Abstract
Maternal aging profoundly affects oocyte quality. This has become common knowledge in industrialized countries and extensive studies addressing the causes and possible countermeasures against age‐associated deterioration of oocytes suggest that mitochondrial dysfunction is a causal factor in infertility. However, almost all studies addressing age‐associated events in oocytes have used mice as an animal model, and the reproductive life of mice is very short, making it difficult to study the gradual decline in fertility observed in humans. In the present review, age‐associated changes in the quality and quantity of bovine oocytes and possible countermeasures related to mitochondrial quality control are introduced.
Keywords: Aging, Cows, Mitochondria, Oocyte, SIRT1
Introduction
Age‐associated decline in fertility is common in mammals. In industrialized countries, the age of womenˈs first birth has increased to approximately 30 years; in women, fertility declines after 35 years of age [1, 2]. The causal factors of age‐associated infertility and possible countermeasures against infertility have become social concerns. Clinical reports from in vitro fertilization have shown that the transfer percentage of women's embryos that result in live birth declines after 35 years, while it remains constant in the case of donor embryos [3], which indicates that the main cause of age‐associated decline in fertility is low number or quality of oocytes and/or embryos. However, ethical and physical restrictions hamper studies of age‐associated events in human oocytes and embryos, and there is, therefore, a need for appropriate animal models.
Cows have a longer reproductive life (approximately 13 years) compared with other model animals, including rodents [4], and they show similar follicular wave, follicle selection, ovulation patterns as well as age‐associated endocrinal changes to humans. [5, 6, 7, 8, 9]. In addition, many oocytes can easily be collected from slaughterhouse‐derived ovaries or in vivo by using ovum pick‐up (OPU) technology. Thus, cows have been suggested to be a good animal model for reproductive aging in humans. Moreover, in Japan, cows are tracked at a high degree of detail, allowing the breeds, age (in months) and farms of origin to be identified at the slaughterhouse. Thus, we have studied age‐associated events in bovine oocytes and embryos. This review introduces age‐associated changes in quality and quantity of bovine oocytes and suggests possible counter measures against age‐associated events in bovine oocytes using mitochondrial quality control.
Follicle number in bovine ovaries
In general, age‐associated infertility is believed to be related to a decline in the oocyte pool; the decline in oocyte reserves occurs on the premise that the follicle pool is established before birth and no de novo synthesis of oocytes occurs during a woman's life. Although the presence of germ cells in epithelial cells of ovaries or bone marrow is inferred [10, 11], the age‐associated decline in follicle number has been supported by the facts that the number of primordial follicles and antral follicles decreases as women age [12, 13, 14] and the follicle reserve is nearly exhausted by the age of 50 [15]. Singh et al. first reported that in cows, the number of small antral follicles with a 4–5‐mm in diameter decreased with aged and the follicle‐stimulating hormone (FSH) concentration in the plasm increases, as seen in aged women [16], and the negative relationship between the reduction in antral follicle number and the age of cows has also been reported in other studies [17, 18]. However, the chronological changes in the number of follicles at various developmental stages have not been clarified in cows. We then collected ovaries from a total of 131 Japanese black cows ranging in age from 25 to 217 months and histologically examined the number of follicles at various developmental stages. Figure 1 shows the relationship between donor age and the number of primordial, early primary, primary and secondary follicles. The number of primordial follicles and early primary follicles decreased as donor age increased and a significantly negative correlation between age of donor and the number of follicles was observed, whereas the negative correlation diminished for primary and secondary follicles. Interestingly, some aged donor cows possessed a large number of secondary follicles. Considering the small number of antral follicles (3–6 mm in diameter) in the ovaries of aged cows [18], it is speculated that development of pre/early antral follicles to antral follicles is inactive in aged cows. In line with this, we examined the diameter of oocytes in antral follicles (AFs, 3–6 mm in diameter) and early antral follicles (EAFs, 0.5–0.7 mm) and compared them between young (25–50 months) and aged (>120 months) cows. In aged cows, the oocytes collected from antral follicles were smaller than those collected from younger cows, whereas oocytes of EAFs were larger than those of their younger counterparts [19]. An age‐associated decline in the size of oocytes from antral follicles has been also reported in mice and humans [20, 21]. From these results, it is suggested that in vivo oocyte development is suspended after some development progression of the EAFs and the development of oocytes to the antral follicle stage is inactive in aged cows.
Figure 1.
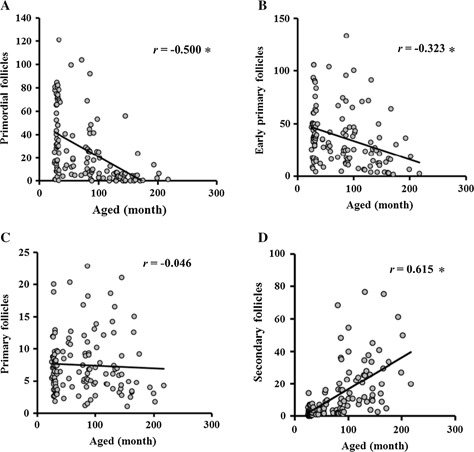
Relationship between donor age and relative number of follicles. The x axis shows donor age in months, and y axis shows the relative number of primordial (a), early primary (b), primary (c) and secondary follicles (d). * P < 0.01. The number of follicles was counted in randomly selected areas under the same microscope, and the average value was multiplied by (the ovary weight)2/3. These figures are modified from Itami et al. 2015 (Japanese Journal of Embryo Transfer 2016 Vol. 37)
Age‐associated decline in oocyte growth and factors underlying the impaired development
In vitro culture of small premature follicles is an easy method to investigate age‐associated effects on oocyte growth. In some species, age‐associated decline in in vitro development of small follicles has been reported. Xue et al. reported that pre‐antral follicles of aged rhesus monkeys develop slower than those of their younger counterparts [22]. Choi et al. reported that the number of follicles in the ovaries of mice decreased with age, and the ability for in vitro follicle formation is lower in aged mice than in young mice [23]. However, in large animals, oocyte growth requires a long period, and a culture system for in vitro growth of oocytes has not been established in cows or in almost all other large animals. EAFs are the final follicular stage, and give rise to a live calf following transfer of embryos derived from oocytes grown in vitro. To examine age‐associated changes in the developmental ability of oocytes derived from EAFs, we collected oocytes and granulosa cell complexes (OGCs) from EAFs of 92 aged (>120 months) and 73 young (25–35 months) cows and cultured them for 16 days. The developmental ability of the OGCs, as determined by antrum formation, fertilization outcome and development to the blastocyst stage, was low for oocytes derived from aged cows, which showed low antrum formation, a high abnormal fertilization rate and a low total cell number of the blastocysts [19]. This result suggests that age‐associated deterioration occurs even at the EAF stage in cows. To investigate the molecular mechanism underlying impaired oocyte growth, we examined the gene expression profiles of the granulosa cells of EAFs collected from 11 young (average 28.3 ± 0.7 months) and aged cows (164 ± 6.1 months) by using next generation sequencing (NGS) technology. To date, no study has reported the standard gene expression profiles in granulosa cells of healthy developing EAFs, and, therefore, we used a data set obtained from previous comparisons of gene expression in granulosa cells between large healthy follicles and subordinated follicles [24, 25, 26, 27, 28, 29, 30] (Fig. 2). The results of gene expression analysis showed that all genes reportedly associated with subordinate follicles were expressed at higher levels in aged cows, whereas many parts of genes associated with the large healthy follicles were expressed at lower levels in aged cows (Fig. 2). The results supports our hypothesis that development of EAFs to AFs is inactivated in aged cows; moreover, the results raises the possibility that the age‐associated decline in oocyte development is partly caused by functional deterioration of surrounding cells. In addition, our comprehensive gene expression analysis showed that expression of genes related to glutathione appeared to be lower in granulosa cells of aged cows and that the glutathione (GSH) content of the EAFs was also lower in aged cows [19]. Similarly, it has been reported that the superoxide dismutase 1 and 2 (SOD1, SOD2) and catalase contents in granulosa cells are low in granulosa cells of aged women and that oxidative stress in granulosa cells, determined by phosphorylated p38 mitogen‐activated protein kinase (MAPK), is higher in aged women compared with their younger counterparts [31, 32]. Moreover, Goto et al. [33] reported another age‐specific feature in granulosa cells, that proliferation activity and global DNA methylation is lower for aged cows than for young cows. Thus, age‐associated decline in granulosa cell quality and function may be a causal factor of age‐associated decline in oocyte growth.
Figure 2.
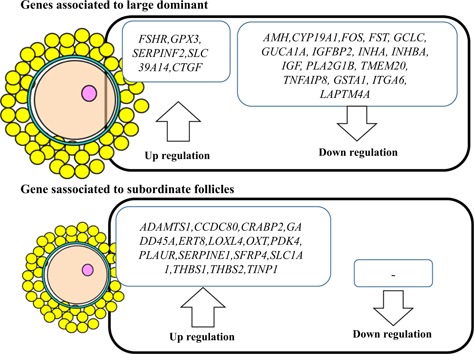
Upregulation or downregulation of genes of granulosa cells derived from aged cows compared with younger cows. Gene expression in granulosa cells of early antral follicles of young and aged cows was examined by using next generation sequencing technology and compared between the two age groups. In the granulosa cells of aged cows, expression of genes associated with subordinate follicles was higher in granulosa cells, and expression of many genes associated with large dominant follicles was lower in granulosa cells compared with the younger counterparts. Summarized from Itami et al. [19]
Age‐associated changes in quality of oocytes derived from antral follicles of aged cows
The decline in oocyte quality has been demonstrated to be a major causal factor of infertility in aged women [34, 35]. However, due to physical and ethical restrictions, it is difficult to compare oocyte quality between young and aged healthy women. Singh et al. compared the oocytes of cows aged 13–16 years (years of age) and their daughters aged 3–6 years (years of age) and reported that follicle number and the responsiveness of ovaries to the hormonal stimulation was lower in aged cows, and that fertilization ability of the oocytes decreased based on the results that the number of oocytes and/or embryos collected was the same between the two age groups, whereas the rate of cleavage was decreased [36]. Su et al. [37] collected oocytes by ovum pick up (OPU) from aged (>15 years) and young (12 months) cows and found that the developmental ability of oocytes to reach the blastocyst stage, and the plasma concentrations of estradiol, were low in the aged groups. We collected oocytes from slaughterhouse‐derived ovaries of Japanese black cows and compared in vitro maturation and fertilization ability between 338 aged cows (average age 156.7 months) and 323 young cows (average age 27.3 months). We observed that oocytes derived from aged cows showed premature progression of nuclear maturation and lower fertilization ability [18]. In addition, we examined the relationship between fertilization ratio and donor age in months using 65 cows ranging from 22 to 174 months and found a significant positive correlation between donor age and rate of abnormal fertilization [38]. After fertilization, embryos derived from aged cows cleaved at a low frequency and the percentage of embryos that reached the 4–8 cell stage at 48 h after fertilization was negatively correlated with donor age [39]. Furthermore, blastocysts derived from oocytes of aged cows had fewer number of blastomeres than those of their younger counterparts [40]. In this study, we also compared gene expression in oocytes and embryos between young and aged cows by using NGS, and revealed that differential expression of genes in oocytes between young and aged cows is related to oxidative phosphorylation and mitochondrial dysfunction. We also showed that at the 8–16 cell stage, when zygotic genome activation occurs in cows, comprehensive gene expression levels were lower in aged cows than in young cows. Furthermore, levels of reactive oxygen species (ROS) were higher in the oocytes of aged cows. These results suggest that mitochondrial function and quality are potential foci for study of age‐associated events in bovine oocytes.
Age‐associated changes in mitochondrial quality and quality in oocytes
Mitochondria are double‐membraned cellular organelles that play crucial roles in cellular energy production, apoptosis, and calcium homeostasis [41]. In addition, mitochondria contain their own circular DNA, consisting of 13 genes that encode respiratory chain enzymes, 22 genes encoding tRNA genes, and 2 genes encoding rRNA and other proteins; while nuclear genes encode other mitochondrial proteins to be transported to mitochondria [42]. Interestingly, mitochondrial DNA (mtDNA) in cells turns over independent of cellular cycles [43], and mitochondria in somatic cells generally contain 2 to 10 copies of their DNA. Unlike in somatic cells, the mitochondrial number can be predicted by determining the DNA copy number on the premise that the mtDNA copy number in mitochondria of oocytes is only one or two [44]. Thus, the mitochondrial number in oocytes has been determined in humans [45], cows [39, 46], and pigs [47], horses [48], and sheep [49]. In the ovaries, almost all oocytes stay in the primordial follicle stage, and the mitochondrial number in oocytes of primordial follicles is expected to be in the thousands [50]. Once oocytes begin to develop, the mitochondrial number, as determined by DNA copy number, increases with oocyte growth, finally reaching approximately 200,000–1,000,000 in oocytes in antral follicles of cows and pigs [38, 51]. The significance of mitochondria for development of oocytes has been evaluated based on the reports that low mitochondrial numbers are associated with oocytes and embryos with low fertilization and developmental ability and ovarian insufficiency [52, 53, 54, 55, 56]. In addition, Wai et al. [57] created germline‐specific heterozygous Tfam knockout mice to reduce the mitochondrial number in oocytes and concluded that a mtDNA copy number below 50,000 copies impairs developmental abilities.
Age‐associated changes in mitochondrial quality and quantity have been used to explain the age‐related deterioration of cellular functions [58, 59]. In older women, oocytes have been reported to show low number of mitochondria, as determined by the fraction of the ooplasma occupied by mitochondria [60]. In line with this, Chan et al. [61] reported an age‐associated decline in mtDNA copy number in human oocytes. Contrary to these reports, Reynier et al. [52] reported that due to the high variation in mitochondrial number among oocytes it is difficult to precisely evaluate the relationship between donor age and mitochondrial number. Furthermore, Barritt et al. [62] examined 87 oocytes collected from 29 women and showed no relationship between donor age and mitochondrial number. A major drawback of this method based on mtDNA is that mitochondrial number varies widely among the cohort of oocytes, even those collected from a single donor. Thus, a large number of oocytes collected from the same donor is needed to precisely predict the mitochondrial copy number for an individual donor. Based on this notion, we collected 20 oocytes from each donor cow and pig and divided them into two groups, each consisting of 10 oocytes. When the average mitochondrial number of oocytes was predicted using the two oocyte groups individually, the two predicted DNA copies were highly correlated, which indicates that using 10 oocytes from an individual donor is sufficient to predict the average mitochondrial number for each donor cow or pig [38, 51]. We then collected 10 oocytes from 180 donor cows ranging from 20 to 197 months of age and determined the average mitochondrial number of in vitro‐matured oocytes for each donor cow. We found that once the age of a donor exceeded 70 months, there was a significantly negative relationship between mtDNA copy number and donor age [38]. A reduction in mtDNA has been reported in mice; oocytes collected from mice older than 300 days contained a lower number of mitochondria; In addition, oocytes from aged females contained differentiated and elongated mitochondria, whereas oocytes from young mice contained immature undifferentiated mitochondria. [63] Similar aged‐associated mitochondrial features were also reported in equine oocytes [48]. Another marker of age‐associated decline in mitochondrial quality is adenosine triphosphate (ATP) content, and a low ATP content in oocytes of aged females has been reported in mice and hamsters [64]. This decrease in mitochondrial function is speculated to be a factor in abnormal meiosis, as mitochondrial dysfunction disturbs spindle integrity [65, 66]. These data indicate that low mitochondrial quantity as well as quality are major causes of age‐associated decline in oocyte quality.
Mitochondrial quality control in cells
Mitochondrial quality is important for cellular homeostasis and is maintained through a quality control system that includes antioxidants, chaperones, proteases, and ubiquitin–proteasomes. [67]. In addition, mitochondrial morphology changes via fission and fusion in response to mitochondrial and cellular conditions [68, 69]. When mitochondria can no longer function, and their function can't be restored through the quality control systems, they are removed from cells via mitophagy. One major mitochondrial removal system is the PARKIN–PINK1 pathway. In normal mitochondria PINK1, a serine/threonine kinase is imported into the inner mitochondrial membrane (IMM) and cleaved by the IMM protease, PARL, and subsequently degraded [70, 71]. However, in dysfunctional mitochondria with a dispersed membrane potential, PINK1 binds to the outer mitochondrial membrane and the activated PINK1 which recruits PARKIN, an E3 ubiquitin ligase, triggering autophagic degeneration of the mitochondria [72, 73]. In line with this, carbonyl cyanide m‐chlorophenyl hydrazine (CCCP) treatment disperses the mitochondrial membrane potential and induces recruitment of PARKIN [74, 75] and autophagy‐related proteins, including p62/SQSTM1, NRB1, LC3, and LC3 family members [76], to the mitochondrial outer membrane. Overexpression of PARKIN removes abnormal mitochondria from the cells and improves mitochondrial functions [77, 78, 79]. Although many studies have been performed on mitochondrial quality control in somatic cells, how mitochondrial quality is maintained in oocytes remains unclear, and most studies have focused on the fate of sperm mitochondria following fertilization [80, 81, 82, 83].
Mitochondrial quality control in oocytes
In somatic cells, accumulating evidence shows that mitochondrial quality and quantity are regulated through mitochondrial fusion and fission, along with de novo synthesis and degradation by proteasomes and mitophagy according to the cellular conditions [84]. In addition, it is believed that the mitochondrial quality control system deteriorates with aging [68]. However, how mitochondrial quality is maintained in oocytes or how maternal aging affects the quality control system is unclear. To examine mitochondrial de novo synthesis and degradation, monitoring the total mitochondrial mass is insufficient. As such, a sophisticated technique that assesses an embryoˈs mitochondria‐targeted green fluorescent protein (mito‐GFP) has been developed [85]. However, this technique can't be used in oocytes of large mammals. Proteasomes play a role in mitochondrial degeneration in cells, and proteasome inhibitors suppress mitochondrial degeneration, increasing the mitochondrial mass in cells [76, 86, 87]. When oocytes are cultured in medium containing MG132 (a proteasome inhibitor), mtDNA copy number as well as ubiquitinated proteins are increased in porcine oocytes [51], indicating that mitochondrial generation can be examined in a medium that inhibits mitochondrial removal. We collected oocytes from porcine ovaries and treated them for 2 h with CCCP which disperses the mitochondrial membrane potential; the ATP content of oocytes was reduced, whereas the amount of ROS increased in CCCP‐treated oocytes. When these oocytes were further incubated in a general maturation medium, mtDNA copy number did not differ between CCCP‐treated and un‐treated oocytes. Whereas, when these oocytes were cultured in medium containing MG132, mtDNA copy number significantly increased in CCCP‐treated oocytes compared with un‐treated oocytes, indicating induced mitochondrial dysfunction stimulates mitochondrial de novo synthesis and degeneration [47]. This notion is supported by the upregulation of the expression level of TFAM and increased puncta of LC3, a marker of autophagosomes, observed in CCCP‐treated oocytes [88]. These data suggest that oocytes possess a mechanism for monitoring mitochondrial functions and/or cellular conditions, and if mitochondrial dysfunction is detected, de novo synthesis and degradation will be up regulated to replenish the mitochondrial pool. In addition, treatment of oocytes with CCCP for 2 h enhanced the expression levels of phosphorylated 5ˈ adenosine monophosphate‐activated protein kinase (AMPK) and silent mating type information regulation 1 (SIRT1) in oocytes. SIRT1 and AMPK are key cellular energy sensors, and SIRT1 has been reported to show protective effect through enhanced mitochondrial generation in ischemic injury [89]. Given these results, we hypothesized that controlling SIRT1 activity may impact the mitochondrial quality control system in oocytes.
Enhanced SIRT1 expression improves the quality of oocytes derived from aged cows
SIRT1 is a sirtuin family member that dependently acetylates proteins (NAD+) and affects various cellular metabolic functions, including regulating mitochondrial biogenesis and degeneration [90, 91]. Resveratrol (trans‐3,4′,5′‐trihydroxystilbene) is a polyphenol present in a wide variety of plants, including grapes (skin), mulberries, and peanuts, and it has attracted much attention for its therapeutic potential. Resveratrol activates SIRT1 and AMPK and triggers mitochondrial biogenesis and autophagy, and regulates glucose and lipid homeostasis as well as immune response [92].
Considering the stimulative effect of resveratrol on mitochondrial biogenesis and degradation, one can speculate that upregulation of mitochondrial replenishment aids in maintenance of cellular viability [93]. To date, several studies have demonstrated that resveratrol mediates the maintenance of cellular functions; for example, resveratrol enhanced SIRT1 and AMPK activity and ameliorated mitochondrial dysfunction through autophagy in SH‐SY5Y cells [94], and resveratrol activated SIIRT1 in H9C2 cells and ameliorated H2O2‐induced cytotoxicity by upregulation of mitochondrial biogenesis through SIRT signaling [95]. Furthermore, resveratrol enhanced survivability of H9c2 cardiac cells by inhibition of mTOR and activation of autophagy [96]. In this context, we examined the effect of resveratrol on oocytes of pigs and cows.
A culture of porcine oocytes in in vitro maturation medium containing 10‐μM resveratrol enhances SIRT1 expression in oocytes and increases generation and degeneration of mitochondria during the maturation period; this effect was diminished by supplementation of the medium with the SIRT1 inhibitor, EX527. Furthermore, treatment of oocytes with resveratrol improved mitochondrial functions, including ATP generation and developmental ability of oocytes to the blastocyst stage [51]. These results indicate that upregulation of SIRT1 results in replenishment of mitochondria in oocytes and, hence, improves oocyte quality. In line with this, supplementation of maturation medium with resveratrol improves fertilization ability of oocytes collected from ovaries of both young and aged cows [40]. Furthermore, SIRT1 closely interacts with AMPK [91], and upregulation of the AMPK activity of oocytes by aminoimidazole carboxamide ribonucleotide (AICAR) treatment improves fertilization outcome and mitochondrial functions of bovine oocytes [97]. Mitochondria are actively generated and increase in number during oocyte growth. As mentioned above, in vitro growth of oocytes derived from EAFs and the developmental ability of oocytes grown in vitro was low in aged cows; in addition, the characteristics of the granulosa cells are similar to those collected from subordinated follicles [19]. Supplementation with resveratrol of an in vitro culture medium of OGCs collected from EAFs of aged cows (>120 months) enhanced the expression level of SIRT1 in both oocytes and granulosa cells, and increased the mtDNA copy number in fully developed oocytes compared with those grown without resveratrol, whereas markers of autophagosomes including LC3 band patterns, as detected by western blot analysis, and the number of LC3‐positive dots following immunostaining were increased by resveratrol treatment. Furthermore, oocytes grown with resveratrol showed greater developmental ability to reach the blastocyst stage than oocytes grown without resveratrol. Gene expression analysis using NGS revealed that the gene expression profiles of granulose cells were improved from the subordinate follicle pattern to the large healthy follicle pattern [98]. These results indicate that upregulation of SIRT1 by resveratrol improves the condition of both granulosa cells and oocytes derived from EAFs of aged cows, partly through replenishment of mitochondria.
Perspective
Mitochondrial dysfunction, including mutation of mtDNA, is a causal factor not only for age‐associated infertility but also for several serious diseases. Pronuclei, spindle, and polar body transfer have been proposed as clinical countermeasures for oocytes with mtDNA mutations [99]. In addition, cryopreservation of oocytes collected at a younger age has been proposed to bypass age‐associated cytosolic deterioration [100]. Here, we propose recuperation of oocytes from aged cows by replenishment of mitochondria through activation of the quality control system (Fig. 3). However, fundamental questions remain, such as; the molecular mechanism underlying the age‐associated deterioration of mitochondria, whether these mitochondrial dysfunctions can be restored through the quality control system, and whether aging affects the mitochondrial quality control system in oocytes. It is clear that further studies are needed to better understand age‐associated events in oocytes.
Figure 3.
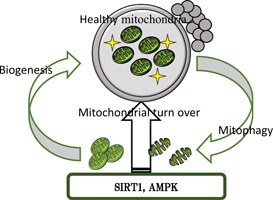
Schematic diagram of regeneration of oocytes through mitochondrial biogenesis and degradation via SIRT1 and/or AMPK activation
Compliance with ethical standards
Conflict of interest
Hisataka Iwata declares that he has no conflicts of interest.
Human studies
This article does not contain any studies with human subjects performed by any of the authors.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1. Hull MG, Fleming CF, Hughes AO, McDermott A. The age‐related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril, 1996, 65, 783–790 10.1016/S0015‐0282(16)58214‐4 [DOI] [PubMed] [Google Scholar]
- 2. Spandorfer SD, Davis OK, Barmat LI, Chung PH, Rosenwaks Z Fertil Steril, 2004, 81, 1265–1269 10.1016/j.fertnstert.2003.09.057 [DOI] [PubMed] [Google Scholar]
- 3.Assisted reproductive technology success rates 2005, national summary and fertility clinic reports. 974208. [DOI] [PubMed]
- 4. Erickson BH, Reynolds RA, Murphree RL. Ovarian characteristics and reproductive performance of the aged cow. Biol Reprod, 1976, 15, 555–560 10.1095/biolreprod15.4.555 [DOI] [PubMed] [Google Scholar]
- 5. Adams GP. Comparative patterns of follicle development and selection in ruminants. J Reprod Fertil Suppl, 1999, 54, 17–32 [PubMed] [Google Scholar]
- 6. Ireland JJ, Mihm M, Austin E, Diskin MG, Roche JF. Historical perspective of turnover of dominant follicles during the bovine estrous cycle: key concepts, studies, advancements, and terms. J Dairy Sci, 2000, 83, 1648–1658 10.3168/jds.S0022‐0302(00)75033‐8 [DOI] [PubMed] [Google Scholar]
- 7. Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod, 2003, 69, 1023–1031 10.1095/biolreprod.103.017772 [DOI] [PubMed] [Google Scholar]
- 8. Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril, 2003, 80, 116–122 10.1016/S0015‐0282(03)00544‐2 [DOI] [PubMed] [Google Scholar]
- 9. Adams GP, Jaiswal R, Singh J, Malhi P. Progress in understanding ovarian follicular dynamics in cattle. Theriogenology, 2008, 69, 72–80 10.1016/j.theriogenology.2007.09.026 [DOI] [PubMed] [Google Scholar]
- 10. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature, 2004, 11 (428) 145–150 10.1038/nature02316 [DOI] [PubMed] [Google Scholar]
- 11. Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction, 2006, 132, 95–109 10.1530/rep.1.01128 [DOI] [PubMed] [Google Scholar]
- 12. Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci, 1963, 22 (158) 417–433 10.1098/rspb.1963.0055 [DOI] [PubMed] [Google Scholar]
- 13. Block E. Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat (Basel)., 1952, 14, 108–123 10.1159/000140595 [DOI] [PubMed] [Google Scholar]
- 14. Schuh‐Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI. Reijo Pera RA. Genetic markers of ovarian follicle number and menopause in women of multiple ethnicities. Hum Genet, 2012, 131, 1709–1724 10.1007/s00439‐012‐1184‐03470691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid‐life: implications for forecasting menopause. Hum Reprod, 1992, 7, 1342–1346 [DOI] [PubMed] [Google Scholar]
- 16. Malhi PS, Adams GP, Singh J. Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics. Biol Reprod, 2005, 73, 45–53 10.1095/biolreprod.104.038745 [DOI] [PubMed] [Google Scholar]
- 17. Cushman RA, Allan MF, Kuehn LA, Snelling WM, Cupp AS, Freetly HC. Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: investigation of influence of stage of the estrous cycle, age, and birth weight. J Anim Sci, 2009, 87, 1971–1980 10.2527/jas.2008‐1728 [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto T, Iwata H, Goto H, Shiratuki S, Tanaka H, Monji Y, Kuwayama T. Effect of maternal age on the developmental competence and progression of nuclear maturation in bovine oocytes. Mol Reprod Dev, 2010, 77, 595–604 10.1002/mrd.21188 [DOI] [PubMed] [Google Scholar]
- 19. Itami N, Kawahara‐Miki R, Kawana H, Endo M, Kuwayama T, Iwata H. Age‐associated changes in bovine oocytes and granulosa cell complexes collected from early antral follicles. J Assist Reprod Genet, 2014, 31, 1079–1088 10.1007/s10815‐014‐0251‐y4130926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manosalva I, González A. Aging changes the chromatin configuration and histone methylation of mouse oocytes at germinal vesicle stage. Theriogenology, 2010, 74, 1539–1547 10.1016/j.theriogenology.2010.06.024 [DOI] [PubMed] [Google Scholar]
- 21. Valeri C, Pappalardo S, Felici M, Manna C. Correlation of oocyte morphometry parameters with woman's age. J Assist Reprod Genet, 2011, 28, 545–552 10.1007/s10815‐011‐9555‐33158254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three‐dimensional culture: effects of gonadotropins and insulin. Reproduction, 2010, 140, 685–697 10.1530/REP‐10‐02843351200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi JK, Ahn JI, Park JH, Lim JM. Derivation of developmentally competent oocytes by in vitro culture of preantral follicles retrieved from aged mice. Fertil Steril, 2011, 15 (95) 1487–1489 10.1016/j.fertnstert.2010.12.062 [DOI] [PubMed] [Google Scholar]
- 24. Greenaway J, Gentry PA, Feige JJ, LaMarre J, Petrik JJ. Thrombospondin and vascular endothelial growth factor are cyclically expressed in an inverse pattern during bovine ovarian follicle development. Biol Reprod, 2005, 72, 1071–1078 10.1095/biolreprod.104.031120 [DOI] [PubMed] [Google Scholar]
- 25. Harlow CR, Bradshaw AC, Rae MT, Shearer KD, Hillier SG. Oestrogen formation and connective tissue growth factor expression in rat granulosa cells. J Endocrinol, 2007, 2007 (192) 41–52 10.1677/joe.1.06689 [DOI] [PubMed] [Google Scholar]
- 26. Assidi M, Dufort I, Ali A, Hamel M, Algriany O, Dielemann S, Sirard MA. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle‐stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod, 2008, 79, 209–222 10.1095/biolreprod.108.067686 [DOI] [PubMed] [Google Scholar]
- 27. Rasmussen LS, Gisvold SE. New author guidelines. Acta Anaesthesiol Scand., 2008, 52, 594–595 10.1111/j.1399‐6576.2008.01662.x [DOI] [PubMed] [Google Scholar]
- 28. Chen AQ, Wang ZG, Xu ZR, Yu SD, Yang ZG. Analysis of gene expression in granulosa cells of ovine antral growing follicles using suppressive subtractive hybridization. Anim Reprod Sci., 2009, 115, 39–48 10.1016/j.anireprosci.2008.10.022 [DOI] [PubMed] [Google Scholar]
- 29. Hayashi KG, Ushizawa K, Hosoe M, Takahashi T. Differential genome‐wide gene expression profiling of bovine largest and second‐largest follicles: identification of genes associated with growth of dominant follicles. Reprod Biol Endocrinol., 2010, 5, 8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mora JM, Fenwick MA, Castle L, Baithun M, Ryder TA, Mobberley M, Carzaniga R, Franks S, Hardy K. Characterization and significance of adhesion and junction‐related proteins in mouse ovarian follicles. Biol Reprod, 2012, 153, 1–14 [DOI] [PubMed] [Google Scholar]
- 31. Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age‐dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod, 2006, 12, 655–660 10.1093/molehr/gal080 [DOI] [PubMed] [Google Scholar]
- 32. Ito M, Miyado K, Nakagawa K, Muraki M, Imai M, Yamakawa N, Qin J, Hosoi Y, Saito H, Takahashi Y. Age‐associated changes in the subcellular localization of phosphorylated p38 MAPK in human granulosa cells. Mol Hum Reprod, 2010, 16, 928–937 10.1093/molehr/gaq076 [DOI] [PubMed] [Google Scholar]
- 33. Goto H, Iwata H, Takeo S, Nisinosono K, Murakami S, Monji Y, Kuwayama T. Effect of bovine age on the proliferative activity, global DNA methylation, relative telomere length and telomerase activity of granulosa cells. Zygote., 2013, 21, 256–264 10.1017/S0967199411000499 [DOI] [PubMed] [Google Scholar]
- 34. Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, Wei YC, Yan J, Wang WH, Shen W, Sun SC, Schatten H, Sun QY. The root of reduced fertility in aged women and possible therapentic options: current status and future perspects. Mol Aspects Med, 2014, 38, 54–85 10.1016/j.mam.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 35. Crawford NM, Steiner AZ. Age‐related infertility. Obstet Gynecol Clin North Am, 2015, 42, 15–25 10.1016/j.ogc.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 36. Malhi PS, Adams GP, Mapletoft RJ, Singh J. Superovulatory response in a bovine model of reproductive aging. Anim Reprod Sci., 2008, 109, 100–109 10.1016/j.anireprosci.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 37. Su L, Yang S, He X, Li X, Ma J, Wang Y, Presicce GA, Ji W. Effect of donor age on the developmental competence of bovine oocytes retrieved by ovum pick up. Reprod Domest Anim, 2012, 47, 184–189 10.1111/j.1439‐0531.2009.01349.x [DOI] [PubMed] [Google Scholar]
- 38. Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev, 2011, 23, 424–432 10.1071/RD10133 [DOI] [PubMed] [Google Scholar]
- 39. Takeo S, Goto H, Kuwayama T, Monji Y, Iwata H. Effect of maternal age on the ratio of cleavage and mitochondrial DNA copy number in early developmental stage bovine embryos. J Reprod Dev., 2013, 59, 174–179 10.1262/jrd.2012‐148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takeo S, Kawahara‐Miki R, Goto H, Cao F, Kimura K, Monji Y, Kuwayama T, Iwata H. Age‐associated changes in gene expression and developmental competence of bovine oocytes, and a possible countermeasure against age‐associated events. Mol Reprod Dev, 2013, 80, 508–521 10.1002/mrd.22187 [DOI] [PubMed] [Google Scholar]
- 41. Ryan MT, Hoogenraad NJ. Mitochondrial‐nuclear communications. Annu Rev Biochem, 2007, 76, 701–722 10.1146/annurev.biochem.76.052305.091720 [DOI] [PubMed] [Google Scholar]
- 42. Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, Meisinger C. The protein import machinery of mitochondria‐a regulatory hub in metabolism, stress, and disease. Cell Metab, 2014, 19, 357–372 10.1016/j.cmet.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 43. Bogenhagen D, Clayton DA. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell, 1977, 11, 719–727 10.1016/0092‐8674(77)90286‐0 [DOI] [PubMed] [Google Scholar]
- 44. Pikó L, Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol, 1976, 49, 1–10 10.1016/0012‐1606(76)90253‐0 [DOI] [PubMed] [Google Scholar]
- 45. Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, Ridout KK, Price LH. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol, 2015, 66, 17–20 10.1016/j.exger.2015.04.0024459604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Endo M, Kimura K, Kuwayama T, Monji Y, Iwata H. Effect of estradiol during culture of bovine oocyte‐granulosa cell complexes on the mitochondrial DNA copies of oocytes and telomere length of granulosa cells. Zygote., 2014, 22, 431–439 10.1017/S0967199412000603 [DOI] [PubMed] [Google Scholar]
- 47. Itami N, Shiratsuki S, Shirasuna K, Kuwayama T, Iwata H. Mitochondrial biogenesis and degradation are induced by CCCP treatment of porcine oocytes. Reproduction, 2015, 150, 97–104 10.1530/REP‐15‐0037 [DOI] [PubMed] [Google Scholar]
- 48. Rambags BP, Boxtel DC, Tharasanit T, Lenstra JA, Colenbrander B, Stout TA. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology, 2014, 81, 959–965 10.1016/j.theriogenology.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 49. Cotterill M, Harris SE, Collado Fernandez E, Lu J, Huntriss JD, Campbell BK, Picton HM. The activity and copy number of mitochondrial DNA in ovine oocytes throughout oogenesis in vivo and during oocyte maturation in vitro. Mol Hum Reprod, 2013, 19, 444–450 10.1093/molehr/gat0133690804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mahrous E, Yang Q, Clarke HJ. Regulation of mitochondrial DNA accumulation during oocyte growth and meiotic maturation in the mouse. Reproduction, 2012, 144, 177–185 10.1530/REP‐12‐0113 [DOI] [PubMed] [Google Scholar]
- 51. Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One, 2014, 9, e94488 10.1371/journal.pone.00944883991605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reynier P, May‐Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, Barrière P, Malthièry Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod, 2001, 7, 425–429 10.1093/molehr/7.5.425 [DOI] [PubMed] [Google Scholar]
- 53. May‐Panloup P, Chrétien MF, Jacques C, Vasseur C, Malthièry Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod, 2005, 20, 593–597 10.1093/humrep/deh667 [DOI] [PubMed] [Google Scholar]
- 54. Santos TA, El Shourbagy S. St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril, 2006, 85, 584–591 10.1016/j.fertnstert.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 55. Mao J, Whitworth KM, Spate LD, Walters EM, Zhao J, Prather RS. Regulation of oocyte mitochondrial DNA copy number by follicular fluid, EGF, and neuregulin 1 during in vitro maturation affects embryo development in pigs. Theriogenology, 2012, 78, 887–897 10.1016/j.theriogenology.2012.04.0024714324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee SK, Zhao MH, Kwon JW, Li YH, Lin ZL, Jin YX, Kim NH, Cui XS. The association of mitochondrial potential and copy number with pig oocyte maturation and developmental potential. J Reprod Dev., 2014, 60, 128–135 10.1262/jrd.2013‐0983999391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod, 2010, 83, 52–62 10.1095/biolreprod.109.0808872888963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chomyn A, Attardi G. MtDNA mutations in aging and apoptosis. Biochem Biophys Res Commun., 2003, 304, 519–529 10.1016/S0006‐291X(03)00625‐9 [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez‐Freire M, de Cabo R, Bernier M, Sollott SJ, Fabbri E, Navas P, Ferrucci L. Reconsidering the Role of Mitochondria in Aging. J Gerontol A Biol Sci Med Sci. 2015 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 60. Bruin JP, Dorland M, Spek ER, Posthuma G, Haaften M, Looman CW, teVelde ER. Age‐related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod, 2004, 70, 419–424 10.1095/biolreprod.103.015784 [DOI] [PubMed] [Google Scholar]
- 61. Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod, 2005, 11, 843–846 10.1093/molehr/gah243 [DOI] [PubMed] [Google Scholar]
- 62. Barritt JA, Kokot M, Cohen J, Steuerwald N, Brenner CA. Quantification of human ooplasmic mitochondria. Reprod Biomed Online, 2002, 4, 243–247 10.1016/S1472‐6483(10)61813‐5 [DOI] [PubMed] [Google Scholar]
- 63. Kushnir VA, Ludaway T, Russ RB, Fields EJ, Koczor C, Lewis W. Reproductive aging is associated with decreased mitochondrial abundance and altered structure in murine oocytes. J Assist Reprod Genet, 2012, 29, 637–642 10.1007/s10815‐012‐9771‐53401248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simsek‐Duran F, Li F, Ford W, Swanson RJ, Jones HW Jr, Castora FJ. Age‐associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One, 2013, 8, e64955 10.1371/journal.pone.00649553669215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takeuchi T, Neri QV, Katagiri Y, Rosenwaks Z, Palermo GD. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol Reprod, 2005, 72, 584–592 10.1095/biolreprod.104.032391 [DOI] [PubMed] [Google Scholar]
- 66. Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H, Shan D, Zhang X, Lv J, Huang C, Dong Q. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev, 2012, 79, 392–401 10.1002/mrd.22042 [DOI] [PubMed] [Google Scholar]
- 67. Held NM, Houtkooper RH. Mitochondrial quality control pathways as determinants of metabolic health. BioEssays, 2015, 37, 867–876 10.1002/bies.201500013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci, 2010, 123, 2533–2542 10.1242/jcs.0704902912461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Youle RJ, Bliek AM. Mitochondrial fission, fusion, and stress. Science, 2012, 337 (6098) 1062–1065 10.1126/science.12198554762028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol, 2010, 8, e1000298 10.1371/journal.pbio.10002982811155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol, 2010, 191, 933–942 10.1083/jcb.2010080842995166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Anand R, Langer T, Baker MJ. Proteolytic control of mitochondrial function and morphogenesis. Biochim Biophys Acta, 2013, 1833, 195–204 10.1016/j.bbamcr.2012.06.025 [DOI] [PubMed] [Google Scholar]
- 73.Hang L, Thundyil J, Lim KL. Mitochondrial dysfunction and Parkinson disease: a Parkin‐AMPK alliance in neuroprotection. Ann N Y Acad Sci. 2015 (Epub ahead of print). [DOI] [PubMed]
- 74. Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol, 2008, 183, 795–803 10.1083/jcb.2008091252592826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Calì T, Ottolini D, Negro A, Brini M. Enhanced parkin levels favor ER‐mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta, 2013, 1832, 495–508 10.1016/j.bbadis.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 76. Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC. Broad activation of the ubiquitin–proteasome system by Parkin is critical for mitophagy. Hum Mol Genet, 2011, 20, 1726–1737 10.1093/hmg/ddr0483071670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Castro Pimenta. I, Costa AC, Lam D, Tufi R, Fedele V, Moisoi N, Dinsdale D, Burman JL, Yu S, Poole AC, Decal RB, Pallanck L. Analysis of neural subtypes reveals selective mitochondrial dysfunction in dopaminergic neurons from parkin mutants. Proc Natl Acad Sci USA., 2012, 109, 10438–10443 10.1073/pnas.1115948108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Deas E, Loh SH, Martins LM. Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster. Cell Death Differ, 2012, 19, 1308–1316 10.1038/cdd.2012.53392634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rana A, Rera M, Walker DW. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci USA., 2013, 110, 8638–8643 10.1073/pnas.12161971103666724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al Rawi S, Louvet‐Vallée S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–7. [DOI] [PubMed]
- 81. Sato M, Sato K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim Biophys Acta, 2013, 1833, 1979–1984 10.1016/j.bbamcr.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 82. Hajjar C, Sampuda KM, Boyd L. Dual roles for ubiquitination in the processing of sperm organelles after fertilization. BMC Dev Biol, 2014, 15 (14) 6 10.1186/1471‐213X‐14‐6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jin YX, Zheng Z, Yu XF, Zhang JB, Namgoong S, Cui XS, Hyun SH, Kim NH. Autophagy and ubiquitin‐mediated proteolysis may not be involved in the degradation of spermatozoon mitochondria in mouse and porcine early embryos. Zygote., 2014, 16, 1–11 [DOI] [PubMed] [Google Scholar]
- 84. Campello S, Strappazzon F, Cecconi F. Mitochondrial dismissal in mammals, from protein degradation to mitophagy. Biochim Biophys Acta, 2014, 1837, 451–460 10.1016/j.bbabio.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 85.Giedt RJ, Pfeiffer DR, Matzavinos A, Kao CY, Alevriadou BR. Mitochondrial dynamics and motility inside living vascular endothelial cells: role of bioenergetics. Ann Biomed Eng. 2012;40:1903–16. [DOI] [PMC free article] [PubMed]
- 86. Margineantu DH, Emerson CB, Diaz D, Hockenbery DM. Hsp90 inhibition decreases mitochondrial protein turnover. PLoS One, 2007, 24, e1066 10.1371/journal.pone.0001066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol, 2010, 191, 1367–1380 10.1083/jcb.2010070133010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Song BS, Yoon SB, Kim JS, Sim BW, Kim YH, Cha JJ, Choi SA, Min HK, Lee Y, Huh JW, Lee SR, Kim SH, Koo DB, Choo YK, Kim HM, Kim SU, Chang KT. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol Reprod, 2012, 87 (8) 1–11 [DOI] [PubMed] [Google Scholar]
- 89. Morris KC, Lin HW, Thompson JW, Perez‐Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab, 2011, 31, 1003–1019 10.1038/jcbfm.2010.2293070983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD‐dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA, 2008, 105, 3374–3379 10.1073/pnas.07121451052265142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brenmoehl J, Hoeflich A. Dual control of mitochondrial biogenesis by sirtuin 1and sirtuin 3. Mitochondrion, 2013, 13, 755–761 10.1016/j.mito.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 92. Kulkarni SS, Cantó C. The molecular targets of resveratrol. Biochim Biophys Acta, 2015, 1852, 1114–1123 10.1016/j.bbadis.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 93. Fu X, Wan S, Lyu YL, Liu LF, Qi H. Etoposide induces ATM‐dependent mitochondrial biogenesis through AMPK activation. PLoS One, 2008, 3, e2009 10.1371/journal.pone.00020092329593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol‐activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals, 2011, 19, 163–174 10.1159/0003285163699815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li YG, Zhu W, Tao JP, Xin P, Liu MY, Li JB, Wei M. Resveratrol protects cardiomyocytes from oxidative stress through SIRT1 and mitochondrial biogenesis signaling pathways. Biochem Biophys Res Commun., 2013, 438, 270–276 10.1016/j.bbrc.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 96. Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res, 2010, 86, 103–112 10.1093/cvr/cvp384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Takeo S, Abe T, Shirasuna K, Kuwayama T, Iwata H. Effect of 5‐aminoimidazole‐4‐carboxamide ribonucleoside on the mitochondrial function and developmental ability of bovine oocytes. Theriogenology, 2015, 84, 490–497 10.1016/j.theriogenology.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 98.Sugiyama M, Kawahara‐Miki R, Kawana H, Shirasuna K, Kuwayama T, Iwata H. Resveratrol‐induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J Reprod Dev 2015 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 99. Wolf DP, Mitalipov N, Mitalipov S. Mitochondrial replacement therapy in reproductive medicine. Trends Mol Med., 2015, 21, 68–76 10.1016/j.molmed.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stoop D, Cobo A, Silber S. Fertility preservation for age‐related fertility decline. Lancet, 2014, 384, 1311–1319 10.1016/S0140‐6736(14)61261‐7 [DOI] [PubMed] [Google Scholar]


