Abstract
Endometriosis is a common chronic benign disease that affects reproductive age women and causes chronic pelvic pain and infertility. Despite its prevalence, the exact mechanisms of the pathogenesis of endometriosis‐associated infertility are unknown, and precise standards of management have not yet been established. Medical and surgical treatments for endometriosis have different effects on the chance of conception, either spontaneously or via assisted reproductive technologies (ART). In this manuscript, we review the literature from years 1979 to 2015 to report on the proposed mechanism of endometriosis‐associated infertility, the staging system of endometriosis for pregnancy outcomes and the current management of patients with endometriosis‐associated infertility.
Keywords: Assisted reproductive technologies, Endometrioma, In vitro fertilization, Staging, Treatment
Introduction
Endometriosis, a common estrogen‐dependent gynecological disorder, affects 10 % of reproductive aged women and causes pelvic pain and infertility. It is defined as the presence of endometrial‐like tissue (glands and stroma) outside the uterus [1]. The ovary, the most common site of endometriosis, may have unilateral or bilateral involvement [2].
Prevalence of endometriosis has increased up to 50 % in women with infertility [3]. Women with endometriosis have a low monthly fecundity rate (MFR) compared with the MFR in fertile controls. Monthly fecundity is 0.02–0.10 in infertile women with endometriosis, while fecundity ranges from 0.15 to 0.20 per month in normal couples [4]. The presence of endometriosis may negatively affect both the spontaneous chance of conception [5, 6] and in vitro fertilization (IVF) pregnancy rates when compared with those of women with unexplained infertility or tubal factor controls [7]. The hypothesis that endometriosis causes infertility is not fully understood. Several mechanisms have been proposed to link the association between endometriosis and infertility. (Table 1)
Table Table 1.
Pathophysiology of endometriosis‐associated infertility
| 1. Ovarian‐tubal dysfunction |
| Anatomical distortion of ovary and tube |
| Ovulation failure |
| Hyperprolactinemia |
| LUF (luteinized un‐ruptured follicle) |
| Abnormal follicle development |
| Reduced follicle development |
| Decreased estrogen production |
| Increased apoptosis of granulosa cells |
| 2. Immunological disorder |
| Anti‐endometrial antibody |
| 3. Abnormal peritoneal environment |
| Increased peritoneal fluids and high concentrations of cytokines |
| Activated macrophage |
| 4. Dysregulated endometrial function |
Pathophysiology of endometriosis‐associated infertility
Ovarian‐tubal dysfunction
Anatomical distortion of ovary and tube
In patients with moderate to severe endometriosis, pelvic adhesion involving ovaries and tubes may impair oocyte release from the ovary, inhibit tubal ovum pick up or ovum transport, and/or block sperm transfer into the fallopian tube [8]. By using hysterosalpingoscintography (HSSG), patients with endometriosis showed a significant reduction in physiologic utero‐tubal transport capacity compared with controls [9].
Ovulation failure
Mechanisms that facilitate normal ovulation are impaired in women with endometriosis. Prolactin (PRL) levels were significantly higher in infertile women with endometriosis when compared with those of women without endometriosis [10]. An elevated level of PRL prevents luteinizing hormone (LH) pulsatility and interferes with hypothalamic function by blocking estrogen receptors, thus producing anovulation [11]. Another cause of ovulation failure is luteinized unruptured follicle syndrome (LUFs). LUF occurs when oocytes become trapped in a luteinizing corpus hemorrhagicum, which is common in patients with mild or minimal endometriosis. This may be one of the causes of endometriosis‐associated infertility [12].
Abnormal follicle development
The number of preovulatory follicles, follicular growth, dominant follicle size, and follicular estradiol concentrations are reduced in the ovaries of endometriosis patients [13, 14, 15, 16]. Further, interleukin‐6 (IL‐6) and its soluble receptor, which are present in the peritoneal fluid of women with endometriosis [17], significantly decreased aromatase gene expression as well as estrogen production via the MAPK signal pathway in human granulosa cells. This may lead to a suboptimum follicular environment [18]. Women with endometriosis also have a higher incidence of apoptotic cells in their granulosa cells, indicating poor oocyte quality [19]. Collectively, these data provide evidence of mechanisms that may cause altered follicular development and ovulatory dysfunction in endometriosis.
Immunological disorder
Aberrant immunological mechanism including production of autoantibodies might be a potent pathophysiological mechanism of endometriosis‐associated infertility. Gleicher et al. (1987) [20] reported the presence of IgG, IgM, and IgA autoantibodies directed against cell‐derived antigens in women with endometriosis. In a study by Gajbhiye et al. (2008), both IgG and IgM anti‐endometrial antibodies (AEA) could be detected in almost 60 % of patients with endometriosis, and it has been suggested that AEAs might be partially responsible for failure of implantation and decreased endometrial receptivity leading to infertility [21].
Abnormal peritoneal environment
Increased peritoneal fluids and high concentration of cytokines
The volume of peritoneal fluid (PF) is significantly elevated in infertile women with endometriosis compared with those without endometriosis [22]. It has been demonstrated that incubation with PF from women with moderate or severe endometriosis caused approximately 40, 50, and 80 % declines in sperm motility [23]. Moreover, PF in patients with endometriosis has a detrimental action on the sperm acrosome reaction [24], mouse embryo growth [25], sperm binding to the zona pellucida [26], and ciliary action in the human Fallopian tube [27]. Studies demonstrated that endometriotic implants secrete estradiol and progesterone, which attract macrophages, vascular endothelial growth factor (VEGF), and interleukin‐8 (IL‐8) [28, 29]. An increased number of activated macrophages also secrete various local products, such as growth factors, and inflammatory cytokines, such as IL‐1, IL‐6, and tumor necrosis factor alpha (TNFα), and angiogenic cytokines, such as IL‐8 and VEGF, which are increased in concentration in PF of patients with endometriosis. We also reported that endometriotic stromal cells produced a significant amount of cytokines [30], such as IL‐6 and IL‐8 [31]. Adding IL‐6 into the culture media reduced mouse embryo development [22, 24] and sperm motility [32]. Thus, an inflammatory state exists, impairing fertility by having a toxic effect on gametes and embryos and impairing tubal motility [17, 30, 31, 32]. Increased IL‐6 associated with endometriosis may not only reduce sperm motility, but may also affect the metaphase‐II oocyte spindle by impairing the microtubule and chromosomal structure, contributing to infertility [33] (Fig. 1).
Figure Fig. 1.
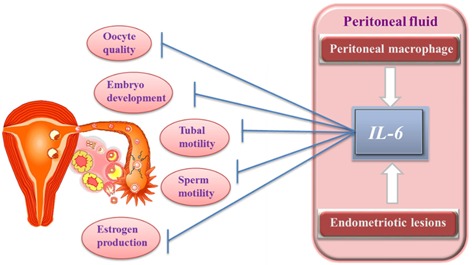
Association of Interleukin‐6 and infertility
Activated macrophage
Activated macrophages may contribute to an unfavorable milieu by producing various kinds of inflammatory cytokines. In the meantime, the increased number of macrophages and their sperm phagocytosis can disturb ovum fertilization by causing damage to oocytes or zygotes and inducing infertility [34]. Interestingly, it has been found that both endometriotic cells and macrophages are responsible for the high concentration of reactive oxygen species in PF of women with endometriosis [35]. These reactive oxygen and nitrogen species may negatively affect embryo implantation and sperm viability in the peritoneal microenvironment. The effects of free radicals on oocytes, sperm, and embryos have also been implicated in poor reproductive outcomes in assisted reproductive technologies (ART) [36].
Dysregulated endometrial function
Several studies have suggested that implantation is not affected in patients with endometriosis. The presence of severe endometriosis [37] or bilateral ovarian endometrioma [36] does not lower implantation or pregnancy rates. Infertility in these patients is not related to an endometrial environment affecting endometrial receptivity [37, 38].
However, endometrial receptivity, which allows the developing embryo to implant, is a complex process involving regulation by hormones, cytokines, adhesion molecules, and other factors [39]. Defective “window of implantation” due to the inadequate expression of various endometrial receptivity molecules may occur in the endometrium of women with endometriosis [40]. Integrins were proposed to be sensitive indicators of the endometrial receptive state. The expression of ανβ3 integrin occurs during the window of implantation in healthy controls and the absence of it is associated with poor reproductive outcomes [41]. Reduced endometrial expression of the αvβ integrin, which may interfere with embryo attachment during the time of implantation, has been described in some women with endometriosis [42, 43] and with unexplained infertility [44]. Moreover, decreased expression of four biomarkers of implantation, such as glycodelin A, osteopontin, lysophosphatidic acid receptor 3, and HOXA10, indicate impaired endometrial receptivity in patients with endometriosis [45]. Some women with endometriosis also exhibit very low levels of an enzyme involved in the synthesis of the endometrial ligand for L‐selectin (a protein that coats the trophoblast on the surface of the blastocyst) [46].
New scoring system for infertility
The American Fertility Society (AFS) (now the American Society for Reproductive Medicine, or ASRM) proposed a classification system in 1979 [47], which was modified in 1985 [48]; it classifies the disease as minimal (Stage I), mild (Stage II), moderate (Stage III) or severe (Stage IV). Currently, revised American Society of Reproductive Medicine Classification (r‐ASRM classification) is the most widely used staging system of endometriosis. Unfortunately, it has a limited predictive ability for pregnancy outcome after surgery [49, 50]. In 2002, Fujishita et al. modified the r‐AFS classification by adding TOP score (fallopian tubes, ovaries, peritoneum and other factors) and assessed the fertility rate. According to TOP classification, the pregnancy rate was mostly affected by tubal condition, and they suggested that individual tubal condition may have a clinically predictive value in assessing the reproductive outcome of women with endometriosis. However, they did not consider the factors affecting pregnancy, e.g., patient age [50].
The new staging system, endometriosis fertility index (EFI), was developed by Adamson and Pasta in 2010 to predict fecundity after endometriosis surgery. EFI is a scoring system that not only assesses the historical factors at the time of surgery (age, duration of infertility, and pregnancy history), but also evaluates least‐function (LF) score (functional score of fallopian tubes, fimbriae, and ovaries bilaterally) and extent of endometriosis (r‐ASRM endometriosis lesion score and total r‐ASRM score) [51]. Tomassetti C et al. [52] also suggested that EFI could be useful as a clinical tool for counseling patients with endometriosis after surgery about their fertility prognosis and eventual need for fertility treatment.
Wang et al. [53] were the first to compare the predictive value of the EFI score with the r‐ASRM classification in the same population of women with endometriosis who had received IVF treatment. They suggested that neither the future of pregnancy nor IVF outcome could be predicted by r‐ASRM classification. Unlike r‐ASRM classification, EFI may have more predictive power, since it includes the assessment of reproductive factors such as age, duration of infertility, pregnancy history, and reproductive potential of pelvic organs by LF score. The clinical pregnancy rate was higher in patients with EFI ≥ 6 score than in those with EFI ≤ 5 score, providing a valuable reference to predict pregnancy outcome after surgery in women with endometriosis.
Evidence of infertility treatment of endometriosis‐associated infertility
COH–IUI
Some randomized, controlled trials have shown that ovulation induction and superovulation (SO) with and without intrauterine insemination (IUI) increases fertility rates in patients without distorted anatomy [54, 55]. There is evidence that controlled ovarian hyperstimulation (COH)–IUI seems to be better than expected management in infertile women with endometriosis. In a study by Tummon et al., treatment with COH–IUI had a better outcome than no treatment for infertility associated with minimal or mild endometriosis. Live births followed 14 of 127 (11 %) superovulation and IUI cycles and four of 184 (2 %) no‐treatment cycles [55].
Notably, most of the studies were assessed in patients with minimal‐mild endometriosis, and there is insufficient evidence to support SO/IUI in patients with severe endometriosis. Gandhi et al. [56] demonstrated that COH+IUI cumulative fertility rate was threefold lower in patients with surgically treated stage III/IV endometriosis. However, according to the European Society of Human Reproduction and Embryology (ESHRE) recommended guideline (2014) [57], IUI is only recommended in subfertile women with minimal‐to‐mild endometriosis, and IUI with controlled ovarian stimulation should be considered within 6 months following surgery in the treatment of infertile women with AFS/ASRM stage I/II endometriosis.
In vitro fertilization (IVF)
IVF is currently the most effective treatment of endometriosis‐associated infertility [58]. It is still uncertain as to how much endometriosis influences IVF success rates. A meta‐analysis by Barnhart et al. (2002), including publications from 1983 to 1998, concluded that women with endometriosis have lower pregnancy rates with IVF than those with tubal infertility (OR 0.56; 95 % CI, 0.44 to 0.70) [59]. However, the limitation in their meta‐analysis is that they did not distinguish between women who had received previous medical and surgical interventions, limiting the applicability of their findings. Similarly, another review by Harb et al. [60] addressing this issue demonstrated that the presence of stage III/IV endometriosis is associated with poor implantation and clinical pregnancy rates in women undergoing IVF treatment.
Interestingly, in contrast to these studies, The Society of Assisted Reproductive Technology reported that over 1400 live births resulted from 5600 IVF cycles in patients with endometriosis. Average delivery rate per retrieval of patients undergoing IVF was 39.1 % in women with endometriosis, compared with 33.2 % in women with all causes of infertility [58]. Barbosa et al. [61] also concluded that women with endometriosis undergoing ART have the same chance of achieving clinical pregnancy and live birth as do women with other causes of infertility. They observed the ART outcomes (live birth [LB], clinical pregnancy [CP], miscarriage and number of oocytes retrieved) in women with endometriosis in different stages and those without endometriosis. In this review, the probability of achieving LB and CP were not relevantly different between women with endometriosis and without endometriosis [LB, RR = 0.99 (95 % CI, 0.92–1.06); CP, RR = 0.95 (95 % CI, 0.89–1.02); miscarriage, RR = 1.31 (95 % CI, 1.07–1.59); number of oocytes retrieved, MD = –1.56 (95 % CI, –2.05 to –1.08)]. In addition, women with stage I/II or III/IV endometriosis had comparable LB and CP rates compared with women with other causes of infertility. No relevant difference was observed in the chance of achieving LB and CP following ART when comparing stage III/IV with Stage I/II endometriosis. [LB, RR = 0.94 (95 % CI, 0.80–1.11); CP, RR = 0.90 (95 % CI, 0.82–1.00); miscarriage, RR = 0.99 (95 % CI, 0.73–1.36); number of oocytes retrieved, MD = –1.03 (95 % CI, –1.67 to –0.39).
A recent study by Hamdan et al. (2015) [62] also evaluated whether the presence and/or severity of endometriosis affects the main ART outcomes, by searching the published articles from 1980 to 2014 in MEDLINE, PubMed, ClinicalTrials.gov, and Cochrane databases. According to the following results, women with endometriosis who underwent ART were found to have the same chance of achieving LB and CP as women without endometriosis. Compared with women with no endometriosis, women with endometriosis undertaking IVF/ICSI have a similar LB rate per woman (OR 0.94, 95 % CI 0.84–1.06), a lower CP rate per woman (OR 0.78, 95 % CI 0.65–0.94), a lower mean number of oocyte retrieved per cycle (MD −1.98, 95 % CI −2.87 to −1.09) and a similar miscarriage rate per woman (OR 1.26, 95 % CI 0.92–1.70). However, in this review, following ART, women with severe endometriosis showed lower LB, CP rates, and lower mean number of oocytes retrieved when compared with women without endometriosis, suggesting that their ovarian reserve may be diminished before IVF/ICSI. On the basis of these findings, patients with endometriosis can be referred for early infertility treatment, including IVF, to increase the chances of conception. Direct IVF should be considered if the women's age is more than 35 years and duration of infertility is long (Fig. 2).
Figure Fig. 2.
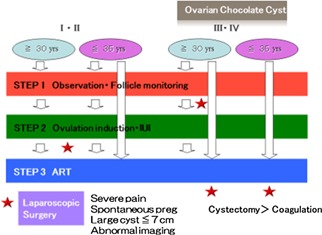
Treatment strategies for women with endometriosis‐associated infertility
Surgical or medical management of endometriosis‐associated infertility
Several studies assessed whether ovulation suppression agents, such as danazol, progestins, and oral contraceptives, could be affective to improve pregnancy outcomes in subfertile women with endometriosis [63]. However, no evidence was found that suppressing ovarian function with hormone therapy improves fertility in the treatment of endometriosis [57, 63]. In addition, there was insufficient evidence to support that the use of hormonal suppression therapy for endometriosis before or after surgery is more effective than surgery alone [64]. In 2010, Matsuzaki et al. [65] identified the risk factors for the removal of normal ovarian tissue during laparoscopic cystectomy for endometriosis, and suggested that pre‐operative medical treatment might predispose to the risk of removing normal ovarian tissue.
In patients with endometriosis‐related infertility, the objective of the surgery is to restore the anatomical relationship and preserve the function of pelvic organs. To establish a favorable pelvic environment, a skilled surgeon must perform a surgical procedure [66]. Duffy et al. (2014) reviewed the effectiveness of laparoscopic surgery in subfertility associated with endometriosis [67]. Laparoscopic removal of minimal‐mild stage endometriosis not only reduces pain, but also increases spontaneous pregnancy rate when compared with diagnostic laparoscopy alone. In infertile women with ovarian endometrioma, operative laparoscopy instead of expectant management may increase spontaneous pregnancy rates [68]. There are various conservative surgical treatments, such as cyst wall ablation, cystectomy, or USG‐guided aspiration for ovarian chocolate cyst. Currently, laparoscopic excision remains as a favored surgical approach to the management of ovarian endometrioma. A Cochrane review concluded that excisional surgery for the endometrioma capsule should be done instead of drainage and electrocoagulation of the endometrioma wall to increase post‐operative spontaneous pregnancy rate [69]. However, inadvertent removal of normal ovarian tissues and recurrence became clinical problems in the surgical management of ovarian endometriosis, as recurrence rate of ovarian endometrioma was significantly increased in women with previous surgery for endometriosis during follow‐up [70, 71, 72, 73, 74]. It is important to note that the ESHRE (2014) [57] guideline recommended that clinicians carefully consider whether to perform surgery if the woman has had previous ovarian surgery, because of the significant damage to ovarian function after repeated surgery.
Regarding medical or surgical treatment prior to ART, the role of medical treatment before IVF was studied by Sallam et al. [75]. They reported that pre‐treatment with gonadotrophin‐releasing hormone (GnRH) agonists at least 3 months prior to IVF increased the pregnancy rate. Moreover, pregnancy outcomes after IVF‐ET using either GnRH agonist or GnRH antagonist were found to be equally effective [76]. With respect to the effectiveness of surgery in women with minimal to mild endometriosis who underwent laparoscopic surgery prior to ART, surgical eradication of endometriosis and associated adhesion improved the result of ART outcome, including live birth rate [77]. Therefore, in infertile women with AFS/ASRM stage I/II endometriosis, complete surgical removal of endometriosis may be considered before treatment with ART. However, in infertile women with endometrioma larger than 3 cm, there was insufficient evidence that cystectomy prior to ART treatment improves pregnancy rate [57, 69, 78, 79]. Thus, the effect of surgery before ART in these women is unclear. Recently, excisional surgery for a cyst has been concerned with damage to ovarian reserve [80]. While some reproductive specialists have advised that endometrioma > 3 cm should be treated by cystectomy before ART, others have suggested the damaging effect of surgery on ovarian reserve. Some studies have demonstrated the possible association between the laparoscopic cystectomy and loss of follicles [81, 82]. Damage to the ovary is more severe when an endometrioma > 4 cm in diameter is excised [83].
In a prospective, randomized study by Demirol et al. (2006) [84], ovarian endometrioma cystectomy before IVF even resulted in decreased ovarian response in the ICSI cycle. In the cystectomy group, the total recombinant follicle‐stimulating hormone (FSH) dose was significantly higher and the mean number of mature oocytes retrieved was significantly lower, although there was no difference in pregnancy outcomes. A meta‐analysis by Benschop et al. [78] also compared the surgery (aspiration or cystectomy) with expectant management in patients with ovarian endometrioma and demonstrated that there were no significant differences in terms of clinical pregnancy, although these reports were conducted in patients with unilateral endometrioma. In a recent report by Streuli et al. [85], findings also suggest that endometrioma per se do not diminish the ovarian response reflected by Anti‐Müllerian hormone (AMH) levels, but that alterations seen in women with endometriosis are a deleterious consequence of endometrioma surgery. According to the results of these studies, cystectomy before ART is not recommended in recent guidelines (Table 2).
Table Table 2.
Guidelines for surgery of endometriosis associated infertility
| ESHRE 2014 | ASRM 2012 | |
|---|---|---|
| Stage I/II | Recommended A Better than diagnostic laproscopy | Recommended Small effect |
| Stage III/IV | Recommended B Better than expected management | Recommended May be beneficial |
| Post‐op medical therapy | Not recommended A Not recommended prior to surgery GPP | Not recommended |
| Prior to IVF | I/II may be considered C No evidence of improvement (3 cm or larger endometrioma) A Only for pain or oocyte PU GPP | No evidence of improved pregnancy rate by cystectomy |
| Cases with recurrence | Not described | Not recommended |
A meta analysis or multiple RTs (of high quality), B meta analysis or multiple RTs (of moderate quality), C single randomized trial, large non‐randomized trial(s) or case control/cohort studies (of moderate quality), GPP (good practice point) based on experts opinion
In contrast to the data on unilateral ovarian endometrioma, despite the use of higher doses of gonadotrophins, the number of follicles, oocytes retrieved, embryos obtained and pregnancy rates were significantly lower in women operated on for bilateral endometrioma [86]. Furthermore, Busacca et al. [87] reported that patients who had been operated on for bilateral endometrioma have a low (2.4 %) but definite risk of premature ovarian failure occurring immediately after surgery. These findings should be taken into account in the decision to operate on endometrioma in women with a desire for future pregnancy.
Treatment strategies for women with endometriosis‐associated infertility
Endometriosis among infertile women is increasingly being detected. The decision about which is the most appropriate treatment for women suffering from endometriosis‐associated infertility is still controversial. Currently, laparoscopic excisional surgery for ovarian endometrioma remains the gold standard. [57]. However, recurrent endometrioma surgery may be more harmful to the ovarian reserve if compared with endomeriomas operated on for the first time [88], and may have the risk of premature ovarian failure occurring immediately after surgery for bilateral endometriomas [89]. Additionally, AMH level was reduced in women with previous endometrioma surgery, not in women suffering from current ovarian endometrioma who had never had previous endometrioma surgery [85], especially in older patients and in the case of bilateral cysts [89]. High cost and post‐operative complications (1.4–7.5 %) are other unfavorable outcomes following surgery [57, 90]. The advantages of surgical treatment include reduction of pain, prevention of the risk of cystic rupture, transvaginal assessment of ovarian follicles, and elimination of the difficulty of ovum pick up (OPU) in ART. Pathological examination also reveals a malignancy rate of 0.7 % [91]. Surgery allows the assessment of the relationship of the fallopian tube with surrounding adhesion.
However, surgical indications for young patients should be limited if pelvic pain is not severe, and they should be counseled about the potential risks of reduced ovarian function after surgery [57]. For young women who have had previous ovarian surgery and a desire for pregnancy, ovarian reserve should be assessed first, and, if the estimated probability of spontaneous conception is low, immediate ART should be considered [92] since there is a lack of evidence that cystectomy prior to ART treatment improves pregnancy rate in infertile women with endometrioma larger than 3 cm [57] (Fig. 2).
Conclusion
Endometriosis, an enigmatic gynecological disease with poorly understood pathogenesis, can affect fertility at different levels. In endometriosis patients, EFI may have more power to predict the IVF outcome than ASRM classification. However, the dilemma in regard to the best approach to manage endometriosis‐associated infertility remains partially unresolved. Before choosing the most appropriate treatment, it is critical for clinicians to consider the severity of clinical symptoms, stage of the disease, age of the patient, infertility duration, and possibility of ART. Clinicians should perform a thorough assessment of ovarian reserve, tubal patency, sperm function, and the uterine cavity before initiating therapy. Indication for surgery for endometrioma should be chosen with caution.
Compliance with ethical standards
Conflict of interest
Yin Mon Khine, Fuminori Taniguchi, and Tasuku Harada declare that they have no conflict of interest.
Human/Animal studies
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1. Ozkan S Murk W Arici A Endometriosis and infertility, epidemiology and evidence‐based treatments. Ann N Y Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007. 10.1196/annals.1434.007 [DOI] [PubMed] [Google Scholar]
- 2.Jenkins S, Olive DL, HaneyAF. Endometriosis: pathogenetic implications of the anatomic distribution. ObstetGynecol. 1986;67:335–338. [PubMed]
- 3. D'Hooghe TM Debrock S Hill JA Meuleman C Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21:243–254. doi: 10.1055/s-2003-41330. 10.1055/s‐2003‐41330 [DOI] [PubMed] [Google Scholar]
- 4. Schwartz D Mayaux MJ Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS. N Engl J Med. 1982;306:404–406. doi: 10.1056/NEJM198202183060706. 10.1056/NEJM198202183060706 [DOI] [PubMed] [Google Scholar]
- 5. Akande VA Hunt LP Cahill DJ Jenkins JM Differences in time to natural conception between women with unexplained infertility and infertile women with minor endometriosis. Hum Reprod. 2004;19:96–103. doi: 10.1093/humrep/deh045. 10.1093/humrep/deh045 [DOI] [PubMed] [Google Scholar]
- 6. Johnson NP Farquhar CM Hadden WE Suckling J Yu Y Sadler L The FLUSH trial—flushing with lipiodol for unexplained (and endometriosis‐related) subfertility by hysterosalpingography: a randomized trial. Hum Reprod. 2004;19:2043–2051. doi: 10.1093/humrep/deh418. 10.1093/humrep/deh418 [DOI] [PubMed] [Google Scholar]
- 7. Barnhart K Dunsmoor‐Su R Coutifaris C Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–1155. doi: 10.1016/s0015-0282(02)03112-6. 10.1016/S0015‐0282(02)03112‐6 [DOI] [PubMed] [Google Scholar]
- 8. Schenken RS Asch RH Williams RF Hodgen GD Etiology of infertility in monkeys with endometriosis: luteinized unruptured follicles, luteal phase defects, pelvic adhesions and spontaneous abortions. Fertil Steril. 1984;41:122–130. doi: 10.1016/s0015-0282(16)47552-7. 10.1016/S0015‐0282(16)47552‐7 [DOI] [PubMed] [Google Scholar]
- 9. Kissler S Hamscho N Zangos S Gatje R Muller A Rody A et al. Diminished pregnancy rates in endometriosis due to impaired uterotubal transport assessed by hysterosalpingoscintigraphy BJOG 2005. 112 1391 1396 10.1111/j.1471‐0528.2005.00676.x [DOI] [PubMed] [Google Scholar]
- 10. Esmaeilzadeh S Mirabi P Basirat Z Zeinalzadeh M Khafri S Association between endometriosis and hyperprolactinemia in infertile women Iran J Reprod Med. 2015. 13 155 1604426155 [PMC free article] [PubMed] [Google Scholar]
- 11. Wang H Gorpudolo N Behr B The role of prolactin‐ and endometriosis‐associated infertility. Obstet Gynecol Surv. 2009;64:542–547. doi: 10.1097/OGX.0b013e3181ab5479. 10.1097/OGX.0b013e3181ab5479 [DOI] [PubMed] [Google Scholar]
- 12. Mio Y Toda T Harada T Terakawa N Luteinized unruptured follicle in the early stages of endometriosis as a cause of unexplained infertility. Am J Obstet Gynecol. 1992;167:271–273. doi: 10.1016/s0002-9378(11)91673-1. 10.1016/S0002‐9378(11)91673‐1 [DOI] [PubMed] [Google Scholar]
- 13. Doody MC Gibbons WE Buttram VC Jr Linear regression analysis of ultrasound follicular growth series: evidence for an abnormality of follicular growth in endometriosis patients. Fertil Steril. 1988;49:47–51. doi: 10.1016/s0015-0282(16)59646-0. 10.1016/S0015‐0282(16)59646‐0 [DOI] [PubMed] [Google Scholar]
- 14. Tummon IS Maclin VM Radwanska E Binor Z Dmowski WP Occult ovulatory dysfunction in women with minimal endometriosis or unexplained infertility. Fertil Steril. 1988;50:716–720. 10.1016/S0015‐0282(16)60304‐7 [PubMed] [Google Scholar]
- 15. Dlugi AM Loy RA Dieterle S Bayer SR Seibel MM The effect of endometriomas on in vitro fertilization outcome. J In Vitro Fert Embryo Transf. 1989;6:338–341. doi: 10.1007/BF01138773. 10.1007/BF01138773 [DOI] [PubMed] [Google Scholar]
- 16. Cahill DJ Wardle PG Maile LA Harlow CR Hull MG Pituitary‐ovarian dysfunction as a cause for endometriosis‐associated and unexplained infertility. Hum Reprod. 1995;10:3142–3146. doi: 10.1093/oxfordjournals.humrep.a135876. [DOI] [PubMed] [Google Scholar]
- 17. Harada T Yoshioka H Yoshida S Iwabe T Onohara Y Tanikawa M Increased interleukin‐6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997;176:593–597. doi: 10.1016/s0002-9378(97)70553-2. 10.1016/S0002‐9378(97)70553‐2 [DOI] [PubMed] [Google Scholar]
- 18. Deura I Harada T Taniguchi F Iwabe T Izawa M Terakawa N Reduction of estrogen production by interleukin‐6 in a human granulosa tumor cell line may have implications for endometriosis‐associated infertility. Fertil Steril. 2005;83(Suppl 1):1086–1092. doi: 10.1016/j.fertnstert.2004.12.014. 10.1016/j.fertnstert.2004.12.014 [DOI] [PubMed] [Google Scholar]
- 19. Toya M Saito H Ohta N Saito T Kaneko T Hiroi M Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil Steril. 2000;73:344–350. doi: 10.1016/s0015-0282(99)00507-5. 10.1016/S0015‐0282(99)00507‐5 [DOI] [PubMed] [Google Scholar]
- 20.Gleicher N, el‐Roeiy A, Confino E. Is endometriosis an autoimmune disease? Obstet Gynecol. 1987;70:115–122. [PubMed]
- 21. Gajbhiye R Suryawanshi A Khan S Multiple endometrial antigens are targeted in autoimmune endometriosis. Reprod Biomed Online. 2008;16:817–824. doi: 10.1016/s1472-6483(10)60147-2. 10.1016/S1472‐6483(10)60147‐2 [DOI] [PubMed] [Google Scholar]
- 22. Harada T Iwabe T Terakawa N Role of cytokines in endometriosis. Fertil Steril. 2001;76:1–10. doi: 10.1016/s0015-0282(01)01816-7. 10.1016/S0015‐0282(01)01816‐7 [DOI] [PubMed] [Google Scholar]
- 23. Oral E Arici A Olive DL Huszar G Peritoneal fluid from women with moderate or severe endometriosis inhibits sperm motility: the role of seminal fluid components. Fertil Steril. 1996;66:787–792. doi: 10.1016/s0015-0282(16)58637-3. 10.1016/S0015‐0282(16)58637‐3 [DOI] [PubMed] [Google Scholar]
- 24. Arumugam K Endometriosis and infertility: raised iron concentration in the peritoneal fluid and its effect on the acrosome reaction. Hum Reprod. 1994;9:1153–1157. doi: 10.1093/oxfordjournals.humrep.a138649. [DOI] [PubMed] [Google Scholar]
- 25. Prough SG Aksel S Gilmore SM Yeoman RR Peritoneal fluid fractions from patients with endometriosis do not promote two‐cell mouse embryo growth. Fertil Steril. 1990;54:927–930. doi: 10.1016/s0015-0282(16)53958-2. 10.1016/S0015‐0282(16)53958‐2 [DOI] [PubMed] [Google Scholar]
- 26. Coddington CC Oehninger S Cunningham DS Hansen K Sueldo CE Hodgen GD Peritoneal fluid from patients with endometriosis decreases sperm binding to the zona pellucida in the hemizona assay: a preliminary report. Fertil Steril. 1992;57:783–786. doi: 10.1016/s0015-0282(16)54959-0. 10.1016/S0015‐0282(16)54959‐0 [DOI] [PubMed] [Google Scholar]
- 27. Lyons RA Djahanbakhch O Saridogan E Naftalin AA Mahmood T Weekes A Peritoneal fluid, endometriosis, and ciliary beat frequency in the human fallopian tube. Lancet. 2002;360:1221–1222. doi: 10.1016/S0140-6736(02)11247-5. 10.1016/S0140‐6736(02)11247‐5 [DOI] [PubMed] [Google Scholar]
- 28. Tseng JF Ryan IP Milam TD Murai JT Schriock ED Landers DV et al. Interleukin‐6 secretion in vitro is up‐regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis J Clin Endocrinol Metab 1996. 81 1118 1122 [DOI] [PubMed] [Google Scholar]
- 29. Shifren JL Tseng JF Zaloudek CJ Ryan IP Mesung YG Ferrara N et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis J Clin Endocrinol Metab 1996. 81 3112 3118 [DOI] [PubMed] [Google Scholar]
- 30. Tsudo T Harada T Iwabe T Tanikawa M Nagano Y Ito M Taniguchi F Terakawa N Altered gene expression and secretion of interleukin‐6 in stromal cells derived from endometriotic tissues. Fertil Steril. 2000;73:205–211. doi: 10.1016/s0015-0282(99)00496-3. 10.1016/S0015‐0282(99)00496‐3 [DOI] [PubMed] [Google Scholar]
- 31. Iwabe T Harada T Tsudo T Tanikawa M Onohara Y Terakawa N Pathogenetic significance of increased levels of interleukin‐8 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1998;69:924–930. doi: 10.1016/s0015-0282(98)00049-1. 10.1016/S0015‐0282(98)00049‐1 [DOI] [PubMed] [Google Scholar]
- 32. Yoshida S Harada T Iwabe T Taniguchi F Mitsunari M Yamauchi N Deura I Horie S Terakawa N A combination of interleukin‐6 and its soluble receptor impairs sperm motility: implications in infertility associated with endometriosis. Hum Reprod. 2004;19:1821–1825. doi: 10.1093/humrep/deh324. 10.1093/humrep/deh324 [DOI] [PubMed] [Google Scholar]
- 33. Banerjee J Sharma R Agarwal A Maitra D Diamond MP Abu‐Soud HM IL‐6 and mouse oocyte spindle. PLoS One. 2012 doi: 10.1371/journal.pone.0035535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muscato JJ Haney AF Weinberg JB Sperm phagocytosis by human peritoneal macrophages: a possible cause of infertility in endometriosis. Am J Obstet Gynecol. 1982;144:503–510. doi: 10.1016/0002-9378(82)90217-4. 10.1016/0002‐9378(82)90217‐4 [DOI] [PubMed] [Google Scholar]
- 35. Osborn BH Haney AF Misukonis MA Weinberg JB Inducible nitric oxide synthase expression by peritoneal macrophages in endometriosis‐associated infertility. Fertil Steril. 2002;77:46–51. doi: 10.1016/s0015-0282(01)02940-5. 10.1016/S0015‐0282(01)02940‐5 [DOI] [PubMed] [Google Scholar]
- 36. Agarwal A Aponte‐Mellado A Premkumar BJ Shaman A Gupta S The effects of oxidative stress on female reproduction: a review Reprod Biol Endocrinol 2012. 10 49 10.1186/1477‐7827‐10‐493527168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Díaz I Navarro J Blasco L Simón C Pellicer A Remohí J Impact of stage III–IV endometriosis on recipients of sibling oocytes: matched case‐control study. Fertil Steril. 2000;74:31–34. doi: 10.1016/s0015-0282(00)00570-7. 10.1016/S0015‐0282(00)00570‐7 [DOI] [PubMed] [Google Scholar]
- 38. Benaglia L Bermejo A Somigliana E Faulisi S Ragni G Fedele L Garcia‐Velasco JA In vitro fertilization outcome in women with unoperated bilateral endometriomas. Fertil Steril. 2013;99(6):1714–1719. doi: 10.1016/j.fertnstert.2013.01.110. 10.1016/j.fertnstert.2013.01.110 [DOI] [PubMed] [Google Scholar]
- 39. Aghajanova L Hamilton A Kwintkiewicz J Vo KC Giudice LC Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis Biol Reprod 2009. 80 105 114 10.1095/biolreprod.108.0703002704986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kokcu Arif Possible effects of endometriosis‐related immune events on reproductive function. Arch Gynecol Obstet. 2013;287:1225–1233. doi: 10.1007/s00404-013-2767-2. 10.1007/s00404‐013‐2767‐2 [DOI] [PubMed] [Google Scholar]
- 41. Revel A Defective endometrial receptivity. Fertil Steril. 2012;97:1028–1032. doi: 10.1016/j.fertnstert.2012.03.039. 10.1016/j.fertnstert.2012.03.039 [DOI] [PubMed] [Google Scholar]
- 42. Kao LC Germeyer A Tulac S Lobo S Yang JP Taylor RN et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease‐based implantation failure and infertility Endocrinology 2003. 144 2870 2881 10.1210/en.2003‐0043 [DOI] [PubMed] [Google Scholar]
- 43. Cakmak H Taylor HS Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone‐hox gene interactions Semin Reprod Med. 2010. 28 69 74 10.1055/s‐0029‐12429963107856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franasiak JM Holoch KJ Yuan L Schammel DP Young SL Lessey BA Prospective assessment of midsecretory endometrial leukemia inhibitor factor expression versus ανβ3 testing in women with unexplained infertility Fertil Steril 2014. 101 1724 1731 10.1016/j.fertnstert.2014.02.0274101991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Q, St Clair JB, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis Fertil Steril. 2009;91:1686–1691. [DOI] [PMC free article] [PubMed]
- 46. Genbacev OD Prakobphol A Foulk RA Krtolica AR Ilic D Singer MS et al. Trophoblast L‐selectin‐mediated adhesion at the maternal‐fetal interface Science 2003. 299 405 408 10.1126/science.1079546 [DOI] [PubMed] [Google Scholar]
- 47.American Fertility Society Classification of endometriosis, authors. Fertil Steril. 1979;32:633–634. 10.1016/S0015‐0282(16)44409‐2 [PubMed] [Google Scholar]
- 48.American Fertility Society Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril. 1985;43:351–352. [DOI] [PubMed]
- 49. Canis M Donnez JG Guzick DS Halme JK Rock JA Schenken RS et al. Revised American Society for Reproductive Medicine classification of endometriosis: 1996 Fertil Steril 1997. 67 817 821 10.1016/S0015‐0282(97)81391‐X [DOI] [PubMed] [Google Scholar]
- 50. Fujishita A Khan KN Masuzaki H Ishimaru T Influence of pelvic endometriosis and ovarian endometrioma on fertility. Gynecol Obstet Invest. 2002;53(suppl 1):40–45. doi: 10.1159/000049423. 10.1159/000049423 [DOI] [PubMed] [Google Scholar]
- 51. Adamson GD Pasta DJ Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94:1609–1615. doi: 10.1016/j.fertnstert.2009.09.035. 10.1016/j.fertnstert.2009.09.035 [DOI] [PubMed] [Google Scholar]
- 52. Tomassetti C Geysenbergh B Meuleman C Timmerman D Fieuws S D'Hooghe T External validation of the endometriosis fertility index (EFI) staging system for predicting non‐ART pregnancy after endometriosis surgery. Hum Reprod. 2013;28:1280–1288. doi: 10.1093/humrep/det017. 10.1093/humrep/det017 [DOI] [PubMed] [Google Scholar]
- 53. Wang W Li R Fang T Huang L Ouyang N Wang L et al. Endometriosis fertility index score maybe more accurate for predicting the outcomes of in vitro fertilisation than r‐AFS classification in women with endometriosis Reprod Biol Endocrinol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fedele L Bianchi S Marchini M Villa L Brioschi D Parazzini F Superovulation with human menopausal gonadotropins in the treatment of infertility associated with minimal or mild endometriosis: a controlled randomized study. Fertil Steril. 1992;58:28–31. doi: 10.1016/s0015-0282(16)55132-2. 10.1016/S0015‐0282(16)55132‐2 [DOI] [PubMed] [Google Scholar]
- 55. Tummon IS Asher LJ Martin JS Tulandi T Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril. 1997;68:8–12. doi: 10.1016/s0015-0282(97)81467-7. 10.1016/S0015‐0282(97)81467‐7 [DOI] [PubMed] [Google Scholar]
- 56. Gandhi AR Carvalho LF Nutter B Falcone T Determining the fertility benefit of controlled ovarian hyperstimulation with intrauterine insemination after operative laparoscopy in patients with endometriosis. J Minim Invasive Gynecol. 2014;21:101–108. doi: 10.1016/j.jmig.2013.07.009. 10.1016/j.jmig.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 57. Dunselman GA Vermeulen N Becker C Calhaz‐Jorge C D'Hooghe T Bie B et al. ESHRE guideline: management of women with endometriosis Hum Reprod 2014. 29 400 1210 10.1093/humrep/det457 [DOI] [PubMed] [Google Scholar]
- 58.Society for Assisted Reproductive Techonology, the American Society for Reproductive Medicine [Internet]. Assisted reproductive technology in the United States: 2010 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproduction registry; 2012. Available from: www.sart.org.
- 59. Barnhart K Dunsmoor‐Su R Coutifaris C Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77:1148–1155. doi: 10.1016/s0015-0282(02)03112-6. 10.1016/S0015‐0282(02)03112‐6 [DOI] [PubMed] [Google Scholar]
- 60. Harb HM Gallos ID Chu J Harb M Coomarasamy A The effect of endometriosis on in vitro fertilisation outcome: a systematic review and meta‐analysis. BJOG. 2013;120:1308–1320. doi: 10.1111/1471-0528.12366. 10.1111/1471‐0528.12366 [DOI] [PubMed] [Google Scholar]
- 61. Barbosa MA Teixeira DM Navarro PA Ferriani RA Nastri CO Martins WP Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2014;44:261–278. doi: 10.1002/uog.13366. 10.1002/uog.13366 [DOI] [PubMed] [Google Scholar]
- 62. Hamdan M Omar SZ Dunselman G Cheong Y Influence of endometriosis on assisted reproductive technology outcomes: a systematic review and meta‐analysis. Obstet Gynecol. 2015;125:79–88. doi: 10.1097/AOG.0000000000000592. 10.1097/AOG.0000000000000592 [DOI] [PubMed] [Google Scholar]
- 63.Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000155. [DOI] [PMC free article] [PubMed]
- 64.Yap C, Furness S, Farquhar C. Pre and postoperative medical therapy for endometriosis surgery. Cochrane Database Syst Rev. 2004;3:CD003678. [DOI] [PMC free article] [PubMed]
- 65. Matsuzaki S Houlle C Darcha C Pouly JL Mage G Canis M Analysis of risk factors for the removal of normal ovarian tissue during laparoscopic cystectomy for ovarian endometriosis. Hum Reprod. 2009;24:1402–1406. doi: 10.1093/humrep/dep043. 10.1093/humrep/dep043 [DOI] [PubMed] [Google Scholar]
- 66. Gizzo S Conte L Gangi S Leggieri C Quaranta M Noventa M Litta P Saccardi C Could surgeon's expertise resolve the debate about surgery effectiveness in treatment of endometriosis‐related infertility? Arch Gynecol Obstet. 2015;292:217–223. doi: 10.1007/s00404-014-3591-z. 10.1007/s00404‐014‐3591‐z [DOI] [PubMed] [Google Scholar]
- 67.Duffy JM, Arambage K, Correa FJ, Olive D, Farquhar C, Garry R, Barlow DH, Jacobson TZ. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014 Apr 3;4:CD011031. doi:10.1002/14651858.CD011031. [DOI] [PubMed]
- 68. Vercellini P Fedele L Aimi G Giorgi O Consonni D Crosignani PG Reproductive performance, pain recurrence and disease relapse after conservative surgical treatment for endometriosis: the predictive value of the current classification system. Hum Reprod. 2006;21:2679–2685. doi: 10.1093/humrep/del230. 10.1093/humrep/del230 [DOI] [PubMed] [Google Scholar]
- 69.Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;2:CD004992. [DOI] [PubMed]
- 70. Koga K Osuga Y Takemura Y Takamura M Taketani Y Front Biosci. 2013;5:676–683. doi: 10.2741/e648. 10.2741/E648 [DOI] [PubMed] [Google Scholar]
- 71. Busacca M Marana R Caruana P Candiani M Muzii L Calia C et al. Recurrence of ovarian endometrioma after laparoscopic excision Am J Obstet Gynecol 1999. 180 519 523 10.1016/S0002‐9378(99)70247‐4 [DOI] [PubMed] [Google Scholar]
- 72. Chapron C Vercellini P Barakat H Vieira M Dubuisson JB Management of ovarian endometriomas. Hum Reprod Update. 2002;8:591–597. doi: 10.1093/humupd/8.6.591. 10.1093/humupd/8.6.591 [DOI] [PubMed] [Google Scholar]
- 73. Vercellini P Chapron C Giorgi O Consonni D Frontino G Crosignani PG Coagulation or excision of ovarian endometriomas? Am J Obstet Gynecol. 2003;188:606–610. doi: 10.1067/mob.2003.7. 10.1067/mob.2003.7 [DOI] [PubMed] [Google Scholar]
- 74. Guo SW Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15:441–461. doi: 10.1093/humupd/dmp007. 10.1093/humupd/dmp007 [DOI] [PubMed] [Google Scholar]
- 75.Sallam HN, Garcia‐Velasco JA, Dias S, Arici A. Long‐term pituitary down‐regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006;1:CD004635. [DOI] [PMC free article] [PubMed]
- 76. Rodriguez‐Purata J Coroleu B Tur R Carrasco B Rodriguez I Barri PN Endometriosis and IVF: are agonists really better? Analysis of 1180 cycles with the propensity score matching. Gynecol Endocrinol. 2013;29:859–862. doi: 10.3109/09513590.2013.808327. 10.3109/09513590.2013.808327 [DOI] [PubMed] [Google Scholar]
- 77. Opøien HK Fedorcsak P Byholm T Tanbo T Complete surgical removal of minimal and mild endometriosis improves outcome of subsequent IVF/ICSI treatment. Reprod Biomed Online. 2011;23:389–395. doi: 10.1016/j.rbmo.2011.06.002. 10.1016/j.rbmo.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 78. Donnez J Wyns C Nisolle M Does ovarian surgery for endometriomas impair the ovarian response to gonadotropin? Fertil Steril. 2001;76(4):662–665. doi: 10.1016/s0015-0282(01)02011-8. 10.1016/S0015‐0282(01)02011‐8 [DOI] [PubMed] [Google Scholar]
- 79.Benschop L, Farquhar C, van der Poel N, Heineman MJ. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst Rev. 2010;11:CD008571. [DOI] [PMC free article] [PubMed]
- 80. Benaglia L Somigliana E Vighi V Ragni G Vercellini P Fedele L Rate of severe ovarian damage following surgery for endometriomas. Hum Reprod. 2010;25:678–682. doi: 10.1093/humrep/dep464. 10.1093/humrep/dep464 [DOI] [PubMed] [Google Scholar]
- 81. Hachisuga T Kawarabayashi T Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17:432–435. doi: 10.1093/humrep/17.2.432. 10.1093/humrep/17.2.432 [DOI] [PubMed] [Google Scholar]
- 82. Muzii L Bianchi A Croce C Manci N Panici PB Laparoscopic excision of ovarian cysts: is the stripping technique a tissue‐sparing procedure? Fertil Steril. 2002;77:609–614. doi: 10.1016/s0015-0282(01)03203-4. 10.1016/S0015‐0282(01)03203‐4 [DOI] [PubMed] [Google Scholar]
- 83. Tang Y Chen SL Chen X He YX Ye DS Guo W et al. Ovarian damage after laparoscopic endometrioma excision might be related to the size of cyst Fertil Steril 2013. 100 464 469 10.1016/j.fertnstert.2013.03.033 [DOI] [PubMed] [Google Scholar]
- 84. Demirol A Guven S Baykal C Gurgan T Effect of endometrioma cystectomy on IVF outcome: a prospective randomized study. Reprod Biomed Online. 2006;12:639–643. doi: 10.1016/s1472-6483(10)61192-3. 10.1016/S1472‐6483(10)61192‐3 [DOI] [PubMed] [Google Scholar]
- 85. Streuli I Ziegler D Gayet V Santulli P Bijaoui G Mouzon J et al. In women with endometriosis anti‐Müllerian hormone levels are decreased only in those with previous endometrioma surgery Hum Reprod 2012. 11 3294 3303 10.1093/humrep/des274 [DOI] [PubMed] [Google Scholar]
- 86. Somigliana E Arnoldi M Benaglia L Iemmello R Nicolosi AE Ragni G IVF‐ICSI outcome in women operated on for bilateral endometriomas. Hum Reprod. 2008;23:1526–1530. doi: 10.1093/humrep/den133. 10.1093/humrep/den133 [DOI] [PubMed] [Google Scholar]
- 87. Busacca M Riparini J Somigliana E Oggioni G Izzo S Vignali M et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas Am J Obstet Gynecol 2006. 195 421 425 10.1016/j.ajog.2006.03.064 [DOI] [PubMed] [Google Scholar]
- 88. Muzii L Achilli C Lecce F Bianchi A Franceschetti S Marchetti C et al. Second surgery for recurrent endometriomas is more harmful to healthy ovarian tissue and ovarian reserve than first surgery Fertil Steril 2015. 103 738 743 10.1016/j.fertnstert.2014.12.101 [DOI] [PubMed] [Google Scholar]
- 89. Alborzi S Keramati P Younesi M Samsami A Dadras N The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometriomas. Fertil Steril. 2014;101:427–434. doi: 10.1016/j.fertnstert.2013.10.019. 10.1016/j.fertnstert.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 90. Chapron C Fauconnier A Goffinet F Breart G Dubuisson J Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta‐analysis. Hum Reprod. 2002;17:1334–1342. doi: 10.1093/humrep/17.5.1334. 10.1093/humrep/17.5.1334 [DOI] [PubMed] [Google Scholar]
- 91. Kobayashi H Sumimoto K Moniwa N Imai M Takakura K Kuromaki T et al. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan Int J Gynecol Cancer 2007. 17 37 43 10.1111/j.1525‐1438.2006.00754.x [DOI] [PubMed] [Google Scholar]
- 92. Gizzo S Andrisani A Esposito F Oliva A Zicchina C Capuzzo D et al. Ovarian reserve test: an impartial means to resolve the mismatch between chronological and biological age in the assessment of female reproductive chances Reprod Sci 2014. 21 632 639 10.1177/1933719113508821 [DOI] [PubMed] [Google Scholar]


