Abstract
Luteinizing hormone (LH) surge stimulates preovulatory follicles to induce the ovulation process, including oocyte maturation, cumulus expansion, and granulosa cell luteinization. The matured oocytes surrounded by an expanded cumulus cell layer are released from follicles to the oviduct. However, LH receptors are dominantly expressed in granulosa cells, but less in cumulus cells and are not expressed in oocytes, indicating that the secondary factors expressed and secreted from LH‐stimulated granulosa cells are required for the induction of the ovulation process. Prostaglandin and progesterone are well‐known factors that are produced in granulosa cells and then stimulate in both granulosa and cumulus cells. The mutant mice of prostaglandin synthase (Ptgs2KO mice) or progesterone receptor (PRKO mice) revealed that the functions were essential to accomplish the ovulation process, but not to induce the ovulation process. To identify the factors initiating the transfer of the stimuli of LH surge from granulosa cells to cumulus cells, M. Conti's lab and our group performed microarray analysis of granulosa cells and identified the epidermal growth factor (EGF)‐like factor, amphiregulin (AREG), epiregulin (EREG), and β‐cellulin (BTC) that act on EGF receptor (EGFR) and then induce the ERK1/2 and Ca2+‐PLC pathways in cumulus cells. When each of the pathways was down‐regulated using a pharmacological approach or gene targeting study, the induction of cumulus expansion and oocyte maturation were dramatically suppressed, indicating that both pathways are inducers of the ovulation process. However, an in vitro culture study also revealed that the EGFR‐induced unphysiological activation of PKC in cumulus cells accelerated oocyte maturation with low cytostatic activity. Thus, the matured oocytes are not arrested at the metaphase II (MII) stage and then spontaneously form pronuclei. The expression of another type of EGF‐like factor, neuregulin 1 (NRG1), that does not act on EGFR, but selectively binds to ErbB3 is observed in granulosa cells after the LH surge. NRG1 supports EGFR‐induced ERK1/2 phosphorylation, but reduces PKC activity to physiological level in the cumulus cells, which delays the timing of meiotic maturation of oocytes to adjust the timing of ovulation. Thus, both types of EGF‐like factor are rapidly induced by LH surge and then stimulate cumulus cells to control ERK1/2 and PKC pathways, which results in the release of matured oocytes with a fertilization competence.
Keywords: Cumulus cells, Granulosa cells, Oocytes, Progesterone, Prostaglandin
Introduction
Mammalian female germ cells form a cluster with their proliferation before birth [1, 2]; however, the number of germ cells dramatically decreases in mice due to the reduction of estrogen and progesterone levels [3, 4, 5]. These dramatic changes induce the meiosis of female germ cells, and the oocytes are arrested at the diplotene stage of the first meiotic prophase by the timing of ovulation [6, 7]. At this stage, the clusters of oocytes are dissolved to form primordial follicles so that one layer of somatic cells surrounds the oocyte [8, 9, 10]. The primordial follicles remain at this stage until the follicles initiate follicular growth to form preovulatory follicles during each estrous cycle [11, 12]. During the early stages of follicle growth, follicular somatic cells are differentiated to granulosa cells and then are proliferated to develop the follicles to secondary follicles [13, 14, 15]. The granulosa cells of secondly follicles are stimulated by FSH secreted from the pituitary gland to proliferate further and form an antrum between the spaces of the granulosa cell layers (mural granulosa cells) interlining the follicular wall and granulosa cell‐derived cumulus cells directly surrounding the oocyte [16, 17, 18]. The cumulus cells transport energy sources and other factors into oocytes via numerous gap junctions, thereby promoting oocyte growth to full development size [19, 20, 21]. The full grown oocytes have the ability to resume meiosis from prophase I to progress to the metaphase II (MII) stage when the oocytes are released from the follicles to in vitro culture medium or when the oocytes are ovulated with the stimulation of LH surge [22].
The size of oocytes reaches the maximum level at the middle size of antral follicles; however, the LH surge can not permit the initiation of oocyte maturation with a progressing ovulation process of middle‐size antral follicles. LH is secreted from the pituitary gland in a similar manner to FSH, and the basal level of LH acts on theca cells of the follicular walls of the antral follicles [23, 24, 25]. The LH surge acts directly on the granulosa cells of preovulatory follicles, which are maximum‐size antral follicles [26, 27, 28]. The ovulation stimuli determine the final differentiation of granulosa cells to form a corpus luteum, cumulus cells to form a hyaluronan‐rich matrix and accumulate the matrix within a cumulus cell layer, called cumulus expansion, and oocytes to resume meiosis to the MII stage [29]. More importantly, although the functions and morphology of oocytes and cumulus cells are dramatically changed by the LH surge, both types of cells either don't express or express fewer LH receptors [30]. Thus, the factors induced and secreted rapidly from LH‐stimulated granulosa cells act on cumulus cells and/or oocytes to induce cumulus expansion and oocyte maturation.
When cumulus oocyte complexes (COCs) were collected and cultured in vitro, the oocytes progressed to the MII stage with fertilization and developmental competence [31]. However, the denuded oocytes predicted low fertilization and developmental competence after maturation [32], suggesting that cumulus cells play a critical role in oocyte maturation. Therefore, the majority of secreted factors from LH‐stimulated granulosa cells act on cumulus cells to induce cell differentiation for impacting not only cumulus expansion, but also the regulation of oocyte maturation. The secondary factors secreted from granulosa cells during the ovulation process to stimulate the cumulus cells of COCs are the focus of the current review.
Progesterone and prostaglandin E2 (PGE2)
The LH surge stops cell proliferation of granulosa cells by decreasing Ccnd2 expression [33, 34]. The molecular mechanism was reported by Richards et al. [35] to be involved in AP‐1 transcription factors. These are regulated by c‐jun or other members of mitogen‐activated protein (MAP) kinase family, including ERK1/2 and then strongly induce marked functional (endocrine, biochemical, and molecular) changes in the preovulatory follicle [35]. Estrogen concentrations decline in follicular fluid as a consequence of the decreased expression of aromatase (Cyp19a1) in granulosa cells [36, 37]. Conversely, progesterone concentrations increase in the follicular fluid in association with the induction of P450scc (Cyp11a1) and StAR (Star) in granulosa cells [38, 39, 40]. These two genes are induced by the activation of cAMP response element binding protein (CREB), the CCAT enhancer binding protein (C/EBP) family, and AP‐1 transcription factors in the cells during the ovulation process [41, 42, 43]. LH increases the cAMP level in granulosa cells, which activates PKA to phosphorylate CREB and E/PAC to activate the RAS‐c‐raf‐MEK1–ERK1/2 pathway, which phosphorylate C/EBP family and the AP‐1 transcription factor family [44, 45, 46]. The in vitro culture study of granulosa cells showed that the induction of cAMP by forskolin treatment rapidly activated the signaling pathway within a few minutes and expressed Cyp11a1 and Star with an increasing level of progesterone in the medium [47, 48].
The roles of progesterone in ovulation have been reported by numerous researchers using animal pharmacological models, in vitro culture studies and the knockout (KO) mice model. Mori et al. [49] injected anti‐progesterone antiserum with hCG to superovulation rats, and the study revealed that ovulation was inhibited by the treatment [49]. The progesterone synthesis inhibitors, aminoglutethimide or epistane, also decreased the number of ovulated oocytes in superovulation rats [50, 51], suggesting that the LH‐induced increase in progesterone is essential for ovulation. Progesterone binds to its receptor, PGR, and the activated receptor moves from the cytoplasm to the nucleus to induce target gene expression [52, 53]. In an in vitro culture study of pig COCs or rat preovulatory follicles, the addition of PGR antagonist, RU486, dramatically suppressed cumulus expansion and oocyte maturation [54, 55, 56, 57]. Female mice null for Pgr (PRKO) failed to ovulate, even in response to exogenous hormone [58, 59]. Thus, LH induction of progesterone‐dependent pathways in granulosa cells and cumulus cells changes gene expression patterns in both cells to mediate critical events during the ovulation process.
Detailed analysis of PRKO mice revealed that follicular development to preovulatory follicles was normal, and the gene expression patterns in cumulus cells and granulosa cells at 2 h after hCG injection were also similar to those in the wild type (WT) mice [60]. Oocyte maturation, cumulus expansion, and corpus luteum formation of KO mice were comparable to those of WT mice; however, matured oocytes were observed in the corpus luteum, but not in the oviduct [58]. The phenotype indicates that although progesterone is a factor secreted from granulosa cells during the ovulation process, its functions appear later during the ovulation process.
One of the other well‐known factors secreted from granulosa cells during the ovulation process is prostaglandin (PG). In LH‐stimulated granulosa cells, prostaglandin synthase (PTGS)‐2, also known as COX‐2, is expressed to produce dominantly PGE2 [61, 62, 63]. Cumulus cells and granulosa cells have PGE receptors (EP2 and EP4), which increase cAMP level to activate PKA and other signaling pathways similar to those by LH [64, 65]. Thus, PGE2 works as a second stimulator of granulosa cells and cumulus cells at a few hours after the LH surge or as a supporter to maintain cAMP level in both cell types during a few periods after the LH surge. In Ptgs2 null mice and Pger2 null mice, the ovulation was significantly suppressed and cumulus expansion was not normal due to decreasing gene expressions of Has2 encoding hyaluronan (HA) synthase 2 and Tnfaip6 encoding HA‐binding protein compared with those in the WT mice [66, 67]. Moreover, the fertilization in vivo by natural mating and in vitro when the matured oocytes were collected from the oviduct was completely suppressed in both KO mice [67]. However, the target gene expressions were decreased at 8 h, but not at 4 h after the injection of hCG [60, 68], indicating that PGE2 also worked later during the ovulation process similar to progesterone but not as secondary factors to initiate the ovulation process.
Ligands for epidermal growth factor (EGF) receptor (EGFR)
Because cumulus cells of the mouse and rat possess few, if any, LH receptors [30], the molecular mechanisms by which LH impacts cumulus cell differentiation to induce cumulus expansion and oocyte maturation have remained unclear. However, earlier studies have indicated that factors other than LH, progesterone or PGE2 can induce cumulus expansion and oocyte maturation in a culture, including growth factors. The addition of epidermal growth factor (EGF) to in vitro culture medium phosphorylates EGF receptor (EGFR) in cumulus cells, but not in oocytes to activate the down‐stream pathway, such as ERK1/2 [69]. The EGF–EGFR‐activated ERK1/2 induces cumulus expansion and oocyte maturation of pigs [70, 71], mice [72], and cattle COCs [73, 74]. Cumulus cells also express insulin‐like growth factor (IGF) receptors and insulin receptors, and these receptors are activated by exogenous IGF‐1 and insulin that impacts cumulus cell expansion and oocyte maturation of COCs in pigs, cattle, and mice [75, 76, 77]. However, the expression level of IGF‐1 is not changed, and the expression of EGF is not induced in granulosa cells during the ovulation process [78, 79], indicating that other types of ligands potentially activating EGFR and/or IGFR are expressed in granulosa cells just after the LH surge.
To confirm the aforementioned hypothesis, Conti and colleagues [80] attempted to perform microarray analysis using RNA recovered from granulosa cells before or after ovulation stimuli. Their observations showed that LH induced the expression of mRNAs encoding the EGF‐like factor, amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC) in mouse preovulatory follicles [80]. EGF‐like factor consists of 13 families that are transmembrane proteins containing a signal sequence, a transmembrane domain and at least one EGF domain [81] (Fig. 1a). Some of these EGF‐like factor function as a ligand to stimulate neighbor cells without releasing the ligand domain from the membrane bound form. However, most are shed by specific protease to release EGF domain to activate specific receptors expressed on not only neighbor cells, but also on separated cells by a paracrine system [82, 83] (Fig. 1c). Granulosa cell‐expressed EGF‐like factor, AREG, EREG, and BTC have a cysteine‐rich domain in the extracellular domain that is a targeted sequence of tumor necrosis factor α‐converting enzyme (TACE) [83]. TACE, which is also known as ADAM17, a disintegrin and metalloprotease family member, is expressed in the granulosa cells of the rat and pig ovary during the ovulation process [71, 84, 85]. The level of expression is dramatically increased in the cells after hCG injection with the induction of EGF‐like factor [71, 86]. When the expression of TACE/ADAM17 was down‐regulated by specific siRNA, the expression level of EGF‐like factor was normal, whereas the phosphorylations of EGFR and ERK1/2 were dramatically decreased [71], suggesting that the secretion from granulosa cells to follicular fluid impacts the functional changes of granulosa cells themselves and cumulus cells to induce a successful ovulation process.
Figure Fig. 1.
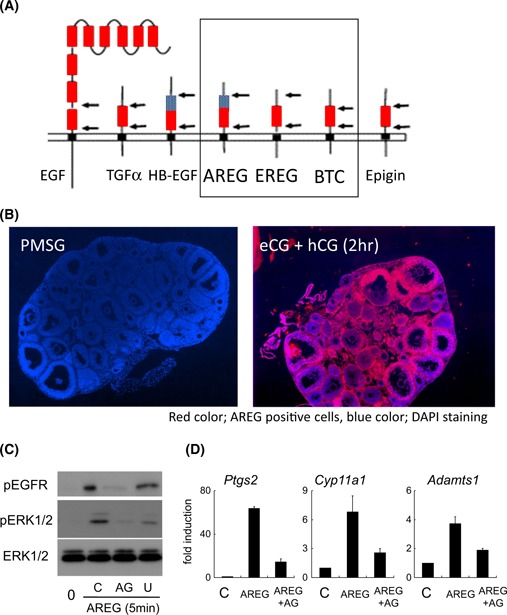
The expression of EGF‐like factor, AREG, EREG, BTC in granulosa cells during the ovulation process. a The typical members of EGF‐like factor that contain a transmembrane domain, protease targeting sequences, heparin‐binding domain and EGF domain, red box EGF domain, blue box heparin‐binding domain, black box transmembrane domain, black arrow protease‐targeting sequence. b The expression and localization of AREG in pre‐ or peri‐ovulatory follicles before or after hCG injection in mice. The localization of AREG was detected by immunohistochemistry using anti‐AREG antibody. AREG positive signals were visualized with Cy3 (red color) and nuclei were stained with DAPI (blue color). c The activation of EGFR and its downstream pathway by AREG in granulosa cells. Mouse granulosa cells recovered from eCG‐stimulated mice were pre‐cultured for overnight and then the cells were treated with AREG and either EGFR tyrosine kinase inhibitor (AG) or MEK1 inhibitor (U) for 5 min. C; Some of pre‐cultured granulosa cells were further cultured with AREG alone for 5 min. 0; The granulosa cells were collected just after pre‐cultivation. d The expression of genes well‐known as ovulation markers in granulosa cells cultured with AREG. Mouse granulosa cells recovered from eCG‐stimulated mice were pre‐cultured for overnight and then the cells were treated with AREG alone (AREG) or AREG + EGFR tyrosine kinase inhibitor (AREG + AG) for 4 h. C; The granulosa cells were collected just after pre‐cultivation
The expression of EGF‐like factor, particularly AREG and EREG is rapidly induced within 2 h after hCG injection in mice granulosa cells [80] (Fig. 1b). An in vitro culture of mouse granulosa cells revealed that the cAMP dependent‐PKA pathway directly activated the promoter activity of both genes. In the promoter region of Areg, cAMP‐responsible element (CRE) is essential for the induction of its gene [87] (Fig. 2a). Phosphorylated CRE‐binding protein (CREB) by PKA is recruited to the promoter region [88, 89], indicating that Areg is one of the rapid target genes of LH surge in granulosa cells. The promoter region of Ereg does not contain any CRE sites and is regulated by Sp1‐binding sites [90]. The transcription factor Sp1 has numerous phosphorylated sites, some of which are phosphorylated by PKA to bind the cytosine‐ and guanine‐rich repeats sequence, Sp1 site of the promoter region [91]. Thus, Ereg promoter in granulosa cells is also directly regulated by LH‐induced signaling during the early phase ovulation process, estimating that AREG and EREG are secondary factors to transfer the signal of LH surge to cumulus cells to progress cumulus cell expansion and oocyte maturation.
Figure Fig. 2.
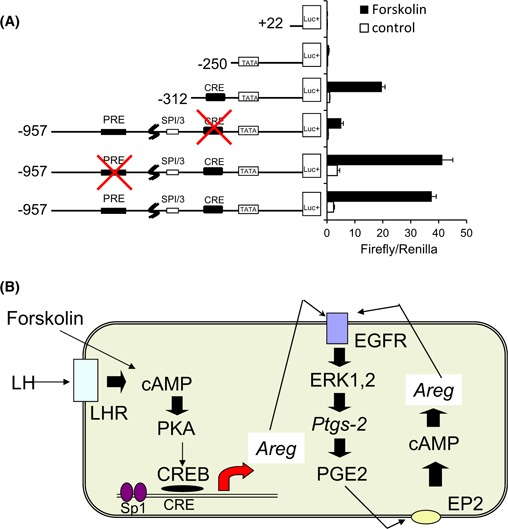
The regulation of Areg expression in granulosa cells during the ovulation process. a The image of Areg promoter and the potential activity of Areg promoter in cultured granulosa cells. The putative Areg promoter has PRE, SP1/3 binding site, CRE and TATA box. Mouse granulosa cells were transiently transfected with the Areg promoter‐luciferase constructs: −957, −312, −250, or +22 bp with or without mutations in PRE or CRE site, and stimulated with or without forskolin for 4 h. Firefly/Renilla; Firefly luciferase activities were normalized by Renilla luciferase activities. Values are mean ± SEM of three replicates. b A schematic diagram with regard to the induction and the maintenance of Areg expression in granulosa cells during the ovulation process. The induction of cAMP by LH or forskolin activates PKA–CREB pathway. The phosphorylated CREB moves to nuclei to bind CRE site of Areg promoter region. The induced AREG acts on its receptor, EGFR, which results in the activation of ERK1/2 pathway. Because one of the target genes of ERK1/2 pathway is Ptgs2 encoding prostaglandin synthase 2, PGE2 production is increased by AREG–EGFR dependent manner. PGE2 acts on its receptor, EP2 to increase the level of cAMP, which induces Areg expression. Thus, the positive feedback loop in the expression of Areg is working to induce ovulation process
Functions of EGF‐like factor in the ovulation process
The addition of AREG or EREG to an in vitro culture medium of preovulatory follicles increased the expression levels of Has2 and Tnfaip6, markers of cumulus expansion, and Cyp11a1 and Star, markers of luteinization of granulosa cells [80, 92] (Fig. 1d). The gene expressions were also observed when COCs or granulosa cells were collected from preovulatory follicles and then cultured with AREG [60]. The inductions were rapidly induced within an hour by the addition of AREG, whereas forskolin or LH took more time to induce gene expression [60, 80, 93, 94]. Moreover, the inductions by the cAMP‐producers were not detected by further treatment with EGFR tyrosine kinase inhibitor, AG1478 [60, 93]. In addition, mutant mice null for Areg and homozygous for Egfr wa2 (Areg −/− Egfr wa2/wa2) exhibited significant decreases in cumulus expansion and oocyte maturation [95]. Thus, EGF‐like factor secreted from granulosa cells are essential initiators to induce a successful ovulation process.
During the ovulation process, multiple signaling pathways are activated in cumulus cells and granulosa cells. EGF‐like factor takes charge of the RAS‐cRAF‐MEK1–ERK1/2 pathway in cumulus cells and granulosa cells [94, 96]. When an EGFR tyrosine kinase inhibitor (AG1478) was added to a culture medium of preovulatory follicles, the induction of EGFR and ERK1/2 phosphorylation was blocked in LH‐stimulated follicles [93]. The addition of AREG or EREG rapidly induced ERK1/2 phosphorylation within 5 min in cultured granulosa cells [60]. Because the release of the EGF domain from the membrane bound form of AREG and EREG is induced by TACE/ADAM17, we examined whether the inhibition of TACE/ADAM17 activity by a specific inhibitor or knockdown of the encoded gene by siRNA suppressed the luteinization of granulosa cells and expansion of cumulus cells. The study clearly revealed that the release of the EGF domain acted on EGFR in both granulosa cells and cumulus cells to activate the ERK1/2 pathway [71]. Moreover, when COCs were cultured with the MEK1 inhibitor (PD98059 or U0126), cumulus expansion was dramatically blocked [69, 97, 98]. In ERK1/2 mutant mice in which both kinases are depleted in granulosa cells and cumulus cells, not only cumulus cell expansion but also oocyte meiotic resumption are completely suppressed [94], suggesting that the EGF‐like factor‐activated ERK1/2 pathway in cumulus cells plays an important role in cumulus expansion and oocyte maturation.
Fan et al. [94] used granulosa cells recovered from an ovary 2 h after hCG injection of ERK1/2KO mice for microarray analysis to comprehensively understand the role of secondary factors during the ovulation process. Because genes encoding EGF‐like factor, namely AREG, EREG, and BTC, are located at close sites of the same chromosome (chromosome 5) [99], it is very difficult to produce triple KO mice to clarify the role of secondary factors in the ovulation process. It has been known that their receptors are EGFR; however, EGF‐like factor also acts on ErbB4 [81], which is other member of the ErbB family, to induce the Ras‐ERK1/2 pathway. The expression of ErbB4 has been reported in the ovary during the ovulation process [100, 101], suggesting that ERK1/2 as the assembly point of EGFR‐ and ErbB4‐downstream pathways are appropriate targets to understand the role of secondary factors in cumulus cells and granulosa cells during the ovulation process. Fan et al. [94] reported that 77 % of genes up‐regulated by LH surge were decreased in ERK1/2KO mice compared with WT mice after hCG injection. Most of the genes that were already reported to be essential for the ovulation process were included in the down‐regulated genes [94]. Some of the genes which decreased after the hCG injection, such as Ccnd2 and Cyp19a1, remained detectable in ERK1/2KO mice. The mechanisms are explained by the ERK1/2‐targeted transcription factors, AP‐1 family, C/EBPα/β, and LRH1 (NR5a2).
Both Fra2 and JunD, present in nuclear extracts purified from bovine granulosa cells, bind to the AP‐1 site of the Tnfaip6 promoter region [35]. AP‐1 also plays an important role in the arrest of the cell cycle due to the expression of cell cycle inhibitor, Kip1 [33, 102]. ERK1/2 also suppresses the expression of Ccnd2 that is a key gene to induce the cell cycle to DNA replication in granulosa cells [94, 96]. The arrest of the cell cycle may play an important role in the differentiation of granulosa cells and cumulus cells during the ovulation process. Other ERK1/2 target transcription factors are C/EBPα/β that are expressed in granulosa cells and cumulus cells during ovulation and that can mediate the induction of selected genes [43, 103]. In C/EBPβ null mice, the number of oocytes ovulated was significantly decreased compared with that in WT mice. Ptgs2 expression level was significantly lower than that of WT mice [104]. Thus, the EGF‐like factor‐induced EGFR–ERK1/2 pathway is mediated to PGE2 production. The sequential production of secreted factors in granulosa cells mediates the ovulation process.
The expression of Areg and Ereg was not affected in Ptgs2KO mice at 2 h after hCG injection, whereas the inductions were significantly decreased at the 4 or 8 h point after hCG injection [60]. The delayed suppressions are explained by the level of cAMP in granulosa cells. At the early time point, cAMP level is induced by LH, whereas the LH receptor is immediately degraded after the binding of ligand to its receptor [44, 105] (Fig. 2b). However, the cAMP level remains high at the 4 h point after the hCG injection because PGE2 produces cAMP in the cells [106] (Fig. 2b). The PGE2‐increased cAMP activates PKA–CREB or PKA–Sp1 pathways to maintain the expression of Areg or Ereg, respectively [60, 87] (Fig. 2b). The positive feedback loop in the expression of EGF‐like factor is also shaped by the progesterone‐PGR pathway. The AP‐1 transcription factors induce the expression of Cyp11a1 and Star, which increases progesterone production in granulosa cells with the induction of Pgr [35, 107]. Although the promoter regions of Areg, Ereg, and Btc do not contain the functional PGR responsible element (PGRE), the overexpression of Pgr dramatically increases the promoter activities of those genes [60]. In PRKO mice, the expressions of Areg, Ereg, and Btc are similar to those in WT mice at 2 or 4 h after the hCG injection, whereas at the 8 h point, the expression levels were completely decreased in KO mice, but remained detected in WT mice [60, 108]. Thus, the progesterone‐PGR pathway does not induce the expression of EGF‐like factor; however, the pathway supports PKA‐induced their expressions. This information supports our understanding of the essential roles of the EGF‐like factor, AREG, EREG, and BTC to not only initiate but also accomplish the ovulation process.
During the follicular development process, granulosa cells and cumulus cells dominantly produce estrogen, but less progesterone. The estrogen that impacts the cell proliferation of cumulus cells and granulosa cells is converted from theca cell‐produced androgen via an aromatase‐dependent manner [109, 110]. The key enzyme aromatase is encoded by Cyp19a1 that is expressed by FSH stimulation [16]. FSH receptor (FSHR) is a member of the G protein coupling receptors and activates adenylyl cyclase, which converts ATP to cAMP similar to LHR [111]. Important questions include the reasons for FSH increasing Cyp19a1 expression to produce estrogen and LH decreasing Cyp19a1 expression to induce the ovulation process. The answer to these questions is that during the follicular development process, both CREB activated by the cAMP–PKA pathway and LRH1 (NR5a2) bind to the promoter region of Cyp19a1 to increase the gene expression [112, 113]. After the LH surge, CREB remains activated by LHR‐produced cAMP and is located in the nucleus, whereas LRH1 is dropped from the nucleus to the cytoplasm due to the phosphorylation by ERK1/2 [87, 114]. The granulosa cell specific Nr5a2 KO mice revealed that the morphology of follicular development appeared normal; however, the ability responded to LH surge was completely lost, indicating that ERK1/2 changed the phase from follicular development to the ovulation process [115]. Thus, EGF‐like factor, AREG, EREG, and BTC are invariable factors to stop follicular growth, initiate, and accomplish the ovulation process.
Neuregulin1: another type of EGF‐like factor
When mouse COCs were cultured with AREG alone, cumulus expansion and oocyte maturation were induced in a similar manner to those of COCs in vivo during the ovulation process. However, the progression of oocyte maturation of COCs was accelerated to approximately 2 h to resume meiosis and emit the first polar body [80, 101]. The matured oocytes have a limited fertilization competence and a low developmental ability to the blastocyst stage after in vitro fertilization [101, 116]. In pig COCs collected from 3 to 5 mm of antral follicles, EGFRs were expressed in cumulus cells, whereas the addition of AREG did not induce cumulus expansion and oocyte maturation [86]. Moreover, the phosphorylation of ERK1/2 in cumulus cells of COCs or cultured granulosa cells was temporally upregulated by the addition of AREG within 15 min, but was transiently downregulated to basal level at the 30‐min point after stimulation [101] (Fig. 3A). In vivo, the phosphorylation of ERK1/2 was markedly increased and was maintained by at least 4 h after hCG injection in both cumulus cells and granulosa cells [94] (Fig. 3a), leading to the hypothesis that other secondary factors would be involved in the differentiation of EGF‐like factor‐stimulated cumulus cells and granulosa cells (Fig. 3b).
Figure Fig. 3.
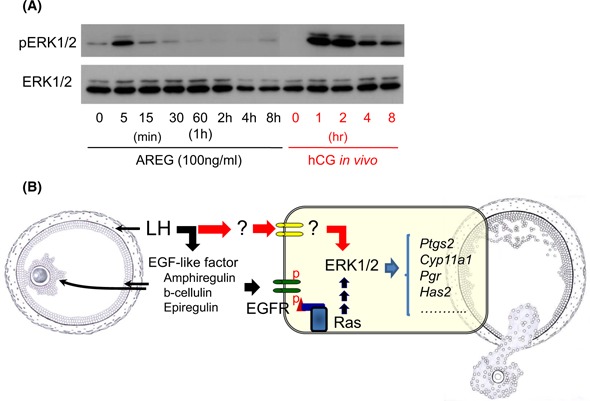
Further unknown secondary factors are required for the induction of the successful ovulation process. a The induction of ERK1/2 phosphorylation in AREG‐stimulated granulosa cells is much lower than that in granulosa cells of hCG‐injected mice. Mouse granulosa cells recovered from eCG‐stimulated mice were pre‐cultured for overnight and then the cells were treated with AREG up to 8 h. Other granulosa cells were collected from periovulatory follicles at 0, 1, 2, 4, and 8 h after hCG injection. These granulosa cells were used for the detection of phosphorylated status of ERK1/2 by western blotting. b Schematic showing the mechanisms how to induce the maximum level of ERK1/2 phosphorylation in granulosa cells during ovulation process
Most of the EGF‐like factor, including AREG, EREG, and BTC bind to EGFR (also known as ErbB1) and then form a dimer to activate EGFR‐containing tyrosine kinase [81, 117]. ErbB1 also forms a dimer with ErbB2 that contains a tyrosine kinase domain, but lacks a ligand‐binding domain and is expressed in both granulosa cells and cumulus cells in preovulatory follicles [81, 101]. Other members of the ErbB family, ErbB3 and ErbB4, are also detected in both cell types, whereas the expression level of ErbB4 is markedly decreased after hCG injection [100, 101]. We performed an immunoprecipitation study to elucidate the activation status of the ErbB family in follicles during the ovulation process, and the data showed that not only EGFR, but also ErbB2 and ErbB3 were activated by the LH surge [101]. ErbB3 has a ligand‐binding domain, but not a tyrosine kinase domain, and binding with ErbB2 is essential for the induction of signaling [117, 118]. Strikingly, ErbB3 formed the heterodimer with ErbB2 with the phosphorylation of both during the ovulation process [101], indicating that the ligands for ErbB3 are expressed and secreted during the ovulation process.
To identify the ligands for ErbB3 expressed in granulosa cells after ovulation stimuli, we searched the open access microarray databases of granulosa cells. However, we did not identify any known ErbB3 ligands encoding genes that were upregulated in granulosa cells after hCG injection. Thus, we constructed each primer set to recognize the ErbB3 ligand encoded genes, Nrg1, Nrg2, and Nrg4 and then examined their expression levels in granulosa cells during the ovulation process in rats and mice. The real‐time PCR studies revealed that the induction of Nrg1 was markedly and rapidly observed in granulosa cells within 4 h after hCG injection [101]. Nrg1 encodes neuregulin 1 (NRG1) that belongs to EGF‐like factor family and has a high affinity to bind to ErbB3, but not to EGFR, and Nrg1 has multiple transcription initiation sites [119, 120] (Fig. 4a). In granulosa cells during the ovulation process, type III Nrg1 that lacks exon 1, but contains a ligand‐binding domain (EGF‐like domain) and transmembrane domain is selectively induced in granulosa cells [101] (Fig. 4b). The Affymetrix Mouse Genome 430 2.0 Array has been commonly used for genome‐wide analysis of the functions of granulosa cells. It has a probe to recognize the exon 1 region that only recognizes type I Nrg1, but does not have any probes for recognizing the common region of Nrg1. Therefore, the researchers who performed the microarray analysis of mouse granulosa cells missed the expression of type III Nrg1.
Figure Fig. 4.
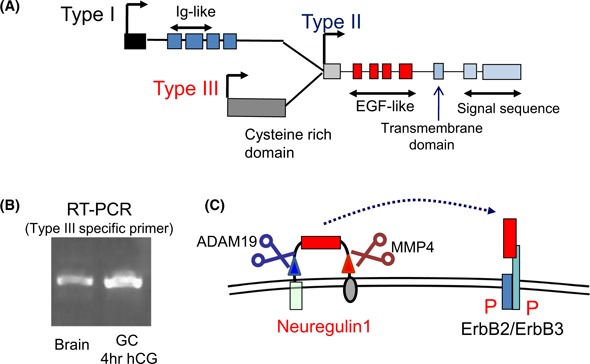
The expression of Nrg1 in granulosa cells during the ovulation process. a The gene construction of Nrg1. Nrg1 has three different transcription start sites. Type I Nrg1 is expressed from exon1, whereas type I Nrg1 mRNA lucks the exon encoding cysteine rich domain via the specific splicing dependent manner. Type II Nrg1 lucks the exons encoding Ig‐like domain and cysteine rich domain. Type III Nrg1 lucks Ig‐like domain, but has a cysteine‐rich domain. All of types of Nrg1 mRNA contain exons encoding EGF‐like domain, transmembrane domain, and signal sequence. b Type III Nrg1 is only induced in granulosa cells during the ovulation process. The specific primer sets to recognize each type of Nrg1 were used for RT‐PCR study. The data revealed that type III Nrg1 was expressed in mouse granulosa cells at 4 h after hCG injection. mRNA recovered from brain was used as positive control. c The construction of NRG1 and the image how to activate its receptor. Type III NRG1 that contains a signal sequence (black box), two transmembrane domains (green box and purple circle) and two protease targeting sequences (blue and red triangles) and a ligand domain (red box) forms a loop structure to present the ligand domain in the extracellular domain. Both proteases, ADAM19 (blue scissors) and MMP4 (brown scissors), are activated by hCG injection, which results in the release of the ligand domain of NRG1 to activate ErbB2/ErbB3 heterodimer
Type III NRG1, which contains a signal sequence, two transmembrane domains, two protease targeting sequences, and a ligand domain forms a loop structure to present the ligand domain in the extracellular domain [121, 122] (Fig. 4c). One of the protease targeting sequences is shed by ADAM19, also expressed in granulosa cells during the ovulation process [101, 123]. MMP4 that has been known to be expressed and activated in follicles during the ovulation process recognizes the C terminal sequence of NRG1 [101, 124, 125]. Both of these proteases are activated by hCG injection with the induction of type III NRG1, indicating that the ligand domain of NRG1 is released to the follicular fluid of periovulatory follicles (Fig. 4c). In fact, the positive signals for antiserum to the ligand domain of NRG1 were detected on the surface of granulosa cells and cumulus cells and in the antrum of follicles at 4 h after hCG injection [126]. On the other hand, the signals for the anti‐C terminal region of NRG1 IgG were only observed on the surface of granulosa cells because this region is located in the cytoplasm [126]. These data show that NRG1 is expressed and secreted from granulosa cells and then acts on both granulosa cells and cumulus cells during the ovulation process.
When mouse granulosa cells were cultured with NRG1, the phosphorylation of ERK1/2 was not induced, and the expression of genes that were observed in granulosa cells during the ovulation process was not increased, similar to those in granulosa cells cultured without any ligands [101]. However, the addition of NRG1 to AREG‐containing medium induced the maximum level of ERK1/2 phosphorylation, and the induction was maintained up to 1 h, whereas AREG alone transiently phosphorylated ERK1/2 at the 15‐min point [101]. In addition, the expression levels of genes involved in the luteinization of granulosa cells were significantly higher in the AREG+NRG1 treatment group than in the AREG alone group [101]. In COCs, the addition of NRG1 increased AREG‐induced ERK1/2 phosphorylation in the cumulus cells, and the oocyte maturation with developmental competence was also improved by the addition of NRG1 to AREG‐containing medium, indicating that NRG1 supported the EGF‐like factor‐induced ovulation process [101].
Reproductive phenotype of Nrg1 null mice
Because the expression of Nrg1 is observed in the brain and heart during embryogenesis, the gene mutation of Nrg1 induces embryo lethality due to heart hypoplasia [127]. Thus, to overcome this severe limitation, we attempted to develop ovarian‐specific Nrg1 mutant mice by the mating of granulosa cell‐specific Cre mice (Cyp19a1Cre mice) with Nrg1 flox/flox mice [126]. A mating test with WT male mice was performed to assess the fertility in the mutant female mice. The number of pups per delivery was significantly decreased to approximately 20 % in Nrg1 flox/flox ;Cyp19a1Cre female mice compared with WT female mice. Although the number of oocytes ovulated by superovulation hormone treatments was more than 50 in both genotypes, the meiotic progression was accelerated to approximately 2 h in Nrg1 flox/flox ;Cyp19a1Cre female mice. The early matured oocytes were arrested at the MII stage by the 16 h point after hCG injection (just after ovulation), whereas most of them spontaneously formed pronuclei without fertilization at 20 h after hCG injection. In other words, the fertilization normally occurred just after ovulation but was abnormal by a few hours after ovulation in Nrg1 flox/flox ;Cyp19a1Cre female mice. When the mating did not occur just after hCG injection, the fertilization was not normally induced, and in most of the sperm‐entered eggs, three pronuclei were observed with a low developmental competence to the blastocyst stage.
To elucidate the relationship between abnormal meiotic progression and abnormal fertilization in Nrg1 flox/flox ;Cyp19a1Cre female mice in more detail, particularly why the number of pups per delivery was decreased by only 20 %, but not completely lost, we focused on the timing of fertilization in each oocyte. Dr. Okabe's group reported that each ovulated oocyte is fertilized sequentially in vivo, but not concurrently [128], estimating that the latter timing of fertilization in Nrg1 flox/flox ;Cyp19a1Cre female mice is outside of the fertilization window. To validate this hypothesis, the mice were assessed for the plug formation every 1 h after hCG injection to determine the timing of mating [126]. The successful fertilization rate was linearly decreased with the time of mating (plug formation) in Nrg1 flox/flox ;Cyp19a1Cre female mice but not in WT mice. Thus, the reason for a decreasing number of pups per delivery in Nrg1 flox/flox ;Cyp19a1Cre female mice is caused by the limiting the duration of oocyte fertilization competence at the MII stage due to the acceleration of meiotic resumption.
Because ErbB2/3 is not localized on the surface of oocytes, but on cumulus cells and granulosa cells [101], and because the maximum level of NRG1 protein is detected at 2 h after hCG injection, the NRG1‐induced signaling pathway and expression of genes at the 2 h point in granulosa cells and cumulus cells may impact on the timing of oocyte meiotic resumption. Microarray analysis was performed for the comprehensive understanding of alternations in both cells in Nrg1 flox/flox ;Cyp19a1Cre female mice (Fig. 5). The clustering analysis of microarray data of granulosa cells in Nrg1 flox/flox ;Cyp19a1Cre female mice showed that the expression pattern of genes induced by hCG injection was similar in WT mice to that in the mutant mice (Fig. 5). More than twofold induction was observed in 1230 genes by hCG than that before hCG injection. In the mutant mice, 221 of upregulated genes at 2 h after hCG injection were further increased to more than twofold than those in WT mice.
Figure Fig. 5.
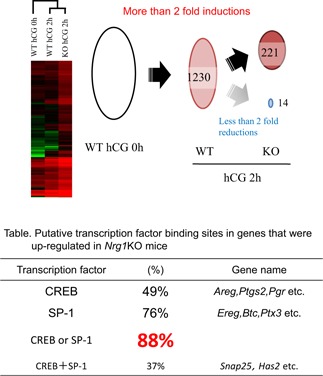
The mutation of Nrg1 increases the expression level of genes that have putative CRE or Sp1‐binding sites in their promoter regions. Image hierarchal clustering analyses were performed on microarray data obtained from granulosa cells and cumulus cells from preovulatory or periovulatory follicles of gcNrg1KO (KO) and WT mice at 0 or 2 h after hCG. Schematic shows the upregulation and downregulation of genes in granulosa/cumulus cells of gcNrg1KO mice. One thousand two hundred and thirty genes were upregulated by hCG, and an additional 221 genes were more highly expressed in cells of the gcNrg1KO mice as compared with those in WT mice at 2 h. Table Putative transcription factor binding sites in genes that were upregulated in granulosa cells of Nrg1 flox/flox ;Cyp19a1Cre mice at 2 h after hCG injection
Most of upregulated genes (88 %) have putative CREB response elements (CRE sites) and/or Sp1 binding sites (Fig. 5). It has been known that CREB and Sp1 have multiple phosphorylation sites, which are targeted by PKA, PKC, etc. [88, 91, 129, 130]. Moreover, the reporter assay study revealed that Pgr promoter containing Sp1‐binding sites was activated in cultured granulosa cells by forskolin, cAMP inducer [54], and that the induction was further increased by additional PMA that was a PKC activator [131]. The promoter region of Snap25, including the further upregulated genes in Nrg1 flox/flox ;Cyp19a1Cre female mice also contains Sp1‐binding sites, and its activity is regulated by both the PKA and PKC pathways [108], thereby estimating that the further induction of genes in Nrg1 flox/flox ;Cyp19a1Cre mice is caused by alternation of PKA or PKC activity. Strikingly, PKC activity similar to PKA activity was significantly increased by hCG injection compared with that in granulosa cells before hCG injection. The PKC, but not PKA, activity was further increased in the mutant mice compared with that in WT mice.
An important question relates to the relationship between erratic PKC activity in cumulus cells and the acceleration of meiotic resumption in oocytes. The first step of oocyte meiotic resumption is known to be the closure of gap junctional communication between the cumulus cells and oocyte [132, 133, 134, 135, 136]. The closure is induced by the phosphorylation of connexin‐43, which results in the decrease of the cGMP transfer level from the cumulus cell to oocyte [137, 138, 139]. The decreased level of cGMP in the oocyte activates phosphodiesterase 3A to decrease the level of cAMP [140, 141, 142, 143]. The phosphorylation of connexin‐43 is induced by ERK1/2 in cumulus cells [144]; however, connexin‐43 contains the phosphorylated site (Ser 368) by PKC [145]. Although the PKC‐induced phosphorylation of connexin‐43 was at a weak level in LH‐stimulated follicles [144], we hypothesized that the unphysiological phosphorylation would induce the acceleration of meiotic resumption in Nrg1 flox/flox ;Cyp19a1Cre female mice. The antibody that recognized only PKC‐phosphorylated connexin‐43 was used for detection in the study [126]. The band shift of the positive signal of total connexin‐43 was detected in both WT and the mutant mice. However, the phosphorylation level of connexin‐43 at Ser 368 was much higher at 2 and 4 h after hCG injection in the mutant mice. In an in vitro culture, with the addition of NRG1 to AREG‐containing medium, NRG1 decreased AREG‐induced Ca2+ level and PKC activity in cumulus cells of COCs, which delayed the timing of meiotic resumption and progression to the MII stage, with a similar timing to those in vivo during the ovulation process in WT mice [126]. Taken together, NRG1 modulates EGF‐like factor‐induced ovulation processes that include not only luteinization and cumulus expansion, but also the timing of the meiotic progression of oocytes via the regulation of PKC activity (Fig. 6).
Figure Fig. 6.
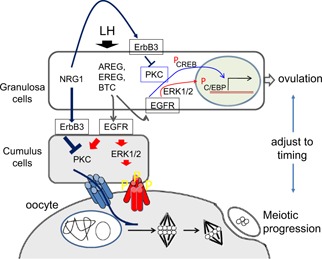
Schematic showing the roles of EGFR ligands and NRG1 in the regulation of timing of ovulation and oocyte maturation. LH induces the expression of EGF‐like factor, AREG, EREG, and BTC in granulosa cells. These factors act on EGFR expressed in both granulosa cells and cumulus cells to stimulate both the ERK1/2 pathway and PKC‐dependent signaling pathway. Both pathways activate gene expressions involved in the ovulation process in granulosa cells. However, the erratic activation of PKC accelerates oocyte meiotic progression due to the early closure of gap junctional communication between cumulus cells and oocyte. LH‐induced NRG1 that selectively acts on ErbB3/2 heterodimer decreases PKC activity to a physiological level. The controlling functions by NRG1 adjust the timing of oocyte maturation to the timing of ovulation
PKC consists of more than ten isoforms classified to three groups: (1) conventional PKC, (2) novel PKC, and (3) atypical PKC [146]. The increase of Ca2+ and diacylglycerol (DG) level induces conventional PKC activity, whereas Ca2+ is not required for the induction of novel PKC activity [147, 148]. In granulosa cells, phorbol 12‐myristate 13‐acetate (PMA) treatment increases the promoter activity of genes that are expressed in a significantly higher level in Nrg1 flox/flox ;Cyp19a1Cre female mice [108, 131, 149]. Because PMA binds to the DG‐binding site of PKC [150], there is a possibility that novel and/or conventional PKC are abnormally activated in granulosa cells of Nrg1 flox/flox ;Cyp19a1Cre female mice and are regulated to a physiological level in WT mice. DG is converted with inositol‐tri‐phosphate (IP3) from phosphatidylinositol‐di‐phosphate (PIP2) in a phospholipase C (PLC)‐dependent manner [151, 152]. IP3 binds to its receptor localized on ER to release Ca2+ [153], and the increased level of Ca2+ in cumulus cells is directly induced by a EGFR‐dependent pathway [154, 155], suggesting that γPLC is activated by the binding to tyrosine phosphorylated EGFR to increase levels of both DG and Ca2+. When mouse COCs were cultured with additional NRG1 to AREG‐containing medium, both PKC activity and Ca2+ level were significantly decreased compared with those in COCs cultured with AREG alone [126]. Thus, NRG1 downregulates AREG–EGFR‐induced PLC activity in cumulus cells and granulosa cells during the ovulation process. ErbB2/3 heterodimer that is the NRG1 receptor contains a γPLC‐binding tyrosine‐phosphorylated site [81], whereas NRG1 has a high affinity for the induction of the PI 3‐kinase‐AKT pathway in granulosa cells and cumulus cells [101]. One possibility is that because PLC and PI 3‐kinase use the same substrate PIP2 to convert IP3 and DG or phosphatidylinositol‐tri‐phosphate (PIP3), respectively, the production of IP3 and DG is competitively suppressed by the activation of PI 3‐kinase [156].
Conclusion
LH surge acts on granulosa cells to induce two types of EGF‐like factor to transfer the stimuli from granulosa cells to cumulus cells, which impacts on cumulus expansion and oocyte maturation. One of these is a conventional EGF‐like factor, AREG, EREG, and BTC that act on EGFR expressed in both granulosa cells and cumulus cells to stimulate both the ERK1/2 pathway and PKC‐dependent signaling pathway. Both pathways are inducers to gene expressions involved in the ovulation process; however, the erratic activation of PKC results in the delicate progression of ovulation being placed out of order. In particular, the timing of oocyte meiotic progression is accelerated by the EGFR‐induced Ca2+‐PKC pathway in cumulus cells. The fringe member of EGF‐like factor, NRG1 that selectively acts on ErbB3/2 heterodimer supports EGFR‐induced ERK1/2 activation, but suppresses the increase of Ca2+ level in cumulus cells, which results in the decrease of PKC activity to a physiological level. Thus, AREG, EREG, and BTC operate as inducers; however, NRG1 plays a modulating role in periovulatory follicles to adjust the timing of oocyte maturation to follicle rupture. The controlling functions by NRG1 are essential for increasing the probability to induce successful fertilization after ovulation. Therefore, both types of EGF‐like factor are notable players that transfer the stimuli of LH surge to cumulus cells and oocyte from granulosa cells to stop the follicular growth stage and then not only initiate but also accomplish the ovulation process.
Acknowledgments
This work was supported in part, by Grant‐in‐Aid for Scientific Research (24688028 and 25132708) from the Japan Society for the Promotion of Science (JSPS) and the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (26066B) (to M.S).
Compliance with ethical standards
Conflict of interest
Masayuki Shimada, Takashi Umehara and Yumi Hoshino declare that they have no conflict of interest.
Human studies
This article does not contain any studies with human subjects performed by any of the authors.
Animal studies
All institutional and national guidelines for the care of use of laboratory animals were followed.
References
- 1. Baker TG Gametogenesis. Acta Endocrinol. 1972;166:18–41. doi: 10.1530/acta.0.071s018. [DOI] [PubMed] [Google Scholar]
- 2. Forabosco A Sforza C Pol A Vizzotto L Marzona L Ferrario VF Morphometric study of the human neonatal ovary. Anat Rec. 1991;231:201–208. doi: 10.1002/ar.1092310208. 10.1002/ar.1092310208 [DOI] [PubMed] [Google Scholar]
- 3. Kezele P Skinner MK Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. 10.1210/en.2002‐0131 [DOI] [PubMed] [Google Scholar]
- 4. Jefferson W Newbold R Padilla‐Banks E Pepling M Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. 10.1095/biolreprod.105.045724 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y Jefferson WN Newbold RR Padilla‐Banks E Pepling ME Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. 10.1210/en.2007‐0088 [DOI] [PubMed] [Google Scholar]
- 6.Eppig JJ. Regulation of mammalian oocyte maturation. In: Adashi E, Leung P, editors. The Ovary. New York: Raven Press, Ltd.; 1993. p. 185–208.
- 7.Molecular mechanisms in ovulation. Molecular biology female reproductive system. San Diego: Academic Press; 1994. pp. 207–258. [Google Scholar]
- 8. Peters H The development of the mouse ovary from birth to maturity. Acta Endocrinol. 1969;62:98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- 9. Pepling ME Spradling AC Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. 10.1006/dbio.2001.0269 [DOI] [PubMed] [Google Scholar]
- 10. Lechowska A Bilinski S Choi Y Shin Y Kloc M Rajkovic A Premature ovarian failure in nobox‐deficient mice is caused by defects in somatic cell invasion and germ cell cyst breakdown J Assist Reprod Genet 2011. 28 583 589316205610.1007/s10815‐011‐9553‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wezel IL Rodgers RJ Morphological characterization of bovine primordial follicles and their environment in vivo. Biol Reprod. 1996;55:1003–1011. doi: 10.1095/biolreprod55.5.1003. 10.1095/biolreprod55.5.1003 [DOI] [PubMed] [Google Scholar]
- 12. Fortune JE The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78:135–163. doi: 10.1016/s0378-4320(03)00088-5. 10.1016/S0378‐4320(03)00088‐5 [DOI] [PubMed] [Google Scholar]
- 13. Hsueh AJ Adashi EY Jones PB Welsh TH Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5:76–127. doi: 10.1210/edrv-5-1-76. 10.1210/edrv‐5‐1‐76 [DOI] [PubMed] [Google Scholar]
- 14. Matzuk MM Burns KH Viveiros MM Eppig JJ Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. 10.1126/science.1071965 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt D Ovitt CE Anlag K Fehsenfeld S Gredsted L Treier AC Treier M The murine winged‐helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. 10.1242/dev.00969 [DOI] [PubMed] [Google Scholar]
- 16. Erickson GF Hsueh AJ Stimulation of aromatase activity by follicle stimulating hormone in rat granulosa cells in vivo and in vitro. Endocrinology. 1978;102:1275–1282. doi: 10.1210/endo-102-4-1275. 10.1210/endo‐102‐4‐1275 [DOI] [PubMed] [Google Scholar]
- 17. Kumar TR Wang Y Lu N Matzuk MM Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. 10.1038/ng0297‐201 [DOI] [PubMed] [Google Scholar]
- 18. Burns KH Yan C Kumar TR Matzuk MM Analysis of ovarian gene expression in follicle‐stimulating hormone beta knockout mice. Endocrinology. 2001;142:2742–2751. doi: 10.1210/endo.142.7.8279. [DOI] [PubMed] [Google Scholar]
- 19. Downs SM Eppig JJ Cyclic adenosine monophosphate and ovarian follicular fluid act synergistically to inhibit mouse oocyte maturation. Endocrinology. 1984;114:418–427. doi: 10.1210/endo-114-2-418. 10.1210/endo‐114‐2‐418 [DOI] [PubMed] [Google Scholar]
- 20. Downs SM Coleman DL Eppig JJ Maintenance of murine oocyte meiotic arrest: uptake and metabolism of hypoxanthine and adenosine by cumulus cell‐enclosed and denuded oocytes. Dev Biol. 1986;117:174–183. doi: 10.1016/0012-1606(86)90359-3. 10.1016/0012‐1606(86)90359‐3 [DOI] [PubMed] [Google Scholar]
- 21. Simon AM Goodenough DA Li E Paul DL Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. 10.1038/385525a0 [DOI] [PubMed] [Google Scholar]
- 22. Eppig JJ O'Brien MJ Comparison of preimplantation developmental competence after mouse oocyte growth and development in vitro and in vivo. Theriogenology. 1998;49:415–422. doi: 10.1016/s0093-691x(97)00413-5. 10.1016/S0093‐691X(97)00413‐5 [DOI] [PubMed] [Google Scholar]
- 23. Magoffin DA Evidence that luteinizing hormone‐stimulated differentiation of purified ovarian thecal‐interstitial cells is mediated by both type I and type II adenosine 3′,5′‐monophosphate‐dependent protein kinases. Endocrinology. 1989;125:1464–1473. doi: 10.1210/endo-125-3-1464. 10.1210/endo‐125‐3‐1464 [DOI] [PubMed] [Google Scholar]
- 24. Palaniappan M Menon KM Regulation of sterol regulatory element‐binding transcription factor 1a by human chorionic gonadotropin and insulin in cultured rat theca‐interstitial cells Biol Reprod 2009. 81 284 292284981510.1095/biolreprod.108.074351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li X Peegel H Menon KM Regulation of high density lipoprotein receptor messenger ribonucleic acid expression and cholesterol transport in theca‐interstitial cells by insulin and human chorionic gonadotropin. Endocrinology. 2001;142:174–181. doi: 10.1210/endo.142.1.7865. [DOI] [PubMed] [Google Scholar]
- 26. Richards JS Ireland JJ Rao MC Bernath GA Midgley AR Jr Reichert LE Jr Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99:1562–1570. doi: 10.1210/endo-99-6-1562. 10.1210/endo‐99‐6‐1562 [DOI] [PubMed] [Google Scholar]
- 27. Uilenbroek JT Richards JS Ovarian follicular development during the rat estrous cycle: gonadotropin receptors and follicular responsiveness. Biol Reprod. 1979;20:1159–1165. doi: 10.1095/biolreprod20.5.1159. 10.1095/biolreprod20.5.1159 [DOI] [PubMed] [Google Scholar]
- 28. Segaloff DL Wang HY Richards JS Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol. 1990;4:1856–1865. doi: 10.1210/mend-4-12-1856. 10.1210/mend‐4‐12‐1856 [DOI] [PubMed] [Google Scholar]
- 29. Richards JS Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. 10.1210/edrv‐15‐6‐725 [DOI] [PubMed] [Google Scholar]
- 30. Peng XR Hsueh AJ LaPolt PS Bjersing L Ny T Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. doi: 10.1210/endo-129-6-3200. 10.1210/endo‐129‐6‐3200 [DOI] [PubMed] [Google Scholar]
- 31. Edwards RG Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–351. doi: 10.1038/208349a0. 10.1038/208349a0 [DOI] [PubMed] [Google Scholar]
- 32. Cross PC Brinster RL In vitro development of mouse oocytes. Biol Reprod. 1970;3:298–307. doi: 10.1093/biolreprod/3.3.298. [DOI] [PubMed] [Google Scholar]
- 33. Robker RL Richards JS Hormone‐induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–940. doi: 10.1210/mend.12.7.0138. 10.1210/mend.12.7.0138 [DOI] [PubMed] [Google Scholar]
- 34. Sicinski P Donaher JL Geng Y Parker SB Gardner H Park MY Robker RL Richards JS McGinnis LK Biggers JD Eppig JJ Bronson RT Elledge SJ Weinberg RA Cyclin D2 is an FSH‐responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. 10.1038/384470a0 [DOI] [PubMed] [Google Scholar]
- 35. Sharma SC Richards JS Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem. 2000;275:33718–33728. doi: 10.1074/jbc.M003555200. 10.1074/jbc.M003555200 [DOI] [PubMed] [Google Scholar]
- 36. Hickey GJ Chen SA Besman MJ Shively JE Hall PF Gaddy‐Kurten D Richards JS Hormonal regulation, tissue distribution, and content of aromatase cytochrome P450 messenger ribonucleic acid and enzyme in rat ovarian follicles and corpora lutea: relationship to estradiol biosynthesis. Endocrinology. 1988;122:1426–1436. doi: 10.1210/endo-122-4-1426. 10.1210/endo‐122‐4‐1426 [DOI] [PubMed] [Google Scholar]
- 37. Hickey GJ Krasnow JS Beattie WG Richards JS Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3′,5′‐monophosphate‐dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5′ genomic DNA. Mol Endocrinol. 1990;4:3–12. doi: 10.1210/mend-4-1-3. 10.1210/mend‐4‐1‐3 [DOI] [PubMed] [Google Scholar]
- 38. Lipner H Wendelken L Inhibition of ovulation by inhibition of steroidogenesis in immature rats. Proc Soc Exp Biol Med. 1971;136:1141–1145. doi: 10.3181/00379727-136-35447. 10.3181/00379727‐136‐35447 [DOI] [PubMed] [Google Scholar]
- 39. Voutilainen R Tapanainen J Chung BC Matteson KJ Miller WL Hormonal regulation of P450scc (20,22‐desmolase) and P450c17 (17 alpha‐hydroxylase/17,20‐lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986;63:202–220. doi: 10.1210/jcem-63-1-202. 10.1210/jcem‐63‐1‐202 [DOI] [PubMed] [Google Scholar]
- 40. Oonk RB Krasnow JS Beattie WG Richards JS Cyclic AMP‐dependent and ‐independent regulation of cholesterol side chain cleavage cytochrome P‐450 (P‐450scc) in rat ovarian granulosa cells and corpora lutea. cDNA and deduced amino acid sequence of rat P‐450scc. J Biol Chem. 1989;264:21934–21942. [PubMed] [Google Scholar]
- 41. Shea‐Eaton W Sandhoff TW Lopez D Hales DB McLean MP Transcriptional repression of the rat steroidogenic acute regulatory (StAR) protein gene by the AP‐1 family member c‐Fos. Mol Cell Endocrinol. 2002;188:161–170. doi: 10.1016/s0303-7207(01)00715-8. 10.1016/S0303‐7207(01)00715‐8 [DOI] [PubMed] [Google Scholar]
- 42. Manna PR Eubank DW Stocco DM Assessment of the role of activator protein‐1 on transcription of the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol. 2004;18:558–573. doi: 10.1210/me.2003-0223. 10.1210/me.2003‐0223 [DOI] [PubMed] [Google Scholar]
- 43. Fan HY Liu Z Johnson PF Richards JS CCAAT/enhancer‐binding proteins (C/EBP)‐α and ‐β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol. 2011;25:253–268. doi: 10.1210/me.2010-0318. 10.1210/me.2010‐0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kolena J Channing CP Stimulatory effects of LH, FSH and prostaglandins upon cyclic 3′,5′‐AMP levels in porcine granulosa cells. Endocrinology. 1972;90:1543–1550. doi: 10.1210/endo-90-6-1543. 10.1210/endo‐90‐6‐1543 [DOI] [PubMed] [Google Scholar]
- 45. Morris JK Richards JS Luteinizing hormone induces prostaglandin endoperoxide synthase‐2 and luteinization in vitro by A‐kinase and C‐kinase pathways. Endocrinology. 1995;136:1549–1558. doi: 10.1210/endo.136.4.7895665. [DOI] [PubMed] [Google Scholar]
- 46. Fan HY Richards JS Minireview: physiological and pathological actions of RAS in the ovary. Mol Endocrinol. 2010;24:286–298. doi: 10.1210/me.2009-0251. 10.1210/me.2009‐0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ainsworth L Tsang BK Downey BR Marcus GJ Armstrong DT Interrelationships between follicular fluid steroid levels, gonadotropic stimuli, and oocyte maturation during preovulatory development of porcine follicles. Biol Reprod. 1980;23:621–627. doi: 10.1095/biolreprod23.3.621. 10.1095/biolreprod23.3.621 [DOI] [PubMed] [Google Scholar]
- 48. Orly J Clemens JW Singer O Richards JS Effects of hormones and protein kinase inhibitors on expression of steroidogenic enzyme promoters in electroporated primary rat granulosa cells. Biol Reprod. 1996;54:208–218. doi: 10.1095/biolreprod54.1.208. 10.1095/biolreprod54.1.208 [DOI] [PubMed] [Google Scholar]
- 49. Mori T Nagasawa H Bern HA Long‐term effects of perinatal exposure to hormones on normal and neoplastic mammary growth in rodents: a review. J Environ Pathol Toxicol. 1979;3:191–205. [PubMed] [Google Scholar]
- 50. Lipner H Greep RO Inhibition of steroidogenesis at various sites in the biosynthetic pathway in relation to induced ovulation. Endocrinology. 1971;88:602–607. doi: 10.1210/endo-88-3-602. 10.1210/endo‐88‐3‐602 [DOI] [PubMed] [Google Scholar]
- 51. Snyder BW Beecham GD Schane HP Inhibition of ovulation in rats with epostane, an inhibitor of 3 beta‐hydroxysteroid dehydrogenase. Proc Soc Exp Biol Med. 1984;176:238–242. doi: 10.3181/00379727-176-41865. 10.3181/00379727‐176‐41865 [DOI] [PubMed] [Google Scholar]
- 52. Evans RM The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. 10.1126/science.3283939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsai MJ O'Malley BW Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. 10.1146/annurev.bi.63.070194.002315 [DOI] [PubMed] [Google Scholar]
- 54. Natraj U Richards JS Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology. 1993;133:761–769. doi: 10.1210/endo.133.2.8344215. [DOI] [PubMed] [Google Scholar]
- 55. Shimada M Yamashita Y Ito J Okazaki T Kawahata K Nishibori M Expression of two progesterone receptor isoforms in cumulus cells and their roles during meiotic resumption of porcine oocytes. J Mol Endocrinol. 2004;33:209–225. doi: 10.1677/jme.0.0330209. 10.1677/jme.0.0330209 [DOI] [PubMed] [Google Scholar]
- 56. Shimada M Nishibori M Yamashita Y Ito J Mori T Richards JS Down‐regulated expression of A disintegrin and metalloproteinase with thrombospondin‐like repeats‐1 by progesterone receptor antagonist is associated with impaired expansion of porcine cumulus‐oocyte complexes. Endocrinology. 2004;145:4603–4614. doi: 10.1210/en.2004-0542. 10.1210/en.2004‐0542 [DOI] [PubMed] [Google Scholar]
- 57. Zavareh S Saberivand A Salehnia M The effect of progesterone on the in vitro maturation and developmental competence of mouse germinal vesicle oocytes. Int J Fertil Steril. 2009;3:21–28. [Google Scholar]
- 58. Lydon JP DeMayo FJ Funk CR Mani SK Hughes AR Montgomery CA Jr Shyamala G Conneely OM O'Malley BW Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. 10.1101/gad.9.18.2266 [DOI] [PubMed] [Google Scholar]
- 59. Robker RL Russell DL Espey LL Lydon JP O'Malley BW Richards JS Progesterone‐regulated genes in the ovulation process: ADAMTS‐1 and cathepsin L proteases Proc Natl Acad Sci USA 2000. 97 4689 46941829410.1073/pnas.080073497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shimada M Hernandez‐Gonzalez I Gonzalez‐Robayna I Richards JS Paracrine and autocrine regulation of epidermal growth factor‐like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. 10.1210/me.2005‐0504 [DOI] [PubMed] [Google Scholar]
- 61. Wong WY Richards JS Induction of prostaglandin H synthase in rat preovulatory follicles by gonadotropin‐releasing hormone. Endocrinology. 1992;130:3512–3521. doi: 10.1210/endo.130.6.1317786. [DOI] [PubMed] [Google Scholar]
- 62. Sirois J Simmons DL Richards JS Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J Biol Chem. 1992;267:11586–11592. [PubMed] [Google Scholar]
- 63. Joyce IM Pendola FL O'Brien M Eppig JJ Regulation of prostaglandin‐endoperoxide synthase 2 messenger ribonucleic acid expression in mouse granulosa cells during ovulation. Endocrinology. 2001;142:3187–3197. doi: 10.1210/endo.142.7.8268. [DOI] [PubMed] [Google Scholar]
- 64. Hizaki H Segi E Sugimoto Y Hirose M Saji T Ushikubi F Matsuoka T Noda Y Tanaka T Yoshida N Narumiya S Ichikawa A Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci USA 1999. 96 10501 105061791810.1073/pnas.96.18.10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kennedy CR Zhang Y Brandon S Guan Y Coffee K Funk CD Magnuson MA Oates JA Breyer MD Breyer RM Salt‐sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–220. doi: 10.1038/5583. 10.1038/7426 [DOI] [PubMed] [Google Scholar]
- 66. Davis BJ Lennard DE Lee CA Tiano HF Morham SG Wetsel WC Langenbach R Anovulation in cyclooxygenase‐2‐deficient mice is restored by prostaglandin E2 and interleukin‐1beta. Endocrinology. 1999;140:2685–2695. doi: 10.1210/endo.140.6.6715. [DOI] [PubMed] [Google Scholar]
- 67. Matsumoto H Ma W Smalley W Trzaskos J Breyer RM Dey SK Diversification of cyclooxygenase‐2‐derived prostaglandins in ovulation and implantation. Biol Reprod. 2001;64:1557–1565. doi: 10.1095/biolreprod64.5.1557. 10.1095/biolreprod64.5.1557 [DOI] [PubMed] [Google Scholar]
- 68. Ochsner SA Russell DL Day AJ Breyer RM Richards JS Decreased expression of tumor necrosis factor‐alpha‐stimulated gene 6 in cumulus cells of the cyclooxygenase‐2 and EP2 null mice. Endocrinology. 2003;144:1008–1019. doi: 10.1210/en.2002-220435. 10.1210/en.2002‐220435 [DOI] [PubMed] [Google Scholar]
- 69. Su YQ Wigglesworth K Pendola FL O'Brien MJ Eppig JJ Mitogen‐activated protein kinase activity in cumulus cells is essential for gonadotropin‐induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/endo.143.6.8845. 10.1210/endo.143.6.8845 [DOI] [PubMed] [Google Scholar]
- 70. Prochazka R Kalab P Nagyova E Epidermal growth factor‐receptor tyrosine kinase activity regulates expansion of porcine oocyte‐cumulus cell complexes in vitro. Biol Reprod. 2003;68:797–803. doi: 10.1095/biolreprod.102.005520. 10.1095/biolreprod.102.005520 [DOI] [PubMed] [Google Scholar]
- 71. Yamashita Y Kawashima I Yanai Y Nishibori M Richards JS Shimada M Hormone‐induced expression of tumor necrosis factor alpha‐converting enzyme/A disintegrin and metalloprotease‐17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology. 2007;148:6164–6175. doi: 10.1210/en.2007-0195. 10.1210/en.2007‐0195 [DOI] [PubMed] [Google Scholar]
- 72. Downs SM Daniel SA Eppig JJ Induction of maturation in cumulus cell‐enclosed mouse oocytes by follicle‐stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96. doi: 10.1002/jez.1402450113. 10.1002/jez.1402450113 [DOI] [PubMed] [Google Scholar]
- 73. Lorenzo PL Illera MJ Illera JC Illera M Enhancement of cumulus expansion and nuclear maturation during bovine oocyte maturation in vitro by the addition of epidermal growth factor and insulin‐like growth factor I. J Reprod Fertil. 1994;101:697–701. doi: 10.1530/jrf.0.1010697. 10.1530/jrf.0.1010697 [DOI] [PubMed] [Google Scholar]
- 74. Vigneron C Perreau C Dupont J Uzbekova S Prigent C Mermillod P Several signaling pathways are involved in the control of cattle oocyte maturation. Mol Reprod Dev. 2004;69:466–474. doi: 10.1002/mrd.20173. 10.1002/mrd.20173 [DOI] [PubMed] [Google Scholar]
- 75. Xia P Tekpetey FR Armstrong DT Effect of IGF‐I on pig oocyte maturation, fertilization, and early embryonic development in vitro, and on granulosa and cumulus cell biosynthetic activity. Mol Reprod Dev. 1994;38:373–379. doi: 10.1002/mrd.1080380404. 10.1002/mrd.1080380404 [DOI] [PubMed] [Google Scholar]
- 76. Izadyar F Tol HT Colenbrander B Bevers MM Stimulatory effect of growth hormone on in vitro maturation of bovine oocytes is exerted through cumulus cells and not mediated by IGF‐I. Mol Reprod Dev. 1997;47:175–180. doi: 10.1002/(SICI)1098-2795(199706)47:2<175::AID-MRD8>3.0.CO;2-J. 10.1002/(SICI)1098‐2795(199706)47:2<175::AID‐MRD8>3.0.CO;2‐J [DOI] [PubMed] [Google Scholar]
- 77. Downs SM Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod. 1989;41:371–379. doi: 10.1095/biolreprod41.2.371. 10.1095/biolreprod41.2.371 [DOI] [PubMed] [Google Scholar]
- 78. Zhou J Chin E Bondy C Cellular pattern of insulin‐like growth factor‐I (IGF‐I) and IGF‐I receptor gene expression in the developing and mature ovarian follicle. Endocrinology. 1991;129:3281–3288. doi: 10.1210/endo-129-6-3281. 10.1210/endo‐129‐6‐3281 [DOI] [PubMed] [Google Scholar]
- 79. Singh B Rutledge JM Armstrong DT Epidermal growth factor and its receptor gene expression and peptide localization in porcine ovarian follicles. Mol Reprod Dev. 1995;40:391–399. doi: 10.1002/mrd.1080400402. 10.1002/mrd.1080400402 [DOI] [PubMed] [Google Scholar]
- 80. Park JY Su YQ Ariga M Law E Jin SL Conti M EGF‐like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. 10.1126/science.1092463 [DOI] [PubMed] [Google Scholar]
- 81. Citri A Yarden Y EGF–ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. 10.1038/nrm1962 [DOI] [PubMed] [Google Scholar]
- 82. Lee DC Sunnarborg SW Hinkle CL Myers TJ Stevenson MY Russell WE Castner BJ Gerhart MJ Paxton RJ Black RA Chang A Jackson LF TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann N Y Acad Sci. 2003;995:22–38. doi: 10.1111/j.1749-6632.2003.tb03207.x. 10.1111/j.1749‐6632.2003.tb03207.x [DOI] [PubMed] [Google Scholar]
- 83. Sahin U Weskamp G Kelly K Zhou HM Higashiyama S Peschon J Hartmann D Saftig P Blobel CP Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands J Cell Biol 2004. 164 769 779217215410.1083/jcb.200307137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yamashita Y Shimada M The release of EGF domain from EGF‐like factors by a specific cleavage enzyme activates the EGFR–MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J Reprod Dev. 2012;58:510–514. doi: 10.1262/jrd.2012-056. 10.1262/jrd.2012‐056 [DOI] [PubMed] [Google Scholar]
- 85. Yamashita Y Okamoto M Ikeda M Okamoto A Sakai M Gunji Y Nishimura R Hishinuma M Shimada M Protein kinase C (PKC) increases TACE/ADAM17 enzyme activity in porcine ovarian somatic cells, which is essential for granulosa cell luteinization and oocyte maturation. Endocrinology. 2014;155:1080–1090. doi: 10.1210/en.2013-1655. 10.1210/en.2013‐1655 [DOI] [PubMed] [Google Scholar]
- 86. Kawashima I Okazaki T Noma N Nishibori M Yamashita Y Shimada M Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation‐related genes that impact oocyte maturation in vivo and in vitro. Reproduction. 2008;136:9–21. doi: 10.1530/REP-08-0074. 10.1530/REP‐08‐0074 [DOI] [PubMed] [Google Scholar]
- 87. Fan HY O'Connor A Shitanaka M Shimada M Liu Z Richards JS Beta‐catenin (CTNNB1) promotes preovulatory follicular development but represses LH‐mediated ovulation and luteinization Mol Endocrinol 2010. 24 1529 1542294046310.1210/me.2010‐0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yamamoto KK Gonzalez GA Biggs WH 3rd Montminy MR Phosphorylation‐induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. 10.1038/334494a0 [DOI] [PubMed] [Google Scholar]
- 89. Gonzalez GA Montminy MR Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. 10.1016/0092‐8674(89)90013‐5 [DOI] [PubMed] [Google Scholar]
- 90. Sekiguchi T Mizutani T Yamada K Yazawa T Kawata H Yoshino M Kajitani T Kameda T Minegishi T Miyamoto K Transcriptional regulation of the epiregulin gene in the rat ovary. Endocrinology. 2002;143:4718–4729. doi: 10.1210/en.2002-220440. 10.1210/en.2002‐220440 [DOI] [PubMed] [Google Scholar]
- 91. Rohlff C Ahmad S Borellini F Lei J Glazer RI Modulation of transcription factor Sp1 by cAMP‐dependent protein kinase. J Biol Chem. 1997;272:21137–21141. doi: 10.1074/jbc.272.34.21137. 10.1074/jbc.272.34.21137 [DOI] [PubMed] [Google Scholar]
- 92. Conti M Hsieh M Park JY Su YQ Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20:715–723. doi: 10.1210/me.2005-0185. 10.1210/me.2005‐0185 [DOI] [PubMed] [Google Scholar]
- 93. Panigone S Hsieh M Fu M Persani L Conti M Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway Mol Endocrinol 2008. 22 924 936227646910.1210/me.2007‐0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fan HY Liu Z Shimada M Sterneck E Johnson PF Hedrick SM Richards JS MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility Science 2009. 324 938 941284789010.1126/science.1171396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hsieh M Lee D Panigone S Horner K Chen R Theologis A Lee DC Threadgill DW Conti M Luteinizing hormone‐dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. 10.1128/MCB.01919‐06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fan HY Shimada M Liu Z Cahill N Noma N Wu Y Gossen J Richards JS Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation Development. 2008. 135 2127 2137354183110.1242/dev.020560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Su YQ Denegre JM Wigglesworth K Pendola FL O'Brien MJ Eppig JJ Oocyte‐dependent activation of mitogen‐activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte‐cumulus cell complex. Dev Biol. 2003;263:126–138. doi: 10.1016/s0012-1606(03)00437-8. 10.1016/S0012‐1606(03)00437‐8 [DOI] [PubMed] [Google Scholar]
- 98. Ochsner SA Day AJ Rugg MS Breyer RM Gomer RH Richards JS Disrupted function of tumor necrosis factor‐alpha‐stimulated gene 6 blocks cumulus cell‐oocyte complex expansion. Endocrinology. 2003;144:4376–4384. doi: 10.1210/en.2003-0487. 10.1210/en.2003‐0487 [DOI] [PubMed] [Google Scholar]
- 99. Pathak BG Gilbert DJ Harrison CA Luetteke NC Chen X Klagsbrun M Plowman GD Copeland NG Jenkins NA Lee DC Mouse chromosomal location of three EGF receptor ligands: amphiregulin (Areg), betacellulin (Btc), and heparin‐binding EGF (Hegfl) Genomics. 1995;28:116–118. doi: 10.1006/geno.1995.1116. 10.1006/geno.1995.1116 [DOI] [PubMed] [Google Scholar]
- 100. Pan B Sengoku K Takuma N Goishi K Horikawa M Tamate K Ishikawa M Differential expression of heparin‐binding epidermal growth factor‐like growth factor in the rat ovary. Mol Cell Endocrinol. 2004;214:1–8. doi: 10.1016/j.mce.2003.12.003. 10.1016/j.mce.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 101. Noma N Kawashima I Fan HY Fujita Y Kawai T Tomoda Y Mihara T Richards JS Shimada M LH‐induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol. 2011;25:104–116. doi: 10.1210/me.2010-0225. 10.1210/me.2010‐0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Robker RL Richards JS Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod. 1998;59:476–482. doi: 10.1095/biolreprod59.3.476. 10.1095/biolreprod59.3.476 [DOI] [PubMed] [Google Scholar]
- 103. Burkart AD Mukherjee A Sterneck E Johnson PF Mayo KE Repression of the inhibin alpha‐subunit gene by the transcription factor CCAAT/enhancer‐binding protein‐beta. Endocrinology. 2005;146:1909–1921. doi: 10.1210/en.2004-0842. 10.1210/en.2004‐0842 [DOI] [PubMed] [Google Scholar]
- 104. Sterneck E Tessarollo L Johnson PF An essential role for C/EBPbeta in female reproduction Genes Dev 1997. 11 2153 216227539410.1101/gad.11.17.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Clayton RN Catt KJ Regulation of pituitary gonadotropin‐releasing hormone receptors by gonadal hormones. Endocrinology. 1981;108:887–895. doi: 10.1210/endo-108-3-887. 10.1210/endo‐108‐3‐887 [DOI] [PubMed] [Google Scholar]
- 106. Tsafriri A Chun SY Zhang R Hsueh AJ Conti M Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1996;178:393–402. doi: 10.1006/dbio.1996.0226. 10.1006/dbio.1996.0226 [DOI] [PubMed] [Google Scholar]
- 107. Park OK Mayo KE Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol. 1991;5:967–978. doi: 10.1210/mend-5-7-967. 10.1210/mend‐5‐7‐967 [DOI] [PubMed] [Google Scholar]
- 108. Shimada M Yanai Y Okazaki T Yamashita Y Sriraman V Wilson MC Richards JS Synaptosomal‐associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–2502. doi: 10.1210/me.2007-0042. 10.1210/me.2007‐0042 [DOI] [PubMed] [Google Scholar]
- 109. Moon YS Tsang BK Simpson C Armstrong DT 17 beta‐Estradiol biosynthesis in cultured granulosa and thecal cells of human ovarian follicles: stimulation by follicle‐stimulating hormone. J Clin Endocrinol Metab. 1978;47:263–267. doi: 10.1210/jcem-47-2-263. 10.1210/jcem‐47‐2‐263 [DOI] [PubMed] [Google Scholar]
- 110. Fisher CR Graves KH Parlow AF Simpson ER Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene Proc Natl Acad Sci USA 1998. 95 6965 69702270310.1073/pnas.95.12.6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Moyle WR Campbell RK Myers RV Bernard MP Han Y Wang X Co‐evolution of ligand‐receptor pairs. Nature. 1994;368:251–255. doi: 10.1038/368251a0. 10.1038/368251a0 [DOI] [PubMed] [Google Scholar]
- 112. Carlone DL Richards JS Evidence that functional interactions of CREB and SF‐1 mediate hormone regulated expression of the aromatase gene in granulosa cells and constitutive expression in R2C cells. J Steroid Biochem Mol Biol. 1997;61:223–231. 10.1016/S0960‐0760(97)80016‐7 [PubMed] [Google Scholar]
- 113. Saxena D Safi R Little‐Ihrig L Zeleznik AJ Liver receptor homolog‐1 stimulates the progesterone biosynthetic pathway during follicle‐stimulating hormone‐induced granulosa cell differentiation. Endocrinology. 2004;145:3821–3829. doi: 10.1210/en.2004-0423. 10.1210/en.2004‐0423 [DOI] [PubMed] [Google Scholar]
- 114. Lee YK Choi YH Chua S Park YJ Moore DD Phosphorylation of the hinge domain of the nuclear hormone receptor LRH‐1 stimulates transactivation. J Biol Chem. 2006;281:7850–7855. doi: 10.1074/jbc.M509115200. 10.1074/jbc.M509115200 [DOI] [PubMed] [Google Scholar]
- 115. Duggavathi R Volle DH Mataki C Antal MC Messaddeq N Auwerx J Murphy BD Schoonjans K Liver receptor homolog 1 is essential for ovulation Genes Dev 2008. 22 1871 1876249273410.1101/gad.472008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ben‐Ami I Komsky A Bern O Kasterstein E Komarovsky D Ron‐El R In vitro maturation of human germinal vesicle‐stage oocytes: role of epidermal growth factor‐like growth factors in the culture medium. Hum Reprod. 2011;26:76–81. doi: 10.1093/humrep/deq290. 10.1093/humrep/deq290 [DOI] [PubMed] [Google Scholar]
- 117. Yarden Y Sliwkowski MX Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 118. Levkowitz G Waterman H Ettenberg SA Katz M Tsygankov AY Alroy I Lavi S Iwai K Reiss Y Ciechanover A Lipkowitz S Yarden Y Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c‐Cbl/Sli‐1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. 10.1016/S1097‐2765(00)80231‐2 [DOI] [PubMed] [Google Scholar]
- 119. Riese DJ 2nd Raaij TM Plowman GD Andrews GC Stern DF The cellular response to neuregulins is governed by complex interactions of the erbB receptor family Mol Cell Biol 1995. 15 5770 577623082910.1128/MCB.15.10.5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Meyer D Yamaai T Garratt A Riethmacher‐Sonnenberg E Kane D Theill LE Birchmeier C Isoform‐specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 121. Leimeroth R Lobsiger C Lüssi A Taylor V Suter U Sommer L Membrane‐bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent Progenitor cells. Dev Biol. 2002;246:245–258. doi: 10.1006/dbio.2002.0670. 10.1006/dbio.2002.0670 [DOI] [PubMed] [Google Scholar]
- 122. Mei L Xiong WC Neuregulin 1 in neural development, synaptic plasticity and schizophrenia Nat Rev Neurosci 2008. 9 437 452268237110.1038/nrn2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shirakabe K Wakatsuki S Kurisaki T Fujisawa‐Sehara A Roles of Meltrin beta/ADAM19 in the processing of neuregulin. J Biol Chem. 2001;276:9352–9358. doi: 10.1074/jbc.M007913200. 10.1074/jbc.M007913200 [DOI] [PubMed] [Google Scholar]
- 124. Curry TE Jr Osteen KG The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. 10.1210/er.2002‐0005 [DOI] [PubMed] [Google Scholar]
- 125. Richards JS Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–79. doi: 10.1016/j.mce.2005.01.004. 10.1016/j.mce.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 126. Kawashima I Umehara T Noma N Kawai T Shitanaka M Richards JS Shimada M Targeted disruption of Nrg1 in granulosa cells alters the temporal progression of oocyte maturation Mol Endocrinol 2014. 28 706 721400477510.1210/me.2013‐1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Meyer D Birchmeier C Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. 10.1038/378386a0 [DOI] [PubMed] [Google Scholar]
- 128. Hasuwa H Muro Y Ikawa M Kato N Tsujimoto Y Okabe M Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp Anim. 2010;59:105–107. doi: 10.1538/expanim.59.105. 10.1538/expanim.59.105 [DOI] [PubMed] [Google Scholar]
- 129. Roberson ED English JD Adams JP Selcher JC Kondratick C Sweatt JD The mitogen‐activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hewson CA Edbrooke MR Johnston SL PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF‐alpha, Ras/Raf, MEK, ERK and Sp1‐dependent mechanisms. J Mol Biol. 2004;344:683–695. doi: 10.1016/j.jmb.2004.09.059. 10.1016/j.jmb.2004.09.059 [DOI] [PubMed] [Google Scholar]
- 131. Sriraman V Sharma SC Richards JS Transactivation of the progesterone receptor gene in granulosa cells: evidence that Sp1/Sp3 binding sites in the proximal promoter play a key role in luteinizing hormone inducibility. Mol Endocrinol. 2003;17:436–449. doi: 10.1210/me.2002-0252. 10.1210/me.2002‐0252 [DOI] [PubMed] [Google Scholar]
- 132. Anderson E Albertini DF Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. 1976;71:680–686. doi: 10.1083/jcb.71.2.680. 10.1083/jcb.71.2.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Eppig JJ The relationship between cumulus cell‐oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev Biol. 1982;89:268–272. doi: 10.1016/0012-1606(82)90314-1. 10.1016/0012‐1606(82)90314‐1 [DOI] [PubMed] [Google Scholar]
- 134. Granot I Dekel N Phosphorylation and expression of connexin‐43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem. 1994;269:30502–30509. [PubMed] [Google Scholar]
- 135. Isobe N Maeda T Terada T Involvement of meiotic resumption in the disruption of gap junctions between cumulus cells attached to pig oocytes. J Reprod Fertil. 1998;113:167–172. doi: 10.1530/jrf.0.1130167. 10.1530/jrf.0.1130167 [DOI] [PubMed] [Google Scholar]
- 136. Shimada M Maeda T Terada T Dynamic changes of connexin‐43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI 3‐kinase during meiotic resumption in porcine oocytes. Biol Reprod. 2001;64:1255–1263. doi: 10.1095/biolreprod64.4.1255. 10.1095/biolreprod64.4.1255 [DOI] [PubMed] [Google Scholar]
- 137. Norris RP Ratzan WJ Freudzon M Mehlmann LM Krall J Movsesian MA Wang H Ke H Nikolaev VO Jaffe LA Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte Development. 2009. 136 1869 1878268011010.1242/dev.035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vaccari S Weeks JL 2nd Hsieh M Menniti FS Conti M Cyclic GMP signaling is involved in the luteinizing hormone‐dependent meiotic maturation of mouse oocytes Biol Reprod 2009. 81 595 604273198110.1095/biolreprod.109.077768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang M Su YQ Sugiura K Xia G Eppig JJ Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes Science 2010. 330 366 369305654210.1126/science.1193573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Richard FJ Tsafriri A Conti M Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod. 2001;65:1444–1451. doi: 10.1095/biolreprod65.5.1444. 10.1095/biolreprod65.5.1444 [DOI] [PubMed] [Google Scholar]
- 141. Masciarelli S Horner K Liu C Park SH Hinckley M Hockman S Nedachi T Jin C Conti M Manganiello V Cyclic nucleotide phosphodiesterase 3A‐deficient mice as a model of female infertility J Clin Invest. 2004. 114 196 20544975210.1172/JCI21804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Han SJ Chen R Paronetto MP Conti M Wee1B is an oocyte‐specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–1676. doi: 10.1016/j.cub.2005.07.056. 10.1016/j.cub.2005.07.056 [DOI] [PubMed] [Google Scholar]
- 143. Han SJ Vaccari S Nedachi T Andersen CB Kovacina KS Roth RA Conti M Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation EMBO J 2006. 25 5716 5725169888010.1038/sj.emboj.7601431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Norris RP Freudzon M Mehlmann LM Cowan AE Simon AM Paul DL Lampe PD Jaffe LA Luteinizing hormone causes MAP kinase‐dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption Development. 2008. 135 3229 3238257222410.1242/dev.025494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lampe PD TenBroek EM Burt JM Kurata WE Johnson RG Lau AF Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication J Cell Biol 2000. 149 1503 1512217513410.1083/jcb.149.7.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Nishizuka Y Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. 10.1126/science.1411571 [DOI] [PubMed] [Google Scholar]
- 147. Takai Y Kishimoto A Kikkawa U Mori T Nishizuka Y Unsaturated diacylglycerol as a possible messenger for the activation of calcium‐activated, phospholipid‐dependent protein kinase system. Biochem Biophys Res Commun. 1979;91:1218–1224. doi: 10.1016/0006-291x(79)91197-5. 10.1016/0006‐291X(79)91197‐5 [DOI] [PubMed] [Google Scholar]
- 148. Kishimoto A Takai Y Mori T Kikkawa U Nishizuka Y Activation of calcium and phospholipid‐dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980;255:2273–2276. [PubMed] [Google Scholar]
- 149. Sriraman V Richards JS Cathepsin L gene expression and promoter activation in rodent granulosa cells. Endocrinology. 2004;145:582–591. doi: 10.1210/en.2003-0963. 10.1210/en.2003‐0963 [DOI] [PubMed] [Google Scholar]
- 150. Kikkawa U Takai Y Tanaka Y Miyake R Nishizuka Y Protein kinase C as a possible receptor protein of tumor‐promoting phorbol esters. J Biol Chem. 1983;258:11442–11445. [PubMed] [Google Scholar]
- 151. Nishizuka Y Turnover of inositol phospholipids and signal transduction. Science. 1984;225:1365–1370. doi: 10.1126/science.6147898. 10.1126/science.6147898 [DOI] [PubMed] [Google Scholar]
- 152. Berridge MJ Irvine RF Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. 10.1038/312315a0 [DOI] [PubMed] [Google Scholar]
- 153. Missiaen L Taylor CW Berridge MJ Spontaneous calcium release from inositol trisphosphate–sensitive calcium stores. Nature. 1991;352:241–244. doi: 10.1038/352241a0. 10.1038/352241a0 [DOI] [PubMed] [Google Scholar]
- 154. O'Donnell JB Jr Hill JL Gross DJ Epidermal growth factor activates cytosolic [Ca2+] elevations and subsequent membrane permeabilization in mouse cumulus‐oocyte complexes. Reproduction. 2004;127:207–220. doi: 10.1530/rep.1.00027. 10.1530/rep.1.00027 [DOI] [PubMed] [Google Scholar]
- 155. Kawashima I Liu Z Mullany LK Mihara T Richards JS Shimada M EGF‐like factors induce expansion of the cumulus cell‐oocyte complexes by activating calpain‐mediated cell movement Endocrinology 2012. 153 3949 3959340434210.1210/en.2012‐1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Czech MP PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. 10.1016/S0092‐8674(00)80696‐0 [DOI] [PubMed] [Google Scholar]


