Abstract
Background
Testicular cancer (TC) is one of the most common malignancies in young men of reproductive age. Although TC is a curable malignancy with a high survival rate, its treatment requires various cytotoxic modalities and negatively impacts spermatogenesis; therefore, the fertility preservation of patients with TC has been studied.
Methods
In order to give an overview of fertility preservation in patients with TC, the literature was reviewed. Original and review articles were identified and examined on the basis of PubMed database searches.
Results
Chemotherapy and radiotherapy damage spermatogenesis and retroperitoneal lymph node dissection negatively impacts ejaculatory function. Testicular sperm extraction facilitates successful sperm retrieval in patients with TC with postchemotherapy azoospermia. Although preserved sperm is used with a very low frequency (8%), the conception rates in those who have used sperm are not inferior.
Conclusion
The number of studies is limited, and because numerous treatment factors affect fertility, outstanding questions remain about preserving the fertility of patients with TC. Further studies are necessary in order to determine the best means of preventing and treating infertility in patients with TC.
Keywords: fertility, semen preservation, sperm banks, survivors, testicular cancer
1. INTRODUCTION
Testicular cancer (TC) is one of the most common malignancies in young adult men, with a peak age range of 20‐44 years.1 Due to advancements in treatment modalities, the 5 year survival rates for TC are currently reported to be >90%.2 Despite this high cure rate, many survivors of TC experience treatment‐induced effects, including short‐ and long‐term sequelae.3 Among the long‐term sequelae, the impact of treatment on fertility is a critical concern for the survivors of TC of reproductive age. Although various studies have examined fertility in the survivors of TC after treatment, the results are inconsistent,4 owing to the complexities of combined treatment modalities, such as orchiectomy, chemotherapy, radiotherapy (RT), and retroperitoneal lymph node dissection (RPLND). Pre‐existing subfertility in patients with TC further complicates the interpretation of the study data. Indeed, the rates of TC and male infertility have increased simultaneously during recent decades.5 Therefore, the fertility of the survivors of TC should be determined on the basis of both pre‐ and post‐treatment evaluations.
Sperm cryopreservation has been available and used broadly to maintain the opportunities for patients with cancer to conceive.6 The American Society of Clinical Oncology (ASCO) guidelines recommend that oncologists address the risk of infertility in patients with cancer of reproductive age and refer them to specialists in fertility treatment.7 However, large‐scale studies have shown that the sperm preservation rates in patients with cancer are low and that the usage rates of preserved sperm are even lower (<10%).8 Although clinical evidence of post‐treatment fertility has been accumulated, this low usage rate remains owing to various sociological and psychological factors.9 Recently, the quality of life (QoL) of survivors of TC has been emphasized and investigated in an increasing number of studies. Some of the results imply that the usage rate of preserved sperm is influenced by QoL‐related factors, such as time, emotional state, patient age, prior children, and cost.10 However, strategies for addressing those factors remain unclear. This article reviews fertility preservation in the survivors of TC by focusing on treatment‐induced infertility and the obstacles to sperm preservation and usage in order to answer questions about the management of patients with TC who are facing anxieties about their upcoming treatments.
2. CLINICAL AND PATHOLOGICAL CHARACTERISTICS OF TESTICULAR CANCER
Testicular cancer is histologically and clinically categorized as seminomatous and non‐seminomatous. Seminomas consist of the pure histological type of seminomatous features, whereas non‐seminomas contain the mixed histological features of embryonal carcinomas, yolk sac tumors, choriocarcinomas, or teratomas.11 Epidemiological studies of TC have shown that it has a unique global distribution and diverse incidence rates that are associated with race and ethnicity. The age‐adjusted incidence rates range from <0.7 per 100 000 men in most Asian and African countries to 12.2 per 100 000 men in Norway.12 The diagnosis and treatment protocols for TC are established in several evidence‐based guidelines. The recent National Comprehensive Cancer Network guideline for TC recommends clinical practices that follow the flow chart in Figure 1.13
Figure 1.
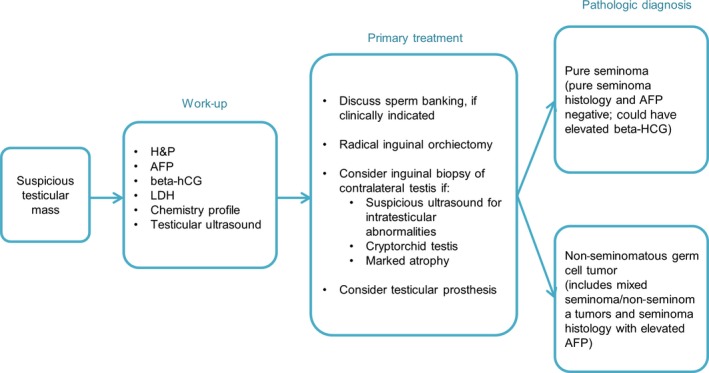
Flow chart showing the work‐up, primary treatment, and pathological diagnosis for testicular cancer, as recommended by the National Comprehensive Cancer Network (NCCN) guidelines. The work‐up includes the measurement of the serum tumor markers that are required for clinical staging. Discussions about sperm banking are recommended at the beginning of the primary treatment. AFP, alpha fetoprotein; hCG, human chorionic gonadotropin; H&P, history and physical examination; LDH, lactate dehydrogenase
The most common presentation of TC is a painless mass within the testis and prompt ultrasound evaluation and examination of the serum tumor markers are required. After a testicular mass is confirmed, radical inguinal orchiectomy is performed, followed by pathological diagnosis. Defining the clinical stage (CS) is recommended based on the Union for International Cancer Control (UICC) classification.14 The cure rate of CS I (localized) seminoma is nearly 100% and is achieved with three treatment options: adjuvant chemotherapy with carboplatin, adjuvant RT, or surveillance with salvage irradiation or chemotherapy at relapse.15 On the contrary, the prognosis of patients with metastatic TC (CS II‐IV) is categorized as “good,” “intermediate,” or “poor,” respectively, according to the classification of the International Germ Cell Cancer Collaborative Group (IGCCCG).16 Individualized treatment strategies are based on both the UICC and IGCCCG classifications and most of these treatment options are harmful to fertility. Therefore, discussing sperm preservation is recommended at the beginning of primary treatment if clinically indicated.13
3. FERTILITY AND TESTICULAR CANCER RISK
Both the pre‐ and post‐treatment reproductive status must be considered in discussions of fertility with patients with TC. Pretreatment fertility in these patients is not fully understood because TC and male fertility often coexist and it is difficult to pinpoint the cause and effect. Some studies have reported that pretreatment fertility in patients with TC is impaired already because they are more likely to have unfavorable semen parameters, compared with other patients with cancer and the general population.17, 18 On the contrary, TC might not be a cause of infertility, but rather an interrelated factor. The results of several large‐scale studies have shown that infertile men have a higher risk of developing TC. For example, a cohort study in the USA evaluated 4549 infertile men in a total of 51 461 couples and found that the men who were seeking infertility treatment had an increased risk of TC (standardized incidence ratio [SIR]: 1.3; 95% confidence interval [CI]: 0.9‐1.9).5
In addition, other cohorts inspected the correlation between semen analysis parameters and the incidence of TC. A study analyzed the semen of 32 442 Danish men, including 89 patients with TC, and reported that the infertile men were 1.6‐fold more likely to develop subsequent TC (SIR: 1.6; 95% CI: 1.3‐1.9).19 This study also showed that a low semen concentration (SIR: 2.3), poor sperm motility (SIR: 2.5), and a high proportion of morphologically abnormal spermatozoa (SIR: 3.0) were associated with an increased risk of TC.19 Another cohort reported a more significant risk of TC in infertile men. A study of 3847 men in the Surveillance, Epidemiology and End Results database showed that, compared with the general population, infertile men with abnormal semen analyses had a 20‐fold greater incidence of TC (SIR: 22.9; 95% CI: 22.4‐23.5).20
These data are consistent with the hypothesis of “testicular dysgenesis syndrome,” which was first proposed by Skakkebæk, Rajpert‐De Meyts, and Main, and describes a single underlying entity that includes poor semen quality, an undescended testis, hypospadias, and TC.21 These authors presented not only epidemiological evidence, but also histopathological findings, that testosterone production is reduced before a TC diagnosis.21 After the association between TC and sperm quality was established, a number of studies investigated the semen parameters of patients with TC.22 Table 1 shows the descriptive studies on pretreatment semen parameters in patients with TC.23, 24, 25, 26, 27, 28, 29 The results of most of the studies indicated that patients with TC show a low sperm concentration and a high rate of abnormal sperm morphology.
Table 1.
Pretreatment semen parameters in patients with testicular cancer (TC)
| Authors Year of study report | Patients with pre‐orchiectomy data | Sperm concentration (×106/mL) (range) | Sperm motility (%) (range) | Morphologically normal sperm (%) (range) |
|---|---|---|---|---|
|
Nijman et al.23
1985 |
14 with TC | 13.20a (0‐63) | 31.0a (0‐70) | 48.0a (30‐58) |
| 59 controls | 73.80a (8‐185) | 50.0a (10‐80) | 58.0a (32‐77) | |
|
Botchan et al.24
1997 |
32 with seminoma | 50.00b (0‐230) | 40.0b (0‐60) | 37.0b (0‐57) |
| 22 with nonseminoma | 17.00b (0‐288) | 35.0b (0‐58) | 30.0b (0‐46) | |
| 190 controls | 175.00b (2‐476) | 50.0b (0‐90) | 44.0b (3‐92) | |
|
Petersen et al.25
1999 |
63 with TC | 15.00b (0‐128) | 66.0b (0‐93) | 41.0b (19‐75) |
| 141 controls | 48.00b (0‐402) | 65.0b (32‐100) | 42.0b (8‐65) | |
|
Williams IV et al.26
2009 |
179 with TC | 32.90a (1.0‐308.5) | 48.5a (1‐85) | N/A |
|
Fraietta et al.27
2010 |
37 with seminoma | 25.98a (0‐145) | 56.3a (0‐88) | 10.0a (2‐21) |
| 63 with nonseminoma | 14.46a (0‐63.3) | 54.2a (1‐89) | 8.2a (1‐21) | |
|
Johnson et al.28
2013 |
134 with TC | 24.80b (0‐203) | 41.5b (0‐96) | N/A |
|
Auger et al.29
2016 |
2315 with TC | 19.60b (5.4‐48.8)c | 45.0b (30‐56) | 33.0b (19‐48) |
N/A, not applicable or not available. aMean; bMedian; cInterquartile range.
On the contrary, the percentage of motile sperm in patients with TC is not significantly lower than the normal value of >40%.25, 26, 27, 28, 29, 30 Indeed, several studies have reported that the conception rates of patients with TC at the time of diagnosis were 35.0%‐46.1%.4, 31, 32, 33 Although the correlation between the motility and the conception rate in patients with TC is unknown, these results might support the hypothesis that poor semen parameters in patients with TC do not always result in infertility. A multicenter study of 451 patients in France showed that 208 patients conceived successfully with their partner, accounting for 46.1% of the total percentage of patients with TC and 91.2% of the patients who were attempting a pregnancy.4 These results might imply that the pretreatment sperm quality in patients with TC is adequate for conception with or without assisted reproductive technology (ART).
4. POST‐TREATMENT FERTILITY
4.1. Chemotherapy
Various factors affect fertility during and after cancer treatment: the modality, treatment dose and intensity, size and location of the radiation field, age, pretreatment fertility, and hormonal insufficiency.34 Identifying patients with TC at high risk for long‐term sequelae remains challenging because it is complicated by the combinations of TC treatment modalities in use.35 Therefore, only a limited number of studies has fairly compared the influence of each treatment modality.36 Among them, chemotherapy might be the most difficult to evaluate because patients with TC who receive toxic amounts of chemotherapy frequently have advanced disease and their fertility is influenced by their physical condition.
The toxicity of chemotherapy on spermatogenesis is considerable because differentiating spermatogenous cells are the most susceptible to cytotoxic agents, which easily reach the Leydig, Sertoli, and spermatogenous cells at the outer rim of the seminiferous tubules. In particular, many chemotherapeutic drugs pass through the Sertoli cell barriers and injure matured germ cells.37 The primary chemotherapeutic regimens for advanced TC consist of cisplatin, etoposide, and bleomycin/ifosfamide,38 all of which pose risks for gonadal dysfunction.7, 39, 40
In evaluating harmful doses of chemotherapy, researchers reviewed five studies and concluded that a cumulative dose of cisplatin of <400 mg/m2 is the determinant factor in the reversibility of impaired spermatogenesis.41 Furthermore, a national multicenter study of 1183 survivors of TC in Norway compared the gonadal function that was associated with cumulative cisplatin doses of <850 mg and >850 mg. The results indicated that hypogonadism (defined as serum testosterone levels of <8 nmol/L, serum luteinizing hormone levels of >12 IU/L, or the use of testosterone supplementation) occurred in 19% of the patients who had received <850 mg of cisplatin (age‐adjusted odds ratio [OR]: 4.8; 95% CI: 2.4‐9.5) and 27% of those who had received >850 mg (age‐adjusted OR: 7.9; 95% CI: 3.6‐17.4), compared with 5% of the healthy controls.42 These researchers also investigated overall 15 year post‐treatment paternity rates by using the same cut‐off value (850 mg cisplatin). The results showed that the paternity rate ranged from 48% (95% CI: 66‐75) in the >850 mg cisplatin group to 92% (95% CI: 78‐98) in the surveillance group (P<.001).43
Similarly, the impact of chemotherapy on the conception rate was investigated in several large‐scale studies. A UK study of 680 survivors of TC compared the conception rates of patients who underwent surveillance, chemotherapy, RT, and chemotherapy+RT. The results showed that the rates of successful conception in the surveillance, chemotherapy, and chemotherapy+RT groups were 85%, 71%, and 67%, respectively.44 Table 2 shows a selected list of relatively large‐scale studies that assessed the impact of chemotherapy on fertility‐related outcomes.4, 42, 43, 44, 45, 46, 47, 48, 49 These results suggest that the cumulative dose of cisplatin is a significant factor, but consensus is difficult to obtain owing to the variety of methodologies. More large‐scale studies, such as the national multicenter survey by Brydøy et al.,43 are required to identify definitive relationships.
Table 2.
Treatment modalities and fertility‐related variables for survivors of testicular cancer (TC)
| Authors Year of study report | Survivors of TC included (N) | Median follow‐up (years) (range) | Treatment modality | Patients (N) | Major variables compared | Main results |
|---|---|---|---|---|---|---|
|
Arai et al.45
1997 |
85 | 7.7 (1.0‐21.8) |
SV CT+RPLND CT‐RPLND RT |
9 19 15 42 |
Post‐treatment sexual function, marital status, fertility distress | Highest rate of infertility distress was observed in patients with CT |
|
Spermon et al.46
2003 |
226 | 7.4 (1.6‐18.7) |
SV RT PRPLND PRPLND+CT CT CT+SRPLND |
20 36 44 42 44 40 |
Patients who attempted and fulfilled fatherhood (%) | 48% (38) of 88 couples conceived within 1 year; treatment modality did not significantly affect conception rates |
|
Nord et al.42
2003 |
1183 | 11.0 (N/A) |
SV RT Cisplatin (<850 mg) Cisplatin (>850 mg) Healthy controls |
52 515 373 96 200 |
Serum sexual hormones, % of men with hypogonadism (defined as serum T<8 nmol/L, LH>12 IU/L, or using T supplementation) | Age‐adjusted odds ratio of hypogonadism was 3.8 (95% CI: 2.0‐7.3) in patients with TC and increased with treatment intensity |
|
Huyghe et al.4
2004 |
451 | 8.0 (3.0‐26.5) |
SV or RPLND CT RT Others |
21 143 171 116 |
Fertility status, including reproductive events | Cumulative conception rates in patients with CT were higher than those with RT |
|
Huddart et al.44
2005 |
680 | 10.2 (0.0‐20.3) |
SV CT CT+RT RT |
169 272 81 158 |
Patients who attempted and succeeded in conception (%), level of gonadal hormones | In CT group, 31% (83) tried to conceive and 75% (62) succeeded with/without infertility treatment; in CT/RT group, 30% (24) tried and 83% (20) succeeded |
|
Brydøy et al.43
2005 |
1433 | 10.6 (4.0‐21.0) |
SV RPLND RT Cisplatin (<850 mg) Cisplatin (>850 mg) |
119 153 610 447 104 |
Patients who attempted and succeeded in conception (%), years from beginning treatment to first‐born child | Success rates of patients who attempted to conceive were 81%, 77%, 65%, 62%, and 38% with each modality, respectively (P<.001) |
|
Gandini et al.47
2006 |
166 | 2.0 (N/A) |
CT RT |
71 95 |
Sperm parameters at 3, 6, 9, 12, and 24 months after treatment | At 2 years after treatment, 3% of CT group and 6% of RT group remained with azoospermia |
|
Brydøy et al.48
2010 |
316 | 12.0 (5.0‐20.0) |
CT (two cycles) CT (three cycles) CT (four cycles) |
20 79 217 |
Sperm count, level of gonadal hormones, % of patients who achieved fatherhood, % of patients with normal ejaculation | Paternity rates for two, three, and four cycles were 100%, 83%, and 76%, respectively (P=.022) |
|
Ping et al.49
2014 |
125 | 10.2 (1.0‐15.0) |
SV RPLND CT RT CT+RT |
36 11 35 28 7 |
Patients who attempted and succeeded in conception (%) | CT, RT, and RPLND were the most highly correlated with a lack of conception |
CI, confidence interval; CT, chemotherapy; LH, luteinizing hormone; N/A, not available; PRPLND, primary RPLND; RPLND, retroperitoneal lymph node dissection; RT, radiotherapy; SRPLND, secondary RPLND; SV, surveillance; T, testosterone.
4.2. Radiotherapy
Radiotherapy is indicated for early‐stage TC after orchiectomy.50 The relapse rate of CS I seminoma is reduced to 1%‐3% by adjuvant RT with a total of 20‐24 Gy to a para‐aortic field with or without ipsilateral iliac lymph nodes.51, 52, 53 For CS IIA/B seminoma, the area of a retroperitoneal metastatic lesion is added to the irradiated area for CS I.50 Two studies of 87 and 126 patients with CS II seminomas showed that relapse‐free survival was achieved in >92% of the CS IIA patients with 30 Gy RT and in 89% of the CS IIB patients with 36 Gy RT.54, 55 Contrary to the favorable therapeutic effect, however, the damage to fertility is substantial because the testis is a highly radiosensitive tissue. The damage is commonly caused by scattered radiation to the neighboring tissues during treatment.56 The recovery of spermatogenesis depends on the radiation dose.57 Other studies have shown that fractionating irradiation with doses of >2.5 Gy causes prolonged azoospermia and doses of 16‐18 Gy cause Sertoli cell‐only syndrome.36, 37, 54
As shown in Table 2, various studies have investigated the conception rates after RT, but with inconsistent results. One assessed 171 patients with TC with CS I and IIA/B who received RT with 25‐35 Gy (median: 28 Gy) and found lower conception rates (~65%) in the patients who received RT, compared with those who received chemotherapy (~85%).4 However, two additional large‐scale studies showed contradictory results. One assessed 158 patients with CS I seminoma, the majority of whom received 30 Gy RT, and reported a conception rate of 85%, which was higher than the 75% that was observed in the patients who received various chemotherapy regimens.44 In contrast, 610 patients were investigated who had CS I‐IIA seminomas and who received RT, with doses ranging from 25 to 40 Gy. There was a post‐treatment conception rate of 65%, which was similar to the rate that was observed in those who had received cisplatin doses of <850 mg (62%).43 Although the effects of RT on fertility have not been elucidated completely, their evaluation might be less difficult than those of chemotherapy because RT is limited to early‐stage seminoma.
4.3. Retroperitoneal lymph node dissection
Retroperitoneal lymph node dissection is indicated in stage I non‐seminoma and is performed selectively in IIA seminoma.50 A randomized phase III trial by the German Testicular Cancer Study Group reported that the recurrence rates of CS I non‐seminoma after one course of chemotherapy or RPLND were 10% and 3%, respectively. As these recurrence rates were not remarkably different, RPLND, an operation that requires invasive procedures, has been more carefully selected recently.58 Retroperitoneal lymph node dissection disrupts the retroperitoneal sympathetic nerve complex that enters the superior hypogastric plexus, which causes retrograde ejaculation.59 Indeed, bilateral RPLND that is performed without a nerve‐sparing technique leads to impaired fertility in >90% of patients with TC.60, 61 In recent decades, nerve‐sparing RPLND has been performed more frequently and a number of studies has indicated that these techniques help to prevent ejaculatory dysfunction (Table 3).62, 63, 64, 65, 66, 67, 68 These results imply that maintaining ejaculatory function depends on the use of nerve‐sparing techniques, narrower dissection templates, and the avoidance of chemotherapy.
Table 3.
Retroperitoneal lymph node dissection and ejaculatory function in survivors of testicular cancer
| Authors Year of study report | Patients who received RPLND (N) | P‐RPLND or PC‐RPLND | Dissection template and/or nerve‐sparing | Patients (N) | Patients with normal ejaculation (N) (%) |
|---|---|---|---|---|---|
|
Coogan et al.62
1996 |
81 | PC‐RPLND | Nerve‐sparing | 81 | 62 (77) |
|
Jacobsen et al.63
1999 |
174 | PC‐RPLND | Modified bilateral template | 89 | 10 (11) |
| Unilateral template | 29 | 22 (76) | |||
| Nerve‐sparing | 56 | 50 (89) | |||
|
Heidenreich et al.64
2003 |
239 | P‐RPLND | Nerve‐sparing (88% unilaterally and 12% bilaterally) | 239 | 223 (93) |
|
Heidenreich et al.65
2009 |
152 | PC‐RPLND | Modified template | 98 | N/A (85) |
| Full bilateral template | 54 | N/A (25) | |||
|
Pettus et al.66
2009 |
136 | PC‐RPLND | Nerve‐sparing and bilateral template | 136 | 107 (79) |
|
Subramanian et al.67
2010 |
208 | P‐RPLND vs PC‐RPLND | P‐RPLND | 70 | 60 (81) |
| PC‐RPLND | 54 | 22 (41) | |||
|
Beck et al.68
2010 |
176 | P‐RPLND | Nerve‐sparing | 135 | 134 (99) |
| Non‐nerve‐sparing | 37 | 33 (89) |
N/A, not available; P‐RPLND, primary RPLND; PC‐RPLND, postchemotherapy RPLND; RPLND, retroperitoneal lymph node dissection.
Several studies have focused on the characteristics of fertility other than ejaculatory function after RPLND. One study analyzed the predictive factors for paternity with a Cox regression multivariate analysis that included the history of cryptorchidism, age at orchiectomy, marital status, fatherhood pretreatment, treatment modality, and dry ejaculation.43 They found that dry ejaculation was the most significant predictor of post‐treatment infertility. This result implies that RPLND eventually causes infertility in the survivors of TC. Indeed, another study showed that the fertility rates of survivors of TC with non‐nerve‐sparing RPLND, with nerve‐sparing RPLND, or without RPLND were 37%, 62%, and 70%, respectively, which suggests that non‐nerve‐sparing RPLND should be avoided if possible.69
4.4. Recovery of fertility
The period between treatment and the recovery of spermatogenesis also has been examined by several studies. One study evaluated the sperm count in 60 patients with TC who underwent an orchiectomy and surveillance and reported that their sexual hormonal levels correlated with their recovery of spermatogenesis.70 The sperm counts in patients with normal levels of serum follicle‐stimulating hormone (FSH) achieved recovery within 1 year after orchiectomy, whereas those with elevated FSH levels were at high risk of insufficient recovery.70 Among the treatment modalities, surgery (including RPLND) is reportedly less toxic than chemotherapy and RT with respect to the long‐term recovery of spermatogenesis. For example, recovery from post‐RPLND retrograde ejaculation is improved when a nerve‐sparing technique is used.66 From the results of a large‐scale survey, it was found that compared with chemotherapy, surgery has different impacts on spermatogenesis and thus patients with TC can attempt to conceive without restrictions from surgery, in terms of fertility.43
On the contrary, the recovery of spermatogenesis after chemotherapy has not been established due to the range of CS, treatment doses, and cycles used in patients with TC. Some studies have indicated that the number of chemotherapy cycles impacts recovery. One study reported that the number of cisplatin cycles was a factor in the recovery of spermatogenesis.71 After four cycles of cisplatin, the chance of spermatogenetic recovery declined to 25% in 3 years and 45% in 5 years.71 The gonadal function of 22 patients with CS I seminoma was prospectively investigated after carboplatin‐only therapy with 400 mg/m2 body surface area scheduled on days 1 and 22.72 Their results showed a favorable recovery rate, with 68% (15) of 22 patients achieving normospermia within 4 years after chemotherapy.72
A study investigated patients with TC with poor‐risk, non‐seminomatous germ cell tumors who underwent four cycles of methotrexate, paclitaxel, ifosfamide, and cisplatin.73 The results showed that 81% (17) of the 21 patients experienced a recovery of spermatogenesis after treatment at a median follow‐up of 2.3 years, whereas one‐third of the recovered patients had oligospermia before treatment.73 The cumulative dose of cisplatin was 400 mg/m2 in this regimen, which might explain the favorable recovery rates. The data on post‐therapeutic recovery remain limited but could provide guidance for patients with TC during decision‐making about their treatment options.
5. QUALITY OF LIFE AND SEXUAL DYSFUNCTION
Although some previous studies have discussed the impact of treatment on the QoL of survivors of TC,74, 75, 76 the impact of QoL on fertility remains under debate. A study compared the survivors of TC who achieved paternity with those who did not and showed that the former had better QoL scores in the assessment areas, such as social functioning, emotional functioning, general QoL, fatigue, pain, sleeping problems, treatment satisfaction, financial satisfaction, sexual problems, and body image problems.77
When the relationship between QoL and infertility is considered, sexual dysfunction, including erectile dysfunction (ED), is an inevitable concern that has been assessed in a few studies. One study surveyed the sexual function and body image of 401 survivors of TC by using six selected questions from the European Organization for the Research and Treatment of Cancer QLQ‐25 questionnaire, which was originally designed for patients with prostate cancer.78 The results showed that 43% of the patients reported reduced sexual activity, 24% had reduced sexual interest, 18% experienced ED, and 17% described changes in body image after treatment. Additionally, the erectile function deteriorated significantly after RPLND, whereas the other sexual functions were not affected according to the modality.78
One study reported a wide‐range frequency of ED (12%‐40%) in patients with TC and attributed it to organic or psychogenic etiologies.79 A cross‐section of 76 patients with TC was evaluated in order to examine their hemodynamics with penile Doppler ultrasonography as an assessment of the organic factors and graded erectile function by using the International Index of Erectile Function (IIEF). The study concluded that the patients had normal erectile hemodynamics, which suggested that their ED could have been psychogenic.79 Another study reported that 25.5% of the 143 survivors of TC experienced ED after chemotherapy, RT, or RPLND and the median time to recovery was 60‐70 months, regardless of the modality, based on IIEF scores.80 Further studies are needed to uncover the correlation among ED, other QoL factors, and fertility in survivors of TC.
6. CRYOPRESERVATION
Securing fertility before treatment is another key issue for patients of reproductive age with TC. The European Germ Cell Cancer Consensus Group strongly recommends that clinicians inform patients about the possibility of cryopreservation before orchiectomy.15 The ASCO guidelines also recommend that oncologists counsel patients with cancer about fertility preservation as part of cancer treatment planning.7, 81 These recommendations were developed after several studies showed alarmingly low rates of sperm preservation in patients with TC. Indeed, a survey of 904 male patients with cancer showed that 77% were childless at diagnosis and 51%‐70% were hoping for paternity in the future; however, only 24% of those hoping for paternity preserved their sperm.82 This study identified a lack of education as a common reason for the low rate of cryopreservation.82 Therefore, detailed counseling that discusses the issues of cell damage, contraception, and storage is recommended.83
One of the common concerns of patients with TC is the quality of the cryopreserved sperm in relation to the types of available ART.83 Recent advances in ART have enabled men who were considered previously to be infertile to father biological offspring.84 For example, the combination of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) allows the injection of a single sperm directly into the cytoplasm of an egg.85 Consequently, studies have reported that unfavorable semen parameters might not affect fertilization or conception rates after cryopreservation with IVF/ICSI, as long as live sperm can be recovered.86 A study showed that the outcome of ICSI was not different between frozen‐thawed and fresh sperm in a comparison of 84 cryopreserved specimens and 85 fresh controls.87
However, insufficient data are available to confirm whether the sperm quality of azoospermic patients with cancer, particularly TC, is sufficient for ART. One study assessed 67 Danish couples with male factor infertility due to cancer who underwent 151 ART cycles that included 55 cycles of intra‐uterine insemination (IUI), 82 of ICSI, and 14 of ICSI–frozen embryo replacement (FER).88 The results showed that the rates of pregnancy/delivery per cycle differed according to the type of ART: 15%/11% after IUI, 39%/31% after ICSI, and 25%/21% after ICSI–FER. By contrast, the use of cryopreserved or fresh sperm did not affect the delivery rate per cycle.88 Although ART‐related data that are specific to the survivors of TC remain insufficient, further studies might support the recommendation of sperm preservation, even for patients with TC with unfavorable sperm parameters.
7. TESTICULAR SPERM EXTRACTION
Testicular sperm extraction (TESE) is an effective method of sperm retrieval from patients with non‐obstructive azoospermia (NOA),18 and has been used also for survivors of cancer with postchemotherapy azoospermia (PCA).89 Two types of method, conventional TESE (cTESE) and microdissection TESE (micro‐TESE), are both widely practiced.90 In the cTESE procedure, the testis is exposed through a small incision and testicular tissue is dissected without identifying focal areas of spermatogenesis.91 In the micro‐TESE procedure, the tunica albuginea is widely opened and the testicular tissue is seen with an operating microscope before dissection. The micro‐TESE procedure enables the surgeon to visualize the tubules that are more likely to contain active spermatogenesis.92
Various studies have reported the improvement of the sperm retrieval rate (SRR) in patients with NOA with micro‐TESE. According to a systematic review in seven eligible studies comparing cTESE and micro‐TESE, the SRR with micro‐TESE ranged from 42.9% to 63%, which is significantly higher than the SRR with cTESE, which ranged from 16.7% to 45%.93 Although evidence of micro‐TESE in the survivors of cancer is still limited, several studies have investigated sperm retrieval by micro‐TESE in patients with PCA. For example, researchers performed micro‐TESE with subsequent ICSI for 73 survivors of cancer with persistent PCA, resulting in an overall SRR of 42.9% (36 of 84 TESE procedures) and the clinical pregnancy rate and the live birth rate were 50% and 42%, respectively.94 One study evaluated 66 Japanese patients with cancer, including 21 patients with TC who received micro‐TESE with ICSI. As a result, the SRR, clinical pregnancy rate, and live birth rate in the patients with TC were 52%, 33%, and 29%, respectively.95 More large‐scale and long‐term studies are needed to verify the effectiveness of micro‐TESE for patients with TC.
Some studies have suggested the efficacy of hormonal treatment for patients with NOA to enhance the recovery of spermatogenesis.89, 96 For example, the benefit of hCG‐based hormonal therapy in patients with NOA who had not achieved sperm retrieval in the first micro‐TESE procedure has been reported. This hormonal therapy involves self‐injections of 5000 IU of hCG three times per week for 3 months prior to a second micro‐TESE procedure.96 The authors administered the hormonal therapy to 26 patients with cancer, including eight patients with TC with PCA, resulting in 75% (six) of the patients with TC achieving sperm retrieval by the combination of hormonal therapy, micro‐TESE, and ICSI.89
In addition, TESE is a possible treatment for patients with advanced TC who underwent postchemotherapy RPLND. Although ejaculatory dysfunction that is caused by RPLND is increasingly avoided by the advancements of techniques, patients with postchemotherapy RPLND are at a higher risk of the sequelae than those who received RPLND without chemotherapy.67 One study suggested a clinical pathway that applied TESE for patients with ejaculatory dysfunction after postchemotherapy RPLND who failed electroejaculation, with which electrical stimulation is emitted from a rectal probe and retrograde ejaculation is collected via a catheter in the bladder. As a result, 81% (21 of 26) of the patients with ejaculatory dysfunction from postchemotherapy RPLND used TESE and 71% (15) of the patients succeeded in sperm retrieval.97
Data on TESE that has been performed before the cancer treatment remain insufficient. Approximately 5% of patients with TC have azoospermia at presentation29 and “onco‐TESE”, the contralateral TESE for patients with TC and/or the ipsilateral TESE, with the removal of cancer tissues followed by ex vivo dissection of the non‐cancerous tissue in the removed testis, has been attempted in some cases.98, 99 A few studies have reported the effectiveness of onco‐TESE. In one study, contralateral onco‐TESE was performed in 14 patients with TC with azoospermia, indicating that 36% (five) of the patients achieved sperm retrieval.98 One study attempted onco‐TESE both contralaterally and bilaterally for five patients with TC and successfully retrieved sperm in 80% (four) of the patients.99 Further studies on onco‐TESE are required to define its efficacy.
8. USAGE RATE OF BANKED SPERM
The usage rate of banked sperm is an issue that might be as important as the sperm quality for patients with TC and determining the significant factors that influence the usage rate should be beneficial for the survivors of TC who are hoping for paternity. A recent systematic review of 30 studies on sperm cryopreservation and reproductive outcomes in male patients with cancer showed that only 8% (95% CI: 8‐9) of 11 798 patients who banked sperm eventually used their sperm.100 Furthermore, the aggregated rate of achieving parenthood was 49% (95% CI: 44‐53) in 488 patients who used their banked sperm.100
Although the data on the usage rates of patients with cancer overall have been fairly accessible, those showing the usage rates in survivors of TC are limited. Researchers evaluated the questionnaires that had been completed by 200 patients with TC who received chemotherapy and found that 30% (61) of the patients cryopreserved their sperm, 18% (11 of 61) used the cryo‐thawed sperm, and 82% (nine of 11) achieved paternity.10 Table 4 lists studies that investigated patients with cancer and patients with TC and their usage and success rates with cryopreserved sperm.10, 31, 43, 46, 101, 102, 103, 104, 105 Although some studies of patients with TC seem to indicate usage rates that are higher than those reported for patients with cancer overall, the implication of this finding is unknown due to the limited number of studies.
Table 4.
Usage and pregnancy or conception rates of the cryopreserved semen of patients with cancer and patients with testicular cancer (TC)
| Authors Year of study report | Patients who cryopreserved sperm (N) | Patients who used sperm for ART (N) | Usage rate (%) | Patients who used sperm with available data (N) | Patients who succeeded (N) | Success rate in pregnancy/paternity (%) |
|---|---|---|---|---|---|---|
| Studies on male patients with cancer overall | ||||||
|
Ragni et al.101
2003 |
686 | 36 | 5.2 | 28 | 10 | 36a |
|
Ishikawa et al.102
2007 |
118 | 4 | 3.4 | 4 | 2 | 50a |
|
van Casteren et al.103
2008 |
557 | 42 | 7.5 | 37 | 18 | 49a |
|
Bizet et al.104
2012 |
931 | 57 | 6.1 | 47 | 22 | 47a |
|
Botchan et al.105
2013 |
682 | 70 | 10.3 | 68 | 27 | 40a |
| Studies on patients with TC | ||||||
|
Spermon et al.46
2003 |
78 | 13 | 16.7 | 13 | 7 | 54b |
|
Magelssen et al.31
2005 |
422 | 29 | 6.9 | 29 | 16 | 55 b |
|
Brydøy et al.43
2005 |
326 | 59 | 18.1 | 59 | 18 | 31 b |
|
Sonnenburg et al.10
2015 |
61 | 11 | 18.0 | 11 | 9 | 82 b |
ART, assisted reproductive technology. aPregnancy rate; bPaternity rate.
One study reported an aggregated rate of 16% (95% CI: 15‐17) of patients discarding their frozen samples.100 This low rate might indicate that most patients do not definitely rule out the possibility of using their cryopreserved semen.100 Moreover, the rate of return for semen analysis after treatment is also low. Another study evaluated a cross‐section of 499 survivors of cancer and showed that 35.8% of them had never sought a semen analysis after cancer treatment.9 A univariate logistic regression analysis showed that the survivors who did not seek this care were more likely to be unemployed, single, had fewer treatment‐related adverse events, and had negative experiences with sperm banking. These patients also believed that their sperm quality was less useful and they had negative attitudes about the disposal of semen.9 These results suggest that the low rate of seeking semen analysis is correlated with psychological and socioeconomic factors. In addition, other researchers pointed out that patients with cancer receive excessive information about cancer and treatment, which results in the failure to understand the long‐term implications of preserving sperm.106
Cost can be another barrier to the rate of sperm usage in a portion of the population. In the USA, the cost of banking sperm is ~US$1000 initially and between US$50 and $300 yearly for continued storage; many insurance companies do not cover those costs.10, 107 Moreover, the per‐cycle cost of IVF ranges from US$7000 to $15 000, which presents a financial burden for infertile couples.108, 109, 110 A study in the USA of 1210 infertile or subfertile women who were included in the National Survey of Family Growth showed that individual income significantly affects the probability of seeking fertility care.111 Notably, a study of 561 infertile women in Massachusetts, USA, showed that less wealthy and less educated persons were less likely to seek fertility care, even in states with comprehensive insurance coverage for such services.112 Therefore, it remains controversial whether economic status is a definitive factor. Although the socioeconomic data were not specified in a study of 200 survivors of TC, 10% of the patients indicated that cost was the reason for not banking sperm.10
A few studies have examined the impact of the time cost on receiving fertility care. A prospective cohort of 319 couples who had received fertility care showed that the average time that was spent on such care over a period of 18 months was 125 hours, which equates to 15.6 days.113 In addition, the time that was spent on fertility care did not differ significantly according to the socioeconomic background but was positively associated with fertility‐related stress.113 These results suggest that time is another significant barrier to receiving fertility care.
9. CONCLUSION
Testicular cancer is among the most curable malignancies that occur in young men of reproductive age and therefore the impact of treatment on fertility is a critical issue for these patients. Although many patients with TC experience azoospermia or oligospermia even before the cancer treatment, it remains controversial whether or not their fertility is impaired. Several studies have shown that their conception rates are not inferior to those of patients without cancer. The treatment strategies for TC, including combinations of modalities, orchiectomy with surveillance, chemotherapy, RT, and RPLND, affect the fertility outcomes. The cumulative doses of cisplatin and radiation define the magnitude of damage to spermatogenesis. On the contrary, RPLND frequently causes ejaculatory dysfunction but nerve‐sparing techniques have remarkably reduced its adverse effects. The recovery of spermatogenesis is a key concern for survivors of TC. Several studies have evaluated the thresholds of treatment doses for the reversibility of lost spermatogenesis. Moreover, the QoL and sexual function after TC treatment are significant issues that can be related to fertility. The TESE procedure is a possible method to retrieve sperm by dissecting the testicular tissues in patients with TC with azoospermia after cytotoxic treatment. Although sperm cryopreservation is recommended for patients with cancer who are receiving highly toxic treatment, only one‐fourth of patients preserve their sperm.83 Furthermore, the usage rates of preserved sperm are only 10% in those who bank sperm.100 This low rate might be influenced by various socioeconomic factors; however, data on the usage rates in survivors of TC remain limited. Further study is crucial to answering questions about how the fertility of survivors of TC should be managed and how their QoL can be improved.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This article does not contain any study with human or animal participants that have been performed by any of the authors.
Hamano I, Hatakeyama S, Ohyama C. Fertility preservation of patients with testicular cancer. Reprod Med Biol. 2017;16:240–251. https://doi.org/10.1002/rmb2.12037
REFERENCES
- 1. SEER cancer statistics review, 1975–2013. National Cancer Institute. http://seer.cancer.gov/csr/1975_2013. Accessed February 14, 2017.
- 2. Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE‐4 data. Lancet Oncol. 2007;8:784‐796. [DOI] [PubMed] [Google Scholar]
- 3. Fosså SD, Oldenburg J, Dahl AA. Short‐ and long‐term morbidity after treatment for testicular cancer. BJU Int. 2009;104:1418‐1422. [DOI] [PubMed] [Google Scholar]
- 4. Huyghe E, Matsuda T, Daudin M, et al. Fertility after testicular cancer treatments: results of a large multicenter study. Cancer. 2004;100:732‐737. [DOI] [PubMed] [Google Scholar]
- 5. Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt KL, Carlsen E, Andersen AN. Fertility treatment in male cancer survivors. Int J Androl. 2007;30:413‐418. [DOI] [PubMed] [Google Scholar]
- 7. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917‐2931. [DOI] [PubMed] [Google Scholar]
- 8. Neal MS, Nagel K, Duckworth J, et al. Effectiveness of sperm banking in adolescents and young adults with cancer: a regional experience. Cancer. 2007;110:1125‐1129. [DOI] [PubMed] [Google Scholar]
- 9. Pacey AA, Merrick H, Arden‐Close E, et al. Monitoring fertility (semen analysis) by cancer survivors who banked sperm prior to cancer treatment. Hum Reprod. 2012;27:3132‐3139. [DOI] [PubMed] [Google Scholar]
- 10. Sonnenburg DW, Brames MJ, Case‐Eads S, Einhorn LH. Utilization of sperm banking and barriers to its use in testicular cancer patients. Support Care Cancer. 2015;23:2763‐2768. [DOI] [PubMed] [Google Scholar]
- 11. Hayes‐Lattin B, Nichols CR. Testicular cancer: a prototypic tumor of young adult. Semin Oncol. 2009;36:432‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, McGlynn KA. International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev. 2010;19:1151‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NCCN clinical practice guidelines in oncology (NCCN guidelines) Testicular Cancer. Version 2. 2017‐December 8, 2016. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf. Accessed February 14, 2017. [DOI] [PubMed]
- 14. Sobin LH, Gospodarowicz ML, Wittekind C, eds. TNM Classification of Malignant Tumours, 7th edn New York, NY: Wiley‐Liss; 2011. [Google Scholar]
- 15. Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European Germ Cell Cancer Consensus Group (EGCCCG): part I. Euro Urol. 2008;53:478‐496. [DOI] [PubMed] [Google Scholar]
- 16. International Germ Cell Cancer Collaborative Group . The International Germ Cell Consensus Classification: a prognostic factor based staging system for metastatic germ cell cancer. J Clin Oncol. 1997;15:594‐603. [DOI] [PubMed] [Google Scholar]
- 17. Hallak J, Kolettis PN, Sekhon VS, Thomas AJ Jr, Agarwal A. Sperm cryopreservation in patients with testicular cancer. Urology. 1999;54:894‐899. [DOI] [PubMed] [Google Scholar]
- 18. Williams DH IV. Sperm banking and the cancer patient. Ther Adv Urol. 2010;2:19‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobsen R, Bostofte E, Engholm G, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raman JD, Nobert CF, Goldstein M. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J Urol. 2005;174:1819‐1822. [DOI] [PubMed] [Google Scholar]
- 21. Skakkebæk NE, Rajpert‐De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972‐978. [DOI] [PubMed] [Google Scholar]
- 22. Djaladat H, Burner E, Parikh PM, Beroukhim KD, Hays K. The association between testis cancer and semen abnormalities before orchiectomy: a systematic review. J Adolesc Young Adult Oncol. 2014;3:153‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nijman JM, Schraffordt Koops H, Kremer J, Willemse PH, Sleijfer DT, Oldhoff J. Fertility and hormonal function in patients with a nonseminomatous tumor of the testis. Arch Androl. 1985;14:239‐246. [DOI] [PubMed] [Google Scholar]
- 24. Botchan A, Hauser R, Yogev L, et al. Testicular cancer and spermatogenesis. Hum Reprod. 1997;12:755‐758. [DOI] [PubMed] [Google Scholar]
- 25. Petersen PM, Skakkebæk NE, Vistisen K, Rørth M, Giwercman A. Semen quality and reproductive hormones before orchiectomy in men with testicular cancer. J Clin Oncol. 1999;17:941‐947. [DOI] [PubMed] [Google Scholar]
- 26. Williams DH IV, Karpman E, Sander JC, Spiess PE, Pisters LL, Lipshultz LI. Pretreatment semen parameters in men with cancer. J Urol. 2009;181:736‐740. [DOI] [PubMed] [Google Scholar]
- 27. Fraietta R, Spaine DM, Bertolla RP, Ortiz V, Cedenho AP. Individual and seminal characteristics of patients with testicular germ cell tumors. Fertil Steril. 2010;94:2107‐2112. [DOI] [PubMed] [Google Scholar]
- 28. Johnson MD, Cooper AR, Jungheim ES, Lanzendorf SE, Odem RR, Ratts VS. Sperm banking for fertility preservation: a 20‐year experience. Eur J Obstet Gynecol Reprod Biol. 2013;170:177‐182. [DOI] [PubMed] [Google Scholar]
- 29. Auger J, Sermondade N, Eustache F. Semen quality of 4480 young cancer and systemic disease patients: baseline data and clinical considerations. Basic Clin Androl. 2016;26:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn Geneva: WHO Press; 2010. [Google Scholar]
- 31. Magelssen H, Haugen TB, von Düring V, Melve KK, Sandstad B, Fosså SD. Twenty years experience with semen cryopreservation in testicular cancer patients: who needs it? Eur Urol. 2005;48:779‐785. [DOI] [PubMed] [Google Scholar]
- 32. Rives N, Perdrix A, Hennebicq S, et al. The semen quality of 1158 men with testicular cancer at the time of cryopreservation: results of the French National CECOS Network. J Androl. 2012;33:1394‐1401. [DOI] [PubMed] [Google Scholar]
- 33. Kanto S, Hiramatsu M, Suzuki K, et al. Risk factors in past histories and familial episodes related to development of testicular germ cell tumor. Int J Urol. 2004;11:640‐646. [DOI] [PubMed] [Google Scholar]
- 34. Magelssen H, Brydøy M, Fosså SD. The effects of cancer and cancer treatments on male reproductive function. Nat Clin Pract Urol. 2006;3:312‐322. [DOI] [PubMed] [Google Scholar]
- 35. Haugnes HS, Bosl GJ, Boer H, et al. Long‐term and late effects of germ cell testicular cancer treatment and implications for follow‐up. J Clin Oncol. 2012;30:3752‐3763. [DOI] [PubMed] [Google Scholar]
- 36. Trottmann M, Becker AJ, Stadler T, et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol. 2007;52:355‐367. [DOI] [PubMed] [Google Scholar]
- 37. Meistrich ML, Vassilopoulou‐Sellin R, Lipshultz LI. Adverse effects of treatment: gonadal dysfunction In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology, 7th edn. Philadelphia: Lippincott Williams & Wilkins; 2005:2560‐2574. [Google Scholar]
- 38. Hinton S, Catalano PJ, Einhorn LH, et al. Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer. 2003;97:1869‐1875. [DOI] [PubMed] [Google Scholar]
- 39. Brydøy M, Fosså SD, Klepp O, et al. Sperm counts and endocrinological markers of spermatogenesis in long‐term survivors of testicular cancer. Br J Cancer. 2012;107:1833‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209‐218. [DOI] [PubMed] [Google Scholar]
- 41. DeSantis M, Albrecht W, Höltl W, Pont J. Impact of cytotoxic treatment on long‐term fertility in patients with germ‐cell cancer. Int J Cancer. 1999;83:864‐865. [DOI] [PubMed] [Google Scholar]
- 42. Nord C, Bjøro T, Ellingsen D, et al. Gonadal hormones in long‐term survivors 10 years after treatment for unilateral testicular cancer. Eur Urol. 2003;44:322‐328. [DOI] [PubMed] [Google Scholar]
- 43. Brydøy M, Fosså SD, Klepp O, et al. Paternity following treatment for testicular cancer. J Natl Cancer Inst. 2005;97:1580‐1588. [DOI] [PubMed] [Google Scholar]
- 44. Huddart RA, Norman A, Moynihan C, et al. Fertility, gonadal and sexual function in survivors of testicular cancer. Br J Cancer. 2005;93:200‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arai Y, Kawakita M, Okada Y, Yoshida O. Sexuality and fertility in long‐term survivors of testicular cancer. J Clin Oncol. 1997;15:1444‐1448. [DOI] [PubMed] [Google Scholar]
- 46. Spermon JR, Kiemeney LA, Meuleman EJ, Ramos L, Wetzels AM, Witjes JA. Fertility in men with testicular germ cell tumors. Fertil Steril. 2003;79:1543‐1549. [DOI] [PubMed] [Google Scholar]
- 47. Gandini L, Sgrò P, Lombardo F, et al. Effect of chemo‐ or radiotherapy on sperm parameters of testicular cancer patients. Hum Reprod. 2006;21:2882‐2889. [DOI] [PubMed] [Google Scholar]
- 48. Brydøy M, Fosså SD, Klepp O, et al. Paternity and testicular function among testicular cancer survivors treated with two to four cycles of cisplatin‐based chemotherapy. Eur Urol. 2010;58:134‐140. [DOI] [PubMed] [Google Scholar]
- 49. Ping P, Gu BH, Li P, Huang YR, Li Z. Fertility outcome of patients with testicular tumor: before and after treatment. Asian J Androl. 2014;16:107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guideline on testicular cancer. European Association of Urology. http://uroweb.org/guideline/testicular-cancer/.2016. Accessed February 14, 2017.
- 51. Fosså SD, Horwich A, Russell JM, et al. Optimal planning target volume for stage I testicular seminoma: a Medical Research Council randomized trial. Medical Research Council Testicular Tumor Working Group. J Clin Oncol. 1999;17:1146. [DOI] [PubMed] [Google Scholar]
- 52. Jones WG, Fosså SD, Mead GM, Roberts JT, Sokal MJ. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I testicular seminoma: a report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328). Clin Oncol. 2005;23:1200‐1208. [DOI] [PubMed] [Google Scholar]
- 53. Melchior D, Hammer P, Fimmers R, Schüller H, Albers P. Long term results and morbidity of paraaortic compared with paraaortic and iliac adjuvant radiation in clinical stage I seminoma. Anticancer Res. 2001;21:2989‐2993. [PubMed] [Google Scholar]
- 54. Classen J, Schmidberger H, Meisner C, et al. Radiotherapy for stages IIA/B testicular seminoma: final report of a prospective multicenter clinical trial. J Clin Oncol. 2003;21:1101‐1106. [DOI] [PubMed] [Google Scholar]
- 55. Chung PW, Gospodarowicz MK, Panzarella T, et al. Stage II testicular seminoma: patterns of recurrence and outcome of treatment. Eur Urol. 2004;45:754‐759. [DOI] [PubMed] [Google Scholar]
- 56. Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005;34:12‐17. [DOI] [PubMed] [Google Scholar]
- 57. Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59:665‐678. [PubMed] [Google Scholar]
- 58. Albers P, Siener R, Krege S, et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966‐2972. [DOI] [PubMed] [Google Scholar]
- 59. Jewett MA, Kong YS, Goldberg SD, et al. Retroperitoneal lymphadenectomy for testis tumor with nerve sparing for ejaculation. J Urol. 1988;139:1220‐1224. [DOI] [PubMed] [Google Scholar]
- 60. Abouassaly R, Fosså SD, Giwercman A, et al. Sequelae of treatment in long‐term survivors of testis cancer. Eur Urol. 2011;60:516‐526. [DOI] [PubMed] [Google Scholar]
- 61. Donohue JP, Rowland RG. Complications of retroperitoneal lymph node dissection. J Urol. 1981;125:338‐340. [DOI] [PubMed] [Google Scholar]
- 62. Coogan CL, Hejase MJ, Wahle GR, et al. Nerve sparing post‐chemotherapy retroperitoneal lymph node dissection for advanced testicular cancer. J Urol. 1996;156:1656‐1658. [PubMed] [Google Scholar]
- 63. Jacobsen KD, Ous S, Waehre H, et al. Ejaculation in testicular cancer patients after post‐chemotherapy retroperitoneal lymph node dissection. Br J Cancer. 1999;80:249‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heidenreich A, Albers P, Hartmann M, et al. Complications of primary nerve sparing retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell tumors of the testis: experience of the German Testicular Cancer Study Group. J Urol. 2003;169:1710‐1714. [DOI] [PubMed] [Google Scholar]
- 65. Heidenreich A, Pfister D, Witthuhn R, Thüer D, Albers P. Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol. 2009;55:217‐224. [DOI] [PubMed] [Google Scholar]
- 66. Pettus JA, Carver BS, Masterson T, Stasi J, Sheinfeld J. Preservation of ejaculation in patients undergoing nerve‐sparing postchemotherapy retroperitoneal lymph node dissection for metastatic testicular cancer. Urology. 2009;73:328‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Subramanian VS, Nguyen CT, Stephenson AJ, Klein EA. Complications of open primary and post‐chemotherapy retroperitoneal lymph node dissection for testicular cancer. Urol Oncol. 2010;28:504‐509. [DOI] [PubMed] [Google Scholar]
- 68. Beck SD, Bey AL, Bihrle R, Foster RS. Ejaculatory status and fertility rates after primary retroperitoneal lymph node dissection. J Urol. 2010;184:2078‐2080. [DOI] [PubMed] [Google Scholar]
- 69. Matos E, Skrbinc B, Zakotnik B. Fertility in patients treated for testicular cancer. J Cancer Surviv. 2010;4:274‐278. [DOI] [PubMed] [Google Scholar]
- 70. Jacobsen KD, Theodorsen L, Fosså SD. Spermatogenesis after unilateral orchiectomy for testicular cancer in patients following surveillance policy. J Urol. 2001;165:93‐96. [DOI] [PubMed] [Google Scholar]
- 71. Lampe H, Horwich A, Norman A, Nicholls J, Dearnaley DP. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol. 1997;15:239‐245. [DOI] [PubMed] [Google Scholar]
- 72. Reiter WJ, Kratzik C, Brodowicz T, et al. Sperm analysis and serum follicle‐stimulating hormone levels before and after adjuvant single‐agent carboplatin therapy for clinical stage I seminoma. Urology. 1998;52:117‐119. [DOI] [PubMed] [Google Scholar]
- 73. Pectasides D, Pectasides E, Papaxoinis G, et al. Testicular function in poor‐risk nonseminomatous germ cell tumors treated with methotrexate, paclitaxel, ifosfamide, and cisplatin combination chemotherapy. J Androl. 2009;30:280‐286. [DOI] [PubMed] [Google Scholar]
- 74. Fosså SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long‐term survivors of testicular cancer. J Clin Oncol. 2003;21:1249‐1254. [DOI] [PubMed] [Google Scholar]
- 75. Fegg MJ, Gerl A, Vollmer TC, et al. Subjective quality of life and sexual functioning after germ‐cell tumour therapy. Br J Cancer. 2003;89:2202‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dahl AA, Mykletun A, Fosså SD. Quality of life in survivors of testicular cancer. Urol Oncol. 2005;23:193‐200. [DOI] [PubMed] [Google Scholar]
- 77. Stoehr B, Schachtner L, Pichler R, et al. Influence of achieved paternity on quality of life in testicular cancer survivors. BJU Int. 2013;111:207‐212. [DOI] [PubMed] [Google Scholar]
- 78. Rossen P, Pedersen AF, Zachariae R, von der Maase H. Sexuality and body image in long‐term survivors of testicular cancer. Eur J Cancer. 2012;48:571‐578. [DOI] [PubMed] [Google Scholar]
- 79. Tal R, Stember DS, Logmanieh N, Narus J, Mulhall JP. Erectile dysfunction in men treated for testicular cancer. BJU Int. 2014;113:907‐910. [DOI] [PubMed] [Google Scholar]
- 80. Capogrosso P, Boeri L, Ferrari M, et al. Long‐term recovery of normal sexual function in testicular cancer survivors. Asian J Androl. 2016;18:85‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2013;31:2500‐2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nangia AK, Krieg SA, Kim SS. Clinical guidelines for sperm cryopreservation in cancer patients. Fertil Steril. 2013;100:1203‐1209. [DOI] [PubMed] [Google Scholar]
- 83. Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880‐1889. [DOI] [PubMed] [Google Scholar]
- 84. Wald M, Ross LS, Prins GS, Cieslak‐Janzen J, Wolf G, Niederberger CS. Analysis of outcomes of cryopreserved surgically retrieved sperm for IVF/ICSI. J Androl. 2006;27:60‐65. [DOI] [PubMed] [Google Scholar]
- 85. Shin D, Lo KC, Lipshultz LI. Treatment options for the infertile male with cancer. J Natl Cancer Inst Monogr. 2005;34:48‐50. [DOI] [PubMed] [Google Scholar]
- 86. Kuczynski W, Dhont M, Grygoruk C, Grochowski D, Wolczynski S, Szamatowicz M. The outcome of intracytoplasmic sperm injection of fresh and cryopreserved ejaculated spermatozoa – a prospective study. Hum Reprod. 2001;16:2109‐2113. [DOI] [PubMed] [Google Scholar]
- 87. Bessonnat J, Brouillet S, Sintzel S, et al. In cryptozoospermia or severe oligozoospermia is sperm freezing useful? Basic Clin Androl. 2014;24:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schmidt KL, Larsen E, Bangsbøll S, Meinertz H, Carlsen E, Andersen AN. Assisted reproduction in male cancer survivors: fertility treatment and outcome in 67 couples. Hum Reprod. 2004;19:2806‐2810. [DOI] [PubMed] [Google Scholar]
- 89. Shiraishi K, Matsuyama H. Microdissection testicular sperm extraction and salvage hormonal treatment in patients with postchemotherapy azoospermia. Urology. 2014;83:100‐106. [DOI] [PubMed] [Google Scholar]
- 90. Bernie AM, Mata DA, Ramasamy R, Schlegel PN. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta‐analysis. Fertil Steril. 2015;104:1099‐1103. [DOI] [PubMed] [Google Scholar]
- 91. Devroey P, Liu J, Nagy Z, et al. Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non‐obstructive azoospermia. Hum Reprod. 1995;10:1457‐1460. [DOI] [PubMed] [Google Scholar]
- 92. Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131‐135. [DOI] [PubMed] [Google Scholar]
- 93. Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non‐obstructive azoospermia: a systematic review. Andrology. 2014;2:20‐24. [DOI] [PubMed] [Google Scholar]
- 94. Hsiao W, Stahl PJ, Osterberg EC, et al. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol. 2011;29:1607‐1611. [DOI] [PubMed] [Google Scholar]
- 95. Shin T, Kobayashi T, Shimomura Y, et al. Microdissection testicular sperm extraction in Japanese patients with persistent azoospermia after chemotherapy. Int J Clin Oncol. 2016;21:1167‐1171. [DOI] [PubMed] [Google Scholar]
- 96. Shiraishi K, Ohmi C, Shimabukuro T, Matsuyama H. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non‐obstructive azoospermia. Hum Reprod. 2012;27:331‐339. [DOI] [PubMed] [Google Scholar]
- 97. Hsiao W, Deveci S, Mulhall JP. Outcomes of the management of post‐chemotherapy retroperitoneal lymph node dissection‐associated anejaculation. BJU Int. 2012;110:1196‐1200. [DOI] [PubMed] [Google Scholar]
- 98. Schrader M, Müller M, Sofikitis N, Straub B, Krause H, Miller K. “Onco‐tese”: testicular sperm extraction in azoospermic cancer patients before chemotherapy – new guidelines? Urology. 2003;2:421‐425. [DOI] [PubMed] [Google Scholar]
- 99. Furuhashi K, Ishikawa T, Hashimoto H, et al. Onco‐testicular sperm extraction: testicular sperm extraction in azoospermic and very severely oligozoospermic cancer patients. Andrologia. 2013;45:107‐110. [DOI] [PubMed] [Google Scholar]
- 100. Ferrari S, Paffoni A, Filippi F, Busnelli A, Vegetti W, Somigliana E. Sperm cryopreservation and reproductive outcome in male cancer patients: a systematic review. Reprod Biomed Online. 2016;33:29‐38. [DOI] [PubMed] [Google Scholar]
- 101. Ragni G, Somigliana E, Restelli L, Salvi R, Arnoldi M, Paffoni A. Sperm banking and rate of assisted reproduction treatment: insights from a 15‐year cryopreservation program for male cancer patients. Cancer. 2003;97:1624‐1629. [DOI] [PubMed] [Google Scholar]
- 102. Ishikawa H, Kaneko S, Miyaji K, Takamatsu K. Cryopreservation of human sperm in patients with malignancy: first 2 years’ experience. Reprod Med Biol. 2007;6:127‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. van Casteren NJ, van Santbrink EJ, van Inzen W, Romijn JC, Dohle GR. Use rate and assisted reproduction technologies outcome of cryopreserved semen from 629 cancer patients. Fertil Steril. 2008;90:2245‐2250. [DOI] [PubMed] [Google Scholar]
- 104. Bizet P, Saias‐Magnan J, Jouve E, et al. Sperm cryopreservation before cancer treatment: a 15‐year monocentric experience. Reprod Biomed Online. 2012;24:321‐330. [DOI] [PubMed] [Google Scholar]
- 105. Botchan A, Karpol S, Lehavi O, et al. Preservation of sperm of cancer patients: extent of use and pregnancy outcome in a tertiary infertility center. Asian J Androl. 2013;15:382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Eiser C, Arden‐Close E, Morris K, Pacey AA. The legacy of sperm banking: how fertility monitoring and disposal of sperm are linked with views of cancer treatment. Hum Reprod. 2011;26:2791‐2798. [DOI] [PubMed] [Google Scholar]
- 107. Nahata L, Cohen LE, Yu RN. Barriers to fertility preservation in male adolescents with cancer: it's time for a multidisciplinary approach that includes urologists. Urology. 2012;79:1206‐1209. [DOI] [PubMed] [Google Scholar]
- 108. Collins J. An international survey of the health economics of IVF and ICSI. Hum Reprod Update. 2002;8:265‐277. [DOI] [PubMed] [Google Scholar]
- 109. Koivurova S, Hartikainen AL, Gissler M, Hemminki E, Klemetti R, Jarvelin MR. Health care costs resulting from IVF: prenatal and neonatal periods. Hum Reprod. 2004;19:2798‐2805. [DOI] [PubMed] [Google Scholar]
- 110. Neumann PJ, Gharib SD, Weinstein MC. The cost of a successful delivery with in vitro fertilization. N Engl J Med. 1994;331:239‐243. [DOI] [PubMed] [Google Scholar]
- 111. Farley Ordovensky Staniec J, Webb NJ. Utilization of infertility services: how much does money matter? Health Serv Res. 2007;42:971‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jain T, Hornstein MD. Disparities in access to infertility services in a state with mandated insurance coverage. Fertil Steril. 2005;84:221‐223. [DOI] [PubMed] [Google Scholar]
- 113. Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: the hidden hardship of building a family. Fertil Steril. 2013;99:2025‐2030. [DOI] [PMC free article] [PubMed] [Google Scholar]


