Abstract
Aim
One of the parameters that greatly affects homeostasis in the body is the pH. Regarding reproductive biology, germ cells, such as oocytes or sperm, are exposed to severe changes in pH, resulting in dramatic changes in their characteristics. To date, the effect of the pH has not been investigated regarding the reprogramming of somatic cells and the maintenance and differentiation of pluripotent stem cells.
Methods
In order to investigate the effects of the pH on cell culture, the methods to produce induced pluripotent stem cells (iPSCs) and to differentiate embryonic stem cells (ESCs) into mesendoderm and neuroectoderm were performed at each medium pH from 6.6 to 7.8. Using the cells of the Oct4‐GFP (green fluorescent protein) carrying mouse, the effects of pH changes were examined on the timing and colony formation at cell reprogramming and on the cell morphology and direction of the differentiation of the ESCs.
Results
The colony formation rate and timing of the reprogramming of the somatic cells varied depending on the pH of the culture medium. In addition, mesendodermal differentiation of the mouse ESCs was enhanced at the high pH level of 7.8.
Conclusion
These results suggest that the pH in the culture medium is one of the key factors in the induction of the reprogramming of somatic cells and in the differentiation of pluripotent stem cells.
Keywords: cell differentiation, embryonic stem cells, induced pluripotent stem cells, pH, reprogramming
1. Introduction
The pH is one of the important parameters in life, specifying the acidity or basicity of an aqueous solution. Variations in the pH influence every biological process at the cellular, tissue, and whole‐body level.1 In reproductive processes, the vaginal pH in women is normally maintained at a pH that ranges between 4.0 and 5.0.2 The semen in men is maintained normally at a pH of >8.0.3 After increasing the vaginal pH within a few seconds by ejaculation,4 the pH recovers to being fairly acidic during pregnancy.5 At the cellular level, an acrosomal reaction results in an increase of the internal pH of sperm at fertilization.6 The intracellular pH during oogenesis and embryogenesis varies at each developmental stage.7, 8 Thus, germ cells and embryos are exposed to pH fluctuations, with dramatic changes in their traits during the development of individuals.9 Regarding the processes of cell differentiation and cellular reprogramming, there is little information concerning the influence of the pH on these phenomena.
The pH affects many molecular mechanisms inside and outside of cells in order to maintain homeostasis. The proton gradient in cells is maintained by pumps and channels, such as Na+/H+ exchangers, HCO3 −/Cl− exchangers, V‐type H+ pumps and voltage‐gated H+ channel on the plasma membrane.1, 10 Active or passive changes by the pH affect cell traits, such as the motility, enzymatic activity, cell cycle, and apoptosis.1, 11 Cell motility is also caused by the constitutional change of the cytoskeleton, which is affected by the environmental pH.12 Recent studies also indicate that actin proteins are essential in transcriptional activation during the differentiation and reprogramming of cells.13, 14 However, the effects of the phenomena on somatic cell reprogramming that are caused by the pH in culture have not been investigated. Regarding the effects of the pH on cell differentiation, mesenchymal stem cells are affected by the pH during cell differentiation into osteogenic and chondrogenic cell lineages.15 Also, the high pH in murine embryonic stem cell (ESC) culture is known to enhance cardiac cell differentiation.16 However, the effect of the pH on the differentiation of ESCs has not been well investigated for a wide range of pH values.
Distinctive changes in the molecular activity of cells can be observed in the processes of cell differentiation and the reprogramming of somatic cells.17 During the course of the reprogramming of fibroblasts, the appearance of cells changes from mesenchymal to epithelial18 and the reverse phenomena of the differentiation of stem cells with major changes in the epigenetic state also occur.19 In this article, the effects of the pH were examined during cellular reprogramming and differentiation by using mouse embryonic fibroblasts (MEFs) and ESCs from transgenic mice that carried the Oct4‐green fluorescent protein (GFP) reporter in vitro: these mice are well suited to estimate pluripotency.20, 21 Cells that have been cultured in media at various pH levels then are observed at the colony formation, at the timing of the reprogramming of the MEFs, and at the differentiation of the ESCs to the mesendoderm (ME) and neuroectoderm (NE).
2. Materials and Methods
2.1. Chemicals and animals
Unless otherwise noted, all of the chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA). The MEFs and ESCs were derived from transgenic mice that carried the Oct4‐GFP reporter (RIKEN BioResource Center, Ibaraki, Japan)22 and were used in experiments for the reprogramming of somatic cells and differentiation. The MEFs and ESCs that originated from the transgenic mice were obtained and treated as described previously.23
For the preparation of the MEFs, embryos were collected at embryonic days 13.5‐15.5, as described previously.23 The isolated embryonic cells were maintained in Dulbecco's modified Eagle medium (DMEM; GIBCO Life Technologies, Grand Island, NY, USA) that contained 10% (v/v) fetal bovine serum (FBS; SAFC Biosciences, Lenexa, KS, USA), 73 IU/mL penicillin (Sigma–Aldrich), and 50 μg/mL streptomycin (Sigma–Aldrich). The fibroblasts with four passages or less were used for the reprogramming of the somatic cells.
For the maintenance of the ESCs, they were cultured in a medium that was mixed equally with Neurobasal medium (GIBCO Life Technologies) and DMEM/F12 that contained 0.5% (v/v) N2 (GIBCO Life Technologies), 0.5% (v/v) B27 (GIBCO Life Technologies), 1% (v/v) L‐glutamine, penicillin, and streptomycin (GIBCO Life Technologies), 0.05% (w/v) bovine serum albumin (BSA, Sigma–Aldrich), and 0.15 mmol/L 1‐thioglycerol (Sigma–Aldrich) that was supplemented with leukemia inhibitory factor (LIF) (1:1000), 1 μmol/L MEK/ERK inhibitor (PD0325901; REAGENTS DIRECT, Encinitas, CA, USA), and 3 μmol/L GSK3β inhibitor (CHIR99021; REAGENTS DIRECT) on human plasma fibronectin (Millipore, Darmstadt, Germany)‐coated dishes. As a source of the LIF, a LIF‐conditioned medium (1:1000 dilution) from COS‐7 or 293FT cell cultures that had been transduced with a mouse LIF‐encoding vector24, 25 was used. The medium was changed every day and the cells were passaged every 2 days by using TrypLE (GIBCO Life Technologies) and reseeded on 35 mm dishes (IWAKI, Tokyo, Japan) at 1 × 106 cells per dish.
2.2. Retroviral transfection
In order to perform the retroviral transfection, pMXs‐based retroviral vectors (Oct4, Sox2, Klf4, and c‐Myc; Addgene plasmid no. 13366, no. 13367, no. 13370, and no. 13375) and a pCMV‐VSV‐G vector (Addgene plasmid no. 8454) were introduced into Plat‐GP cells (Cell Biolabs, Inc., San Diego, CA, USA)26 by using the lipofectamine LTX transfection reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's recommendations. The medium was changed the following day. After another 24 hours of incubation, virus‐containing supernatants that had been derived from the Plat‐GP cell cultures were filtered through a 0.45 μm cellulose acetate filter (Schleicher & Schuell, Dassel, Germany).
The transfection of the retroviruses into the MEFs was performed on RetroNectin (Takara, Shiga, Japan)‐coated dishes according to the manufacturer's recommendations. The virus that had been recovered from the Plat‐GP culture was added to the RetroNectin‐coated dishes and was incubated at 37°C. After 6 hours of incubation, the dish was washed with phosphate buffered saline (PBS) that contained 0.25% (v/v) BSA. The MEFs that had been cultured in the somatic cell medium were trypsinized and passaged on virus‐containing dishes at a density of 2 × 105 cells per well in the fresh somatic cell medium.
2.3. Adjusting and monitoring the pH in the culture media
Each medium pH condition was controlled by changing the concentration of NaHCO3 (Wako, Osaka, Japan) under 5% (v/v) CO2 according to the Henderson–Hasselbalch equation method that was applied to tissue culture vessels.27 The fresh ESC medium was pre‐incubated for 24 hours in 5% (v/v) CO2 in air and the pH of the medium was measured within 0.1 of standard error against the predicted pH. Each medium pH before and after the medium change was checked by a pH meter (B‐212; HORIBA, Kyoto, Japan). The stale medium was changed immediately to a fresh medium after checking the pH every day (Figs 1A and S1).
Figure 1.
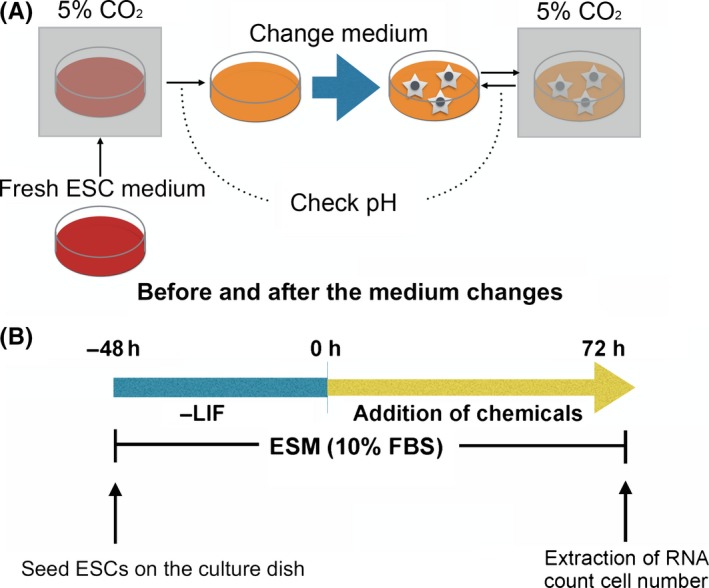
A, Schemes of pH adjustment of the medium and B, differentiation of mouse embryonic stem cells (ESCs). ESM, embryonic stem cell medium; FBS, fetal bovine serum; LIF, leukemia inhibitory factor
2.4. Induction and estimation of the reprogramming of the mouse embryonic fibroblasts
After 4 days of viral transfection, the transfected cells (~1.6‐4.8 × 103 cells/cm2) were reseeded on mitomycin‐treated STO feeders (~2.5 × 104 cells/cm2) (RCB0536; RIKEN BioResource Center) that were cultured in embryonic stem cell medium (ESM); Glasgow minimum essential medium (Sigma–Aldrich) that contained 15% (v/v) Knockout serum replacement (KSR; GIBCO Life Technologies), 0.3% (v/v) FBS (SAFC Biosciences), 2 mmol/L L‐glutamine (MP Biomedicals, Tokyo, Japan), 1 mmol/L sodium pyruvate (Sigma–Aldrich), 1 × MEM non‐essential amino acids (Invitrogen Life Technologies), 0.1 mmol/L 2‐mercaptoethanol (Wako), 73 IU/mL penicillin (Sigma–Aldrich), and 50 μg/mL streptomycin (Sigma–Aldrich) that had been supplemented with LIF. The cultured cells were observed by using an inverted microscope (DIAPHOT 300; Nikon, Tokyo, Japan) and the photographs were acquired by using a COOLPIX P6000 camera (Nikon).
2.5. Estimation of the embryonic stem cell proliferation
The estimation of the cell proliferation of the ESCs in various pH conditions was performed in the ESM that contained 15% KSR with LIF. The ESCs were passaged on STO feeders or gelatin‐coated dishes and were cultured for 3 days and then the cell number in each medium pH was counted.
2.6. Alkaline phosphatase activity in the pluripotent cell culture and immunofluorescence staining
The cells were fixed with 3.7% paraformaldehyde (Wako) in PBS for 10 minutes at room temperature. After washing with PBS three times, the level of alkaline phosphatase (AP) activity was detected by using the Vector Alkaline Phosphatase Substrate kit III (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions.
For the immunofluorescence analysis, cells were fixed with PBS that contained 3.7% paraformaldehyde for 10 minutes at room temperature. After washing with PBS, the cells were blocked with PBS that contained 5% BSA (Sigma–Aldrich) and 0.1% Triton X‐100 (Sigma–Aldrich) for 45 minutes at room temperature. Then, they were incubated overnight at 4°C with a primary antibody T (Brachyury, 1:1000, ab20680; Abcam, Tokyo, Japan). Alexa Fluor 594‐conjugated goat anti‐mouse immunoglobulin G (1:500; Invitrogen Life Technologies) was used as the secondary antibody. The nuclei were stained with 1 μg/mL Hoechst 33342 (Sigma–Aldrich).
2.7. Reverse transcription–polymerase chain reaction
The total RNA was prepared by using the TRIzol reagent (Ambion Life Technologies, Foster City, CA, USA) according to the manufacturer's instructions. DNase (Roche, Indianapolis, IN, USA) was added to the preparations in order to avoid genomic contamination. First‐strand cDNA was synthesized by using reverse transcriptase (ReverTra Ace; Toyobo, Osaka, Japan) and random primers (Invitrogen Life Technologies) according to the manufacturer's instructions. The polymerase chain reaction (PCR) was performed by using ExTaq (Takara) according to the manufacturer's instructions. The transcription levels were normalized by the Gapdh expression level.
For the quantitative PCR (qPCR), THUNDEBIRD SYBR qPCR Mix (Toyobo) was used according to the manufacturer's instructions. The PCR reaction was performed under two‐step cycles: denaturation at 95°C for 3 seconds and annealing and extension at 60°C for 30 seconds. Each expression of the genes was normalized by the expression of Gapdh. The sequences of primers that was used for the experiments are shown in Table 1.
Table 1.
Sequences of the primers for the reverse transcription–polymerase chain reaction
| Gene | Primer sequence | Anealing temperature (°C) | Product size (bp) | Source |
|---|---|---|---|---|
| Mixl1 | 5′‐GCACGTCGTTCAGCTCGGAGC‐3′5′‐AGTCATGCTGGGATCCGGAACGTGG‐3′ | 55 | 305 | Jackson et al. (2010)27 |
| Nestin | 5′‐ GGAGAGTCGCTTAGAGGTGC‐3′5′‐AGGTGCTGGTCCTCTGGTAT‐3′ | 55 | 375 | NM_016701.3 |
| T | 5′‐TGCTGCCTGTGAGTCATAAC‐3′5′ TCCAGGTGCTATATATTGCC‐3′ | 55 | 948 | Jackson et al. (2010)27 |
| Sox2 | 5′‐TAGACTGCACATGGCCCAGCACT‐3′5′‐TTGCCTTAAACAAGACCACGAAA‐3′ | 55 | 778 | NM_011443.3 |
| Oct3/4 | 5′‐AAGTGCCCGAAGCCCTCCCTACAG‐3′5′‐CAGAGGGAAAGGCCTCGCCCTCAG‐3′ | 55 | 289 | NM_001252452.1 |
| Gapdh | 5′‐ACGGCACAGTCAAGGCAGAG‐3′5′‐GTGATGGCGTGGACAGTGGT‐3′ | 55 | 376 | NM_001289726 |
| For real time | ||||
| Mixl1 | 5′‐CAGTTGCTGGAGCTCGTCTT‐3′5′‐TCCGGAACGTGGTTCACATC‐3′ | 60 | 266 | NM_013729.3 |
| Nestin | 5′‐GGGGCTACAGGAGTGGAAAC‐3′5′‐GACCTCTAGGGTTCCCGTCT‐3′ | 60 | 213 | NM_016701.3 |
| T | 5′‐GGCTGGGAGCTCAGTTCTTT‐3′5′‐TGTCCACGAGGCTATGAGGA‐3′ | 60 | 179 | NM_009309.2 |
| Sox2 | 5′‐GATCAGCATGTACCTCCCCG‐3′5′‐TCCTCTTTTTGCACCCCTCC‐3′ | 60 | 212 | NM_011443.3 |
| Oct3/4 | 5′‐CCAATCAGCTTGGGCTAGAG‐3′5′‐CCTGGGAAAGGTGTCCCTGT‐3′ | 60 | 130 | NM_001252452.1 |
| Gapdh | 5′‐CGTGTTCCTACCCCCAATGTG‐3′5′‐TGTCATCATACTTGGCAGGTTTC‐3′ | 60 | 73 | NM_001289726 |
2.8. Induction of the differentiation of the embryonic stem cells in vitro
In order to induce the differentiation of the ESCs, the cells were passaged onto gelatin‐coated 35 mm dishes at a density of 6 × 104 cells per dish in 10% FBS that contained ESM without LIF, PD0325901, and CHIR99021. Two days after culture, the following chemicals were added to each differentiation medium:28 for neuroectodermal differentiation, 500 nmol/L retinoic acid (Sigma–Aldrich) and 5 ng/mL human basic fibroblast growth factor (bFGF; Wako or ReproCELL, Yokohama, Japan); for mesendodermal differentiation, 3 μmol/L CHIR99021 (REAGENTS DIRECT) and 10 ng/mL activin A (R&D Systems, Minneapolis, MN, USA) (Fig. 1b). At the induction of each germ lineage, the pH of the medium was adjusted to a pH of 6.8, 7.4, and 7.8.
2.9. Statistical analysis
The statistical significance of the difference between the sample means was determined by using the Student's t‐test.
3. Results
3.1. Effects of the external pH on colony formation during the reprogramming of the mouse somatic cells
In order to investigate the effects of the pH during the reprogramming of the mouse somatic cells, the Oct4‐GFP‐positive colonies were counted at each medium pH. The pH was checked every day and was confirmed to be within 0.1 of standard error, indicating little change in the pH throughout the culture (Fig. S1).
The GFP‐positive colonies appeared from day 5 to day 10 of culture and were counted at day 17 for each medium pH (Figs 2A, 3A, and S2C). The highest number of GFP‐positive colonies was obtained at a pH of 7.4 (Fig. 2A). No colony, however, was observed at a pH of 6.6. The maximum difference in the colony number at a pH of 6.8 was 15‐fold lower than that at a pH of 7.4. The average colony number at each medium pH was divided by the colony number at a pH of 7.4 and each ratio was found to be significantly different than those at a pH of 7.4. Interestingly, only a 0.2 point difference of pH caused a significant decrease in the colony formation number (Fig. 2A). In order to examine the effects on the established pluripotent stem cells, murine ESCs were cultured at each medium pH from 6.8 to 7.8. After 3 days of culture in each pH, the highest number of cells was obtained at a pH of 7.4 (Fig. 2B) and the lowest number was observed at a pH of 6.8. Between a pH of 7.4 and 7.6, however, there was no significant difference in the cell number.
Figure 2.
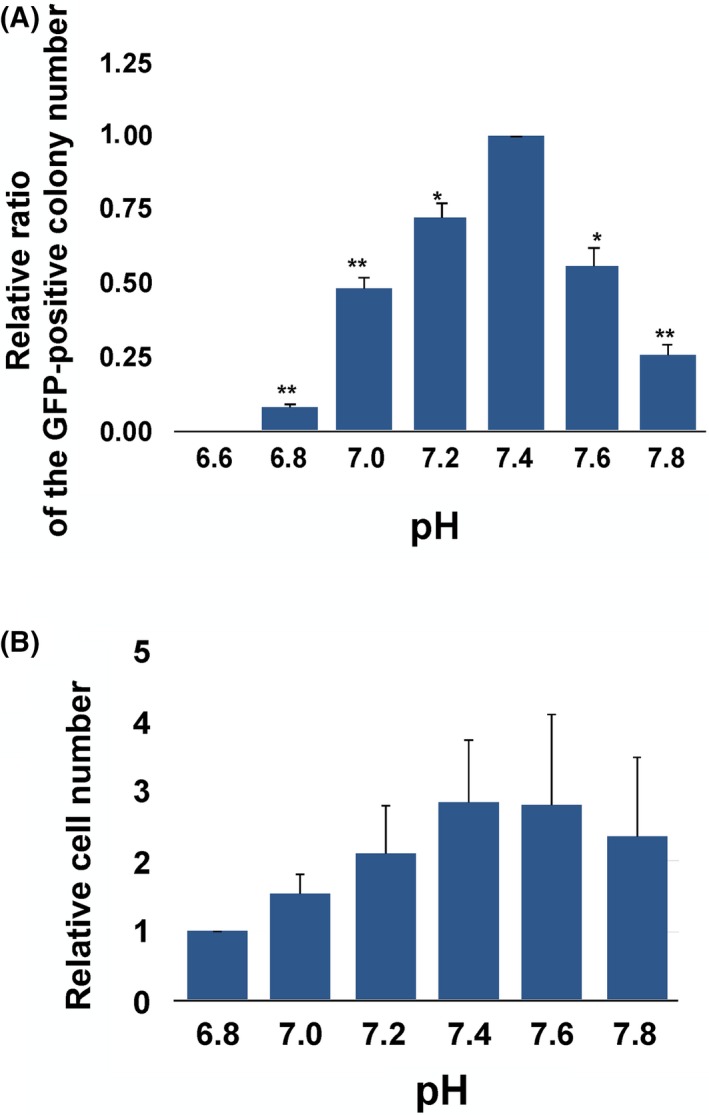
Effects of the pH on the colony formation of the mouse somatic cell reprogramming and the proliferation of the mouse embryonic stem cells (ESCs). A, The relative colony formation rates under different pH conditions. The green fluorescent protein (GFP)‐positive colony number was counted after 17 days of the induction of reprogramming. The relative ratio of the colony in each treatment group was obtained by dividing the colony number in each group by the number at a pH of 7.4. For the statistical analysis, each ratio of the treated group was compared with the ratio at a pH of 7.4. The asterisks indicate significant differences, compared with the relative ratio at a pH of 7.4 (.01<*P<.05 and **P<.01, according to the Student's t‐test). The data are presented as the means±standard error of the mean. The experiments were repeated three times independently. B, The number of cells of each treatment group that was obtained after 4 days of culture was divided by the values that were obtained at a pH of 6.8. The experiment was independently repeated three times
Figure 3.
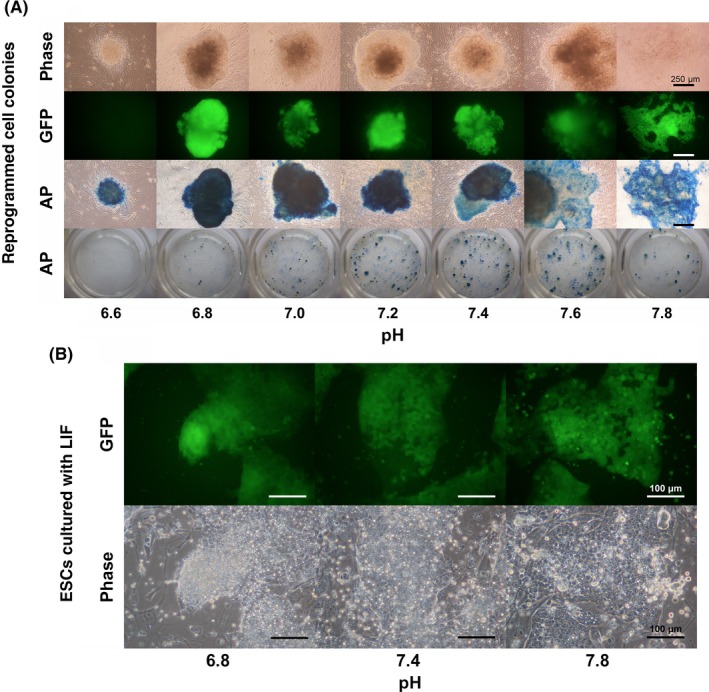
Variation in green fluorescent protein (GFP) expression and alkaline phosphatase (AP) activity in the colonies in the culture media with different pH conditions. A, Phase‐contrast (phase), expression of GFP, and AP activity in each culture dish were examined in the colonies that were cultured under different pH conditions for 17 days for the induction of somatic cell reprogramming in mice. B, The morphology of the colony formation of mouse embryonic stem cells under different pH conditions in the presence of leukemia inhibitory factor (in particular, the GFP signals of the Oct4 reporter and phase‐contrast images) were examined. ESC, embryonic stem cells; LIF, leukemia inhibitory factor
In order to elucidate the effects of the pH on somatic cell reprogramming, the ESCs were replated as single cell at a low cell density (Fig. S3). A similar number of colonies among the various pH ranges then was obtained.
3.2. Effects of the pH on the timing of reprogramming
During the reprogramming of the somatic cells at each medium pH, the number of GFP‐positive colonies was counted every day. The fastest appearance of the GFP‐positive colonies was observed at 5 days of culture at a pH of 7.8, but the latest appearance of the colonies was at 11 days in the culture at a pH of 6.8 (Table 2).
Table 2.
Effect of the pH on the timing of the somatic cell reprogramming
| Trial 1 | Time (day) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 6.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| 7.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 7 | 9 | 9 |
| 7.2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 8 | 10 | 12 | 15 |
| 7.4 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 6 | 8 | 10 | 16 | 18 |
| 7.6 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 3 | 4 | 4 | 6 |
| 7.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 2 | Time (day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 6.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 7 |
| 7.2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | 8 | 10 |
| 7.4 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 6 | 8 | 10 |
| 7.6 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 3 | 4 |
| 7.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 3 | Time (day) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 6.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6.8 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 7.0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 4 | 4 | 6 | 5 |
| 7.2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 8 | 6 | 8 | 7 | 8 |
| 7.4 | 0 | 0 | 0 | 0 | 2 | 1 | 4 | 5 | 4 | 5 | 6 | 10 |
| 7.6 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 5 | 2 | 4 | 5 | 6 |
| 7.8 | 0 | 0 | 0 | 0 | 1 | 3 | 2 | 1 | 1 | 2 | 2 | 1 |
Oct3/4‐green fluorescent protein (GFP)‐positive colonies were counted in each pH condition every day during the culture of the transfected mouse embryonic fibroblasts on the somatic cell reprogramming. The background shading indicates the number of GFP‐positive colonies.
3.3. Effects of the pH on the colony morphology of the induced pluripotent stem cells and the embryonic stem cells
During the somatic cell reprogramming, the morphology of the iPSC colonies differed depending on the pH (Figs 3A, and S2A–C). The colonies that were obtained between a pH of 7.0 and 7.4 had compact morphologies with strong GFP expression and AP activity (Figs 3A and S2A). In contrast, the colonies that were obtained at a pH of 6.6 had a compact morphology with weak GFP expression and AP activity. The colonies that were obtained at a pH of 7.6 and at a pH of 7.8 were dispersed and flat in appearance, compared with the colonies that were obtained in the other pH treatment groups.
When the ESCs were cultured at various pH values, morphological differences in the colonies were observed at each pH (Figs 3B and S3A). The cell distance among the neighbor ESCs in a colony was different depending on the pH. At a pH of 6.8, the cells were located close to each other and the cell boundary was not clear. In contrast, at a pH of 7.8, the cells were more dispersed and could be observed as single cells.
3.4. Effects of the pH on the pluripotency and differentiation of the embryonic stem cells
The ESCs were cultured in the differentiation‐inducing medium, leading to ME in the culture medium at a pH of 6.8, 7.4, and 7.8. For 3 days of culture in the presence of CHIR and activin A, the endodermal markers of Mixl1 and T were expressed in the cells at a pH of 7.4 and 7.8, but not at a pH of 6.8 (Fig. S4A). Nestin expression, which is a neural marker, was slightly expressed at a pH of 6.8 but was not observed at a pH of 7.4 and 7.8. In order to confirm the further effects of the pH on ESC differentiation, the cells were cultured in neural differentiation medium in the presence of bFGF and retinoic acid. For 3 days of culture, Nestin was expressed at a pH of 6.8 and 7.4, but not at a pH of 7.8, in the cells (Fig. S4A). Under the mesendodermal differentiation medium, the qPCR analysis also showed a high expression of mesendodermal differentiation genes in the high pH condition (Fig. 4A).
Figure 4.
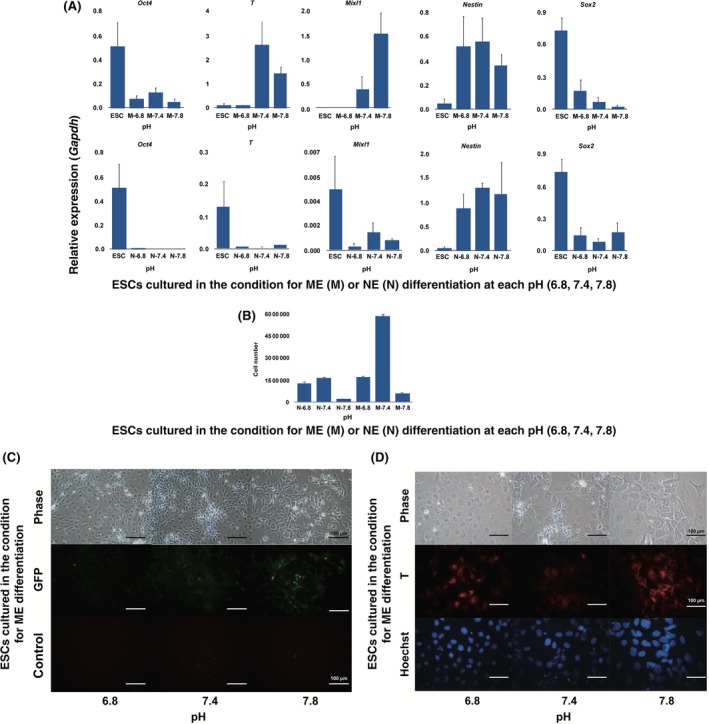
Effects of the pH on the cell differentiation of mouse embryonic stem cells (ESCs). A, Quantitative real‐time polymerase chain reaction analysis of neuroectodermal and mesendodermal gene expression in differentiated ESCs under different pH conditions. The cells were cultured at a pH of 6.8, 7.4, and 7.8. Each expressed gene was normalized by the expression of Gapdh. Y axis means relative expressions normalized by the expression of Gapdh. M, the induction medium for mesendodermal differentiation; N, the induction medium for neuroectodermal differentiation. The experiments were independently performed three times. B, The number of cells in each treatment group at day 3 in each differentiation induction medium. The experiments were independently performed three times. C, Phase‐contrast (phase) and green fluorescent protein (GFP) expression of the microscopic images of the ESCs that were cultured for 3 days in the mesendodermal differentiation induction medium under various pH conditions. The control means that the micrographs were observed with a red filter to eliminate the possibility of self‐fluorescence. D, Immunofluorescent staining of the T protein (T) and DNA (Hoechst) of the ESCs that were cultured for 3 days in the mesendodermal differentiation induction medium under various pH conditions
At the same time, the cell number was estimated: the lowest number was observed at a pH of 6.8 in the mesendodermal differentiation medium, which was less than one‐third of the value at a pH of 7.4 (Fig. 4B). In contrast, in the neural differentiation medium, there was no major difference in the number at a pH of 6.8 and 7.4. The cell number at a pH of 7.8 was the lowest both in the mesendodermal and neuroectodermal differentiation media (Fig. 4B). In a pH of 6.8, each cell was scattered and the Oct4‐GFP expression in the cells was weak for 3 days after the treatment with the chemicals for mesendodermal differentiation (Fig. 4C). In contrast, the Oct4‐GFP expression still remained at a pH of 7.8 (Fig. 4C). Also, the T protein, which is the early mesendodermal marker, was highly expressed at a pH of 7.8 (Fig. 4D).
4. Discussion
The pH is well known to fluctuate significantly in cells and tissues and affects many biological phenomena. In this study, it was found that the fluctuation of pH in culture medium has an effect on the processes that occur during the somatic cell reprogramming and differentiation of ESCs.
The medium that contains NaHCO3 that is stored at 4°C in air usually indicates a higher pH, with a 0.03% CO2 concentration in air, than the predicted pH under 5% CO2 in the incubator at chemical equilibrium. A culture of pluripotent stem cells with high metabolic activity that is dependent on glycolysis results in an immediate decrease in the pH due to the supply of lactic acid to the culture. Therefore, the fresh medium was pre‐incubated and the culture medium was changed every day in the process of somatic cell reprogramming to maintain a stable pH in the culture.
An acidic pH is known to suppress the cell cycle.29 The proliferation of ESCs at a lower pH (6.8‐7.2) was decreased, compared with that at a pH of 7.4, indicating that a lower pH inhibits the cell proliferation of ESCs (Fig. 2B). During somatic cell reprogramming, another 4‐8 days were needed at a pH of 6.8 and 7.0 for the appearance of GFP‐positive cells (Table 2). A high proliferation rate of cells is necessary for the induction of cell reprogramming and the maintenance of pluripotent stem cells.30 This finding was considered to be caused by the acidic pH of the medium. These results indicate that the delay in the appearance of colonies and the lower number of colonies at an acidic pH could be caused by the inhibition of the cell cycle, resulting in a slow or inefficient induction of cell reprogramming.
Morphological differences in the colonies at different pH values were observed during the induction of cell reprogramming and the maintenance of the ESCs, in which the cells were dispersed in morphology at a high pH (7.6‐7.8) and compacted at a low pH (6.6‐7.2) (Figs 3, S2C and S3A). In oligodendrocytic precursor cells, an acidic pH has effects on cell migration.31 Additionally, in cancer cells, migration is affected by the pH, but vesicle trafficking, contraction, invasion, and metastasis also are affected.12 The compacted morphology of the colonies in the acidic pH might be caused by the inhibition of cell migration.
The variation of pH in the medium affected early cell differentiation into ME, which is the precursor of the mesoderm and endoderm, and into NE. Mixl1 and Brachyury (T), which allow cells to differentiate into the mesoderm and endoderm, are observed in the primitive streak of embryos at the gastrula stage.32, 33, 34, 35 It has been known that the Oct4 and Sox2 genes are inducers for mesendodermal and neuroectodermal cell differentiation, respectively, at early ESC differentiation.28 In this experiment, Oct4‐GFP expression at a pH of 7.8 also indicates the direction of cell differentiation to the mesendodermal cells (Fig. 4C). Previously, it was shown that a high pH in culture has effects on cardiac differentiation at a pH of 7.1 and 7.4, rather than at a pH of 6.8.16 In this study, the cells showed a much higher expression of Mixl1 at a pH of 7.8. In addition, under the mesendodermal differentiation condition, Sox2 and Nestin expression were relatively higher at a lower pH. However, under the neuroectodermal differentiation condition, the expression of Nestin was not inhibited in any pH range, compared to mesendodermal differentiation (Fig. 4A). These results indicate that a broad range of pH levels in culture affects cell differentiation, as well as the specific inhibitory effects of a low pH on ESC differentiation into the mesendoderm. The differentiation direction of cells is determined by their environment as to whether to differentiate into the progenitors of ME or NE.28 These results suggest that pluripotent stem cells define the direction of cell differentiation in culture and that the environmental pH is one of the cues that determines the directional property.
The present study indicates that the extracellular pH affects cell reprogramming and cell differentiation. There are many pathways in which the pH affects these processes. For example, a low pH down‐regulates cell proliferation by inducing p53 activation and p53‐dependent cell cycle inhibition.29, 36 Furthermore, the inhibition of p53 supports the establishment of iPSCs.37 Previous articles29, 36, 37 thus have indicated that there is a close relationship between the effects of the pH on cell reprogramming and the cell cycle. In addition, it also should be considered that the effects of the intracellular pH on cell physiology might act through organelles, such as the nucleus, mitochondria, and endoplasmic reticulum, as well as through internal epigenetic regulation.10 Although this study examined the effects of the pH in vitro, fluctuations in pH also are considered to affect cells in vivo.
Disclosure
Conflicts of interest: The authors declare no conflict of interest. Human rights statement and informed consent: This article does not contain any study with human participants that was performed by any of the authors. Animal studies: All the institutional and national guidelines for the care and use of laboratory animals were followed.
Supporting information
Acknowledgements
The materials that were used for the experiments were kindly provided by the following persons: the mouse LIF‐encoding vector came from Takashi Yokota (Kanazawa University, Japan) and Jun‐ichi Miyazaki (Osaka University, Japan); the pMXs vectors came from Shinya Yamanaka (Kyoto University, Japan); and the pCMV‐VSV‐G vector came from Bob Weinberg (Whitehead Institute for Biomedical Research, Cambridge, MA, USA). This research was supported by a grant from the Research Fellowship Program of the Japan Society for the Promotion of Science to NK and a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 24380172 and 26292168) to HI.
Kim, N. , Minami, N. , Yamada, M. and Imai, H. (2017), Immobilized pH in culture reveals an optimal condition for somatic cell reprogramming and differentiation of pluripotent stem cells. Reproductive Medicine and Biology, 16:58–66. doi: 10.1002/rmb2.12011
References
- 1. Occhipinti R, Boron WF. Mathematical modeling of acid–base physiology. Prog Biophys Mol Biol. 2015;117:43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caillouette JC, Sharp CF, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270–1275, discussion 5–7. [DOI] [PubMed] [Google Scholar]
- 3. Haugen TB, Grotmol T. pH of human semen. Int J Androl. 1998;21:105–108. [DOI] [PubMed] [Google Scholar]
- 4. Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio‐telemetry of vaginal pH during human coitus. J Reprod Fertil. 1973;33:69–75. [DOI] [PubMed] [Google Scholar]
- 5. Hauth JC, Macpherson C, Carey JC, et al. Early pregnancy threshold vaginal pH and Gram stain scores predictive of subsequent preterm birth in asymptomatic women. Am J Obstet Gynecol. 2003;188:831–835. [DOI] [PubMed] [Google Scholar]
- 6. Darszon A, Labarca P, Nishigaki T, Espinosa F. Ion channels in sperm physiology. Physiol Rev. 1999;79:481–510. [DOI] [PubMed] [Google Scholar]
- 7. Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod. 1998;13:964–970. [DOI] [PubMed] [Google Scholar]
- 8. FitzHarris G, Baltz JM. Regulation of intracellular pH during oocyte growth and maturation in mammals. Reproduction. 2009;138:619–627. [DOI] [PubMed] [Google Scholar]
- 9. Irie N, Tang WW, Azim Surani M. Germ cell specification and pluripotency in mammals: a perspective from early embryogenesis. Reprod Med Biol. 2014;13:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. [DOI] [PubMed] [Google Scholar]
- 11. McBrian MA, Behbahan IS, Ferrari R, et al. Histone acetylation regulates intracellular pH. Mol Cell. 2013;49:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol. 2013;4:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyamoto K, Gurdon JB. Transcriptional regulation and nuclear reprogramming: roles of nuclear actin and actin‐binding proteins. Cell Mol Life Sci. 2013;70:3289–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo J, Wang Y, Sachs F, Meng F. Actin stress in cell reprogramming. Proc Natl Acad Sci USA. 2014;111:E5252–E5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moghadam FH, Tayebi T, Dehghan M, et al. Differentiation of bone marrow mesenchymal stem cells into chondrocytes after short term culture in alkaline medium. Int J Hematol Oncol Stem Cell Res. 2014;8:12–19. [PMC free article] [PubMed] [Google Scholar]
- 16. Teo A, Mantalaris A, Lim M. Influence of culture pH on proliferation and cardiac differentiation of murine embryonic stem cells. Biochem Eng J. 2014;90:8–15. [Google Scholar]
- 17. Teperek M, Miyamoto K. Nuclear reprogramming of sperm and somatic nuclei in eggs and oocytes. Reprod Med Biol. 2013;12:133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 19. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brambrink T, Foreman R, Welstead GG, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshimizu T, Sugiyama N, De Felice M, et al. Germline‐specific expression of the Oct‐4/green fluorescent protein (GFP) transgene in mice. Dev Growth Differ. 1999;41:675–684. [DOI] [PubMed] [Google Scholar]
- 23. Tsukiyama T, Asano R, Kawaguchi T, et al. Simple and efficient method for generation of induced pluripotent stem cells using piggyBac transposition of doxycycline‐inducible factors and an EOS reporter system. Genes Cells. 2011;16:815–825. [DOI] [PubMed] [Google Scholar]
- 24. Niwa H, Yamamura K, Miyazaki J. Efficient selection for high‐expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. [DOI] [PubMed] [Google Scholar]
- 25. Yoshida‐Koide U, Matsuda T, Saikawa K, et al. Involvement of Ras in extraembryonic endoderm differentiation of embryonic stem cells. Biochem Biophys Res Commun. 2004;313:475–481. [DOI] [PubMed] [Google Scholar]
- 26. Morita S, Kojima T, Kitamura T. Plat‐E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. [DOI] [PubMed] [Google Scholar]
- 27. pH and Pressure in Closed Tissue Culture Vessels. Thermo Fisher Scientific Web site. https://www.thermoscientific.jp/content/dam/tfs/Country%20Specific%20Assets/ja-ja/LPG/LCD/Application-Notes/Cell-Culture/Technical%20Bulletin%2005.pdf Published 2010. Accessed 30 June 2012.
- 28. Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor IW, Hodson PJ. Cell cycle regulation by environmental pH. J Cell Physiol. 1984;121:517–525. [DOI] [PubMed] [Google Scholar]
- 30. Ruiz S, Panopoulos AD, Herrerías A, et al. A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Curr Biol. 2011;21:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jagielska A, Wilhite KD, Van Vliet KJ. Extracellular acidic pH inhibits oligodendrocyte precursor viability, migration, and differentiation. PLoS ONE. 2013;8:e76048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ng ES, Azzola L, Sourris K, Robb L, Stanley EG, Elefanty AG. The primitive streak gene Mixl1 is required for efficient haematopoiesis and BMP4–induced ventral mesoderm patterning in differentiating ES cells. Development. 2005;132:873–884. [DOI] [PubMed] [Google Scholar]
- 33. Tada S, Era T, Furusawa C, et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. [DOI] [PubMed] [Google Scholar]
- 34. Kubo A, Shinozaki K, Shannon JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. [DOI] [PubMed] [Google Scholar]
- 35. Yasunaga M, Tada S, Torikai‐Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. [DOI] [PubMed] [Google Scholar]
- 36. Williams AC, Collard TJ, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene. 1999;18:3199–3204. [DOI] [PubMed] [Google Scholar]
- 37. Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature. 2009;460:1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jackson SA, Schiesser J, Stanley EG, Elefanty AG. Differentiating embryonic stem cells pass through ‘temporal windows’ that mark responsiveness to exogenous and paracrine mesendoderm inducing signals. PLoS One. 2010;5:e10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


