Abstract
Aim
Several studies have indicated that the cause of the increased birthweight of frozen‐thawed embryos was associated with assisted reproductive technology (ART) procedures, such as cryopreservation. In the present study, the mean birthweight of singletons was compared between the ovulatory and hormone replacement therapy (HRT) cycles in order to investigate the primary factor that leads to higher birthweights from frozen‐thawed embryo transfer (FET).
Methods
This retrospective study was carried out from January 2011 to December 2014 on 2738 singletons who were born at 37‐41 weeks’ gestation, following ART in a single facility. The mean birthweight of the singletons who were born after a fresh embryo transfer (ET) was compared to the mean birthweight of the singletons who were born after a FET. In the FET cycles, the mean birthweight of the singletons was compared between the ovulatory and HRT cycles.
Results
The mean birthweight of the singletons who were born after a FET was significantly higher than that of the singletons who were born after a fresh ET. In the FET cycles, the birthweight from the HRT cycles was significantly higher than that from the ovulatory cycles. Among the HRT cycles, there was no difference between the birthweight of the singleton who were born from a blastocyst transfer and those who were born from a cleavage‐stage ET.
Conclusion
The primary factor to affect the birthweight of singletons might be the pre/postET hormonal environment of the endometrium and not the stage of the transferred embryo nor the frozen‐thawed procedure itself.
Keywords: assisted reproductive technology, birthweight, frozen‐thawed embryo transfer, hormone replacement cycle, spontaneous cycle
1. Introduction
Since the first successful pregnancy was achieved with assisted reproduction technology (ART), such as in vitro fertilization (IVF) and embryo transfer (ET), >4 million babies have been born using these technologies.1 However, it is well known that ART pregnancies are associated with maternal and fetal health risks, including preterm birth (<37 gestational weeks), low birthweight (<2500 g), and small for gestational age (SGA, <10th percentile), compared to naturally conceived births.2, 3
Several studies have shown that the frozen‐thawed embryo transfer (FET) is a better method than the fresh ET for fetal growth and a reduced risk of a low birthweight.4, 5 It also has been studied that singletons who were born after a FET had a higher risk of being large for their gestational age (LGA, >90th percentile), compared to singletons who were born after a fresh ET.6, 7 However, it is not clear whether these risk factors are associated with the ART procedures or maternal and/or paternal backgrounds.
The birthweight is an important factor for children's future health. A low birthweight and SGA have been associated with an increased risk of adult chronic diseases, including hypertension and cardiovascular disease.8 In contrast, LGA children remain taller and heavier throughout childhood and have a higher risk of adulthood obesity, compared to babies that were an appropriate birthweight for their gestational age.9 In addition, both SGA and LGA infants are indicated to have a higher risk of an adverse cardio‐metabolic profile during childhood and adolescence, referring to an increased risk of cardiovascular disease later in life.10 Therefore, it is very important to confirm the possible risk factors for the occurrence of SGA and LGA newborns.
Regarding ART, some studies have investigated the effect of the duration of the embryo in an in vitro culture on their birthweight. One study demonstrated that an extended culture of embryos was associated with an increasing proportion of LGA children.11 In another study, it was shown that a higher birthweight and more LGA children were born after blastocyst transfer, compared to singletons who were born after cleavage‐stage (day 3) ET.12 More recently, research discovered that the mean birthweight of boys who were born after a frozen‐thawed blastocyst transfer was significantly higher than the mean birthweight of boys who were born after a cleavage‐stage ET.13
These studies indicated that the cause of an increased birthweight from frozen‐thawed embryos was associated with IVF procedures, such as cryopreservation and/or the duration of an embryo culture in vitro. However, the answer still was not clear because all of them compared the birthweight from a fresh ET and a FET, which included both spontaneous (ovulatory) and hormone replacement therapy (HRT) cycles. In these FET cycles, the approaches to preparing the endometrium for the ET are different. In ovulatory cycles, the endometrium for implantation is prepared with the endogenous female hormone that is produced by growing follicles in the ovary. In addition, the timing of the ET will be scheduled according to the spontaneous luteinizing hormone (LH) surge and confirmation of ovulation. In contrast, in the HRT cycles, the preparation of the endometrium will be arranged by means of exogenous estradiol (E2) and progesterone.
In the present study, the mean birthweight of singletons was compared between the ovulatory cycles and the HRT cycles in order to assess the primary factor that leads to higher birthweights from a FET. Additionally, in order to assess the embryonic factor(s) that affects the birthweight, including the duration of the pre‐implantation embryo culture prior to the ET and the stage and quality of the embryos, the embryos were divided into cleavage‐stage and blastocyst‐stage embryos and scored on a morphological scale for each cycle.
2. Materials and Methods
2.1. Patients
In the time period from January 2011 to December 2014, 2738 singleton births at full‐term delivery (defined as more than or equal to 37 weeks) were investigated. All the pregnancies were a transferred single embryo in fresh ET and/or FET cycles.
2.2. Ovarian stimulation
In the oocyte retrieval cycle, ovarian stimulation was carried out by using standard gonadotropin‐releasing hormone agonist/follicle‐stimulating hormone (FSH) protocols or an antagonist/FSH protocol.14 The ovarian follicular development was monitored by using serum E2 levels and/or transvaginal ultrasound measurements. Ovulation induction was triggered when the second‐leading follicle was >18 mm in diameter. Ultrasound‐guided transvaginal oocyte retrieval was performed 35‐36 hours later.
2.3. Culture conditions
Immediately on retrieval, the oocytes were placed in Universal IVF Medium (Origio a/s, Jyllinge, Denmark) that was overlaid with mineral oil (Irvine Scientific, St. Ana, CA, USA). The oocytes were inseminated 3‐5 hours after using conventional insemination procedures or intracytoplasmic sperm injection (ICSI), depending on the semen parameters. Successful fertilization was confirmed 16‐18 hours (day 1) after insemination and the normally fertilized zygotes were cultured in global medium (LifeGlobal, Guelph, Canada). One‐to‐four embryos were cultured in a 50 μL droplet of culture medium under mineral oil. These embryos were cultured in a reduced oxygen atmosphere (6% CO2/5% O2/89% N2) at 37°C.
Cleavage‐stage embryos (day 2) and blastocyst‐stage embryos (days 5‐6) were graded according to the Veeck scale15 and Gardner criteria,16 respectively. The authors defined a cleavage‐stage embryo as high quality if the embryo had 4 cells on day 2 and no or negligible fragmentation (≥grade 2) and a blastocyst‐stage embryo as high quality if the cavity filled the embryo and there were cohesiveness of the inner cell mass and a trophectoderm (≥grade 3BB).
2.4. Cryopreservation protocols
All the procedures were performed by using a hand‐made vitrifying/warming solution and standard vitrification method. Modified human tubal fluid (m‐HTF; Irvine Scientific) medium that was supplemented with 20% serum substitute (Irvine Scientific) was used as the basic medium. The embryos were equilibrated in 7.5% (v/v) EG (Sigma‐Aldrich, St. Louis, MO, USA) and 7.5% (v/v) DMSO (Sigma‐Aldrich) for a maximum of 12 min for the cleavage‐stage embryos or 15 min for the blastocyst‐stage embryos. Following equilibration, the embryos were transferred into a vitrification solution that consisted of 15% (v/v) EG and 15% (v/v) DMSO, supplemented with 0.5 mol/L sucrose (Sigma‐Aldrich) for 60 s for the cleavage‐stage embryos or 90 s for the blastocyst‐stage embryos. These embryos were placed in a cryopreservation device immediately, and then gently plunged into liquid nitrogen. In the warming procedure, these embryos were immediately warmed in 1 mol/L sucrose at 37°C for 1 minute. Then, the thawed embryos were transferred into various concentrations of sucrose (0.75 mol/L, 0.5 mol/L, 0.25 mol/L, 0.125 mol/L, and 0 mol/L) and equilibrated for 1.5 minutes, 1.5 minutes, 2.5 minutes, 2.5 minutes, and 5 minutes at room temperature, respectively. The surviving embryos were cultured in global medium (LifeGlobal) until the ET.
2.5. Frozen‐thawed embryo transfer
The FET was categorized into two groups, spontaneous (ovulatory) cycles and HRT cycles. A single embryo was transferred by using ultrasound guidance. In both cycles, the clinical pregnancy was examined by transvaginal ultrasound 3 weeks after the ET.
In the ovulatory cycles, the ET was performed after spontaneous ovulation that was detected by means of a urinary LH test. The ET usually was carried out 2 days (cleavage‐stage embryo) or 5 days (blastocyst‐stage embryo) after the confirmation of ovulation. Luteal support with E2 and progesterone was initiated at the ET and continued until a clinical pregnancy was confirmed.
In the HRT cycles, 2.16 mg of E2 was administered daily from cycle day 2 to day 8. The dose was increased gradually to 4.32 mg until the ET and decreased to 2.16 mg thereafter. Vaginal progesterone (1200 mg/day) was started when the endometrial thickness was ≥7‐8 mm on cycle day 15 and continued until day 30. In cases of pregnancy, both E2 and progesterone were continued until the eighth gestational week.
2.6. Statistical analyses
The means were compared with the Student's t test and the proportions with the chi‐square test. A statistical analysis was performed by using StatMate III software (ATMS, Inc., Tokyo, Japan).
3. Results
3.1. Maternal and newborn characteristics
The maternal characteristics and perinatal outcomes are shown in Table 1. The maternal age and Body Mass Index were not different between the fresh ET and the FET (P=.077 and P=.23, respectively) groups and between the ovulatory cycle and the HRT cycle groups with the FET (P=.081 and P=.20, respectively). In the FET group, ICSI was used frequently as a fertilization method, compared to the fresh ET group. In the fresh ET group, the cleavage‐stage embryos were selected frequently as the transferred embryo. The ratio of embryo quality was categorized as high or similarly in all groups. In this study, only full‐term deliveries were included. In common with all groups, approximately half of the deliveries occurred at 39 and 40 weeks’ gestation. The sex ratio of the newborns was also similar in all groups (fresh ET vs FET, P=.87; ovulatory cycles vs HRT cycles, P=.11).
Table 1.
Characteristics of the mothers and the newborns
| Characteristic | Fresh embryo transfer | FET | P‐value | Frozen‐thawed embryo transfer (FET) | P‐value | |
|---|---|---|---|---|---|---|
| Ovulatory cycle | HRT cycle | |||||
| N (%) | n=323 | n=2415 | n=234 | n=2181 | ||
| Age of the mother (years) (mean±SD) | 36.9±3.6 | 35.1±3.8 | .077 | 35.8±3.8 | 35.0±3.8 | .0810 |
| Body Mass Index (kg/m2) (mean±SD) | 20.8±2.8 | 20.6±3.0 | .230 | 20.5±2.9 | 20.6±2.8 | .6000 |
| Insemination | ||||||
| In vitro fertilization | 121 (37.5) | 1712 (70.9) | 166 (70.9) | 1546 (70.9) | ||
| Intracytoplasmic sperm injection | 201 (62.5) | 703 (19.1) | 68 (29.1) | 635 (29.1) | ||
| Embryo transfer | ||||||
| Cleavage stage | 304 (94.1) | 240 (10.0) | <.001 | 38 (16.2) | 202 (9.3) | <.0010 |
| High quality | 256 (84.2) | 199 (82.9) | .690 | 30 (78.9) | 169 (83.7) | .4800 |
| Low quality | 48 (36.8) | 41 (17.1) | 8 (21.1) | 33 (16.3) | ||
| Blastocyst stage | 19 (5.9) | 2175 (90.0) | <.001 | 196 (83.8) | 1979 (90.7) | <.0010 |
| High quality | 12 (63.2) | 1360 (62.5) | .960 | 124 (63.3) | 1236 (62.5) | .8200 |
| Low quality | 7 (36.8) | 815 (37.5) | 72 (36.7) | 743 (37.5) | ||
| Week of delivery | ||||||
| 37 | 53 (16.4) | 341 (14.1) | .100 | 34 (14.5) | 307 (14.1) | |
| 38‐39 | 142 (44.0) | 967 (40.0) | 114 (48.7) | 853 (39.1) | ||
| 40‐41 | 128 (39.6) | 1107 (45.8) | 86 (36.8) | 1021 (46.8) | .0084 | |
| Sex of the newborn | ||||||
| Boy | 168 (52.0) | 1268 (52.5) | .870 | 135 (57.7) | 1133 (51.9) | .1100 |
| Girl | 155 (48.0) | 1147 (47.5) | 99 (42.3) | 1048 (48.1) | ||
HRT, hormone replacement therapy; SD, standard deviation.
3.2. Frozen‐thawed embryo transfer and fresh embryo transfer
The birthweight of the babies who were born from the FET was significantly higher than that of the babies who were born from the fresh ET (3118.0±374.9 g vs 3031.9±369.3 g, P<.001) (Table 2). Even when the sex of these babies was taken into account, the difference in the birthweight remained in the boys (3169.5±375.9 g vs 3061.9±367.3 g, P <.001) and in the girls (3061.3±365.7 g vs 2991.1±369.9 g, P=.024) (Table 2).
Table 2.
Mean birthweights of the singletons who were born from fresh embryo transfer (ET) and frozen‐thawed embryo transfer (FET)
| Singleton | Mean birthweight | P‐value | ||
|---|---|---|---|---|
| Fresh ET g (SD) | FET g (SD) | Difference (g) | ||
| All | 3031.9 (369.3) | 3118.0 (374.9) | 86.1 | <.001 |
| Boys | 3061.9 (367.3) | 3169.5 (375.9) | 107.6 | <.001 |
| Girls | 2991.1 (369.9) | 3061.3 (365.7) | 70.2 | .024 |
SD, standard deviation.
As seen in Figure 1, the mean birthweights of the singletons at a gestational age of 37 weeks, 38‐39 weeks, and 40‐41 weeks were significantly higher from the FET, compared to those from the fresh ET (respectively 2886.8±335.7 g vs 2788.4±363.7 g, P=.025; 3068.7±356.3 g vs 3001.6±365.2 g, P=.018; 3232.9±362.7 g vs 3162.2±318.6 g, P=.010).
Figure 1.
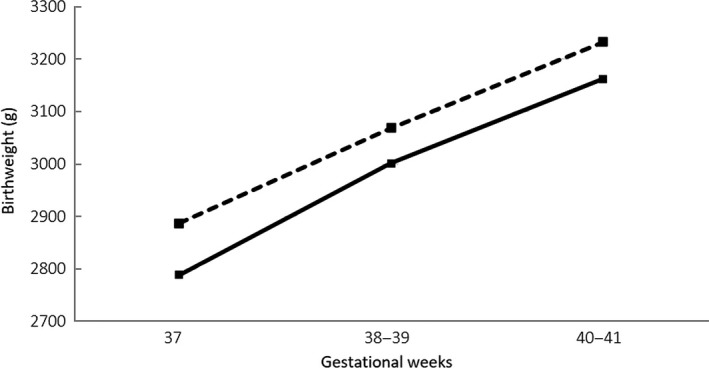
Mean birthweight of singletons who were born from a fresh embryo transfer (fresh ET; solid line) and a frozen‐thawed embryo transfer (FET; dotted line) following term gestation. The mean birthweight at 37 weeks, 38‐39 weeks, and 40‐41 weeks of gestational age was significantly higher in the singletons from the FET, compared to the fresh ET (respectively 2886.8±335.7 vs 2788.4±363.7 g, P=.025; 3068.7±356.3 g vs 3001.6±365.2 g, P=.018; 3232.9±362.7 g vs 3162.2±318.6 g, P=.010)
3.3. Ovulatory cycles and hormone replacement therapy cycles in the frozen‐thawed embryo transfer
The mean birthweight of the frozen‐thawed babies who were born from the HRT cycles was significantly higher than that of the babies who were born from the ovulatory cycles (3127.7±368.5 g vs 3028.0±368.5 g, P<.001). The difference was statistically significant both in the boys (3182.6±374.6 g vs 3061.0±371.0 g, P<.001) and in the girls (3068.7±365.3 g vs 2982.9±362.2 g, P=.013) (Table 3).
Table 3.
Mean birthweights of the singletons who were born from ovulatory and hormone replacement therapy (HRT) cycles
| Singleton | Mean birthweight | P‐value | ||
|---|---|---|---|---|
| Ovulatory cycle g (SD) | HRT cycle g (SD) | Difference (g) | ||
| All | 3028.0 (368.5) | 3127.7 (368.5) | 99.7 | <.001 |
| Boys | 3061.0 (371.0) | 3182.6 (374.6) | 121.6 | <.001 |
| Girls | 2982.9 (362.2) | 3068.7 (365.3) | 85.8 | .013 |
SD, standard deviation.
As seen in Figure 2, the birthweights of the neonates from the HRT cycles were higher than those from the ovulatory cycles at each gestational age. At a gestational age of 38‐39 weeks and 40‐41 weeks, the average weight of the HRT cycle group was significantly higher than that of the ovulatory cycle group (respectively 3079.2±349.0 g vs 2990.9±399.5 g, P=.013; 3241.2±364.2 g vs 3134.8±331.3 g, P=.0045).
Figure 2.
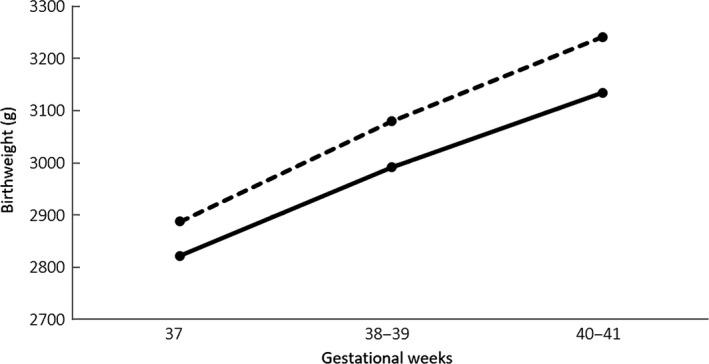
Mean birthweight of fresh frozen‐thawed babies who were born from hormone replacement treatment (HRT; dotted line) cycles and that from spontaneous (ovulatory; solid line) cycles following term gestation. At a gestational age of 38‐39 weeks and 40‐41 weeks, the average birthweight of the singletons in the HRT cycle group was significantly higher than that of the singletons in the ovulatory cycle group (respectively 3079.2±349.0 g vs 2990.9±399.5 g, P=.013; 3241.2±364.2 g vs 3134.8±331.3 g, P=.0045)
3.4. Developmental stage and embryo quality in each frozen‐thawed embryo transfer cycle
Table 4 shows the mean birthweight of singleton births, sorted by each ET cycle by the developmental stage (cleavage or blastocyst stage) and the embryo quality (high or low). The birthweight of the babies who were born from the high‐grade and the low‐grade embryos was not different between the cleavage stage and the blastocyst stage in the ovulatory cycles (3057.3±331.9 g vs 3022.3±375.7 g, P=.28) or in the HRT cycles (3109.7±361.7 g vs 3129.5±375.7 g, P=.24). In the ovulatory‐cycle‐transferred‐cleavage embryos, no weight difference was detected between the high‐grade and the low‐grade embryos (3056.4±350.9 g vs 3061.0±265.6 g, P=.49), nor at the blastocyst stage (3013.1±355.2 g vs 3038.1±410.7 g, P=.33). Similar results were detected in the HRT cycles (cleavage stage: 3111.9±375.7 g vs 3098.1±323.6 g, P=.42; blastocyst stage: 3120.1±371.3 g vs 3145.2±382.7 g, P=.077).
Table 4.
Mean birthweight of the babies as classified by the developmental stage and the embryo quality
| Mean birthweight | P‐value | ||
|---|---|---|---|
| High gradeg (SD) | Low gradeg (SD) | ||
| Ovulatory cycle | |||
| Cleavage stage | 3056.4 (350.9) | 3061.0 (268.6) | .490 |
| Blastocyst stage | 3013.1 (355.2) | 3038.1 (410.7) | .330 |
| P‐value | .27 | .42 | |
| HRT cycle | |||
| Cleavage stage | 3111.9 (375.7) | 3098.1 (323.6) | .420 |
| Blastocyst stage | 3120.1 (371.3) | 3145.2 (382.7) | .077 |
| P‐value | .39 | .24 | |
HRT, hormone replacement therapy; SD, standard deviation.
4. Discussion
In the present study, the birthweights of the singletons from the FET were significantly higher than those from the fresh ET, regardless of the sex of the newborn. Similar results are shown in other studies, in that the newborns from FET are heavier than those from fresh ET.4, 5, 17 These studies showed the possibility that the frozen‐thawed procedure was a factor that increased the birthweight. Other studies demonstrated that the vitrification procedure also might have an impact on increasing the newborn's birthweight. In one study, the birthweight of babies, who as embryos had been vitrified at cleavage stage, was higher than that of the babies who came from slow‐freezing embryos.18 In another study, the birthweight of babies who came from embryos who had been vitrified at cleavage stage was significantly higher than that of the babies who came from fresh embryos.19 Interestingly, research showed that the gestational sac diameters at the point in time of 21 days after fertilization already were larger from FET, compared to from fresh ET.20 However, most studies that were categorized into FET, in addition to the above studies, did not compare the ovulatory cycles and the HRT cycles in relation to the birthweight.
In the present study, the most important finding was the significant difference of birthweight between the HRT cycles and the ovulatory cycles in FET, even though the frozen‐thawed procedure was used in both cycles. These differences in birthweight were seen, regardless of the sex of the newborn, in each group. Similarly, the birthweight difference was obtained at each gestation term that was categorized into 37, 38‐39, and 40‐41 weeks.
In several studies that compared the effect of the embryo culture length on the birthweight, they failed to find any difference in the mean weight of the newborns.21, 22, 23 In this study, there was also no difference between the cleavage‐stage and the blastocyst‐stage embryos in the FET. Furthermore, in the present study, no difference was found in the mean weight of the babies, when comparing the effect of the embryo quality on the newborn's weight. The current results showed that the birthweight differences between HRT cycles and ovulatory cycles was not associated with cryopreservation, the developmental stage of the embryo, nor the quality of the embryo.
In this study, the difference between the ovulatory cycle and the HRT cycle in the FET can be described as follows: (1) the development of the follicle and/or corpus luteum formation, which produce endogenous hormones, are present during ovulatory cycles, even when the endometrium is artificially prepared by the exogenous hormones during HRT cycles; and (2) the term of hormone supplementation for luteal support was longer in the HRT cycles, compared to the ovulatory cycles. Based on the present study, the primary factor to increase the birthweight is assumed to be caused by the pre‐ or postET hormonal environment of the endometrium and not the frozen‐thawed procedure itself. This assumption was confirmed by the results in the present study that the birthweight of those who were born after a fresh ET was not different from that who were born after the ovulatory cycles in a FET (3031.9±369.3 g vs 3028.0±368.5 g, P=.45). From these results, the frozen‐thawed procedure would not directly affect the birthweight.
Progesterone is a steroid hormone that modulates different biological processes and is an essential steroid hormone in different reproductive events, such as ovulation, uterine and mammary gland development, and placentation. From the seventh week of gestation, the placenta takes over the steroid hormone production and becomes the main source of progesterone until the end of the pregnancy.20, 24 In addition, the placenta initiates the diffusion of nutrients and oxygen from the maternal blood to the fetal blood. Therefore, the placenta is a key organ for fetal growth. From these reports, the current results that a higher birthweight in HRT cycles, compared to ovulatory cycles, has been obtained indicates the possibility that the supplementation of steroid hormones for luteal support might be implicated in the uterine development, placental formation, subsequent fetal growth, and heavier birthweight after implantation. Further studies are required in order to understand the relationship between fetal growth, placentation, and the term of exogenous hormone supplementation for luteal support.
The strengths of this study were that all of the pregnancies from the FET cycles, including spontaneous ovulation and HRT cycles, were adjusted to be treated with luteal support by E2 and progesterone supplementation in a single facility and by the fact that these results regarding neonate birthweights were obtained from only singleton full‐term pregnancies. However, this study was limited by the retrospective design, which prevented the collection of data about the parental characteristics that affect birthweight, such as the smoking history,25 presence of hypertension,26 pre‐eclampsia,27 and other relevant factors.
In summary, the study's finding was that the primary factor to increase the birthweight is the pre‐ or postET hormonal environment of the endometrium, not the frozen‐thawed procedure itself. To the authors’ knowledge, the present study is the first report to show the birthweight difference between ovulatory cycles and HRT cycles. However, the reason for the weight differences among newborns remains unclear. Further studies are required to reveal the factor(s) that increases the birthweight of singletons from HRT cycles.
Disclosures
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This article does not contain any study with human or animal participants that have been performed by any of the authors. This study was approved by the Ethics Committee of Hanabusa Women's Clinic, Kobe, Japan.
Tsuji Y, Otsuki J, Iwasaki T, et al. Retrospective comparative study of the factors affecting birthweights in frozen‐thawed embryo transfer, compared to fresh embryo transfer. Reprod Med Biol. 2017;16:283–289. https://doi.org/10.1002/rmb2.12038
References
- 1. Biggers JD. IVF and embryo transfer: historical origin and development. Reprod Biomed Online. 2012;25:118‐127. [DOI] [PubMed] [Google Scholar]
- 2. Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonald SD, Han Z, Mulla S, et al. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta‐analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138‐148. [DOI] [PubMed] [Google Scholar]
- 4. Belva F, Henriet S, Van den Abbeel E, et al. Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtained by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum Reprod. 2008;23:2227‐2238. [DOI] [PubMed] [Google Scholar]
- 5. Nakashima A, Araki R, Tani H, et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the National Assisted Reproduction Registry of Japan. Fertil Steril. 2013;99:450‐455. [DOI] [PubMed] [Google Scholar]
- 6. Sazonova A, Källen K, Thurin‐Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod. 2012;27:1343‐1350. [DOI] [PubMed] [Google Scholar]
- 7. Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29:618‐627. [DOI] [PubMed] [Google Scholar]
- 8. Perälä MM, Männistö S, Kaartinen NE, et al. Body size at birth is associated with food and nutrient intake in adulthood. PLoS ONE. 2012;7:e46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity – a vicious circle across generations. Int J Obes (Lond). 2012;36:1320‐1324. [DOI] [PubMed] [Google Scholar]
- 10. Chiavaroli V, Marcovecchio ML, de Giorgis T, Diesse L, Chiarelli F, Mohn A. Progression of cardio‐metabolic risk factors in subjects born small and large for gestational age. PLoS ONE. 2014;9:e104278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mäkinen S, Söderström‐Anttila V, Vainio J, Suikkari AM, Tuuri T. Does long in vitro culture promote large for gestational age babies? Hum Reprod. 2013;28:828‐834. [DOI] [PubMed] [Google Scholar]
- 12. Zhu J, Lin S, Li M, et al. Effect of in vitro culture period on birthweight of singleton newborns. Hum Reprod. 2014;29:448‐454. [DOI] [PubMed] [Google Scholar]
- 13. Kaartinen NM, Kananen KM, Rodriguez‐Wallberg KA, Tomás CM, Huhtala HS, Tinkanen HI. Male gender explains increased birthweight in children born after transfer of blastocysts. Hum Reprod. 2015;30:2312‐2320. [DOI] [PubMed] [Google Scholar]
- 14. Goto S, Kadowaki T, Hashimoto H, Kokeguchi S, Shiotani M. Stimulation of endometrium embryo transfer (SEET): injection of embryo culture supernatant into the uterine cavity before blastocyst transfer can improve implantation and pregnancy rates. Fertil Steril. 2007;88:1339‐1343. [DOI] [PubMed] [Google Scholar]
- 15. Veeck L. Abnormal morphology of human oocytes and conceptus In: Veeck L, ed. Atlas of the Human Oocyte and Early Conceptus. Vol. 2. Boca Raton, FL: CRC Press; 1999:151‐179. [Google Scholar]
- 16. Gardner DK, Lane M, Stevens J, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155‐1158. [DOI] [PubMed] [Google Scholar]
- 17. Shih W, Rushford DD, Bourne H, et al. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644‐1653. [DOI] [PubMed] [Google Scholar]
- 18. Liu SY, Teng B, Fu J, Li X, Zheng Y, Sun XX. Obstetric and neonatal outcomes after transfer of vitrified early cleavage embryos. Hum Reprod. 2013;28:2093‐2100. [DOI] [PubMed] [Google Scholar]
- 19. Shi W, Xue X, Zhang S, et al. Perinatal and neonatal outcomes of 494 babies delivered from 972 vitrified embryo transfers. Fertil Steril. 2012;97:1338‐1342. [DOI] [PubMed] [Google Scholar]
- 20. Nishi O, Miyata H, Kinoshita Y, Tominaga T. At what stage does the embryo begin to grow larger in frozen‐thawed embryo transfer?: fetal development from the standpoint of gestational sac diameter and birth weight. J Mamm Ova Res. 2015;32:109‐113. [Google Scholar]
- 21. Szekeres‐Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. Progesterone as an immunomodulatory molecule. Int Immunopharmacol. 2001;1:1037‐1048. [DOI] [PubMed] [Google Scholar]
- 22. Ferando D, Halliday JL, Breheny S, Healy DL. Outcomes of singleton births after blastocyst versus nonblastocyst transfer in assisted reproductive technology. Fertil Steril. 2012;97:579‐584. [DOI] [PubMed] [Google Scholar]
- 23. De Vos A, Janssens R, Van de Velde H, et al. The type of culture medium and the duration of in vitro culture do not influence birthweight of ART singletons. Hum Reprod. 2015;30:20‐27. [DOI] [PubMed] [Google Scholar]
- 24. Malek A. Role of IgG antibodies in association with placental function and immunologic diseases in human pregnancy. Expert Rev Clin Immunol. 2013;9:235‐249. [DOI] [PubMed] [Google Scholar]
- 25. Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health. 2007;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Churchill D, Perry IJ, Beevers DG. Ambulatory blood pressure in pregnancy and fetal growth. Lancet. 1997;349:7‐10. [DOI] [PubMed] [Google Scholar]
- 27. Xiong X, Demianczuk NN, Buekens P, Saunders LD. Am J Obstet Gynecol. 2000;183:148‐155. [DOI] [PubMed] [Google Scholar]


