Abstract
It has been widely accepted that the age of women plays a fundamental role in fecundity, and age‐related fertility decline has one of the most significant and detrimental effects on the success rate of infertility treatment. Therefore, treatment cycles of non‐in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) treatment for infertile women of advanced aged have been limited due to their lack of efficacy, and they are often optimized, compared to IVF/ICSI treatment. Recent trends in infertility treatment apparently indicate that IVF/ICSI treatment, including egg donation, is frequently offered to aged women for first‐line management, despite its heavy burden, but hasty IVF/ICSI treatment should be avoided, considering its socioeconomic problems. It is important to distinguish women who could conceive by non‐IVF/ICSI treatment, although the optimization of non‐IVF/ICSI treatment protocols remains poorly understood. This review focuses on extracting aged patients who have higher chance of conceiving with non‐IVF/ICSI treatment and providing necessary and sufficient infertility treatment. After initial evaluation for fertility, including tubal factor, male factor, the presence of endometriosis and/or adenomyosis, and ovarian reserve, the outcomes of fertility treatment can be predicted to some extent in aged infertile women.
Keywords: Advanced reproductive age, Anti‐Müllerian hormone, Infertility, Intrauterine insemination, Treatment protocol
Abbreviations
- AFC
Antral follicle count
- AMH
Anti‐Müllerian hormone
- ART
Assisted reproductive technology
- ASRM
American society for reproductive medicine
- BMI
Body mass index
- CC
Clomiphene citrate
- CPR
Cumulative pregnancy rate
- GS
Gestational sac
- hMG
Human menopausal gonadotropin
- ICSI
Intracytoplasmic sperm injection
- IUI
Intrauterine insemination
- IVF
In vitro fertilization
- RCT
Randomized controlled trial
- rFSH
Recombinant FSH
- PR
Pregnancy rates
Introduction
Although it is widely known that advanced age plays fundamental roles in infertility treatment [1, 2], more women in developed countries tend to delay childbearing until their late 30s and 40s [3]. This trend is resulting in an increasing number of patients undergoing infertility treatment. Intrauterine insemination (IUI) has been used to treat infertility for more than 90 years [4], and is usually offered as a first line therapy after initial infertility evaluations have been fulfilled. Patients who fail to conceive using IUI are generally counseled to undergo in vitro fertilization (IVF) and/or intracytoplasmic sperm injection (ICSI) [5, 6, 7] treatment, because it will be favorable for infertile couples and clinicians that the treatment is stepwise. Initial treatment should be inexpensive and less invasive, and then clinicians should move to treatments requiring greater resources. Considering the treatment procedure, age is a major limiting factor that significantly affects future fecundity of reproductive aged women. For women > 43 years, recently more clinicians are providing IVF/ICSI treatment, including egg donation, as first‐line treatment [8], because controlled ovarian stimulation together with IUI is generally not effective [9] and the IVF/ICSI success rate with the woman's own oocytes is limited [10]. However, gynecologists should remember that IVF/ICSI treatment is a large burden physically, socially and also financially [11]. Therefore, it is important for clinicians to provide appropriate infertility treatment, and unnecessary IVF/ICSI should be avoided in patients who have a certain chance of becoming pregnant without IVF/ICSI measures. Meanwhile, patients with advanced age have lower chance to conceive with non‐IVF/ICSI treatment, and assisted reproductive technologies (ART) should be provided appropriately at an earlier step.
Many studies have shown that advanced age is a crucial factor that leads to a declined cumulative pregnancy rate (CPR) in IUI treatment [12, 13], and other factors, such as tubal infertility, endometriosis and/or adenomyosis, male infertility, decreases in ovarian reserve/ovulatory disorders, uterine fibroids, developmental anomalies, pelvic surgery and menstrual abnormalities, are commonly known to be representative factors that affect fecundity significantly [14], but few clinical studies have discussed the most detrimental infertility factors. We believe that the combination of these infertility factors might have an impact on future fecundity. Therefore, we previously conducted a retrospective study [15] to offer a suitable treatment strategy for aged infertile women, and we focused on extracting those aged patients with a greater likelihood of conceiving by non‐IVF/ICSI treatment and providing the necessary and sufficient infertility treatment. By analyzing a combination of crucial factors that affect CPR in non‐IVF/ICSI treatment, we attempted to determine appropriate treatments for aged infertile women. We extracted representative infertility factors from infertile patients undergoing timed intercourse (TI) or IUI treatment. Ovarian stimulation methods were mainly divided into unstimulated, clomiphene citrate (CC), and human menopausal gonadotropin (hMG) cycles. Per‐cycle pregnancy rates (PRs) tended to decline after several cycles [16], so we also considered the number of cycles of treatment. This study retrospectively analyzed 1392 non‐IVF/ICSI treatment cycles for 372 infertile patients aged 38 years old or older, and we initially considered eight representative infertility factors. These factors were: (1) ‘advanced female age’, defined as ≧ 42 years old at the time of the first visit (n = 75); (2) ‘endometriosis/adenomyosis’, defined as a past or present history of endometriosis and/or adenomyosis, based on clinical images (n = 57); (3) ‘tubal infertility’, defined as an obstructed fallopian tube, based on hysterosalpingography, hysterofiberscopy, or past tubal surgery (n = 49); (4) ‘male infertility’, defined as sperm abnormality, based on semen analysis [17] (n = 208); (5) ‘decrease in ovarian reserve’, defined as a serum FSH level exceeding 10 mIU/mL in the early follicular phase (n = 147); (6) ‘primigravida’ (n = 213); (7) ‘uterine fibroid’, defined as a past or present history of uterine fibroids, based on clinical images (n = 131); and (8) ‘positive serum Chlamydia antibodies’, defined as serum Chlamydia antibody immunoglobulin IgA and/or IgG greater than a cutoff index of 0.9 (n = 64) implying the present of past infection of Chlamydia trachomatis. These eight groups were stratified into 24 subgroups by the three methods of ovarian stimulation: unstimulated, CC, and hMG cycle. The 1392 cycles of treatment yielded 54 pregnancies, and the overall CPR per cycle was 3.88 %. The overall outcomes of the four groups—(A) ‘advanced female age’, (E) ‘endometriosis/adenomyosis’, (T) ‘tubal infertility’, and (M) ‘male infertility’—were extremely poor, and each CPR per cycle was less than 2 % (0.86, 1.01, 1.37, and 1.80 %, respectively). To analyze each combination of the four factors, the patients were divided into two groups: patients with at least one of the analyzed factors; and patients with none of the factors. Totally, 11 combinations (A/E, A/T, A/M, E/T, E/M, T/M, A/E/T, A/E/M, A/T/M, E/T/M, A/E/T/M) were analyzed and the four groups without (M) ‘male infertility’ showed approximately 1 % CPR per cycle, and the CPR improved as the number of excluded factors increased. Ninety‐five patients with none of these four factors underwent 394 treatment cycles, and the CPR per cycle was 9.14 %, showing the strong influence of the four factors on infertility treatment outcomes. This study shows the importance of extracting poor prognostic factors, and although the protocol is not yet evident, the combination of poor factors tends to decrease the pregnancy chance in non‐IVF/ICSI treatment, so patients with multiple factors and who are willing to undergo IVF/ICSI treatment should be offered the treatment after a few cycles of TI/IUI treatment. Because the number of patients enrolled in this study was not large, further accumulation of data is expected. Factor (5), ‘decrease in ovarian reserve’, showed a CPR of 2.55 % per cycle in this study, which was higher than with the four factors stated above, so it was excluded from the combination of factors. However, only serum FSH level was considered in this study, and using other ovarian reserve markers, such as antral follicle count (AFC) and anti‐Müllerian hormone (AMH), it might yield different results.
Since this retrospective study was published, a few reports have focused on the infertility factors that affect the fecundity of women of advanced reproductive age. Herein, we would like to review the current topics of endometriosis/adenomyosis, ovarian reserve, treatment cycle, and method of ovarian stimulation in non‐IVF/ICSI treatment for women of advanced age.
Endometriosis/adenomyosis
The presence of endometriosis and/or adenomyosis and infertility evidently have some relationships, but the clinical management of endometriosis and adenomyosis during infertility treatment remains a matter of debate. The severity of endometriosis is classified into four stages (I—minimal, II—mild, III—moderate, and IV—severe) using the revised American Society for Reproductive Medicine (ASRM) staging, depending on the location, extent, and depth of endometriotic implants, the presence and severity of adhesions, and the presence and size of ovarian endometriomas [18]. A Canadian randomized controlled trial (RCT) of infertile women with stage I‐II disease with or without laparoscopic surgery followed the women without treating the endometriosis, and the CPR was significantly higher in the surgically managed patients [19]. Two current reviews [20, 21] show that patients with stage I‐II endometriosis are good candidates for IUI treatment, but there have been no RCTs randomizing stage III‐IV patients to determine the outcomes of IUI treatment. A study showed that pelvic endometriosis with peritoneal fluid reduced the pregnancy rate in patients undergoing IUI treatment [22]. Endometriosis and adenomyosis progresses with age [23, 24], and aged patients undergoing infertility treatment tend to have a higher possibility of having advanced stages of these diseases, which might be a reason for the poorer outcomes with IUI treatment. The presence of uterine fibroids, which are another representative estrogen‐dependent disease, has recently been identified as an independent risk factor for endometriosis [25, 26, 27], and patients who were diagnosed with both were more likely to be infertile [28]. The study was interesting in that the size of the dominant leiomyoma might have had a correlation with the existence of endometriosis, and the majority of cases of endometriosis (76/105 cases, 72.4 %) were detected in patients with a dominant leiomyoma < 8 cm. Although more cases must be analyzed, the size of the largest leiomyoma might help to provide clues for evaluating coexistent endometriosis.
Ovarian reserve
AMH is a new ovarian reserve marker [29], and a study has shown that patients attaining a successful live birth in the first cycle or cumulatively in three cycles of IUI treatment had significantly higher AMH concentrations than those failing treatment, and after controlling for age, body mass index (BMI), AFC, and FSH, AMH remained the only significant predictor of cumulative live birth [30]. AMH is less cycle dependent than FSH and AFC [31] and less operator dependent than AFC. Therefore, AMH is currently regarded as a superior marker and is helpful when counselling couples before they undergo intensive fertility treatment including ART. More clinicians are measuring AMH in infertility treatment, and the accumulation of data during non‐IVF/ICSI treatment is expected, but clinicians should be careful in translating the meaning of AMH levels, because their predictive accuracy for future conception remains elusive. It is a matter of debate when counseling women undergoing fertility treatment regarding pregnancy rates, particularly women with decreased ovarian reserves.
Treatment cycles
The CPR per cycle was stated in the previous study [15], but the number of cycles and the cycle that succeeded in conceiving were not reported. We have clinically experienced that there seems to be an upper limit to the number of successful treatment cycles [32], and the number of treatments that should be conducted is important because aging women might have limited overall opportunities for treatment.
A large retrospective study of 4199 CC‐IUI cycles in 1738 patients indicated the importance of limited treatment cycles [33]. The study showed that the CPRs per cycle in the following patient ages were, respectively, younger than 35 years old, 11.5 %, 35–37 years old, 9.2 %, 38–40 years old, 7.3 %, 41–42 years old, 4.3 %, and older than 43 years old, 1.0 %; as predicted, the CPR per cycle decreased as the patients aged. The remarkable data shown here were that 95 % of the patients who became pregnant in this study initiated four cycles of treatment or fewer (maximum of nine cycles). The percentages of pregnancies achieved within 3–4 cycles were, respectively, younger than 35 years old, 89.5 → 94.5 %, 35–37 years old, 91.0 → 92.3 %, 38–40 years old, 95.0 → 97.5 %, 41–42 years old, 66.7 → 83.3 %, and older than 43 years old, 100 → 100 %; these data indicate a rationale that the number of IUI treatment cycles should be limited to fewer than four. Only one patient of 55 who were older than 43 years old ever achieved a pregnancy, demonstrating the absence of efficacy of CC/IUI for patients of advanced age, so patients who are willing to undergo IVF/ICSI treatment should be promptly counseled. Another study [34] examined 1117 cycles of IUI treatment, focusing on women aged 40–42 years old. A total of 106 IUI cycles in patients ≧40 years old resulted in a CPR of 17.9 % per cycle, which seemed to be a higher percentage than in other studies. However, the miscarriage rate presented in this study was high (52.6 %), and the live birth rate per cycle was 8.5 %. Because the term ‘pregnancy’ varies according to studies, from the detection of a gestational sac (GS) by transvaginal ultrasound to live birth, this endpoint should be considered carefully. Nonetheless, the high rates of spontaneous pregnancy in aged women [35] should always be considered. Only one patient ≧43 years old conceived in the study, so the data above seem to suggest less intent to treat patients ≧43 years.
Ovarian stimulation
One review assessed the efficacy of ovarian stimulation in IUI treatment of aged women [36]. In summary, there was no evidence for the use of CC, hMG, or recombinant FSH (rFSH). A Cochrane review [37] showed the efficacy of CC, compared to natural cycles. However, in women ≧40 years old, there were no data supporting the efficacy of ovarian stimulation [16, 33, 34, 36, 37, 38, 39]. Our study [15] indicated that there was no significant difference due to ovarian stimulation (unstimulated, CC, hMG) in treatment outcomes in the IUI treatment of patients ≧38 years old. Another study [34] examined various methods of ovarian stimulation in IUI treatment for patients ≧40 years old, and there were also no significant differences among the treatments, which included unstimulated, CC, CC‐rFSH, rFSH, and gonadotropin‐releasing hormone (GnRH) agonist‐rFSH. This finding might indicate the possibility of the low efficacy of ovarian stimulation in aged patients, and because ovarian stimulation treatment, especially injections, is a large physical and social burden on patients, clinicians might eventually shift to unstimulated treatment in IUI procedures.
Conclusions
The number of patients undergoing infertility treatment is increasing yearly, but the treatment protocol remains unclear. Because the majority of patients are in their late 30s or at least 40 years old, treatment cycles are limited; therefore, cautious choice of treatment is indispensable. The efficacy of non‐IVF/ICSI treatment is often optimized, but by selecting patients properly, some patients might be able to avoid undergoing IVF/ICSI treatment. Treatment protocols have not yet been defined, but the extraction of infertility factors could be useful (Fig. 1).
Figure Fig. 1.
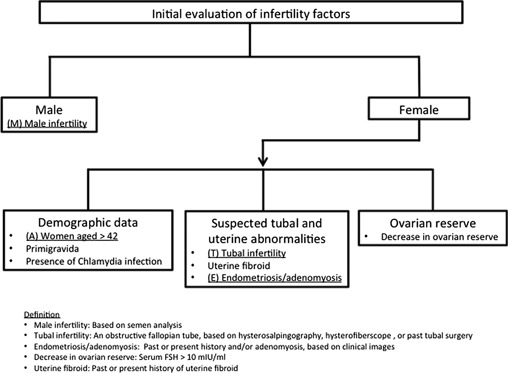
Outlines to evaluate infertility factors. Crucial detrimental factors (male infertility, tubal infertility, endometriosis/adenomyoais) are underlined
The decision tree (Fig. 2) might help to provide proper infertility treatment. After the extraction of infertility factors, we must bear in mind that the combination of poor factors, especially ‘endometriosis/adenomyosis’, ‘tubal infertility’, and ‘male infertility’, leads to the failure of IUI treatment in aged patients. It is true that the NICE guideline for infertility (2013) [40] does not recommend IUI for patients with unexplained infertility, mild endometriosis or mild male factor infertility, and does not recommend oral ovarian stimulation to women with unexplained infertility. Although there is no obvious evidence that ovarian stimulation and concomitant IUI apparently improves the pregnancy outcomes, mild ovarian stimulation treatment using CC could be considered [37], because the response to CC is thought to reflect the patients’ ovarian reserve, and placing high concentration sperm could enhance the likelihood of becoming pregnant [41, 42]. Among ovarian reserve markers, AMH is regarded as a superior one compared to FSH and AFC. Therefore, measurement of AMH could be useful for predicting the patients’ treatment prognosis. IUI treatment cycles should be limited to fewer than four cycles because the majority of patients who conceive by IUI will do so within four cycles. A few additional cycles could be allowed for patients younger than 40 years old, but because the pregnancy rate by IUI is not sufficiently high for patients older than 40 years old, patients who would like to conceive should not persist in IUI treatment. For patients older than 42 years old, if the patient is willing to proceed with IVF/ICSI treatment, skipping IUI treatment because the pregnancy rate is extremely low and undergoing IVF/ICSI treatment constitute a good option. Using this protocol, predicting the most suitable timing for IVF/ICSI treatment in aged infertile women might become possible, and excessive treatment could be avoided. It could be intriguing if we could employ RCT using this decision tree considering the efficacy, cost‐balance, and patients’ satisfaction, but a large population study might be necessary.
Figure Fig. 2.
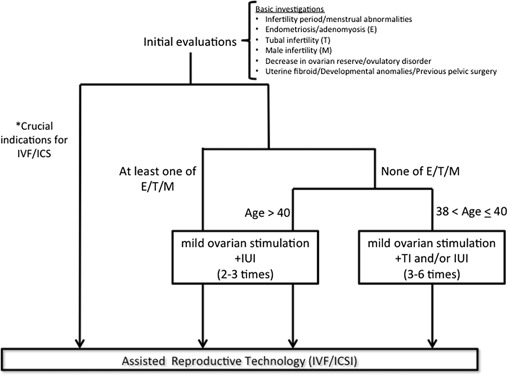
Proposal of infertility treatment for women of advanced reproductive age. Asterisk representative crucial indications for IVF/ICSI include severe oligoasthenozoospermia and bilateral obstruction of the fallopian tubes
Acknowledgments
This report was supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science and Culture, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, and The Nakatomi Foundation. This report was also supported by the Japan Society for Reproductive Medicine. The authors would like to thank American Journal Experts (http://www.aje.com) for the English language review.
Compliance with ethical standards
Conflicts of interest
These authors declare that they have no conflicts of interest to disclose.
Human rights statements and informed consent
This article does not contain any studies with human subjects performed by any of the authors.
Animal rights
This article does not contain any studies with animal subjects performed by the any of the authors.
References
- 1.ACOG Committee Opinion, authors. Age‐related fertility decline. Obstet Gynecol. 2008;112:409–411. doi: 10.1097/AOG.0b013e318183fbe6. 10.1097/AOG.0b013e318183fbe6 [DOI] [PubMed] [Google Scholar]
- 2. Dew JE Don RA Hughes GJ Johnson TC Steigrad SJ The influence of advanced age on the outcome of assisted reproduction J Assist Reprod Genet 1998. 15 210 214 10.1023/A:10230045036973454935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziebe S Devroey P Assisted reproductive technologies are an integrated part of national strategies addressing demographic and reproductive challenges. Hum Reprod Update. 2008;14:583–592. doi: 10.1093/humupd/dmn038. 10.1093/humupd/dmn038 [DOI] [PubMed] [Google Scholar]
- 4. Barton M Walker K Wiesner BP Artificial insemination Br Med J. 1945. 1 40 3 10.1136/bmj.1.4384.402056529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frederick JL Denker MS Rojas A Horta I Stone SC Asch RH Balmaceda JP Is there a role for ovarian stimulation and intra‐uterine insemination after age 40? Hum Reprod. 1994;9:2284–2286. doi: 10.1093/oxfordjournals.humrep.a138438. [DOI] [PubMed] [Google Scholar]
- 6. Lass A Croucher C Duffy S Dawson K Margara R Winston RM One thousand initiated cycles of in vitro fertilization in women > or = 40 years of age. Fertil Steril. 1998;70:1030–1034. doi: 10.1016/s0015-0282(98)00353-7. 10.1016/S0015‐0282(98)00353‐7 [DOI] [PubMed] [Google Scholar]
- 7. Tsafrir A Simon A Revel A Reubinoff B Lewin A Laufer N Retrospective analysis of 1217 IVF cycles in women aged 40 years and older. Reprod Biomed Online. 2007;14:348–355. doi: 10.1016/s1472-6483(10)60878-4. 10.1016/S1472‐6483(10)60878‐4 [DOI] [PubMed] [Google Scholar]
- 8. Marinakis G Nikolaou D National survey of the current management of infertility in women aged 40 and over in the UK. J Obstet Gynaecol. 2012;32:375–378. doi: 10.3109/01443615.2012.663424. 10.3109/01443615.2012.663424 [DOI] [PubMed] [Google Scholar]
- 9. Corsan G Trias A Trout S Kemmann E Ovulation induction combined with intrauterine insemination in women 40 years of age and older: is it worthwhile? Hum Reprod. 1996;11:1109–1112. doi: 10.1093/oxfordjournals.humrep.a019306. 10.1093/oxfordjournals.humrep.a019306 [DOI] [PubMed] [Google Scholar]
- 10.Japan.Society.of.Reproductive Medicine, http://plaza.umin.ac.jp/~jsog‐art/, Accessed 3 7 2016.
- 11. Brandes M Steen JO Bokdam SB Hamilton CJ Bruin JP Nelen WL Kremer JA When and why do subfertile couples discontinue their fertility care? A longitudinal cohort study in a secondary care subfertility population, Hum Reprod. 2009;24:3127–3135. doi: 10.1093/humrep/dep340. [DOI] [PubMed] [Google Scholar]
- 12. Speyer BE Abramov B Saab W Doshi A Sarna U Harper JC Serhal P Factors influencing the outcome of intrauterine insemination (IUI): age, clinical variables and significant thresholds. J Obstet Gynaecol. 2013;33:697–700. doi: 10.3109/01443615.2013.810199. 10.3109/01443615.2013.810199 [DOI] [PubMed] [Google Scholar]
- 13. Westerlaken LA Naaktgeboren N Helmerhorst FM Evaluation of pregnancy rates after intrauterine insemination according to indication, age, and sperm parameters J Assist Reprod Genet 1998. 15 359 364 10.1023/A:10225768316913455020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nuojua‐Huttunen S Tomas C Bloigu R Tuomivaara L Martikainen H Intrauterine insemination treatment in subfertility: an analysis of factors affecting outcome. Hum Reprod. 1999;14:698–703. doi: 10.1093/humrep/14.3.698. 10.1093/humrep/14.3.698 [DOI] [PubMed] [Google Scholar]
- 15. Isono W Wada‐Hiraike O Shirane A Fujimoto A Osuga Y Yano T Taketani Y Alternative strategies to in vitro fertilization/intracytoplasmic sperm injection treatment for aged infertile women. Reprod Med Biol. 2011;11:69–72. doi: 10.1007/s12522-011-0107-4. 10.1007/s12522‐011‐0107‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickey RP Taylor SN Lu PY Sartor BM Rye PH Pyrzak R Effect of diagnosis, age, sperm quality, and number of preovulatory follicles on the outcome of multiple cycles of clomiphene citrate‐intrauterine insemination. Fertil Steril. 2002;78:1088–1095. doi: 10.1016/s0015-0282(02)04212-7. 10.1016/S0015‐0282(02)04212‐7 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization, WHO laboratory manual for the examination of human semen and sperm‐cervical mucus interaction Cambridge University Press, 2010.
- 18.American Society for Reproductive Medicine, Endometriosis, A Guide for Patients https://www.asrm.org/BOOKLET_Endometriosis Accessed 3 7 2016.
- 19. Marcoux S Maheux R Berube S Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis, N Engl J Med. 1997;337:217–222. doi: 10.1056/NEJM199707243370401. [DOI] [PubMed] [Google Scholar]
- 20. Ozkan S Murk W Arici A Endometriosis and infertility: epidemiology and evidence‐based treatments. Ann NY Acad Sci. 2008;1127:92–100. doi: 10.1196/annals.1434.007. 10.1196/annals.1434.007 [DOI] [PubMed] [Google Scholar]
- 21. Buyalos RP Agarwal SK Endometriosis‐associated infertility. Curr Opin Obstet Gynecol. 2000;12:377–381. doi: 10.1097/00001703-200010000-00006. 10.1097/00001703‐200010000‐00006 [DOI] [PubMed] [Google Scholar]
- 22. Wu HM Tzeng CR Chen CH Chen PH Pelvic endometriosis with peritoneal fluid reduces pregnancy rates in women undergoing intrauterine insemination. Taiwan J Obstet Gynecol. 2013;52:512–515. doi: 10.1016/j.tjog.2013.10.010. 10.1016/j.tjog.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 23. Eskenazi B Warner ML Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. 10.1016/S0889‐8545(05)70302‐8 [DOI] [PubMed] [Google Scholar]
- 24. Moradi M Parker M Sneddon A Lopez V Ellwood D Impact of endometriosis on women's lives: a qualitative study BMC Womens Health 2014. 14 123 10.1186/1472‐6874‐14‐1234287196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dueholm M Lundorf E Hansen ES Ledertoug S Olesen F Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002;186:409–415. doi: 10.1067/mob.2002.121725. 10.1067/mob.2002.121725 [DOI] [PubMed] [Google Scholar]
- 26. Huang JQ Lathi RB Lemyre M Rodriguez HE Nezhat CH Nezhat C Coexistence of endometriosis in women with symptomatic leiomyomas. Fertil Steril. 2010;94:720–723. doi: 10.1016/j.fertnstert.2009.03.052. 10.1016/j.fertnstert.2009.03.052 [DOI] [PubMed] [Google Scholar]
- 27. Hemmings R Rivard M Olive DL Poliquin‐Fleury J Gagne D Hugo P Gosselin D Evaluation of risk factors associated with endometriosis. Fertil Steril. 2004;81:1513–1521. doi: 10.1016/j.fertnstert.2003.10.038. 10.1016/j.fertnstert.2003.10.038 [DOI] [PubMed] [Google Scholar]
- 28. Isono W Wada‐Hiraike O Osuga Y Yano T Taketani Y Diameter of dominant leiomyoma is a possible determinant to predict coexistent endometriosis. Eur J Obstet Gynecol Reprod Biol. 2012;162:87–90. doi: 10.1016/j.ejogrb.2012.01.018. 10.1016/j.ejogrb.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 29. Marca A Sighinolfi G Radi D Argento C Baraldi E Artenisio AC Stabile G Volpe A Anti‐Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. 10.1093/humupd/dmp036 [DOI] [PubMed] [Google Scholar]
- 30. Li HWR Yeung WSB Lau EYL Ho PC Ng EHY Evaluating the performance of serum antimullerian hormone concentration in predicting the live birth rate of controlled ovarian stimulation and intrauterine insemination. Fertil Steril. 2010;94:2177–2181. doi: 10.1016/j.fertnstert.2009.12.059. 10.1016/j.fertnstert.2009.12.059 [DOI] [PubMed] [Google Scholar]
- 31. Marca A Volpe A Anti‐Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol. 2006;64:603–610. doi: 10.1111/j.1365-2265.2006.02533.x. 10.1111/j.1365‐2265.2006.02533.x [DOI] [PubMed] [Google Scholar]
- 32. Plosker SM Jacobson W Amato P Predicting and optimizing success in an intra‐uterine insemination programme. Hum Reprod. 1994;9:2014–2021. doi: 10.1093/oxfordjournals.humrep.a138385. [DOI] [PubMed] [Google Scholar]
- 33. Dovey S Sneeringer RM Penzias AS Clomiphene citrate and intrauterine insemination: analysis of more than 4100 cycles. Fertil Steril. 2008;90:2281–2286. doi: 10.1016/j.fertnstert.2007.10.057. 10.1016/j.fertnstert.2007.10.057 [DOI] [PubMed] [Google Scholar]
- 34. Haebe J Martin J Tekepety F Tummon I Shepherd K Success of intrauterine insemination in women aged 40–42 years. Fertil Steril. 2002;78:29–33. doi: 10.1016/s0015-0282(02)03168-0. 10.1016/S0015‐0282(02)03168‐0 [DOI] [PubMed] [Google Scholar]
- 35. Wyatt PR Owolabi T Meier C Huang T Age‐specific risk of fetal loss observed in a second trimester serum screening population. Am J Obstet Gynecol. 2005;192:240–246. doi: 10.1016/j.ajog.2004.06.099. 10.1016/j.ajog.2004.06.099 [DOI] [PubMed] [Google Scholar]
- 36. Tsafrir A Simon A Margalioth EJ Laufer N What should be the first‐line treatment for unexplained infertility in women over 40 years of age—ovulation induction and IUI, or IVF? Reprod Biomed Online. 2009;19(Suppl 4):4334. [PubMed] [Google Scholar]
- 37. Veltman‐Verhulst SM Cohlen BJ Hughes E Heineman MJ Intra‐uterine insemination for unexplained subfertility. The Cochrane database of systematic reviews. 2012;9:Cd001838. doi: 10.1002/14651858.CD001838.pub4. [DOI] [PubMed] [Google Scholar]
- 38. Ferrara I Balet R Grudzinskas JG Intrauterine insemination with frozen donor sperm. Pregnancy outcome in relation to age and ovarian stimulation regime, Hum Reprod. 2002;17:2320–2324. doi: 10.1093/humrep/17.9.2320. [DOI] [PubMed] [Google Scholar]
- 39. Belloc S Cohen‐Bacrie P Benkhalifa M Cohen‐Bacrie M Mouzon J Hazout A Menezo Y Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online. 2008;17:392–397. doi: 10.1016/s1472-6483(10)60223-4. 10.1016/S1472‐6483(10)60223‐4 [DOI] [PubMed] [Google Scholar]
- 40.W.s. National Collaborating Centre for, H. Children's, National Institute for Health and Clinical Excellence: Guidance, Fertility: Assessment and Treatment for People with Fertility Problems, Royal College of Obstetricians and Gynaecologists National Collaborating Centre for Women's and Children's Health, London, 2013.
- 41. Practice M Committee of the American Society for Reproductive. Use of clomiphene citrate in infertile women: a committee opinion, Fertil Steril. 2013;100:341–348. doi: 10.1016/j.fertnstert.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 42.UpToDate, http://www.uptodate.com/contents/unexplained‐infertility, Accessed 3 7 2016.


