Abstract
The number of ovulated oocytes is different among mammals but does not vary much within the same species. In order to sustain periodic ovulation, follicular development must be coordinated at the tissue level. Elucidating the regulatory mechanisms of follicular development is difficult because the ovary has a complicated structure and it takes a long time for primordial follicles to develop into Graafian follicles. Therefore, it is not possible to observe follicular development by conventional experiments. The authors previously developed a new ovarian tissue culture method that enabled the observation of follicular development from the early follicle stage. These findings indicated that follicular interactions are important in regulating follicular development and ovulation. This review describes the current methods of observing follicular development in the ovary and the regulatory mechanisms of follicular development.
Keywords: follicle culture, follicular development, ovarian tissue culture, time‐lapse image, ultrasonography
1. Introduction
Follicle development and ovulation are controlled by follicle‐stimulating hormone (FSH) and luteinizing hormone (LH), which are secreted from the pituitary gland. The secretion of FSH and LH is controlled by estradiol, progesterone, and inhibin, which are produced by the ovary. Therefore, communication between the hypothalamus, pituitary gland, and ovary is important for periodic ovulation.1, 2, 3, 4, 5, 6 In some mammals, inhibin regulates follicular development and the number of oocytes that are released during ovulation.7, 8, 9, 10, 11 The inhibin that is secreted from growing follicles suppresses the secretion of FSH from the pituitary gland and a dominant follicle develops.12 In humans, there are two types of inhibin: inhibin A is secreted from the corpus luteum and inhibin B is secreted from the follicles. Changes in inhibin B serum concentrations are similar in humans and other mammals.8 The neutralization of inhibin increases the number of growing follicles and ovulated oocytes but does not affect ovulation.13, 14, 15 This indicates that inhibin controls the number of dormant follicles in different species. However, the mechanisms of dormant follicular selection remain to be elucidated. In addition, they are not known how the number of follicles is regulated during each wave of development and how each wave of follicular development is initiated during the estrus cycle.
In order to answer these questions, the dynamics of follicular development must be observed in the ovary. Ultrasonography has been used to observe follicular development in the ovary of domestic animals.12, 16, 17, 18 Using this approach, it was possible to visualize follicular growth and measure the follicle sizes. However, it was not possible to observe smaller follicles with this method, including primordial, primary, and early secondary follicles. During these follicular stages, growth does not depend on gonadotrophin and is controlled by other regulatory mechanisms in the ovary. In order to define these mechanisms, new methods are required to observe the dynamics of follicular development. This review describes the current methods of observing the dynamics and regulatory mechanisms of follicular development in the ovary.
2. Methods of Observing theDynamics and Regulatory Mechanisms of Follicular Development in the Ovary
2.1. Ultrasonography
Ultrasonography is both a diagnostic and a research tool. It was first used to study domestic animal reproduction in pregnant sheep.19 Since then, ultrasonography has been used in various fields of reproductive research, including follicular dynamics, ovulation, corpus luteum development, uterine monitoring, and fetal imaging.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 Tracing follicular development is important in order to elucidate regulatory mechanisms. However, it is difficult to trace the dynamics of a single follicle in the ovary of a live animal. Ultrasonography has been used to observe follicular development in the ovary of domestic animals. These studies revealed a periodic wave of development in each estrus cycle, during which a group of recruited follicles grew.16, 17, 18, 25, 37, 38, 39, 40, 41 A different number of developmental waves was detected in different animals: two waves were observed in cows and three waves in heifers.24, 25 These developmental waves consisted of three events: the recruitment of growing follicles, selection of dominant follicles, and ovulation.12 The waves of follicular development are initiated by gonadotropin.42 The number of follicles per developmental wave is different among species: >50 in pigs, 5‐10 in cattle, and 1‐4 in horses. From the recruited follicles, one dominant follicle is selected in cattle and horses, while 12 dominant follicles are selected in pigs. Generally, the follicular size is considered to be an important parameter for the selection of a dormant follicle; however, the mechanism of selection has not been fully defined.42 There are two possible mechanisms: (1) sensitivity to FSH selects the dominant follicle; and (2) the largest follicles inhibit the development of other follicles by an unknown factor. The selected dominant follicles then grow and mature, while the other follicles regress. During this phase, new follicles are not recruited.39 Follicular dynamics can be observed during these stages by ultrasonography.
In order to understand the mechanisms that sustain periodic ovulation, it is important to observe the successive waves of follicular development and to analyze the mechanisms that control developmental waves in the ovary. The dynamics of follicular development in the ovary of cattle have been traced by ultrasound examination.43 The follicles were traced by the sectional method in order to observe the development of single follicles. Follicles with a diameter of >4 mm were identified, but follicles with a diameter of 1‐3 mm could not be monitored for long periods because they grew and changed position. In order to monitor these follicles, it was necessary to change the plane of ultrasound imaging. To trace the small follicles, the researchers generated sectional maps of the ovary and compared the images that had been recorded by ultrasound. With this method, they were able to trace the development of specific follicles over a long period. They found that the dominant follicle could be identified during the early antral follicular stage when the diameter was 1 mm. This was earlier than previous reports and demonstrated that the future dominant follicle was already larger than the other follicles and already might be selected in the early antral follicular stage.44, 45, 46 These findings demonstrated the importance of monitoring the dynamics of follicular development in the ovary in order to elucidate how ovulation is coordinated.
2.2. New method to trace follicular development in the mouse ovary
Ultrasonography can show the development of antral follicles in the ovaries of live animals; however, it is difficult to reliably observe primordial, primary, and secondary follicles because they are smaller (<1 mm). Observing these follicles is important in order to understand their development; thus, the authors developed a new method of mouse ovarian tissue culture and time‐lapse analysis (Fig. 1).47 Under these culture conditions, follicular development was observed from the primordial (or primary) to the antral follicular stages. In addition, ovulation and follicular atresia were observed (Fig. 2). In order to analyze follicular development in the cultured ovary, the follicular area was measured in time‐lapse images at 24 hours intervals with ImageJ (National Institutes of Health, Maryland, USA) and the follicular stage was identified based on the area (Fig. 3). In the bright field image, it was difficult to distinguish the primordial and primary follicles, so these follicles were described as primordial‐to‐primary. The follicles in the cultured ovary were classified into three groups: primordial‐to‐primary, secondary, and antral follicles.47 Time‐lapse images of the cultured ovarian tissues were captured at 30 minutes intervals for 4 weeks. The area of each follicle was measured to trace the follicular development. These experiments showed that the follicles grew in a developmental wave during the same period between the LH surge as one cohort and made a wave of follicular development in the tissue in the cultured mouse ovary (Fig. 3), which has not been demonstrated before in rodents. Rodents have a short estrus cycle and can be manipulated genetically, which makes them a better model than domestic animals for studying follicular development. However, rodents are too small to study follicular development by ultrasonography. Therefore, the ovarian culture and time‐lapse analysis methods have made it possible to study follicular development in the mouse. The physiology of the mouse ovary was reproduced in these cultures.
Figure 1.
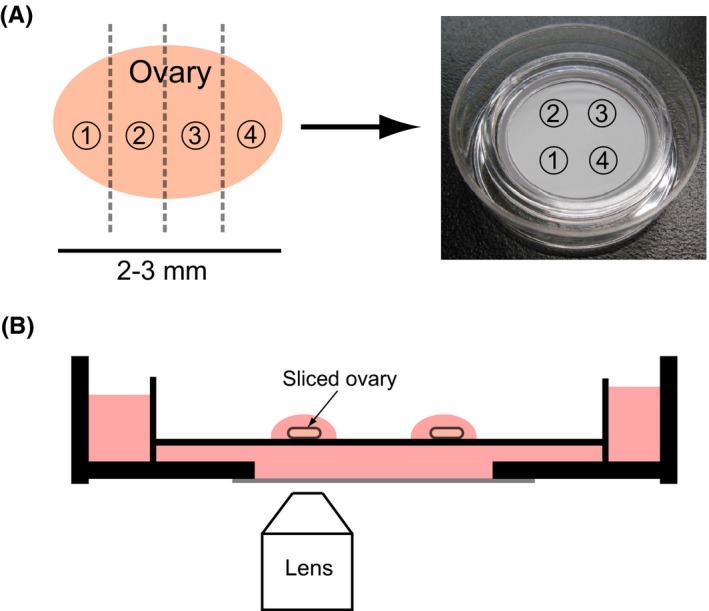
Ovarian tissue culture method. A, Slices of ovarian tissue from 4 week old mice were cultured in culture inserts. B, Time‐lapse images of cultured ovarian tissue were captured by using confocal microscopy [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
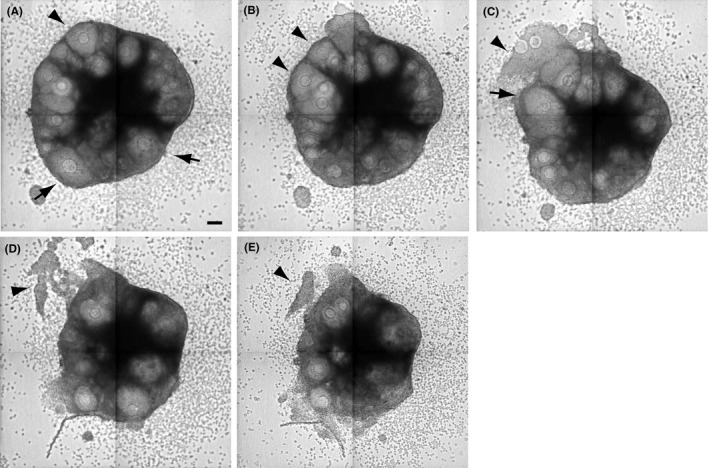
Time‐lapse imaging of ovarian cultures. The culture time for each image is (A) 151 hours, (B) 169 hours, (C) 209 hours, (D) 277 hours, and (E) 325 hours. The arrowheads in (A) and (B) indicate the follicles that released oocytes after several hours and the arrowheads in (C–E) indicate the granulosa cells that returned to their original position after ovulation. The arrows indicate the regressed follicles. Scale bar = 100 μm
Figure 3.
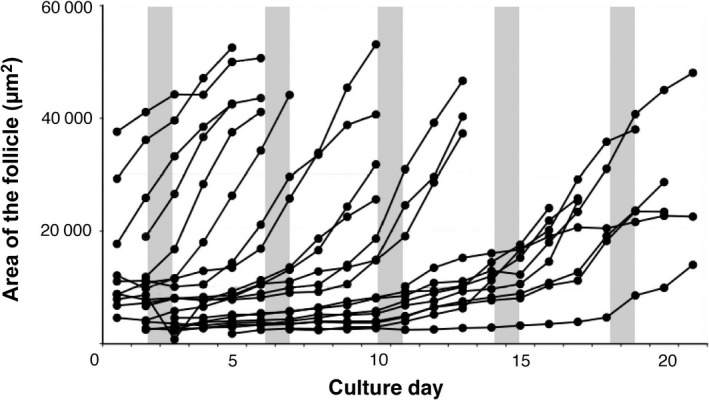
Changes in the follicular area in the cultured ovarian tissue, which was measured in time‐lapse images at 24 hours intervals. The gray lines indicates the period of luteinizing hormone (LH) surges. This graph presents the follicular area measurements from one ovarian tissue slice
2.3. Follicular development in mouse ovarian cultures
Ovarian tissue is dynamic during the estrus cycle. Follicular development and ovulation are followed by formation of the corpus luteum.48, 49, 50 Follicular development, ovulation, and follicular atresia were observed in the ovarian cultures.47 After ovulation, the granulosa cells that were released from the ovulated follicle into the culture medium returned to their original position. However, the corpus luteum was not formed (Fig. 2).
In order to trace the development of single follicles in the cultured tissue, the follicular area was measured.47 These analyses revealed waves of follicular development in the cultured ovarian tissues, which synchronized with the tissue pieces from the same ovary (Fig. 3). The FSH and LH regulate follicular development after the antral follicular stage and their concentration in the blood changes during the estrus cycle.51, 52, 53 However, the concentration of FSH did not change under the culture conditions. Therefore, the authors’ observations can be explained in two ways: (1) a LH surge coordinated the developmental waves in the cultured ovarian tissue; or (2) the biological rhythm (e.g. the circadian clock) controlled the developmental wave and this mechanism was inherent to each cultured ovarian tissue or follicle.54, 55, 56, 57, 58, 59 The timing of ovulation and the number of released oocytes were different in the ovaries from different mice. However, there were few differences between the right and left ovaries from the same mouse. Therefore, the authors believe that the inherent biological rhythm of each mouse was sustained under the culture conditions and was able to influence follicular growth and ovulation. Previous reports have shown that LH controls the expression of core oscillator elements that control the circadian clock. Therefore, the inherent biological rhythm of individual ovaries might synchronize in culture.47, 54
2.4. Interaction of follicles in the ovary to coordinate follicular development
Many factors control follicular development and these have been extensively reviewed.60, 61, 62, 63, 64, 65, 66, 67, 68 This review focuses on follicular interactions in the ovary. Studying individual follicles is important in investigating follicular development and many researchers have cultured isolated follicles to elucidate the regulatory mechanism of follicular development.69, 70, 71, 72, 73, 74, 75, 76 These studies revealed interactions among oocytes, granulosa cells, and theca cells and the factors that are produced by these cells.61, 65, 66 These results described the regulatory mechanisms of follicular development within a follicle, but were not able to determine the mechanism in the ovary.
In one study, co‐cultures of two mouse ovarian follicles were prepared in order to determine how the dominant follicle was selected.77 Two growing follicles were co‐cultured with or without physical contact. Only one of the two follicles with physical contact developed into antral follicles, while the growth of the other was suppressed. However, the two follicles without physical contact developed similarly. This indicated that contact between two follicles is important for selection of the dominant follicle. The ovary contains many follicles at various developmental stages; therefore, various regulatory mechanisms in addition to physical contact mediate follicular interactions. A regulatory mechanism was reported for primordial follicular development.78 The focus was on the position of primordial and growing follicles and analyzing the positioning of the follicles within the ovary. The number of growing follicles that were surrounded by other primordial follicles or that were located near the edge of the ovarian surface was lower than at other locations. The researchers concluded that the primordial follicles and the ovarian epithelium inhibited the development of primordial follicles nearby. It also was reported that a distance of 10‐20 μm was most effective for the inhibitory effect. These findings indicated that an inhibitory factor might be released from the primordial follicles and ovarian epithelial cells. However, the exact mechanisms remain undefined. This approach investigated systematic mechanisms in an ovary that contained follicles at various stages of development, stromal cells, ovarian epithelial cells, and others. However, only the early postnatal ovaries were used; therefore, it is not clear whether the identified mechanisms play a role during reproduction. The structure of the postnatal ovary is transient. In adult mice, the follicles mainly grow near the ovarian surface. In early postnatal mice, the follicles start to grow in the inner part of the cortex.79 Therefore, more primordial follicles accumulate near the ovarian epithelium in early postnatal mice, compared with adult mice. In addition to the inhibitory mechanisms of the primordial follicles and ovarian epithelial cells, there could be other regulatory mechanisms in the ovary of pubertal mice because the follicles are distributed differently than in adult mice. Furthermore, in adult mice, the corpus luteum and vascular plexus develop, which could affect follicular development.
Controlling follicular growth is important for continuous and periodic ovulation. Growth differentiation factor‐9 is expressed in oocytes from the primary follicular stage and stimulates granulosa cell proliferation and androgen production by theca cells.80, 81, 82 Bone morphogenetic protein‐4 and protein‐7 are present in the theca cells of the primary and secondary follicles and stimulate the transition from the primordial to the primary follicle.83, 84 Basic fibroblast growth factor is expressed in the oocytes of primordial and primary follicles in some species and promotes primordial follicular development.85, 86, 87 These factors also can influence the neighboring follicles by diffusion. The authors previously showed that leukemia inhibitory factor (LIF) diffused from the follicles where it was produced and repressed the growth of the neighboring primary, secondary, and antral follicles.47 In this experiment, recombinant LIF and a neutralizing anti‐LIF antibody were added to the medium of ovarian tissue cultures. Neutralizing anti‐LIF antibody promoted the growth of secondary and antral follicles. If LIF were to act only in the follicle where it was produced, then the antibody would not be able to neutralize LIF because the antibody would be too large to enter the follicle. These results indicated that LIF was released from the follicle into the culture medium and repressed the growth of the surrounding follicles. It is likely that other factors are released from the follicle and the concentration gradient of these factors could change during ovulation, follicular atresia, and the formation of the corpus luteum. These changes might control follicular growth at various stages of development. In cultured ovarian tissue, the growth of multiple follicles was observed by time‐lapse imaging. With this approach, it is possible to analyze the regulatory mechanisms of follicular development.
3. Conclusion
Leukemia inhibitory factor has been shown to promote the transition of primordial follicles to primary follicles. This was observed in the ovarian cultures of postnatal day 4 rats.88 After 2 weeks in culture, recombinant LIF and neutralizing anti‐LIF antibody were added to the medium and the numbers of primordial and growing follicles were counted. There were mainly primordial and primary follicles; therefore, it was difficult to elucidate the effect of LIF on the other stages of follicular development. The development of primordial, primary, secondary, and antral follicles must be coordinated in the ovary in order to ensure a supply of oocytes for ovulation. Therefore, it is necessary to observe the development of individual follicles simultaneously in order to elucidate the regulatory mechanism of follicular development in the ovary.
Ovarian physiology is complicated and it takes a long time for primordial follicles to become Graafian follicles. Therefore, it is difficult to determine the regulatory mechanisms of follicular development. The methods to observe follicular development in the ovary need to be improved in the future in order to enhance reproductive techniques.
Disclosures
Conflict of interest: The authors declare no conflict of interest. Human rights statement and informed consent: This article does not contain any study with human participants. Animal rights: All the institutional and national guidelines for the care and use of animals were followed.
Acknowledgements
The authors thank Satoru Masubuchi and Akira Iwase for their support to write this review. The authors also would like to thank Enago (www.enago.jp) for the English‐language review.
Komatsu, K. and Masubuchi, S. (2017), Observation of the dynamics of follicular development in the ovary. Reproductive Medicine and Biology, 16: 21–27. doi: 10.1002/rmb2.12010.
References
- 1. Kaneko H, Watanabe G, Taya K, Sasamoto S. Changes in peripheral levels of bioactive and immunoreactive inhibin, estradiol‐17 beta, progesterone, luteinizing hormone, and follicle‐stimulating hormone associated with follicular development in cows induced to superovulate with equine chorionic gonadotropin. Biol Reprod. 1992;47:76–82. [DOI] [PubMed] [Google Scholar]
- 2. Kishi H, Taya K, Watanabe G, Sasamoto S. Follicular dynamics and secretion of inhibin and oestradiol‐17 beta during the oestrous cycle of the hamster. J Endocrinol. 1995;146:169–176. [DOI] [PubMed] [Google Scholar]
- 3. Knight PG. Roles of inhibins, activins, and follistatin in the female reproductive system. Front Neuroendocrinol. 1996;17:476–509. [DOI] [PubMed] [Google Scholar]
- 4. Kishi H, Itoh M, Ohshima K, Wang MW, Watanabe G, Taya K. Regulations of gonadotropin secretion by circulating inhibin, estradiol, and progesterone in cyclic hamsters. Am J Physiol. 1999;277:E876–E882. [DOI] [PubMed] [Google Scholar]
- 5. Muttukrishna S, Tannetta D, Groome N, Sargent I. Activin and follistatin in female reproduction. Mol Cell Endocrinol. 2004;225:45–56. [DOI] [PubMed] [Google Scholar]
- 6. Roa J, Tena‐Sempere M. KiSS‐1 system and reproduction: comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol. 2007;153:132–140. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe G, Taya K, Sasamoto S. Dynamics of ovarian inhibin secretion during the oestrous cycle of the rat. J Endocrinol. 1990;126:151–157. [DOI] [PubMed] [Google Scholar]
- 8. Groome NP, Illingworth PJ, O'Brien M, et al. Measurement of dimeric inhibin B throughout the human menstrual cycle. J Clin Endocrinol Metab. 1996;81:1401–1405. [DOI] [PubMed] [Google Scholar]
- 9. Ohshima K, Kishi H, Itoh M, et al. Secretion of inhibin A, inhibin B and inhibin pro‐alphaC during the oestrous cycle of the golden hamster (Mesocricetus auratus). J Endocrinol. 1999;162:451–456. [DOI] [PubMed] [Google Scholar]
- 10. Shi F, Ozawa M, Komura H, et al. Secretion of ovarian inhibin and its physiologic roles in the regulation of follicle‐stimulating hormone secretion during the estrous cycle of the female guinea pig. Biol Reprod. 1999;60:78–84. [DOI] [PubMed] [Google Scholar]
- 11. Medan MS, Watanabe G, Sasaki K, Sharawy S, Groome NP, Taya K. Ovarian dynamics and their associations with peripheral concentrations of gonadotropins, ovarian steroids, and inhibin during the estrous cycle in goats. Biol Reprod. 2003;69:57–63. [DOI] [PubMed] [Google Scholar]
- 12. Driancourt MA. Regulation of ovarian follicular dynamics in farm animals. Implications for manipulation of reproduction. Theriogenology. 2001;55:1211–1239. [DOI] [PubMed] [Google Scholar]
- 13. Kaneko H, Nakanishi Y, Akagi S, et al. Immunoneutralization of inhibin and estradiol during the follicular phase of the estrous cycle in cows. Biol Reprod. 1995;53:931–939. [DOI] [PubMed] [Google Scholar]
- 14. Kishi H, Okada T, Kawazu S, et al. Effects of passive immunization against oestradiol‐17β and inhibin on the secretion of gonadotrophin in the cyclic golden hamster (Mesocricetus auratus). Reprod Fertil Dev. 1997;9:447–453. [DOI] [PubMed] [Google Scholar]
- 15. Takedomi T, Kaneko H, Aoyagi Y, et al. Effects of passive immunization against inhibin on ovulation rate and embryo recovery in holstein heifers. Theriogenology. 1997;47:1507–1518. [DOI] [PubMed] [Google Scholar]
- 16. Pierson RA, Ginther OJ. Ultrasonography of the bovine ovary. Theriogenology. 1984;21:495–504. [DOI] [PubMed] [Google Scholar]
- 17. Schrick FN, Surface RA, Pritchard JY, Dailey RA, Townsend EC, Inskeep EK. Ovarian structures during the estrous cycle and early pregnancy in ewes. Biol Reprod. 1993;49:1133–1140. [DOI] [PubMed] [Google Scholar]
- 18. Ginther OJ, Kot K. Follicular dynamics during the ovulatory season in goats. Theriogenology. 1994;42:987–1001. [DOI] [PubMed] [Google Scholar]
- 19. Lindahl IL. Detection of pregnancy in sheep by means of ultrasound. Nature. 1966;212:642–643. [DOI] [PubMed] [Google Scholar]
- 20. Taverne MA, Szenci O, Szetag J, Piros A. Pregnancy diagnosis in cows with linear‐array real‐time ultrasound scanning: a preliminary note. Vet Q. 1985;7:264–270. [DOI] [PubMed] [Google Scholar]
- 21. Guilbault LA, Dufour JJ, Thatcher WW, Drost M, Haibel GK. Ovarian follicular development during early pregnancy in cattle. J Reprod Fertil. 1986;78:127–135. [DOI] [PubMed] [Google Scholar]
- 22. Pierson RA, Ginther OJ. Ovarian follicular populations during early pregnancy in heifers. Theriogenology. 1986;26:649–659. [DOI] [PubMed] [Google Scholar]
- 23. Okano A, Tomizuka T. Ultrasonic observation of postpartum uterine involution in the cow. Theriogenology. 1987;27:369–376. [DOI] [PubMed] [Google Scholar]
- 24. Pierson RA, Ginther OJ. Ultrasonographic appearance of the bovine uterus during the estrous cycle. J Am Vet Med Assoc. 1987;190:995–1001. [PubMed] [Google Scholar]
- 25. Sirois J, Fortune JE. Ovarian follicular dynamics during the estrous cycle in heifers monitored by real‐time ultrasonography. Biol Reprod. 1988;39:308–317. [DOI] [PubMed] [Google Scholar]
- 26. Grasso F, Guilbault LA, Roy GL, Lussier JG. Ultrasonographic determination of ovarian follicular. Development in superovulated heifers pretreated with FSH‐P at the beginning of the estrous cycle. Theriogenology. 1989;31:1209–1220. [DOI] [PubMed] [Google Scholar]
- 27. Kahn W. Sonographic fetometry in the bovine. Theriogenology. 1989;31:1105–1121. [DOI] [PubMed] [Google Scholar]
- 28. Kastelic JP, Ginther OJ. Fate of conceptus and corpus luteum after induced embryonic loss in heifers. J Am Vet Med Assoc. 1989;194:922–928. [PubMed] [Google Scholar]
- 29. Rajamahendran R, Robinson J, Desbottes S, Walton JS. Temporal relationships among estrus, body temperature, milk yield, progesterone and luteinizing hormone levels, and ovulation in dairy cows. Theriogenology. 1989;31:1173–1182. [DOI] [PubMed] [Google Scholar]
- 30. Carroll DJ, Pierson RA, Hauser ER, Grummer RR, Combs DK. Variability of ovarian structures and plasma progesterone profiles in dairy cows with ovarian cysts. Theriogenology. 1990;34:349–370. [DOI] [PubMed] [Google Scholar]
- 31. Farin PW, Youngquist RS, Parfet JR, Garverick HA. Diagnosis of luteal and follicular ovarian cysts in dairy cows by sector scan ultrasonography. Theriogenology. 1990;34:633–642. [DOI] [PubMed] [Google Scholar]
- 32. Kahn W. Sonographic imaging of the bovine fetus. Theriogenology. 1990;33:385–396. [DOI] [PubMed] [Google Scholar]
- 33. Pieterse MC, Taverne MA, Kruip TA, Willemse AH. Detection of corpora lutea and follicles in cows: a comparison of transvaginal ultrasonography and rectal palpation. Vet Rec. 1990;126:552–554. [PubMed] [Google Scholar]
- 34. Savio JD, Boland MP, Hynes N, Roche JF. Resumption of follicular activity in the early post‐partum period of dairy cows. J Reprod Fertil. 1990;88:569–579. [DOI] [PubMed] [Google Scholar]
- 35. Rajamahendran R, Taylor C. Follicular dynamics and temporal relationships among body temperature, oestrus, the surge of luteinizing hormone and ovulation in Holstein heifers treated with norgestomet. J Reprod Fertil. 1991;92:461–467. [DOI] [PubMed] [Google Scholar]
- 36. Reis A, Staines ME, Watt RG, Dolman DF, McEvoy TG. Embryo production using defined oocyte maturation and zygote culture media following repeated ovum pick‐up (OPU) from FSH‐stimulated Simmental heifers. Anim Reprod Sci. 2002;72:137–151. [DOI] [PubMed] [Google Scholar]
- 37. diZerega GS, Hodgen GD. Folliculogenesis in the primate ovarian cycle. Endocr Rev. 1981;2:27–49. [DOI] [PubMed] [Google Scholar]
- 38. Ginther OJ, Knopf L, Kastelic JP. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J Reprod Fertil. 1989;87:223–230. [DOI] [PubMed] [Google Scholar]
- 39. Ko JC, Kastelic JP, Del Campo MR, Ginther OJ. Effects of a dominant follicle on ovarian follicular dynamics during the oestrous cycle in heifers. J Reprod Fertil. 1991;91:511–519. [DOI] [PubMed] [Google Scholar]
- 40. Evans AC, Adams GP, Rawlings NC. Follicular and hormonal development in prepubertal heifers from 2 to 36 weeks of age. J Reprod Fertil. 1994;102:463–470. [DOI] [PubMed] [Google Scholar]
- 41. Ahmad N, Townsend EC, Dailey RA, Inskeep EK. Relationships of hormonal patterns and fertility to occurrence of two or three waves of ovarian follicles, before and after breeding, in beef cows and heifers. Anim Reprod Sci. 1997;49:13–28. [DOI] [PubMed] [Google Scholar]
- 42. Ginther OJ, Wiltbank MC, Fricke PM, Gibbons JR, Kot K. Selection of the dominant follicle in cattle. Biol Reprod. 1996;55:1187–1194. [DOI] [PubMed] [Google Scholar]
- 43. Jaiswal RS, Singh J, Adams GP. Developmental pattern of small antral follicles in the bovine ovary. Biol Reprod. 2004;71:1244–1251. [DOI] [PubMed] [Google Scholar]
- 44. Adams GP, Matteri RL, Kastelic JP, Ko JC, Ginther OJ. Association between surges of follicle‐stimulating hormone and the emergence of follicular waves in heifers. J Reprod Fertil. 1992;94:177–188. [DOI] [PubMed] [Google Scholar]
- 45. Adams GP. Comparative patterns of follicle development and selection in ruminants. J Reprod Fertil Suppl. 1999;54:17–32. [PubMed] [Google Scholar]
- 46. Ginther OJ, Bergfelt DR, Kulick LJ, Kot K. Selection of the dominant follicle in cattle: establishment of follicle deviation in less than 8 hours through depression of FSH concentrations. Theriogenology. 1999;52:1079–1093. [DOI] [PubMed] [Google Scholar]
- 47. Komatsu K, Koya T, Wang J, Yamashita M, Kikkawa F, Iwase A. Analysis of the effect of leukemia inhibitory factor on follicular growth in cultured murine ovarian tissue. Biol Reprod. 2015;93:1–8. [DOI] [PubMed] [Google Scholar]
- 48. Smith MF, Ricke WA, Bakke LJ, Dow MP, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol. 2002;191:45–56. [DOI] [PubMed] [Google Scholar]
- 49. Acosta TJ, Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci. 2004;82–83:127–140. [DOI] [PubMed] [Google Scholar]
- 50. Bachelot A, Binart N. Corpus luteum development: lessons from genetic models in mice. Curr Top Dev Biol. 2005;68:49–84. [DOI] [PubMed] [Google Scholar]
- 51. Butcher RL, Collins WE, Fugo NW. Altered secretion of gonadotropins and steroids resulting from delayed ovulation in the rat. Endocrinology. 1975;96:576–586. [DOI] [PubMed] [Google Scholar]
- 52. Meijs‐Roelofs HM, Uilenbroek JT, De Greef WJ, De Jong FH, Kramer P. Gonadotrophin and steroid levels around the time of first ovulation in the rat. J Endocrinol. 1975;67:275–282. [DOI] [PubMed] [Google Scholar]
- 53. Docke F, Rohde W, Stahl F, Smollich A, Dorner G. Serum levels of FSH, LH and estradiol‐17 beta in female rats around the time of puberty onset. Exp Clin Endocrinol. 1984;83:6–13. [DOI] [PubMed] [Google Scholar]
- 54. Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod. 2006;75:624–632. [DOI] [PubMed] [Google Scholar]
- 55. He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol Cell Biochem. 2007;302:111–118. [DOI] [PubMed] [Google Scholar]
- 56. He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. The disruption of circadian clockwork in differentiating cells from rat reproductive tissues as identified by in vitro real‐time monitoring system. J Endocrinol. 2007;193:413–420. [DOI] [PubMed] [Google Scholar]
- 57. Chu G, Yoshida K, Narahara S, et al. Alterations of circadian clockworks during differentiation and apoptosis of rat ovarian cells. Chronobiol Int. 2011;28:477–487. [DOI] [PubMed] [Google Scholar]
- 58. Shimizu T, Hirai Y, Murayama C, Miyamoto A, Miyazaki H, Miyazaki K. Circadian clock genes Per2 and clock regulate steroid production, cell proliferation, and luteinizing hormone receptor transcription in ovarian granulosa cells. Biochem Biophys Res Commun. 2011;412:132–135. [DOI] [PubMed] [Google Scholar]
- 59. Chen H, Zhao L, Chu G, et al. FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43‐dependent pathway. Am J Physiol Endocrinol Metab. 2013;304:E566–E575. [DOI] [PubMed] [Google Scholar]
- 60. Knight PG, Glister C. Local roles of TGF‐beta superfamily members in the control of ovarian follicle development. Anim Reprod Sci. 2003;78:165–183. [DOI] [PubMed] [Google Scholar]
- 61. Knight PG, Glister C. TGF‐beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. [DOI] [PubMed] [Google Scholar]
- 62. Craig J, Orisaka M, Wang H, et al. Gonadotropin and intra‐ovarian signals regulating follicle development and atresia: the delicate balance between life and death. Front Biosci. 2007;12:3628–3639. [DOI] [PubMed] [Google Scholar]
- 63. McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11. [DOI] [PubMed] [Google Scholar]
- 64. Binelli M, Murphy BD. Coordinated regulation of follicle development by germ and somatic cells. Reprod Fertil Dev. 2010;22:1–12. [DOI] [PubMed] [Google Scholar]
- 65. Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–2954. [DOI] [PubMed] [Google Scholar]
- 66. Palma GA, Arganaraz ME, Barrera AD, Rodler D, Mutto AA, Sinowatz F. Biology and biotechnology of follicle development. Sci World J. 2012;2012:938138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Prizant H, Gleicher N, Sen A. Androgen actions in the ovary: balance is key. J Endocrinol. 2014;222:R141–R151. [DOI] [PubMed] [Google Scholar]
- 68. McGee EA, Raj RS. Regulators of ovarian preantral follicle development. Semin Reprod Med. 2015;33:179–184. [DOI] [PubMed] [Google Scholar]
- 69. Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–276. [DOI] [PubMed] [Google Scholar]
- 70. Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. [DOI] [PubMed] [Google Scholar]
- 71. Hartshorne GM. In vitro culture of ovarian follicles. Rev Reprod. 1997;2:94–104. [DOI] [PubMed] [Google Scholar]
- 72. Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction. 2002;123:185–202. [DOI] [PubMed] [Google Scholar]
- 73. Desai N, Alex A, AbdelHafez F, et al. Three‐dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 2010;8:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brito IR, Lima IM, Xu M, Shea LD, Woodruff TK, Figueiredo JR. Three‐dimensional systems for in vitro follicular culture: overview of alginate‐based matrices. Reprod Fertil Dev. 2014;26:915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zuccotti M, Merico V, Rebuzzini P, et al. 3D culture of ovarian follicles: a system towards their engineering? Int J Dev Biol. 2015;59:211–216. [DOI] [PubMed] [Google Scholar]
- 76. Shiomi‐Sugaya N, Komatsu K, Wang J, Yamashita M, Kikkawa F, Iwase A. Regulation of secondary follicle growth by theca cells and insulin‐like growth factor 1. J Reprod Dev. 2015;61:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Spears N, de Bruin JP, Gosden RG. The establishment of follicular dominance in co‐cultured mouse ovarian follicles. J Reprod Fertil. 1996;106:1–6. [DOI] [PubMed] [Google Scholar]
- 78. Da Silva‐Buttkus P, Marcelli G, Franks S, Stark J, Hardy K. Inferring biological mechanisms from spatial analysis: prediction of a local inhibitor in the ovary. Proc Natl Acad Sci USA. 2009;106:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Byskov AG, Guoliang X, Andersen CY. The cortex‐medulla oocyte growth pattern is organized during fetal life: an in‐vitro study of the mouse ovary. Mol Hum Reprod. 1997;3:795–800. [DOI] [PubMed] [Google Scholar]
- 80. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor‐9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. [DOI] [PubMed] [Google Scholar]
- 81. Nilsson EE, Skinner MK. Growth and differentiation factor‐9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod. 2002;67:1018–1024. [DOI] [PubMed] [Google Scholar]
- 82. Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte–granulosa–theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein‐7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994–999. [DOI] [PubMed] [Google Scholar]
- 84. Nilsson EE, Skinner MK. Bone morphogenetic protein‐4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod. 2003;69:1265–1272. [DOI] [PubMed] [Google Scholar]
- 85. van Wezel IL, Umapathysivam K, Tilley WD, Rodgers RJ. Immunohistochemical localization of basic fibroblast growth factor in bovine ovarian follicles. Mol Cell Endocrinol. 1995;115:133–140. [DOI] [PubMed] [Google Scholar]
- 86. Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. [DOI] [PubMed] [Google Scholar]
- 87. Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. [DOI] [PubMed] [Google Scholar]
- 88. Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188:65–73. [DOI] [PubMed] [Google Scholar]


