Abstract
The female reproductive organ undergoes dynamic morphological changes under the influence of hormonal stimuli, and particularly those mediated by estrogen and progesterone. The uterus changes both its morphological appearance and its functional movements in function of these influences. Functionally, the uterus is known to exert two kinds of inherent contractility: sustained uterine contractions and uterine peristalsis. The former is focal and consists of the sporadic bulging of the myometrium, while the latter is rhythmic and manifests itself as the subtle stripping movement in the subendometrial myometrium. The mechanisms underlying these uterine movements, their relationship and their correlation to age, pharmaceutical administration, and a variety of gynecologic and obstetrical problems remain under discussion. Cine MR imaging may offer the potential to directly observe the changes undergone by the uterus in relation to important functions such as fertility and menstrual problems.
Keywords: Cine MRI, Contraction, MRI, Peristalsis, Uterus
Introduction
The physiological changes undergone by the uterus have been extensively studied and very well described in human anatomy books, including changes according to the age, hormonal drug intake, and so on. Of the several imaging modalities currently available, magnetic resonance (MR) imaging holds a superior tissue contrast compared to ultrasonography or computer tomography (CT) in addition to being devoid of the use of ionizing radiation. Reliant on an excellent tissue contrast, MR imaging allows visualization of uterine zonal anatomy and those changes clearly, and is required to evaluate the local disease extent of gynecological disorders in both benign and malignant cases. Positron emission tomography (PET)/CT had been the only modality to visualize functional aspects of the body. Recent progress in MR techniques has allowed the acquisition of functional information using modalities such as cine MR imaging, diffusion‐weighted images (DWI) or dynamic contrast‐enhanced (DCE) MR images. Cine MR imaging can be applied to the evaluation of the physiology of uterine contractions and pelvic floor dysfunction [1, 2].
This article reviews the uterine anatomy and its function with a focus on uterine contractions using cine MR imaging of female pelvic lesions.
Normal anatomy of the uterus in MRI
During the reproductive period, the uterus shows three distinct zonal layers in the uterine corpus and the cervix on T2‐weighted images (WI). These include the endometrium characterized by its high signal intensity, the inner myometrium presenting a low signal intensity, and also called the junctional zone (JZ), and the outer myometrium associated with a relatively high signal intensity (Fig. 1) [3]. The cervix also consists of three separate layers on T2WI: first, the cervical mucosa associated with a high signal intensity, the cervical stroma exhibiting a distinct low signal intensity due to the presence of fibrous connective tissue, and the outer layer characterized by its medium to high signal intensity [3]. The appearance of these structures changes under the effect of ovarian hormones including estrogen and progesterone. As such, the zonal differentiation of the corpus is often indistinct in premenarchal girls and postmenopausal women. In addition, the thickness or signal intensity of each zone shows cyclic changes according to the menstrual cycle phases and age (Fig. 2) [4, 5, 6].
Figure Fig. 1.
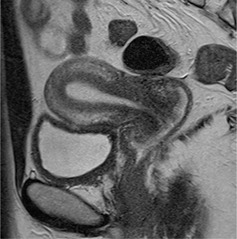
Representative case of a 34‐year‐old woman with a normal uterus. On T2‐weighted images, the uterus shows three distinct layers: a high signal endometrium, a low signal junctional zone, and an intermediate‐signal outer myometrium
Figure Fig. 2.
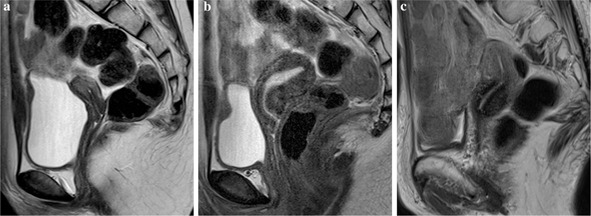
Changes in uterine MR appearance in function of age. a Uterus of a 9‐year‐old girl at premenarcheal state showing a small uterine body, which has almost the same length as that of the uterine cervix. The endometrium is difficult to recognize. b Uterus of a 12‐year‐old girl, the same subject with a. As menstruation starts, the uterine body becomes bigger and the zonal anatomy is clearly recognized with a thickened endometrium. c Representative images of a normal uterus in a 73‐year‐old woman. The uterine volume shrinks down and returns to its indistinct zonal appearance
A number of studies have reported that the JZ was recognized in MR imaging while it was not in hematoxylin and eosin‐stained sections [7, 8, 9, 10, 11, 12]. Correlation with endovaginal ultrasound (US) also revealed a discrepancy with the subendometrial halo (subendometrial hypoechoic layer of the myometrium) on US [8]. Hricak et al. reported JZ as the stratum basale of the endometrium, and described it as a vascular or physiochemical phenomenon [3]. By morphological analysis, an increased water content, nuclear area, and vascular density were reported to be characteristic of the JZ when compared to the outer myometrium [7, 9, 10]. In contrast, Lee et al. suggested that the JZ might be a physiologic phenomenon rather than a morphologic zone on the basis of MR imaging of the uterus ex vivo [11]. Recent progress in the acquisition speed of MR allowed for the visualization of changes in JZ thickness in a normal uterus [12, 13]. These physiological uterine changes of both slow and relatively fast movement will be introduced in the next section.
Physiological changes of the uterus
The zonal appearance of the uterus changes significantly in response to various effects such as hormonal intake, menstrual cycle changes, and contractions. As for menstrual cycle changes, the thickness of the uterine endometrium gradually increases after menstruation and is at its thickest during the secretory phase [5, 14]. The signal intensity of the outer myometrium also increased during the secretory phase on T2‐WI, and the highest signal intensity is observed in the mid‐secretory phase, possibly due to the edematous myometrium [1]. In patients using oral contraceptives (OCs), a characteristic appearance is observed on T2WI. In addition to the thinning of the endometrium, well characterized on ultrasound, the JZ thickness also becomes thinner while the myometrium becomes thicker and present a brighter signal on T2WI compared to normal uterine images [15]. Patients taking GnRH analogs present a smaller uterus reflecting myometrial and endometrial atrophy [14]. The signal intensity of the myometrium also decreases upon intake of GnRH analog. Similar changes were observed in patients with cervical cancer subjected to neoadjuvant chemotherapy (NACT). In pre‐menopausal patients, both the uterine and the ovarian volumes, as well as the signal intensity of the myometrium decrease to levels similar to those seen in post‐menopausal women following NACT. This is due to ovarian toxicity and secondary hormonal changes [16]. Tamoxifen citrate is an antiestrogen agent that binds to estrogen receptors. It acts as an antiestrogen on breast tissue, but shows a weak estrogen agonistic activity in postmenopausal endometrial tissues. Two MR imaging patterns are characteristic of postmenopausal patients treated with tamoxifen [17]. The endometrium shows a homogenously high signal on T2WI with enhancement or a heterogeneously high signal with enhancement at the endometrial–myometrial interface and a lattice‐like enhancement [17].
Uterine contraction commonly occurs during pregnancy as Braxton Hicks contraction. It is commonly known that Braxton Hicks contractions appear unpredictably and sporadically and usually in a non‐rhythmic fashion, but increase prior to delivery [18]. On MR images, similar sporadic contraction may be observed in the non‐gravid uterus, and is commonly coined sustained contraction (Fig. 3) [19]. This type of contraction frequently involves the entire layer of the myometrium and appears as transient focal masses of low signal intensity on T2WI, bulging into the endometrium [19]. They may last several minutes, occasionally up to 20–30 min. These focal contractions are known as pseudolesions resembling those observed in disorders such as fibroids and adenomyosis [19].
Figure Fig. 3.
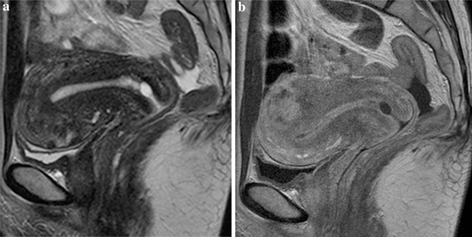
Normal uterus of a 48‐year‐old woman. a On T2‐WI, the zonal anatomy of the uterine body can be recognized, but small and irregular low signal areas are observed within the anterior wall, indicating sporadic uterine contractions. b Ten minutes later (represented in a) the uterus appearance, imaged in contrast‐enhanced T1‐WI, shows differences from that seen on T2‐WI. A focal myometrial lesion with irregular enhancement has emerged in the posterior wall. In contrast, the thickness of the anterior wall looks reduced. These findings mean that the sporadic contraction of the anterior wall shown in a has disappeared and emerged in the posterior wall
In a non‐gravid myometrium, another type of myometrial contraction occurs and is known as uterine peristalsis. Uterine peristalsis is identifiable on US as rhythmic and subtle wave‐like endometrial movements associated with contractions of the inner myometrium [20, 21]. The frequency, height, and direction of the peristaltic waves were reported to vary throughout the phases of the menstrual cycle or in function of hormonal variations [20, 21]. For example, the direction of the contractions is usually retrograde (cervix to fundus) during the mid‐cycle and anterograde during menstruation. As such, uterine peristalsis is considered to play a role in sperm transport, discharge of menstrual blood, and conservation of the gestational sac during the early stages of pregnancy [20, 21]. Because their average frequency of occurrence is 2–3 contractions per minute, they are difficult to visualize on conventional T2‐weighted MRI images due to insufficient time resolution [20]. For this reason, cine MR imaging using single‐shot fast spin‐echo (SSFSE)/half‐Fourier acquisition single‐shot turbo spin‐echo (HASTE) sequence is the preferred method for visualizing uterine peristalsis for the evaluation of pelvic floor dysfunction [2].
Subtle and rhythmic uterine contractions: uterine peristalsis on MRI imaging techniques for cine MR
Nakai et al. have shown that cine MR might be superior to trans‐vaginal US (TVUS) in the evaluation of uterine contractility for its noninvasiveness (i.e., the method does not induce any artificial contractility) and its excellent tissue contrast allowing for the clear delineation of wave conduction in the subendometrial myometrium [22]. Another advantage of MR is its ability to visualize the wave extension going through the myometrium and into the outer myometrium [22]. As for the MR sequence, a T2‐weighted image does not offer adequate time resolution, as mentioned above. Instead, SSFSE sequences may be used to obtain relatively motion‐free breath‐hold sequences with tissue contrast similar to that obtained when using more conventional fast spin‐echo T2‐weighted images [23]. The drawback of this sequence is the inherently low signal‐to‐noise ratio (SNR) and contrast‐to‐noise ratio (CNR) in the uterus [23, 24]. Therefore, Nakai et al. examined the optimal scan interval for the evaluation of uterine peristalsis and the best performance was obtained with a 4‐s scan interval, which yielded a superior CNR and peristaltic detection compared with the 2‐ or 3‐s scan intervals [25].
Another important point in the evaluation of uterine peristalsis is the display of these images in cine‐mode i.e., at 5‐ to 12‐fold the regular speed, since the uterine contractile movement is slow and subtle compared with contractile movements such as heartbeat or bowel peristalsis.
Definition and evaluation of uterine peristalsis
On cine MR imaging, uterine peristalsis, which is observed as a stripping movement of the endometrium on US, may be depicted as a low‐intensity conduction within the subendometrial myometrium (often within the JZ), usually associated with a stripping movement of the endometrium [1, 12, 20, 21]. The low‐intensity conduction seems to directly display wavy conductions of the subtle and rhythmic contractions of the subendometrial myometrium. Quantitatively, this movement is reflected as a change in JZ thickness and signal intensity [13]. The only peristaltic movement that can be visualized on MR imaging is the conduction of low signal intensity toward the outer myometrium (outer myometrial conduction, OMC) [26].
The assessment of uterine peristalsis starts visually but automated evaluation methods have also been developed. Semi‐automated or fully automated methods of evaluation were created utilizing software based on a contour‐tracing method or based on the identification of neighboring areas showing similar patterns of signal intensity decrease in a different timing [27, 28, 29, 30]. Watanabe et al. evaluated the agreement between the number of peristaltic waves in the visual evaluation and the fully automated software was assessed using weighted kappa statistics and resulted in kappa values ranging from 0.89 to 0.95, indicating a good correlation [29].
Although peristaltic images were, at first, mainly obtained on the sagittal plane, we also tried to visualize the coronal plane of the uterus. The key benefit of the coronal plane is that it includes the uterine fundus and allows for the assessment of the correlation between ovulation side and peristaltic direction. In a study using hysterosalpingoscintigraphy, considerable ascension of the technetium‐labeled sperm, which was directed preferentially into the tube ipsilateral to the dominant follicle, was observed during the late follicular phase [31]. Then, rapid transport of the spermatozoa through the female genital tract is under the endocrine control of the dominant follicle [31]. In a study by Shitano et al., the side of ovulation was estimated in ten out of 31 subjects. Since the confirmation of ovulation side by transvaginal ultrasound was difficult, the ovulation side was estimated from the MR imaging findings. The dominant follicle was defined as having a size of about 20 mm, found only on one side of the ovaries. Corpus luteum on T2‐weighted image was defined as a nodule or cystic structure, which had a thick and irregular low signal‐intensity wall [32]. However, peristaltic waves conducted toward the possible ovulation side were observed in only three of the ten subjects. Further study will be expected. Another advantage of coronal planes resides in the fact that peristaltic directions and outer myometrial conduction (OMC) are more distinctively and more frequently recognized in the coronal plane compared to the sagittal plane [32]. However, the inevitable problem with obtaining coronal cine MR is the movement of the uterus itself, especially in the cephalo‐caudal direction due to the bowel movements. As such, it is possible that the endometrial–myometrial junctions of the fundus might slide off the focus of the coronal cine MRI and result in failed images.
Normal uterine cycles, circadian rhythm, and changes during menstrual phases
Peristaltic frequency and direction vary according to the menstrual cycle phases under the influence of hormonal variations [20, 21]. Uterine peristalsis is most active during the periovulatory phase and almost absent during the luteal phase. The dominant peristaltic direction is retrograde (cervix to fundus) during the periovulatory phase (Fig. 4) and anterograde (fundus to cervix) during the menstrual phase [1, 12, 20, 21]. These movements are believed to be regulated by the secretion of estrogen and progesterone and play a role in the very early stages of reproduction, including activities such as sperm transport, early preservation of the gestational sac, and discharge of menstrual blood [1, 12, 20, 21]. In contrast to menstrual cycle changes, peristalsis does not fluctuate significantly within the course of the day [33]. Because serum estradiol and luteinizing hormones are known to lack circadian rhythm or show only small diurnal variations, they do not affect the uterine movement [34, 35]. Anticholinergic agents also do not affect uterine peristalsis [26]. Compared to the small intestine, suppression of peristalsis was weaker, with an earlier onset and a shorter duration in the uterus [26]. On the contrary, uterine peristalsis may be suppressed by the occurrence of sustained contraction. Even during the peri‐ovulation with frequent movement, its frequency was reduced in the presence of sustained contraction, either concealed or suppressed by strong myometrial contractions [33]. Dramatic hormonal changes occur upon delivery, including a decrease in the level of estrogen and progesterone [36]. These hormonal changes cause the uterus to shrink down to a size similar to that observed during a menopausal state and peristalsis is scarcely observed [37].
Figure Fig. 4.
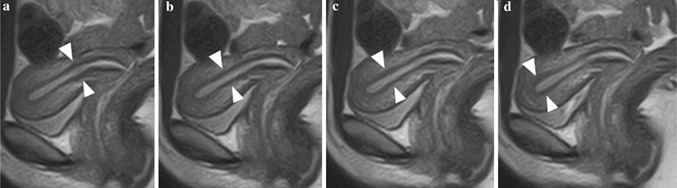
Normal uterus of a 20‐year‐old subject. Serial images of uterine peristalsis captured with a 3‐s interval. a JZ at the area of internal ostium shows a thicker and darker signal than that in other areas. b, c The thick and dark JZ has moved with time and has reached the fundus. d This signal change means that subtle contraction of the subendometrial myometrium has occurred and may help convey the sperm from the cervix to the fundus
Evaluation of dysmenorrhea and treatment
Primary dysmenorrhea is defined as a pain occurring during menses in the absence of an identifiable pathologic lesion and is a major cause of activity restriction leading to school and work absence in adolescent girls [38]. The severe pain is believed to be caused by an increased production of prostaglandin by the endometrium leading to uterine contractions [39, 40]. Increased secretion of prostaglandin results in higher uterine tone with high‐amplitude contractions resulting in decreased uterine blood flow [41]. However, evaluation of these measurements was achieved invasively through measurement of the intrauterine pressure by inserting a catheter into the uterine cavity. The pain severity level is thus a very subjective evaluation. Because of these limitations and the challenge in assessing these types of pains, the clinical evaluation of dysmenorrhea has stagnated.
As such, MR imaging represents a potential tool for evaluating dysmenorrhea using both static and cine MR. On MR imaging, remarkable changes of the uterine appearances were observed during the cycle date of 1–3 [42]. The degree of pain was significantly associated with the thickness of the inner low‐signal‐intensity myometrial layer and the degree of endometrial distortion at fundus on T2‐WI [42]. The area of the uterine myometrium is significantly smaller during cycle days 1–3 in the dysmenorrheic group, as compared with that in the eumenorrheic group (p = 0.010) [42]. Uterine peristalsis was undetectable when the pain was severe or moderate [42]. The decrease in myometrial signal intensity on T2‐WI is the result of the squeezing of the blood out of the myometrium, while the thicker inner low‐signal‐intensity zone (JZ) seems to reflect lower blood content in this area due to the squeezing effect of the contractions [19, 42]. Decreased uterine corpus size and severe endometrial distortion also directly reflect the effects of myometrial contractility aimed at discharging menstrual blood from the cavity. All of these findings seemed to directly relate to the degree of myometrial contraction.
While OCs were initially developed to prevent conception, they are now widely used for the treatment of primary dysmenorrhea in women [38]. Of the several mechanisms reported in the pain relief of dysmenorrhea, the effective reduction of prostaglandin (PG) production by OCs is believed to be one of the most probable ones [38]. Then, the next question is as to how the MR findings are observed in dysmenorrhea subjects with OCs users. The results were opposite to those observed in dysmenorrhea patients. In other words, endometrial distortion was significantly less prominent and the subendometrial low‐intensity area was significantly thinner in the OCs group [43]. The uterine myometrial area tended to be larger in OCs users compared to the controls. Also, the degree of pain was significantly lower than in the control patients [43]. Ekström et al. reported a reduction in the intrauterine pressure in OCs users compared to subjects not taking any OCs. These MR findings are thought to reflect reduced uterine contractility [44].
Uterine contractility and contraceptives [OCs and intrauterine device (IUD)]
As mentioned above, OCs were initially developed to prevent conception. Their mechanism of action resides in the inhibition of ovulation through suppression of the basal secretion level of follicle‐stimulating hormone (FSH) and luteinizing hormone (LH) [45]. OCs diminish the ability of the pituitary gland to synthesize gonadotropin upon stimulation by hypothalamic GnRH [45, 46]. These hormonal changes affect uterine contractility and morphology. Uterine peristalsis was markedly decreased in the OC users down to about 1.0/min in mid‐cycle compared to the normal uterus of 2.3/min during the periovulatory phase. This is consistent with previous reports using TVUS [15, 47]. Suppression of peristalsis may have an adverse effect on conception, possibly disturbing the upward transport of sperm. As for the uterine morphology, the endometrium and JZ were significantly thinner, and the myometrium was thicker in the OC users compared to controls [15]. The signal intensity of the myometrium and the cervical mucus was also significantly higher in the OC users. All of these findings reflect the histological findings, i.e., endometrial atrophy and myometrial edema together with vasodilatation and viscous cervical mucus [48, 49].
Another means of contraception is the use of IUDs, although their mechanism is still debated. One reported mechanism is the interference of IUDs with implantation of the fertilized ovum, and another mechanism that has been proposed is that IUDs exert a spermicidal effect via inhibition of sperm transport [50, 51]. On TVUS, it has been documented that there is a significant decrease in the wave frequency of the cervix to fundus‐directed uterine contractions in women bearing IUD on mid‐cycle [47]. The same pattern was observed on MR imaging. Characteristic waves during peri‐ovulation period from cervix to fundus (CF) was observed in very few subjects, but a fundo‐cervical (FC)‐directed peristaltic wave was identified in four out of 11 IUD‐bearing subjects in contrast to only one of 12 subjects in the control group [52]. In addition, FC waves extended through more than half of the thickness of the myometrium [52]. We could at least say that this direction of peristaltic waves was not supportive of sperm transport but could be supportive of an attempt to expel the IUD from the uterine cavity. The endometrium of IUD users is known to release prostaglandins (PGs), especially PGE, which induces strong contractions of the myometrium [53]. We speculated that strong FC waves represented contractions regulated by PGs, while subtle CF waves observed at mid‐cycle were regulated by estrogen [20].
Correlation with infertility problems
Infertility rates have seen an increasing trend, with 10–15 % of couples in the United States of America diagnosed as infertile [54]. Endometriosis and fibroids represents two of the most important causes of infertility. Numerous other factors have been considered as potential etiologies for infertility, including decreased ovarian reserve, tubal injury/adhesion, uterine factors, and immunologic aberrations [54].
The association between infertility and endometriosis is complex and controversial. Although various mechanisms, such as ovulatory dysfunction, immune system abnormalities, pelvic scarring/adhesion, and intraperitoneal inflammation, have been suggested, the precise mechanisms are still obscure [55]. As for the relationship with uterine peristalsis, Leyendecker and Kunz et al. reported hyper‐frequent peristalsis in patients with endometriosis compared to control subjects in every menstrual phase based on TVUS findings [56]. In addition, “dysperistalsis” is observed in women presenting endometriosis, which might lead to a reduction in uterine transport capacity [56, 57]. “Dysperistalsis” is defined as contractions originating in the middle portion of the uterus and spreading simultaneously to the fundus and the cervix, or contractions starting simultaneously at different sites, creating a convulsive appearance in uterine activity, with some waves vanishing before reaching the uterine fundus [56]. On the contrary, on MR imaging, uterine peristalsis was observed less frequently during the periovulation phase [58]. There is a potential relationship with the increased sustained contraction in endometriosis patients during this phase [58].
Although the exact effect of fibroids on peristalsis has not been clearly established, it is possible that some forms of fibroids, by either mechanical or functional means, may suppress or disrupt normal peristaltic wave propagation in the inner myometrium [59, 60, 61]. Nishino et al. reported that submucosal fibroids produced focal movement or dysfunctional contraction, in an attempt to expel the fibroids [59]. In a previous study including symptomatic fibroid patients, candidate for uterine arterial embolization, uterine peristalsis was significantly decreased in fibroid patients compared to normal controls [61]. Peristalsis frequency in fibroid patients was also lower than that of normal subjects. However, there was no significant relationship between fibroid characteristics—such as uterine volume, index fibroid volume, index fibroid location—and fibroid number in fibroid patients with or without peristalsis.
Transcatheter uterine artery embolization (UAE) is a minimally invasive, safe, and effective treatment option for patients with symptomatic uterine fibroids. The long‐term outcome for women undergoing UAE is comparable to that for myomectomy and hysterectomy [62, 63, 64]. In 20 cases of fibroids patients who were scheduled for UAE, peristalsis was observed in only 2–4 cases before UAE [65]. The presence and frequency of uterine peristalsis also increased after UAE, but significance was only found in the presence of uterine peristalsis [65]. That said, peristalsis newly emerged in six of the patient cases who underwent UAE. Uterine volumes prior to UAE were significantly smaller in these six cases in comparison with the remaining 14 cases, though no significant difference was found in the reduction rate of the uterus or fibroid volumes [65].
Gynecologist groups examined the uterine peristalsis of patients with intramural fibroids during the mid‐luteal phase, in other words, the implantation period and most scanty peristalsis phase. Their results showed that the patients with a higher frequency of uterine peristalsis (≥2 times/3 min) during the mid‐luteal phase did not achieve pregnancy compared to 10/29 patients in the low‐frequency group [66]. Therefore, high‐frequency peristalsis during the mid‐luteal phase might be one of the causes of infertility associated with intramural‐type fibroids [66]. On the other hand, myomectomy will lead to a favorable outcome in these fibroids patients both in terms of uterine peristalsis and fertility. Of 15 fibroid patients presenting hyperperistalsis during the implantation phase, the frequency of uterine peristalsis was normalized (0 or 1 time/3 min) in 14 of the patients [67]. In addition, six of the 15 patients achieved pregnancy [67]. The presence of uterine fibroids may induce abnormal uterine peristalsis in some patients, leading to infertility, and myomectomy may thus improve fertility in these patients [67].
Ijland et al. explored the relationship between uterine peristalsis and fecundability in spontaneous menstrual cycles using TVUS [68]. Their observations that fundo‐cervical peristaltic direction is more frequent in the infertile group during the periovulatory phase agrees with our MRI findings [69]. Uterine peristalsis during the in vitro fertilization (IVF) cycle was reported to be more frequently identified and prominent compared with a normal cycle, according to a TVUS‐based study [70, 71]. In addition, Fanchin et al. reported that the fewer the uterine peristaltic contraction, the higher the pregnancy rate after IVF‐ET even though the progressive decrease in uterine contractility, during the luteal phase, was observed in IVF‐cycle [72].
Conclusions
MR imaging allows the non‐invasive visualization of the changes undergone by the female reproductive organ under the influence of hormonal stimuli, both morphologically and functionally. Cine MR imaging offers the potential to directly observe the alteration and impairment of uterine functions in a variety of conditions and gynecologic disorders.
Compliance with ethical standards
Conflict of interest
Aki Kido and Kaori Togashi declare that they have no conflicts of interest.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients included in the study.
Animal studies
This article does not contain any studies with animal subjects performed by any of the authors.
References
- 1. Togashi K Nakai A Sugimura K Anatomy and physiology of the female pelvis: MR imaging revisited. J Magn Reson Imaging. 2001;13(6):842–849. doi: 10.1002/jmri.1120. [DOI] [PubMed] [Google Scholar]
- 2. Sayed RF et al. Pelvic floor dysfunction: assessment with combined analysis of static and dynamic MR imaging findings Radiology 2008. 248 2 518 530 [DOI] [PubMed] [Google Scholar]
- 3. Hricak H et al. Magnetic resonance imaging of the female pelvis: initial experience AJR Am J Roentgenol 1983. 141 6 1119 1128 [DOI] [PubMed] [Google Scholar]
- 4. McCarthy S Tauber C Gore J Female pelvic anatomy: MR assessment of variations during the menstrual cycle and with use of oral contraceptives. Radiology. 1986;160(1):119–123. doi: 10.1148/radiology.160.1.3715022. [DOI] [PubMed] [Google Scholar]
- 5. Haynor DR et al. Changing appearance of the normal uterus during the menstrual cycle: MR studies Radiology 1986. 161 2 459 462 [DOI] [PubMed] [Google Scholar]
- 6. Hauth EA et al. MR imaging of the uterus and cervix in healthy women: determination of normal values Eur Radiol 2007. 17 3 734 742 [DOI] [PubMed] [Google Scholar]
- 7. McCarthy S et al. Uterine junctional zone: MR study of water content and relaxation properties Radiology 1989. 171 1 241 243 [DOI] [PubMed] [Google Scholar]
- 8. Mitchell DG et al. Zones of the uterus: discrepancy between US and MR images Radiology 1990. 174 3 Pt 1 827 831 [DOI] [PubMed] [Google Scholar]
- 9. Scoutt LM et al. Junctional zone of the uterus: correlation of MR imaging and histologic examination of hysterectomy specimens Radiology 1991. 179 2 403 407 [DOI] [PubMed] [Google Scholar]
- 10. Brosens JJ Souza NM Barker FG Uterine junctional zone: function and disease. Lancet. 1995;346(8974):558–560. doi: 10.1016/s0140-6736(95)91387-4. [DOI] [PubMed] [Google Scholar]
- 11. Lee JK et al. The uterus: in vitro MR‐anatomic correlation of normal and abnormal specimens Radiology 1985. 157 1 175 179 [DOI] [PubMed] [Google Scholar]
- 12. Nakai A et al. Junctional zone on magnetic resonance imaging: continuous changes on ultrafast images J Women's Imaging 2001. 3 3 89 93 [Google Scholar]
- 13. Masui T et al. Changes in myometrial and junctional zone thickness and signal intensity: demonstration with kinematic T2‐weighted MR imaging Radiology 2001. 221 1 75 85 [DOI] [PubMed] [Google Scholar]
- 14. Demas BE Hricak H Jaffe RB Uterine MR imaging: effects of hormonal stimulation. Radiology. 1986;159(1):123–126. doi: 10.1148/radiology.159.1.3952297. [DOI] [PubMed] [Google Scholar]
- 15. Kido A et al. Oral contraceptives and uterine peristalsis: evaluation with MRI J Magn Reson Imaging 2005. 22 2 265 270 [DOI] [PubMed] [Google Scholar]
- 16. Himoto Y et al. MR imaging‐based evaluation of morphological changes in the uterus and ovaries of patients following neoadjuvant chemotherapy for cervical cancer Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med 2015. 14 1 65 72 [DOI] [PubMed] [Google Scholar]
- 17. Ascher SM Imaoka I Lage JM Tamoxifen‐induced uterine abnormalities: the role of imaging. Radiology. 2000;214(1):29–38. doi: 10.1148/radiology.214.1.r00ja4429. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham FG, editor. Williams obstetrics. New York: McGraw‐Hill; 2001. Section 2 maternal and fetal anatomy and physiology; pp. 14–173. [Google Scholar]
- 19. Togashi K et al. Sustained uterine contractions: a cause of hypointense myometrial bulging Radiology 1993. 187 3 707 710 [DOI] [PubMed] [Google Scholar]
- 20. Vries K et al. Contractions of the inner third of the myometrium Am J Obstet Gynecol 1990. 162 3 679 682 [DOI] [PubMed] [Google Scholar]
- 21. Lyons EA et al. Characterization of subendometrial myometrial contractions throughout the menstrual cycle in normal fertile women Fertil Steril 1991. 55 4 771 774 [DOI] [PubMed] [Google Scholar]
- 22. Nakai A et al. Uterine peristalsis shown on cine MR imaging using ultrafast sequence J Magn Reson Imaging 2003. 18 6 726 733 [DOI] [PubMed] [Google Scholar]
- 23. Outwater EK Ultrafast MR imaging of the pelvis. Eur J Radiol. 1999;29(3):233–244. doi: 10.1016/s0720-048x(98)00165-x. [DOI] [PubMed] [Google Scholar]
- 24. Sugimura H et al. Comparison of conventional fast spin echo, single‐shot two‐dimensional and three‐dimensional half‐fourier RARE for T2‐weighted female pelvic imaging J Magn Reson Imaging JMRI 2004. 19 3 349 355 [DOI] [PubMed] [Google Scholar]
- 25. Nakai A et al. Optimizing cine MRI for uterine peristalsis: a comparison of three different single shot fast spin echo techniques J Magn Reson Imaging JMRI 2013. 38 1 161 167 [DOI] [PubMed] [Google Scholar]
- 26. Daido S et al. Anticholinergic agents result in weaker and shorter suppression of uterine contractility compared with intestinal motion: time course observation with cine MRI J Magn Reson Imaging JMRI 2013. 38 5 1196 1202 [DOI] [PubMed] [Google Scholar]
- 27. Nishiura M Yuasa M Watanabe M Active contour extraction method using partial shape constraint contour model. Syst Comput Jpn. 2000;131(14):20–28. [Google Scholar]
- 28. Hozumi T et al. Echocardiographic estimation of left ventricular cavity area with a newly developed automated contour tracking method J Am Soc Echocardiogr 1997. 10 8 822 829 [DOI] [PubMed] [Google Scholar]
- 29.Watanabe K, et al. Automated detection and measurement of uterine peristalsis in cine MR images. J Magn Reson Imaging JMRI. 2014. [DOI] [PubMed]
- 30. Kido A et al. A semiautomated technique for evaluation of uterine peristalsis J Magn Reson Imaging 2005. 21 3 249 257 [DOI] [PubMed] [Google Scholar]
- 31. Kunz G et al. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy Hum Reprod 1996. 11 3 627 632 [DOI] [PubMed] [Google Scholar]
- 32.Shitano F, et al. Evaluation of uterine peristalsis using cine MRI on the coronal plane in comparison with the sagittal plane. Acta Radiol. 2015. [DOI] [PubMed]
- 33. Kido A et al. Investigation of uterine peristalsis diurnal variation Magn Reson Imaging 2006. 24 9 1149 1155 [DOI] [PubMed] [Google Scholar]
- 34. Baird DT Guevara A Concentration of unconjugated estrone and estradiol in peripheral plasma in nonpregnant women throughout the menstrual cycle, castrate and postmenopausal women and in men. J Clin Endocrinol Metab. 1969;29(2):149–156. doi: 10.1210/jcem-29-2-149. [DOI] [PubMed] [Google Scholar]
- 35. Reame N et al. Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin‐releasing hormone secretion J Clin Endocrinol Metab 1984. 59 2 328 337 [DOI] [PubMed] [Google Scholar]
- 36.Cunningham FG, editor. Williams obstetrics. New York: McGraw‐Hill; 2001. The Puerperium; pp. 403–421. [Google Scholar]
- 37.Daido SN, Kido A, Fujimoto A, Kusahara K, Togashi K. Uterine appearance and uterine peristalsis during lactation on MR imaging. In: 19th Annual Meeting, International Society for Magnetic Resonance in Medicine. Montreal, Canada. 2011.
- 38. Davis AR Westhoff CL Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol. 2001;14(1):3–8. doi: 10.1016/s1083-3188(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 39. Pulkkinen MO Prostaglandins and the non‐pregnant uterus. The pathophysiology of primary dysmenorrhea. Acta Obstet Gynecol Scand Suppl. 1983;113:63–67. doi: 10.3109/00016348309155200. [DOI] [PubMed] [Google Scholar]
- 40.Berek J, editor. Novak's gynecology. Philadelphia: Lippincott Williams and Wilkins & Wilkins; 2002. Pelvic pain and dysmenorrhea; pp. 421–452. [Google Scholar]
- 41. Akerlund M Stromberg P Forsling ML Primary dysmenorrhoea and vasopressin. Br J Obstet Gynaecol. 1979;86(6):484–487. doi: 10.1111/j.1471-0528.1979.tb10794.x. [DOI] [PubMed] [Google Scholar]
- 42. Kataoka M et al. Dysmenorrhea: evaluation with cine‐mode‐display MR imaging‐initial experience Radiology 2005. 235 1 124 131 [DOI] [PubMed] [Google Scholar]
- 43. Kido A et al. The effect of oral contraceptives on uterine contractility and menstrual pain: an assessment with cine MR imaging Hum Reprod 2007. 22 7 2066 2071 [DOI] [PubMed] [Google Scholar]
- 44. Ekstrom P et al. Effect of an oral contraceptive in primary dysmenorrhea—changes in uterine activity and reactivity to agonists Contraception 1989. 40 1 39 47 [DOI] [PubMed] [Google Scholar]
- 45.Berek J, editor. Novak's gynecology. Philadelphia: Lippincott Williams and Wilkins & Wilkins; 2002. Family planning; pp. 231–293. [Google Scholar]
- 46. Diczfalusy E Mode of action of contraceptive drugs. Am J Obstet Gynecol. 1968;100(1):136–163. doi: 10.1016/s0002-9378(15)33651-6. [DOI] [PubMed] [Google Scholar]
- 47. Maslow KD Lyons EA Effect of oral contraceptives and intrauterine devices on midcycle myometrial contractions. Fertil Steril. 2003;80(5):1224–1227. doi: 10.1016/s0015-0282(03)01174-9. [DOI] [PubMed] [Google Scholar]
- 48. Rivera R Yacobson I Grimes D The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181(5 Pt 1):1263–1269. doi: 10.1016/s0002-9378(99)70120-1. [DOI] [PubMed] [Google Scholar]
- 49. Bartoli JM et al. The normal uterus on magnetic resonance imaging and variations associated with the hormonal state Surg Radiol Anat 1991. 13 3 213 220 [DOI] [PubMed] [Google Scholar]
- 50. Stanford JB Mikolajczyk RT Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol. 2002;187(6):1699–1708. doi: 10.1067/mob.2002.128091. [DOI] [PubMed] [Google Scholar]
- 51. Spinnato JA 2nd Mechanism of action of intrauterine contraceptive devices and its relation to informed consent. Am J Obstet Gynecol. 1997;176(3):503–506. doi: 10.1016/s0002-9378(97)70537-4. [DOI] [PubMed] [Google Scholar]
- 52. Kido A et al. Intrauterine devices and uterine peristalsis: evaluation with MRI Magn Reson Imaging 2008. 26 1 54 58 [DOI] [PubMed] [Google Scholar]
- 53. Hillier K Kasonde JM Prostaglandin E and F concentrations in human endometrium after insertion of intrauterine contraceptive device. Lancet. 1976;1(7949):15–16. doi: 10.1016/s0140-6736(76)92909-3. [DOI] [PubMed] [Google Scholar]
- 54.Berek J, editor. Novak's gynecology. Philadelphia: Lippincott Williams and Wilkins & Wilkins; 2002. Infertility; pp. 973–1066. [Google Scholar]
- 55.Berek J, editor. Novak's gynecology. Philadelphia: Lippincott Williams and Wilkins & Wilkins; 1996. Chapter 26 endometriosis; pp. 887–914. [Google Scholar]
- 56. Leyendecker G et al. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility Hum Reprod 1996. 11 7 1542 1551 [DOI] [PubMed] [Google Scholar]
- 57. Leyendecker G et al. Endometriosis: a dysfunction and disease of the archimetra Hum Reprod Update 1998. 4 5 752 762 [DOI] [PubMed] [Google Scholar]
- 58. Kido A et al. Cine MR imaging of uterine peristalsis in patients with endometriosis Eur Radiol 2007. 17 7 1813 1819 [DOI] [PubMed] [Google Scholar]
- 59. Nishino M et al. Uterine contractions evaluated on cine MR imaging in patients with uterine leiomyomas Eur J Radiol 2005. 53 1 142 146 [DOI] [PubMed] [Google Scholar]
- 60. Orisaka M et al. A comparison of uterine peristalsis in women with normal uteri and uterine leiomyoma by cine magnetic resonance imaging Eur J Obstet Gynecol Reprod Biol 2007. 135 1 111 115 [DOI] [PubMed] [Google Scholar]
- 61. Kido A et al. 3 T MRI uterine peristalsis: comparison of symptomatic fibroid patients versus controls Clin Radiol 2014. 69 5 468 472 [DOI] [PubMed] [Google Scholar]
- 62. Spies JB et al. Outcome of uterine embolization and hysterectomy for leiomyomas: results of a multicenter study Am J Obstet Gynecol 2004. 191 1 22 31 [DOI] [PubMed] [Google Scholar]
- 63. Spies JB et al. Long‐term outcome of uterine artery embolization of leiomyomata Obstet Gynecol 2005. 106 5 Pt 1 933 939 [DOI] [PubMed] [Google Scholar]
- 64. Mara M et al. Uterine fibroid embolization versus myomectomy in women wishing to preserve fertility: preliminary results of a randomized controlled trial Eur J Obstet Gynecol Reprod Biol 2006. 126 2 226 233 [DOI] [PubMed] [Google Scholar]
- 65. Kido A et al. Comparison of uterine peristalsis before and after uterine artery embolization at 3‐T MRI AJR Am J Roentgenol 2011. 196 6 1431 1435 [DOI] [PubMed] [Google Scholar]
- 66. Yoshino O et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids Hum Reprod 2010. 25 10 2475 2479 [DOI] [PubMed] [Google Scholar]
- 67. Yoshino O et al. Myomectomy decreases abnormal uterine peristalsis and increases pregnancy rate J Minim Invasive Gynecol 2012. 19 1 63 67 [DOI] [PubMed] [Google Scholar]
- 68. Mm IJ et al. Relation between endometrial wavelike activity and fecundability in spontaneous cycles Fertil Steril 1997. 67 3 492 496 [DOI] [PubMed] [Google Scholar]
- 69. Kido A et al. Uterine peristalsis in women with repeated IVF failures: possible therapeutic effect of hyoscine bromide J Obstet Gynaecol Can 2009. 31 8 732 735 [DOI] [PubMed] [Google Scholar]
- 70. Mm IJ et al. Endometrial wave direction switch and the outcome of in vitro fertilization Fertil Steril 1999. 71 3 476 481 [DOI] [PubMed] [Google Scholar]
- 71. Fanchin R et al. Uterine contractions at the time of embryo transfer alter pregnancy rates after in vitro fertilization Hum Reprod 1998. 13 7 1968 1974 [DOI] [PubMed] [Google Scholar]
- 72. Fanchin R et al. Uterine contractility decreases at the time of blastocyst transfers Hum Reprod 2001. 16 6 1115 1119 [DOI] [PubMed] [Google Scholar]


