 9, 175–187 10.1089/jir.1989.9.175 [DOI] [PubMed] [Google Scholar]
- 16. Pontzer CH, Bazer FW, Johnson HM. Antiproliferative activity of a pregnancy recognition hormone, ovine trophoblast protein‐1. Cancer Res, 1991, 51, 5304–5307 [PubMed] [Google Scholar]
- 17. Pontzer CH, Yamamoto JK, Bazer FW, Ott TL, Johnson HM. Potent anti‐feline immunodeficiency virus and anti‐human immunodeficiency virus effect of IFN‐τ. J Immunol., 1997, 158, 4351–4357 [PubMed] [Google Scholar]
- 18. Pestka S, Krause CD, Walter MR. Interferons, interferon‐like cytokines, and their receptors. Immunol Rev, 2004, 202, 8–32 10.1111/j.0105‐2896.2004.00204.x [DOI] [PubMed] [Google Scholar]
- 19. Rosenfeld CS, Han CS, Alexenko AP, Spencer TE, Roberts RM. Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the ovine uterus. Biol Reprod, 2002, 67, 847–853 10.1095/biolreprod.102.004267 [DOI] [PubMed] [Google Scholar]
- 20. Imakawa K, Tamura K, Lee R‐S, Ji Y, Kogo H, Sakai S, Christenson RK. Temporal expression of type I interferon receptor in the peri‐implantation ovine extra‐embryonic membranes: demonstration that human IFN‐alpha can bind to this receptor. Endocr J, 2002, 49, 195–205 10.1507/endocrj.49.195 [DOI] [PubMed] [Google Scholar]
- 21. Ott TL, Yin J, Wiley AA, Kim HT, Gerami‐Naini B, Spencer TE, Bartol FF, Burghardt RC, Bazer FW. Effects of the estrous cycle and early pregnancy on uterine expression of Mx protein in sheep (Ovis aries). Biol Reprod, 1998, 59, 784–794 10.1095/biolreprod59.4.784 [DOI] [PubMed] [Google Scholar]
- 22. Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross‐reactive protein in the ovine uterus. Biol Reprod, 1999, 61, 312–318 10.1095/biolreprod61.1.312 [DOI] [PubMed] [Google Scholar]
- 23. Hicks BA, Etter SJ, Carnahan KG, Joyce MM, Assiri AA, Carling S, Kodali K, Johnson GA, Hansen TR, Mirando MA, Woods GL, Vanderwall DK, Ott TL. Expression of the uterine Mx protein in cyclic and pregnant cows, gilts, and mares. J Anim Sci, 2003, 81, 1552–1562 [DOI] [PubMed] [Google Scholar]
- 24. Shirasuna K, Nitta A, Sineenard J, Shimizu T, Bollwein H, Miyamoto A. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domest Anim Endocrinol, 2012, 43, 198–211 10.1016/j.domaniend.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 25. Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Ann Rev Biochem., 1998, 67, 227–264 10.1146/annurev.biochem.67.1.227 [DOI] [PubMed] [Google Scholar]
- 26. Kim S, Choi Y, Bazer FW, Spencer TE. Identification of genes in the ovine endometrium regulated by interferon tau independent of signal transducer and activator of transcription 1. Endocrinology, 2003, 144, 5203–5214 10.1210/en.2003‐0665 [DOI] [PubMed] [Google Scholar]
- 27. Chen Y, Antoniou E, Liu Z, Hearne LB, Roberts RM. A microarray analysis for genes regulated by interferon‐τ in ovine luminal epithelial cells. Reproduction, 2007, 134, 123–135 10.1530/REP‐07‐0387 [DOI] [PubMed] [Google Scholar]
- 28. Spencer TE, Sandra O, Wolf E. Genes involved in conceptus‐endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction, 2008, 135, 165–179 10.1530/REP‐07‐0327 [DOI] [PubMed] [Google Scholar]
- 29. Gray CA, Adelson DL, Bazer FW, Burghardt RC, Meeusen EN, Spencer TE. Discovery and characterization of an epithelial‐specific galectin in the endometrium that forms crystals in the trophectoderm. Proc Natl Acad Sci USA, 2004, 101, 7982–798741954310.1073/pnas.0402669101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohamed OA, Jonaert M, Labelle‐Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/β‐catenin signaling is required for implantation. Proc Natl Acad Sci USA, 2005, 102, 8579–8584115082010.1073/pnas.0500612102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagaoka K, Sakai A, Nojima H, Suda Y, Yokomizo Y, Imakawa K, Sakai S, Christenson RK. Expression of a chemokine, IFN‐gamma‐inducible protein 10 kDa, is stimulated by IFN‐τ in the ovine endometrium. Biol Reprod, 2003, 68, 1413–1421 10.1095/biolreprod.102.008912 [DOI] [PubMed] [Google Scholar]
- 32. Imakawa K, Imai M, Sakai A, Suzuki M, Nagaoka K, Sakai S, Lee S‐R, Chang K‐T, Echterrnkamp SE, Christenson RK. Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol Reprod Dev., 2006, 73, 850–58 10.1002/mrd.20496 [DOI] [PubMed] [Google Scholar]
- 33. Nagaoka K, Nojima H, Watanabe F, Christenson RK, Sakai S, Imakawa K. Regulation of blastocyst migration, apposition and initial adhesion by a chemokine, IFN‐γ‐inducible protein 10 kDa (IP‐10), during early gestation. J Biol Chem, 2003, 278, 29048–29056 10.1074/jbc.M300470200 [DOI] [PubMed] [Google Scholar]
- 34. Imakawa K, Nagaoka K, Nojima H, Hara Y, Christenson RK. Changes in immune cell distribution and IL‐10 production are regulated through endometrial IP‐10 expression in the goat uterus. Am J Reprod Immunol, 2005, 53, 54–64 10.1111/j.1600‐0897.2004.00243.x [DOI] [PubMed] [Google Scholar]
- 35. Aplin JD, Hey NA, Graham RA. Human endometrial MUC1 carries keratan sulfate: characteristic glycoforms in the luminal epithelium at receptivity. Glycobiology, 1998, 8, 269–276 10.1093/glycob/8.3.269 [DOI] [PubMed] [Google Scholar]
- 36. Armant DR. Blastocysts don't go it alone. Extrinsic signals fine‐tune the intrinsic developmental program of trophoblast cells. Dev Biol, 2005, 280, 260–280271529610.1016/j.ydbio.2005.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell, 1996, 9, 181–186 [PubMed] [Google Scholar]
- 38. MacIntyre DM, Lim HC, Ryan K, Kimmins S, Small JA, MacLaren LA. Implantation‐associated changes in bovine uterine expression of integrins and extracellular matrix. Biol Reprod, 2002, 66, 1430–1436 10.1095/biolreprod66.5.1430 [DOI] [PubMed] [Google Scholar]
- 39. Pfarrer C, Hirsch P, Guillomot M, Leiser R. Interaction of integrin receptors with extracellular matrix is involved in trophoblast giant cell migration in bovine placentomes. Placenta, 2003, 24, 588–597 10.1016/S0143‐4004(03)00059‐6 [DOI] [PubMed] [Google Scholar]
- 40. Pfarrer CD. Characterization of the bovine placenta by cytoskeleton, integrin receptors, and extracellular matrix. Methods Mol Med, 2006, 121, 323–335 [DOI] [PubMed] [Google Scholar]
- 41. Yamakoshi S, Bai R, Chaen T, Ideta A, Aoyagi Y, Sakurai T, Konno T, Imakawa K. Expression of mesenchymal‐related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction, 2012, 143, 377–387 10.1530/REP‐11‐0364 [DOI] [PubMed] [Google Scholar]
- 42. Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi‐Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine‐induced endothelial protein that binds to lymphocytes. Cell, 1989, 59, 1203–1211 10.1016/0092‐8674(89)90775‐7 [DOI] [PubMed] [Google Scholar]
- 43. May MJ, Entwistle G, Humphries MJ, Ager A. VCAM‐1‐1 is a CS1 peptide‐inhibitable adhesion molecule expressed by lymph node high endothelium. J Cell Sci., 1993, 106, 109–119 [DOI] [PubMed] [Google Scholar]
- 44. Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM‐1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol, 2003, 9, 1409–1414461547310.3748/wjg.v9.i7.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM‐1 gene: essential role of VCAM‐1 in chorioallantoic fusion and placentation. Genes Dev., 1995, 9, 1–14 10.1101/gad.9.1.1 [DOI] [PubMed] [Google Scholar]
- 46. Konac E, Alp E, Onen HI, Korucuoglu U, Biri AA, Menevse S. Endometrial mRNA expression of matrix metalloproteinases, their tissue inhibitors and cell adhesion molecules in unexplained infertility and implantation failure patients. Reprod Biomed Online., 2009, 19, 391–397 10.1016/S1472‐6483(10)60174‐5 [DOI] [PubMed] [Google Scholar]
- 47. Rahman AN, Snibson KJ, Lee CS, Meeusen EN. Effects of implantation and early pregnancy on the expression of cytokines and vascular surface molecules in the sheep endometrium. J Reprod Immunol, 2004, 64, 45–58 10.1016/j.jri.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 48. Henninger DD, Panés J, Eppihimer M, Russell J, Gerritsen M, Anderson DC, Granger DN. Cytokine‐induced VCAM‐1 and ICAM‐1 expression in different organs of the mouse. J Immunol., 1997, 158, 1825–1832 [PubMed] [Google Scholar]
- 49. Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T‐cell homing to lymphoid and non‐lymphoid sites: getting there and staying there. Crit Rev Immunol, 2009, 29, 87–109274446310.1615/CritRevImmunol.v29.i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of β 1 integrins in mice results in inner cell mass failure and peri‐implantation lethality. Genes Develop., 1995, 9, 1883–1895 10.1101/gad.9.15.1883 [DOI] [PubMed] [Google Scholar]
- 51. Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development., 1995, 121, 549–560 [DOI] [PubMed] [Google Scholar]
- 52. Bai R, Bai H, Kuse M, Ideta A, Aoyagi Y, Fujiwara H, Okuda K, Imakawa K, Sakurai T. Involvement of VCAM1 in the bovine conceptus adhesion to the uterine endometrium. Reproduction, 2014, 148, 119–127 10.1530/REP‐13‐0655 [DOI] [PubMed] [Google Scholar]
- 53. Sakurai T, Bai H, Bai R, Arai M, Iwazawa M, Zhang J, Konno T, Godkin JD, Okuda K, Imakawa K. Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biol Reprod, 2012, 87, 1–11 10.1095/biolreprod.112.100180 [DOI] [PubMed] [Google Scholar]
- 54. Biggers JD, Bell JE, Benos DL. Mammalian blastocyst: transport functions in a developing epithelium. Am J Physiol, 1988, 255, C419–C432 [DOI] [PubMed] [Google Scholar]
- 55. Kang HM, Kim K, Kwon HB, Cho WK. Regulation of laminin gene expression in the expansion of mouse blastocysts. Mol Reprod Dev., 1990, 27, 191–199 10.1002/mrd.1080270303 [DOI] [PubMed] [Google Scholar]
- 56. Fleming TP, Sheth B, Fesenko I. Cell adhesion in the preimplantation mammalian embryo and its role in trophectodermal differentiation and blastocyst morphogenesis. Front Biosci, 2000, 6, 1000–1007 10.2741/Fleming [DOI] [PubMed] [Google Scholar]
- 57. Denker HW. Implantation: a cell biological paradox. J Exp Zool, 1993, 266, 541–558 10.1002/jez.1402660606 [DOI] [PubMed] [Google Scholar]
- 58. Wathes DC, Wooding FB. An electron microscopic study of implantation in the cow. Am J Anat., 1980, 159, 285–306 10.1002/aja.1001590305 [DOI] [PubMed] [Google Scholar]
- 59. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype. Nat Rev Cancer, 2007, 7, 415–428 10.1038/nrc2131 [DOI] [PubMed] [Google Scholar]
- 60. Wang X, Zheng M, Liu G, Xia W, McKeown‐Longo PJ, Hung MC, Zhao I. Küppel‐like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res, 2007, 67, 7184–7193 10.1158/0008‐5472.CAN‐06‐4729 [DOI] [PubMed] [Google Scholar]
- 61. Yang J, Weinberg RA. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Develop Cell., 2008, 14, 818–829 10.1016/j.devcel.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 62. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelium‐mesenchymal transitions in development and disease. Cell, 2009, 139, 871–890 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 63. Mess A, Carter AM. Evolutionary transformations of fetal membrane characters in Eutheria with special reference to Afrotheria. J Exp Zool B Mol Dev Evol., 2006, 306, 140–163 10.1002/jez.b.21079 [DOI] [PubMed] [Google Scholar]
- 64. Mess A. Evolutionary transformations of chorioallantoic placental characters in Rodentia with special reference to hystricognath species. J Exp Zool A Comp Exp Biol., 2003, 299, 78–98 10.1002/jez.a.10292 [DOI] [PubMed] [Google Scholar]
- 65. Welsh AO, Enders AC. Trophoblast‐decidual cell interactions and establishment of maternal blood circulation in the parietal yolk sac placenta of the rat. Anat Rec, 1987, 217, 203–219 10.1002/ar.1092170213 [DOI] [PubMed] [Google Scholar]
- 66. Enders AC, Blankenship TN, Conley AJ, Jones CJ. Structure of the midterm placenta of the spotted hyena, Crocuta crocuta, with emphasis on the diverse hemophagous regions. Cells Tissues Organs, 2006, 183, 141–155 10.1159/000095988 [DOI] [PubMed] [Google Scholar]
- 67.Enders AC, Carter AM. What can comparative studies of placental structure tell us?—a review. Placenta. 2004;25 Suppl A:S3–9. [DOI] [PubMed]
- 68. Parseval N, Lazar V, Casella JF, Benit L, Heidmann T. Survey of human genes of retroviral origin: identification and transcriptome of the genes with coding capacity for complete envelope proteins. J Virol, 2003, 77, 10414–1042222846810.1128/JVI.77.19.10414‐10422.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blaise S, Parseval N, Heidmann T. Functional characterization of two newly identified Human Endogenous Retrovirus coding envelope genes. Retrovirology., 2005, 2, 1955574610.1186/1742‐4690‐2‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC, McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature, 2000, 403, 785–789 10.1038/35001608 [DOI] [PubMed] [Google Scholar]
- 71. Blaise S, Parseval N, Benit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelops identifies syncytins 2, a gene conserved on primate evolution. Proc Natl Acad Sci USA, 2003, 100, 13013–1301824073610.1073/pnas.2132646100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Be L, Kanellopoulos C, Sapin V, Dupressoir A, Marceau G, Heidmann T. Placenta‐specific murine envelope genes of retroviral origin conserved in Muridae. Science, 2005, 102, 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arnaud F, Caporale M, Varela M, Biek R, Chessa B, Alberti A, Golder M, Mura M, Zhang YP, Yu L, Pereira F, Demartini JC, Leymaster K, Spencer TE, Palmarini M. A paradigm for virus‐host coevolution: sequential counter‐adaptations between endogenous and exogenous retroviruses. PLoS Pathog, 2007, 11, e170 10.1371/journal.ppat.0030170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heidmann O, Vernochet C, Dupressoir A, Heidmannn T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta‐specific expression in the rabbit: a new “syncytins” in a third order of mammals. Retrovirology., 2009, 6, 107278905310.1186/1742‐4690‐6‐107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cornelis G, Heidmann O, Bernard‐Stoecklin S, Reynaud K, Véron G, Mulot B, Dupressoir A, Heidmann T. Ancestral capture of syncytin‐Car1, a fusogenic endogenous retroviral envelope gene involved in placentation and conserved in Carnivora. Proc Natl Acad Sci USA, 2012, 109, E432–E441328938810.1073/pnas.1115346109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koshi K, Suzuki Y, Nakaya Y, Imai K, Hosoe M, Takahashi T, Kizaki K, Miyazawa T, Hashizume K. Bovine trophoblastic cell differentiation and binucleation involves enhanced endogenous retrovirus element expression. Reprod Biol Endocrinol., 2012, 10, 41341908210.1186/1477‐7827‐10‐41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cornelis G, Heidmann O, Degrelle SA, Vernochet C, Lavialle C, Letzelter C, Bernard‐Stoecklin S, Hassanin A, Mulot B, Guillomot M, Hue I, Heidmann T, Dupressoir A. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc Natl Acad Sci USA, 2013, 110, E828–E837358726310.1073/pnas.1215787110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cornelis G, Vernochet C, Carradec Q, Souquere S, Mulot B, Catzeflis F, Nilsson MA, Menzies BR, Renfree MB, Pierron G, Zeller U, Heidmann O, Dupressoir A, Heidmann T. Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials. Proc Natl Acad Sci USA, 2015, 112, E487–E496432125310.1073/pnas.1417000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boeke JD, Stoye JP Coffin JM. Hughes SH, Varmus HE. Retrotransposons, Endogenous Retroviruses, and the Evolution of Retroelements. Retroviruses, 1997. Cold Spring Harbor (NY) Cold Spring Harbor Laboratory Press; [PubMed] [Google Scholar]
- 80. Best S, Tissier PR, Stoye JP. Endogenous retroviruses and the evolution of resistance to retroviral infection. Trends Microbiol, 1997, 5, 313–318 10.1016/S0966‐842X(97)01086‐X [DOI] [PubMed] [Google Scholar]
- 81. Harris JR. Placental endogenous retrovirus (ERV): structural, functional, and evolutionary significance. BioEssays, 1998, 20, 307–316 10.1002/(SICI)1521‐1878(199804)20:4<307::AID‐BIES7>3.0.CO;2‐M [DOI] [PubMed] [Google Scholar]
- 82. Rawn SM, Cross JC. The evolution, regulation, and function of placenta‐specific genes. Annu Rev Cell Dev Biol, 2008, 24, 159–181 10.1146/annurev.cellbio.24.110707.175418 [DOI] [PubMed] [Google Scholar]
- 83. Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth, Singer O, Trono D, Pfaff SL. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature, 2012, 487, 57–633395470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huppertz B, Kaufmann P, Kingdom J. Trophoblast turnover in health and diseases. Fetal Matern Med Rev., 2002, 13, 103–118 10.1017/S0965539502000220 [Google Scholar]
- 85. Dupressoir A, Marceau G, Vernochet C, Bénit L, Kanellopoulos C, Sapin V, Heidmann T. Syncytin‐A and syncytin‐B, two fusogenic placenta‐specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci USA, 2005, 102, 725–73054554010.1073/pnas.0406509102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schubert SW, Lamoureux N, Kilian K, Klein‐Hitpass L, Hashemolhosseini S. Identification of integrin‐alpha4, Rb1, and syncytin a as murine placental target genes of the transcription factor GCMa/Gcm1. J Biol Chem, 2008, 283, 5460–5465 10.1074/jbc.M710110200 [DOI] [PubMed] [Google Scholar]
- 87. Schreiber J, Riethmacher‐Sonnenberg E, Riethmacher D, Tuerk EE, Enderich J, Bösl MR, Wegner M. Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol Cell Biol., 2000, 20, 2466–24748543910.1128/MCB.20.7.2466‐2474.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mangeney M, Renard M, Schlecht‐Louf G, Bouallaga I, Heidmann O, Letzelter C, Richaud A, Ducos B, Heidmann T. Placental syncytins: genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci USA, 2007, 104, 20534–20539215446610.1073/pnas.0707873105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wooding FB, Beckers JF. Trinucleate cells and the ultrastructural localization of bovine placental lactogen. Cell Tissue Res, 1987, 247, 667–673 10.1007/BF00215761 [DOI] [PubMed] [Google Scholar]
- 90. Dunlap KA, Palmarini M, Varela M, Burghardt RC, Hayashi K, Farmer JL, Spencer TE. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc Natl Acad Sci USA, 2006, 103, 14390–14395159997310.1073/pnas.0603836103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baba K, Nakaya Y, Shojima T, Muroi Y, Kizaki K, Hashizume K, Imakawa K, Miyazawa T. Identification of novel endogenous betaretroviruses which are transcribed in the bovine placenta. J Virol, 2011, 85, 1237–1245302049510.1128/JVI.01234‐10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nakaya Y, Koshi K, Nakagawa S, Hashizume K, Miyazawa T. Fematrin‐1 is involved in fetomaternal cell‐to‐cell fusion in Bovinae placenta and has contributed to diversity of ruminant placentation. J Virol, 2013, 87, 10563–10572380741910.1128/JVI.01398‐13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wooding FB. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta, 1992, 13, 101–113 10.1016/0143‐4004(92)90025‐O [DOI] [PubMed] [Google Scholar]
- 94.Carter AM. Evolution of placental structure and function in ruminants. In: Juengel JE, Miyamoto A, Price C, Reynolds LP, Smith MF, Webb R, editors. Reproduction in domestic ruminants VIII. England: Context Products Ltd. 2014. p. 387–99.
- 95. Nakagawa S, Bai H, Sakurai T, Nakaya Y, Konno T, Miyazawa T, Gojobori T, Imakawa K. Dynamic evolution of endogenous retrovirus‐derived genes expressed in bovine conceptuses during the period of placentation. Genome Biol Evol., 2013, 5, 296–306359076510.1093/gbe/evt007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ratajczak J, Wysoczynski M, Hayek F, Janowska‐Wieczorek A, Ratajczak MZ. Membrane‐derived microvesicles: important and underappreciated mediators of cell‐to‐cell communication. Leukemia, 2006, 20, 1487–1495 10.1038/sj.leu.2404296 [DOI] [PubMed] [Google Scholar]
- 97. Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Lett, 2009, 583, 1792–1799 10.1016/j.febslet.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 98. Mincheva‐Nilsson L, Baranov V. Placenta‐derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol, 2014, 72, 440–457 10.1111/aji.12311 [DOI] [PubMed] [Google Scholar]
- 99.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte‐'t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. [DOI] [PMC free article] [PubMed]
- 100. Mincheva‐Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol, 2010, 63, 520–533 10.1111/j.1600‐0897.2010.00822.x [DOI] [PubMed] [Google Scholar]
- 101. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta, 2012, 820, 940–948 10.1016/j.bbagen.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 102. Gong R, Peng X, Kang S, Feng H, Huang J, Zhang W, Lin D, Tien P, Xiao G. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family W. Biochem Biophys Res Commun., 2005, 331, 1193–1200 10.1016/j.bbrc.2005.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Record M. Intercellular communication by exosomes in placenta: a possible role in cell fusion?. Placenta, 2014, 35, 297–302 10.1016/j.placenta.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 104. Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent‐Schneider H, Lankar D, Salles JP, Bonnerot C, Perret B, Record M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett, 2004, 572, 11–14 10.1016/j.febslet.2004.06.082 [DOI] [PubMed] [Google Scholar]
- 105. Record M, Subra C, Silvente‐Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol, 2011, 81, 1171–1182 10.1016/j.bcp.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 106. Huppertz B, Borges M. Placenta trophoblast fusion. Methods Mol Biol, 2008, 475, 135–147 10.1007/978‐1‐59745‐250‐2_8 [DOI] [PubMed] [Google Scholar]
- 107.Record M. Exosomal lipids in cell–cell communication. In: Zhang H‐G, editor. Emerging concepts of tumor exosome‐mediated cell–cell communication. USA: Springer; 2012. p. 47–68.


