Abstract
Background
In recent years, uterus transplantation (UTx) has been applied as the treatment for patients with uterine factor infertility worldwide. Thus, the clinical application of UTx in Japan should be considered through both the history of UTx technology development in the world and future prospects.
Methods
Recent information on UTx was collected via a literature survey and the Internet.
Results
Basic research using various animals has been done mainly since 2000. In 2014, the world's first UTx baby was born in Sweden. In total, 24 UTx procedures have been performed at 10 institutes in nine countries and five births were obtained (as of May, 2017). In Japan, the “Project Team for Uterus Transplantation” initiated UTx experiments in 2008 and the “Japan Society for Uterus Transplantation” was organized in March, 2014. In the rest of the world, the “International Society for Uterus Transplantation” was established in January, 2016.
Conclusion
Uterus transplantation is still under development as a reproductive medicine tool and organ transplant procedure. A collaborative system that is not limited by facilities and specialties should strive to build an “all‐Japan” team.
Keywords: ethical issues, Mayer–Rokitansky–Küster–Hauser syndrome, surrogacy, uterine factor infertility, uterus transplantation
1. INTRODUCTION
In recent years, assisted reproductive technology (ART) procedures, such as in vitro fertilization (IVF), intracytoplasmic sperm injection, and embryo cryopreservation, have led to successful pregnancy and childbirth in many infertile couples worldwide. In Japan, 47 322 babies were born in 2014 by using ART, which constituted one‐in‐21 newborns. However, in some cases, it is not possible to achieve conception with this technology. This is primarily related to uterine factor infertility (UFI).1 The acquired gynecologic causes of UFI are uterine leiomyoma, endometrial polyposis, chronic endometritis, Asherman's syndrome, severe adenomyosis,2 intractable endometriosis, and uterine malignancy3, 4 that requires a hysterectomy. Obstetric causes of UFI include intractable post‐partum hemorrhage and malplacentation, wherein a hysterectomy is required following the failure of standard therapy. A congenital absence or anatomical defect of the uterus, such as uterine hypoplasia, Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome,5 uterine malformations, and Müllerian anomalies6 also can contribute to UFI. It is estimated that there are between 60 000 and 70 000 patients in Japan with UFI of reproductive age (20‐40 years old).
For ART treatment in patients with UFI, IVF surrogacy has been applied recently.7 However, in this third‐party ART, the following problems regarding ethical, legal, and social issues (ELSI) exist: (i) ethical: handover and takeover refusal; (ii) legal: custody and childcare rights; and (iii) social: commercialization and medical tourism. In a 2016 survey,8 only 38% of countries permitted gestational carrier arrangements and 56% did not. In recent times, strong restrictions on surrogacy have been placed by countries on patients who are not citizens of that country. Additionally, a notice from the Japan Society of Obstetrics and Gynecology (JSOG, Opinion for Surrogacy; http://www.jsog.or.jp/about_us/view/html/kaikoku/H15_4.html (Japanese)) states that “It is not permitted to implement surrogate pregnancy.”
In October 2014, a significant news report about a new successful treatment for UFI was issued in Sweden: “Birth of the world's first uterus transplantation (UTx) baby.”9 In this review, the clinical application of UTx in Japan through the history of UTx technology development in the world and the present situation and future prospects in Japan are considered.
2. BASIC ANIMAL RESEARCH
The first clinical implementation of UTx was performed in Saudi Arabia in April, 2000.10 The recipient was a 26 year old woman whose uterus was excised due to post‐partum hemorrhage and the donor was a third‐party 46 year old woman who was hysterectomized with a resection of bilateral ovarian cysts. Although the transplant surgery itself succeeded, the transplanted uterus had to be removed due to uterine necrosis, with thrombus formation on the 99th postoperative day. The insufficiency of basic studies in this field was pointed out,11 and since then, basic research using animal species was started. The animal experiments regarding UTx have been conducted on small animals, such as mice,12, 13, 14, 15, 16 rats,17, 18, 19, 20, 21, 22 and rabbits,23, 24, 25, 26 large animals (including pigs27, 28, 29, 30, 31 and sheep32, 33, 34, 35, 36, 37, 38, 39, 40), and non‐human primates like monkeys41, 42, 43, 44, 45, 46, 47, 48 and baboons.49, 50, 51, 52 Many results regarding transplantation techniques, postoperative management, immunosuppression methods, and so on have been obtained.53, 54, 55, 56, 57, 58, 59
However, the significance of basic research using animals might be attenuated because the case number of human UTx is increasing steadily in the world and clinical knowledge has been accumulating.60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 Although the animal experiments might be useful for developing adequate surgical techniques that are associated with UTx, the implementation limits from the viewpoint of animal welfare should be considered further.
3. CLINICAL DEVELOPMENT
Based on the fundamental research results mentioned above, the clinical application of UTx again was initiated abroad. In August, 2011, a Turkish team (Akdeniz University Hospital, Antalya, Turkey) reported a UTx from a brain‐dead woman.71, 72, 73, 74 The donor was a 22 year old multi‐organ donor who died in a traffic accident and the recipient was a 21 year old woman with MRKH syndrome. A successful pregnancy was achieved by cryopreserved‐thawed embryo transfer (ET) in April, 2013, which resulted in a spontaneous abortion. The pregnancies did not result in the birth of babies, even after two trials of ET.
In September, 2012, a UTx between a mother and a daughter was performed at Sahlgrenska University Hospital in Gothenburg, Sweden.75, 76 Ultimately, UTx procedures from nine living donors were performed. Eight of the recipients had MRKH syndrome (27‐38 years old) and one had postoperative cervical carcinoma (33 years old). As donors, five cases were mothers, and a sister, an aunt, a mother‐in‐law, and a friend of the mother were involved. The average age of the donors was 53 years (37‐62 years), five were postmenopausal (three of them had been postmenopausal for ≥5 years). The mean time of the hysterectomy for the donors was 11 h, 37 min (10 h, 17 min to 13 h, 8 min) and the average bleeding volume was 920 mL (300‐2400 mL). The mean time that was required for transplantation to the recipient was 4 h, 46 min (4 h, 10 min to 5 h, 56 min) and the average bleeding volume was 670 mL (250‐1600 mL). A bladder–vaginal fistula occurred as a postoperative complication in a donor, but it was repaired. Two of the transplanted uteri had to be removed due to thrombosis and repetitive intrauterine infections.
In the postoperative course, menstruation was observed in all patients and rejection was observed in five patients, but it was relieved by steroid administration.77 From March, 2014, cryopreserved embryos were thawed and transferred sequentially and the first infant was born in September, 2014.9, 78 In the recipient of this case with MRKH syndrome, pre‐eclampsia occurred at 31 weeks of gestation and a 1775 g boy was born via an emergency Cesarean section. Since then, five births have been officially reported to date as of April, 2017.
4. ETHICAL, LEGAL, AND SOCIAL ISSUES REGARDING UTERUS TRANSPLANTATION
Uterus transplantation is a medical treatment with two aspects: reproductive medicine and an organ transplant. When discussing the ELSI for UTx, it must be remembered that the concepts of third‐party ART and organ transplantation are different from culture to culture due to diversified philosophical and religious traditions79 and that the feelings toward reproduction are different from person to person, even within the same culture. In addition, the ELSI for progressive medical performance will change over time. Furthermore, uterine tissue engineering has been tried in rat models.80, 81, 82 If the artificial uterus or placenta is able to regenerate, UTx might become unnecessary for the treatment of UFI.
Although many articles from various regions around the world have been published so far,83, 84, 85, 86, 87, 88, 89, 90 the ELSI of UTx discussed here are applicable to the present situation in Japan.
4.1. Ethics
From the standpoint of reproductive medicine involving a third party, UTx is comparable with surrogacy.91, 92, 93, 94 As a kind of organ transplant, the problem of transplantation that is unrelated to life‐threatening organ use is faced. Although many ethical problems exist regarding the clinical application of UTx, ~80% of Japanese general citizens have accepted UTx ethically, which was higher than the acceptance rate of surrogacy,95, 96, 97 similar to other countries.98, 99
4.2. Legislation
Japan is a unique country, in which no law is available for reproductive medicine. This medicine is regulated only by a notice of JSOG that consists of doctors belonging to a special medical field. Organ transplantation involves hearts, lungs, livers, pancreases, kidneys, and small intestines from deceased donors and is regulated by the Organ Transplant Law that went into effect in 1997. However, the transplant implementation from living donors is only regulated by the ethical guidelines of The Japan Society for Transplant (JST). In other words, UTx from a living donor can be performed without it being illegal today in Japan. Of course, it goes without saying that comprehensive legal development should be necessary.
4.3. Social consensus
In order to obtain the social consensus for UTx, the authors organized a society concerning UTx and underwent several meetings, as mentioned below. However, the recognition rate of UTx by general citizens was 10%‐15%, even after the successful UTx and live birth in Sweden was reported.96, 97 Compared to this, the term “surrogacy” was well known to >80% of them.
Although three other guidelines than those indicated below have been proposed (those of the International Federation of Gynecology and Obstetrics Committee,100 The Montreal Criteria,1 and The Indianapolis consensus101, 102), they must be revised in order to adapt to the current conditions of clinical UTx research.103
5. CURRENT SITUATION IN JAPAN
5.1. Project Team for Uterus Transplantation
Six individuals belonging to the Department of Obstetrics and Gynecology, Keio Gijuku University School of Medicine, Tokyo, the Department of Plastic, Reconstructive and Aesthetic Surgery, The University of Tokyo Hospital, Tokyo, or the Department of Human Health Sciences, Kyoto University Graduate School of Medicine, Kyoto, Japan, organized the “Project Team for Uterus Transplantation” (PTUTx) (www.pt-ut.org) in 2008 and started autografting experiments by using cynomolgus macaques in January 2009.41, 42 As a result, a natural pregnancy was achieved in the sixth case and the child was acquired in March, 2012.43, 44 Even after that, allogeneic transplant experiments have been carried out as a clinical approach to UTx.45, 46, 47, 48
Furthermore, because ethical and social problems could be important for actual clinical application,104, 105 in September, 2012, an ethics committee with seven outside parties was established in the PTUTx. This committee includes not only obstetricians and gynecologists, doctors in other departments, and health professionals, such as a midwife, a nurse, and a clinical psychologist, but also specialists in research ethics and bioethics and a representative of the infertility patients’ association. After eight discussions over 2 years, in December, 2014, the “Guidelines for Clinical Research on Uterus Transplantation” was published (Appendix 1). This guideline was sent to JSOG, the Japan Society for Reproductive Medicine (JSRM), and JST for review. At JSOG, a subcommittee on UTx was established within the Ethics Committee and discussions were made. The JSRM replied that the society would follow JSOG's policy.
In addition, because the authors thought that it was necessary to provide information to the society in general, to discuss the UTx situation, to investigate the consciousness of donors, recipients, and general citizens, and to obtain social consensus, a specific academic society was organized, as stated below.
5.2. Japan Society for Uterus Transplantation
As a place to deepen the social understanding and discussion of UTx, the “Japan Society for Uterus Transplantation” (JSUTx) (www.js-ut.org) was established in March, 2014. The executive committee of JSUTx consists of experts in each field that is related to UTx as four advisers, 23 directors, three secretaries, and one auditor. Six board meetings already have been held, consisting of academic and public lectures. At the fourth meeting on November 3, 2015, four women with MRKH syndrome themselves stated the intention for UTx as recipients. At the sixth meeting on April 9, 2017, a woman with MRKH syndrome and a hysterectomized survivor of cervical cancer as recipient candidates and a man with gender identity disorder (female‐to‐male) as a donor candidate also talked about their mental troubles and expectations of UTx.
6. TRENDS IN THE REST OF THE WORLD
6.1. Trends in foreign countries
Following the success in Sweden, the clinical application of UTx is planned and performed in many countries in the world. In September, 2015, planned UTx using deceased donors to 10 recipients (18‐36 years old) was revealed at Imperial College, London, UK.106 Likewise, in November of the same year, for eight recipients (25‐35 years old) at Limoges Hospital, Limoges, France,107 and 10 recipients (21‐39 years old) at Cleveland Clinic, Cleveland, OH, USA, UTx was planned by using deceased donors.108
Furthermore, in November, 2015, Xijing Hospital in Xi'an, China, announced that a UTx was performed successfully between a 43 year old mother and a 22 year old daughter.109 The UTx procedures involved 38 doctors with 11 areas of expertise and took 14 h with the help of robotic surgical tools. In 2016, the Cleveland Clinic performed its first UTx in February, but the transplanted uterus was removed 2 weeks after surgery due to complications.108 In September, four UTx procedures using living donors were performed at Baylor University Medical Center, Dallas, TX, USA, but three procedures were unsuccessful.110 Furthermore, UTx procedures from two living and two deceased donors in the Czech Republic (Motol University Hospital, Prague, Czech Republic)111 and a UTx from a living donor in Germany (Tübingen University Hospital, Tübingen, Germany)112 were performed in October, 2016. A team in Serbia also completed a UTx in March, 2017 (M. Brännström, 2017, personal communication). Most recently, an Indian team (Galaxy Care Hospital, Pune, India) performed a UTx procedure between a mother and a daughter in May, 2017.113
Table 1 shows the summary of clinical UTx procedures that were performed before May, 2017. In total, 24 procedures were performed from 20 living and four deceased donors. Twenty‐two (92%) of the recipients were women with MRKH syndrome. Eight (33%) uteri had to be removed due to failure; therefore, it is hard to state that UTx is a reliable procedure.
Table 1.
Clinical application of uterus transplantation worldwide (n=24 cases, as of May 2017)
| Year | Country | Donor (N) | Recipient (N) |
|---|---|---|---|
| 2000 | Saudi Arabia | Living (1) | Post‐partum hemorrhage (1) |
| 2011 | Turkey | Deceased (1) | MRKH syndrome (1) |
| 2012‐2013 | Sweden | Living (9) | MRKH syndrome (8)/cervical cancer (1) |
| 2015 | China | Living (1) | MRKH syndrome (1) |
| 2016 | USA (Cleveland) | Deceased (1) | MRKH syndrome (1) |
| 2016 | USA (Baylor) | Living (4) | MRKH syndrome (4) |
| 2016 | Czech Republic | Living (2)/deceased (2) | MRKH syndrome (4) |
| 2016 | Germany | Living (1) | MRKH syndrome (1) |
| 2017 | Serbia | Living (1) | MRKH syndrome (1) |
| 2017 | India | Living (1) | MRKH syndrome (1) |
| Total | 10 institutes in 9 countries | Living: 20 | MRKH syndrome: 22 |
| Deceased: 4 | Others: 2 |
MRKH, Mayer–Rokitansky–Küster–Hauser.
6.2. International Society for Uterus Transplantation
With the call of Professor Mats Brännström of Sahlgrenska University in Sweden, a founding meeting of the “International Society for Uterus Transplantation” (ISUTx) (www.isutx.org) was held in Gothenburg on January 8‐9, 2016. Seventy researchers from 20 institutions in 17 countries gathered together. After reporting the circumstances of each country, the articles of by laws, membership, global case registration system, and schedule of academic congresses were discussed. For the executive committee of ISUTx, Professor Brännström as President, Dr. Suganuma as Vice‐President, two secretaries, and two treasurers were chosen. The names of 12 board members from all over the world and two advisory members also were suggested.
The specialized medical fields of the 71 founding members of ISUTx differ widely (Table 2). Although most are gynecologists, transplantation surgeons, reproductive medical doctors, and specialists in other fields are involved. The team leader in each institute or country also is distributed. This means that UTx includes different medical aspects for third‐party ART and organ transplantation.
Table 2.
Founding members of the International Society for Uterus Transplantation
| Country | Gynecology | Reproductive medicine | Transplantation surgery | Others (N) |
|---|---|---|---|---|
| Sweden | ④ | 2 | 3 | Obstetrics (1) |
| Nephrology (1) | ||||
| Anesthesiology (2) | ||||
| Phycology (1) | ||||
| Pathology(1) | ||||
| Medical film photography (1) | ||||
| Argentina | 1 | ① | ||
| Australia | ① | |||
| Belgium | ① | 1 | Nephrology (1) | |
| China | ② | Nursing (1) | ||
| Colombia | ③ | |||
| Czech Republic | 2 | ① | ||
| France (Limoges) | ③ | Nephrology (1) | ||
| France (Paris) | ③ | 1 | ||
| Germany | 1 | ① | ||
| India | ① | |||
| Japan | ① | 1 | ||
| Mexico | ② | |||
| Singapore | ② | Plastic and reconstructive surgery (3) | ||
| Spain | ② | |||
| Turkey | 1 | Plastic and reconstructive surgery (②) | ||
| UK | ② | Phycology (1) | ||
| USA (Boston) | ① | |||
| USA (Cleveland) | 2 | ① | ||
| USA (Dallas) | 1 | ② | Obstetrics (1) | |
| USA (Omaha) | 1 | ② | Nursing (1) | |
| Total [number of team leaders] | 31[12] | 10 [2] | 12 [6] | 18 [1] |
○, the field to which the team leader belongs, according to the institute.
It was decided that the first international meeting would be held in Gothenburg on September 18‐19, 2017, with a precongress course to demonstrate the techniques of live‐donation UTx on September 17. The program will consist of symposia that discuss several relevant topics of UTx: (i) the surgical techniques of organ procurement in live‐donor UTx; (ii) the surgical techniques of organ procurement in deceased‐donor UTx; (iii) venous outflow options; (iv) the surgical technique of UTx for the recipient; (v) immunosuppression at UTx; (vi) rejection diagnosis and grading; (vii) IVF before and after UTx; (viii) obstetric monitoring after UTx; (ix) psychology regarding UTx; and (x) the ethics around UTx. These items seem to involve actual issues for the clinical application of UTx.
7. FUTURE PROSPECTS FOR CLINICAL APPLICATION IN JAPAN
For patients with UFI, there is no doubt that UTx can be an alternative to surrogacy and adoption. However, this medical treatment requires not only gynecologists and transplant surgeons, but also many medical staff members (Figure 1). Psycological support for the recipients and donors of UTx is essential.114, 115, 116, 117, 118
Figure 1.
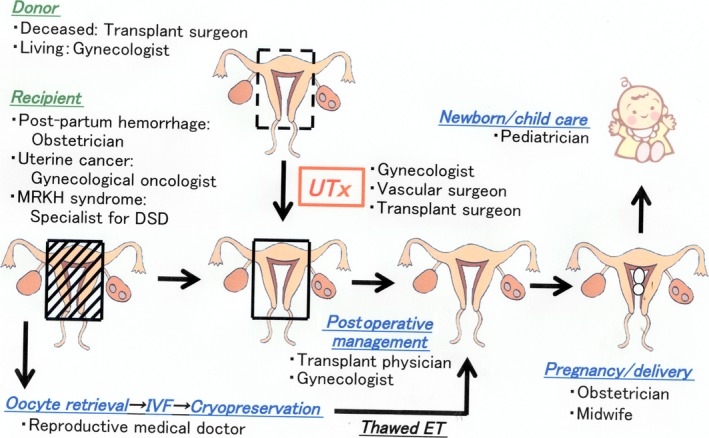
Medical fields and staff members that are involved in the clinical process of uterus transplantation. DSD, disorders of sexual development; ET, embryo transfer; IVF: in vitro fertilization; MRKH, Mayer–Rokitansky–Küster–Hauser; UTx, uterus transplantation
Of course, even in Japan, UTx cannot be carried out only by the authors’ team. The facility that will actually perform UTx needs to form a UTx group therein. At that time, PTUTx will join as an advisory board on the surgical procedures that have been obtained through animal experiments, clinical management, and ethical considerations so far. In doing so, approval of each institutional ethics committee is necessary. Additionally at that time, because JSUTx consists of many occupational types and experts involved in UTx, its ethics committee can suggest the approach for technical problems and ELSI through discussion among the committee members, as well as consultation with various academic societies, such as JSOG, JSRM, and JST through JSUTx.
8. CONCLUSION
The present situation and prospects of UTx have been outlined. Based on these, it cannot be denied that UTx is still under development as a reproductive medicine and organ transplant procedure. Future efforts will be required to treat patients with UFI successfully.101, 119, 120, 121, 122, 123, 124, 125, 126 Additonally, in Japan, a collaborative system that is not limited by facilities and specialties should strive to build an “all‐Japan” team.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This article does not contain any study with human or animal participants that has been performed by any of the authors.
ACKNOWLEDGEMENTS
Parts of this manuscript were presented as “overviews” at symposia entitled “Present situation of UTx” at the IFFS/JSRM International Meeting 2015 (April 26‐29, 2015, Yokohama, Japan) and “Treatment for women with UFI in our country: UTx vs surrogacy” at The 61st Congress of Japan Society for Reproductive Medicine (November 3‐4, 2016, Yokohama, Japan). This work was partially supported by a Japan Society for the Promotion of Science KAKENHI Grant No. 15K15852, Tokyo, Japan. We would like to thank Editage for English‐language editing.
APPENDIX 1. Guidelines For Clinical Research On Uterus Transplantation (By the Project Team for Uterus Transplantation, December 17, 2014.)
1.1.
Uterus transplantation, unlike the transplantation of traditional life‐sustaining organs, in view of the reproductive health and rights of women, is positioned as organ transplantation for the purpose of the improvement of quality of life by allowing pregnancy and childbirth.
Any living and deceased donors are considered as UTx candidates, as well as for other organ transplants. Their dignity, rights, and safety must be prioritized above anything else. In a living donor, any intimidation must be eliminated and the provision of voluntary decisions must be ensured. Also, in order to relieve any mental burden, a support system of continued counseling is required.
The recipients of UTx are women with UFI who are absolutely unable to get pregnant medically and who strongly hope to bear children. Noting their physical risk and burden, it is necessary to provide sufficient information to them. In addition, a support system is required to relieve any mental burden through counseling of the recipients, as well as of the donors.
The rights and welfare of the children who are born by UTx must be guaranteed to the maximum.
Before UTx implementation, the treatment procedures, including the problems and disadvantages to be expected, should be explained in advance to the recipient and the donor by using a document that fully describes these risks, sufficient understanding of the participant to voluntarily consent should be obtained, and the ability to store the consent document should be possible.
Regarding the costs involved in UTx, it is necessary to obtain an agreement between the patient and the practitioner in advance. Additionally, mediation of commercial uterus provision or a similar action should never be taken.
In clinical studies of UTx, in addition to compliance with the laws and regulations, guidelines, and ethical principles, compliance with the guidelines of various medical societies needs to come into force with the approval of the implementation facility's ethics committee.
Basic experiments on animals, including non‐human primates, are needed to train to achieve adequate surgical techniques for the implementation of UTx.
It is recognized that the UTx procedure uses medicine across different fields. A medical team that includes a wide range of professionals must be enforced.
In order to obtain a social consensus on the clinical application of UTx, information provision and opinion collection must be performed in addition to continuous research.
Suganuma N, Hayashi A, Kisu I, Banno K, Hara H, Mihara M. Uterus transplantation: Toward clinical application in Japan. Reprod Med Biol. 2017;16:305‐313. https://doi.org/10.1002/rmb2.12048
REFERENCES
- 1. Lefkowitz A, Edwards M, Balayla J. The Montreal Criteria for the Ethical Feasibility of Uterine Transplantation. Transpl Int. 2012;25:439‐447. [DOI] [PubMed] [Google Scholar]
- 2. Geirsson RT. Adenomyosis to uterine transplantation. Acta Obstet Gynecol Scand. 2010;89:1372‐1373. [DOI] [PubMed] [Google Scholar]
- 3. Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S43‐S103. [DOI] [PubMed] [Google Scholar]
- 4. Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105‐S143. [DOI] [PubMed] [Google Scholar]
- 5. Oppelt P, Renner SP, Kellermann A, et al. Clinical aspects of Mayer–Rokitansky–Kuester–Hauser syndrome: recommendations for clinical diagnosis and staging. Hum Reprod. 2006;21:792‐797. [DOI] [PubMed] [Google Scholar]
- 6. Cheroki C, Krepischi‐Santos AC, Szuhai K, et al. Genomic imbalances associated with mullerian aplasia. J Med Genet. 2008;45:228‐232. [DOI] [PubMed] [Google Scholar]
- 7. Brinsden PR. Gestational surrogacy. Hum Reprod Update. 2003;9:483‐491. [DOI] [PubMed] [Google Scholar]
- 8. International Federation of Fertility Societies . IFFS Surveilance 2016. Global Reprod Health. 2016;1:1‐143. [Google Scholar]
- 9. Brännström M, Johannesson L, Bokström H, et al. Livebirth after uterus transplantation. Lancet. 2015;385:607‐616. [DOI] [PubMed] [Google Scholar]
- 10. Fageeh W, Raffa H, Jabbad H, Marzouki A. Transplantation of the human uterus. Int J Gynaecol Obstet. 2002;76:245‐251. [DOI] [PubMed] [Google Scholar]
- 11. Kandela P. Uterine transplantation failure causes Saudi Arabian government clampdown. Lancet. 2000;356:838. [PubMed] [Google Scholar]
- 12. Racho El‐Akouri R, Kurlberg G, Dindelegan G, Mölne J, Wallin A, Brännström M. Heterotopic uterine transplantation by vascular anastomosis in the mouse. J Endocrinol. 2002;174:157‐166. [DOI] [PubMed] [Google Scholar]
- 13. Racho El‐Akouri R, Kurlberg G, Brännström M. Successful uterine transplantation in the mouse: pregnancy and post‐natal development of offspring. Hum Reprod. 2003;18:2018‐2023. [DOI] [PubMed] [Google Scholar]
- 14. Racho El‐Akouri R, Wranning CA, Mölne J, Kurlberg G, Brännström M. Pregnancy in transplanted mouse uterus after long‐term cold ischaemic preservation. Hum Reprod. 2003;18:2024‐2030. [DOI] [PubMed] [Google Scholar]
- 15. El‐Akouri RR, Mölne J, Groth K, Kurlberg G, Brännström M. Rejection patterns in allogeneic uterus transplantation in the mouse. Hum Reprod. 2006;21:436‐442. [DOI] [PubMed] [Google Scholar]
- 16. Wranning CA, El‐Akouri RR, Groth K, Mölne J, Parra AK, Brännström M. Rejection of the transplanted uterus is suppressed by cyclosporine A in a semi‐allogeneic mouse model. Hum Reprod. 2007;22:372‐379. [DOI] [PubMed] [Google Scholar]
- 17. Jiga LP, Lupu CM, Zoica BS, Ionac M. Experimental model of heterotopic uterus transplantation in the laboratory rat. Microsurgery. 2003;23:246‐250. [DOI] [PubMed] [Google Scholar]
- 18. Wranning CA, Akhi SN, Kurlberg G, Brännström M. Uterus transplantation in the rat: model development, surgical learning and morphological evaluation of healing. Acta Obstet Gynecol Scand. 2008;87:1239‐1247. [DOI] [PubMed] [Google Scholar]
- 19. Wranning CA, Akhi SN, Diaz‐Garcia C, Brännström M. Pregnancy after syngeneic uterus transplantation and spontaneous mating in the rat. Hum Reprod. 2011;26:553‐558. [DOI] [PubMed] [Google Scholar]
- 20. Akhi SN, Diaz‐Garcia C, El‐Akouri RR, Wranning CA, Mölne J, Brännström M. Uterine rejection after allogeneic uterus transplantation in the rat is effectively suppressed by tacrolimus. Fertil Steril. 2013;99:862‐870. [DOI] [PubMed] [Google Scholar]
- 21. Díaz‐García C, Akhi SN, Martínez‐Varea A, Brännström M. The effect of warm ischemia at uterus transplantation in a rat model. Acta Obstet Gynecol Scand. 2013;92:152‐159. [DOI] [PubMed] [Google Scholar]
- 22. Díaz‐García C, Johannesson L, Shao R, Bilig H, Brännström M. Pregnancy after allogeneic uterus transplantation in the rat: perinatal outcome and growth trajectory. Fertil Steril. 2014;102:1545‐1552. [DOI] [PubMed] [Google Scholar]
- 23. Sieunarine K, Doumplis D, Kuzmin E, et al. Uterine allotransplantation in the rabbit model using a macrovascular patch technique. Int Surg. 2008;93:288‐294. [PubMed] [Google Scholar]
- 24. Saso S, Hurst S, Chatterjee J, et al. Test of long‐term uterine survival after allogeneic transplantation in rabbits. J Obstet Gynaecol Res. 2014;40:754‐762. [DOI] [PubMed] [Google Scholar]
- 25. Saso S, Petts G, Chatterjee J, et al. Uterine allotransplantation in a rabbit model using aorto‐caval anastomosis: a long‐term viability study. Eur J Obstet Gynecol Reprod Biol. 2014;182:185‐193. [DOI] [PubMed] [Google Scholar]
- 26. Saso S, Petts G, David AL, et al. Achieving an early pregnancy following allogeneic uterine transplantation in a rabbit model. Eur J Obstet Gynecol Reprod Biol. 2015;185:164‐169. [DOI] [PubMed] [Google Scholar]
- 27. Sieunarine K, Zakaria FB, Boyle DC, et al. Possibilities for fertility restoration: a new surgical technique. Int Surg. 2005;90:249‐256. [PubMed] [Google Scholar]
- 28. Wranning CA, El‐Akouri RR, Lundmark C, et al. Auto‐transplantation of the uterus in the domestic pig (Sus scrofa): surgical technique and early reperfusion events. J Obstet Gynaecol Res. 2006;32:358‐367. [DOI] [PubMed] [Google Scholar]
- 29. Sieunarine K, Boyle DC, Corless DJ, et al. Pelvic vascular prospects for uterine transplantation. Int Surg. 2006;91:217‐222. [PubMed] [Google Scholar]
- 30. Avison DL, DeFaria W, Tryphonopoulos P, et al. Heterotopic uterus transplantation in a swine model. Transplantation. 2009;88:465‐469. [DOI] [PubMed] [Google Scholar]
- 31. Hurst SA, Smith JR, Del Priore G. Experiences in uterine transplantation. Transplantation. 2010;89:769. [DOI] [PubMed] [Google Scholar]
- 32. Ramirez ER, Ramirez DK, Pillari VT, Vasquez H, Ramirez HA. Modified uterine transplant procedure in the sheep model. J Minim Invasive Gynecol. 2008;15:311‐314. [DOI] [PubMed] [Google Scholar]
- 33. Wranning CA, Dahm‐Kähler P, Mölne J, Nilsson UA, Enskog A, Brännström M. Transplantation of the uterus in the sheep: oxidative stress and reperfusion injury after short‐time cold storage. Fertil Steril. 2008;90:817‐826. [DOI] [PubMed] [Google Scholar]
- 34. Dahm‐Kähler P, Wranning C, Lundmark C, et al. Transplantation of the uterus in sheep: methodology and early reperfusion events. J Obstet Gynaecol Res. 2008;34:784‐793. [DOI] [PubMed] [Google Scholar]
- 35. Dittrich R, Maltaris T, Mueller A, et al. Uterus cryopreservation in the sheep: one step closer to uterus transplantation. In Vivo. 2010;24:629‐634. [PubMed] [Google Scholar]
- 36. Ramirez ER, Ramirez Nessetti DK, Nessetti MB, et al. Pregnancy and outcome of uterine allotransplantation and assisted reproduction in sheep. J Minim Invasive Gynecol. 2011;18:238‐245. [DOI] [PubMed] [Google Scholar]
- 37. Gauthier T, Bertin F, Fourcade L, et al. Uterine allotransplantation in ewes using an aortocava patch. Hum Reprod. 2011;26:3028‐3036. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez‐Pinto IM, Tryphonopoulos P, Avison DL, et al. Uterus transplantation model in sheep with heterotopic whole graft and aorta and cava anastomoses. Transplant Proc. 2013;45:1802‐1804. [DOI] [PubMed] [Google Scholar]
- 39. Wei L, Xue T, Yang H, et al. Modified uterine allotransplantation and immunosuppression procedure in the sheep model. PLoS ONE. 2013;8:e81300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saso S, Petts G, Thum MY, et al. Achieving uterine auto‐transplantation in a sheep model using iliac vessel anastomosis: a short‐term viability study. Acta Obstet Gynecol Scand. 2015;94:245‐252. [DOI] [PubMed] [Google Scholar]
- 41. Mihara M, Kisu I, Hara H, et al. Uterus autotransplantation in cynomolgus macaques: intraoperative evaluation of uterine blood flow using indocyanine green. Hum Reprod. 2011;26:3019‐3027. [DOI] [PubMed] [Google Scholar]
- 42. Kisu I, Banno K, Mihara M, et al. Indocyanine green fluorescence imaging for evaluation of uterine blood flow in cynomolgus macaque. PLoS ONE. 2012;7:e35124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mihara M, Kisu I, Hara H, et al. Uterine autotransplantation in cynomolgus macaques: the first case of pregnancy and delivery. Hum Reprod. 2012;27:2332‐2340. [DOI] [PubMed] [Google Scholar]
- 44. Kisu I, Mihara M, Banno K, et al. Uterus allotransplantation in cynomolgus macaque: a preliminary experience with non‐human primate models. J Obstet Gynaecol Res. 2014;40:907‐918. [DOI] [PubMed] [Google Scholar]
- 45. Kisu I, Banno K, Mihara M, et al. A surgical technique using the ovarian vein in non‐human primate models of potential living‐donor surgery of uterus transplantation. Acta Obstet Gynecol Scand. 2015;94:942‐948. [DOI] [PubMed] [Google Scholar]
- 46. Obara H, Kisu I, Kato Y, et al. Surgical technique for allogeneic uterus transplantation in macaques. Sci Rep 2016;6:35989 https://doi.org/10.1038/srep35989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adachi M, Kisu I, Nagai T, et al. Evaluation of allowable time and histopathological changes in warm ischemia of the uterus in cynomolgus monkey as a model for uterus transplantation. Acta Obstet Gynecol Scand. 2016;95:991‐998. [DOI] [PubMed] [Google Scholar]
- 48. Kisu I, Kato Y, Yamada Y, et al. Organ perfusion for uterus transplantation in non‐human primates with assumed procurement of a uterus from a brain‐dead donor. Transplant Proc. 2016;48:1266‐1269. [DOI] [PubMed] [Google Scholar]
- 49. Enskog A, Johannesson L, Chai DC, et al. Uterus transplantation in the baboon: methodology and long‐term function after auto‐transplantation. Hum Reprod. 2010;25:1980‐1987. [DOI] [PubMed] [Google Scholar]
- 50. Johannesson L, Enskog A, Dahm‐Kähler P, et al. Uterus transplantation in a non‐human primate: long‐term follow‐up after autologous transplantation. Hum Reprod. 2012;27:1640‐1648. [DOI] [PubMed] [Google Scholar]
- 51. Johannesson L, Enskog A, Mölne J, et al. Preclinical report on allogeneic uterus transplantation in non‐human primates. Hum Reprod. 2013;28:189‐198. [DOI] [PubMed] [Google Scholar]
- 52. Tryphonopoulos P, Tzakis AG, Tekin A, et al. Allogeneic uterus transplantation in baboons: surgical technique and challenges to long‐term graft survival. Transplantation. 2014;98:e51‐e56. [DOI] [PubMed] [Google Scholar]
- 53. Brännström M, Wranning CA. Uterus transplantation: how far away from human trials? Acta Obstet Gynecol Scand. 2008;87:1097‐1100. [DOI] [PubMed] [Google Scholar]
- 54. Del Priore G, Schlatt S, Malanowska‐Stega J. Uterus transplant techniques in primates: 10 years’ experience. Exp Clin Transplant. 2008;6:87‐94. [PubMed] [Google Scholar]
- 55. Brännström M, Wranning CA, Altchek A. Experimental uterus transplantation. Hum Reprod Update. 2010;16:329‐345. [DOI] [PubMed] [Google Scholar]
- 56. Brännström M, Diaz‐Garcia C, Hanafy A, Olausson M, Tzakis A. Uterus transplantation: animal research and human possibilities. Fertil Steril. 2012;97:1269‐1276. [DOI] [PubMed] [Google Scholar]
- 57. Díaz‐García C, Johannesson L, Enskog A, Tzakis A, Olausson M, Brännström M. Uterine transplantation research: laboratory protocols for clinical application. Mol Hum Reprod. 2012;18:68‐78. [DOI] [PubMed] [Google Scholar]
- 58. Kisu I, Banno K, Mihara M, Suganuma N, Aoki D. Current status of uterus transplantation in primates and issues for clinical application. Fertil Steril. 2013;100:280‐294. [DOI] [PubMed] [Google Scholar]
- 59. Kisu I, Banno K, Mihara M, et al. Uterine transplantation in primates: a mini‐review of the literature. Transplant Proc. 2014;46:1212‐1216. [DOI] [PubMed] [Google Scholar]
- 60. Wranning CA, Mölne J, El‐Akouri RR, Kurlberg G, Brännström M. Short‐term ischaemic storage of human uterine myometrium – basic studies towards uterine transplantation. Hum Reprod. 2005;20:2736‐2744. [DOI] [PubMed] [Google Scholar]
- 61. Del Priore G, Stega J, Sieunarine K, Ungar L, Smith JR. Human uterus retrieval from a multi‐organ donor. Obstet Gynecol. 2007;109:101‐104. [DOI] [PubMed] [Google Scholar]
- 62. Sieunarine K, Lindsay I, Ungar L, Del Priore G, Smith JR. Cold ischaemic preservation of human uterine tissue. Int Surg. 2008;93:366‐372. [PubMed] [Google Scholar]
- 63. Díaz‐García C, Akhi SN, Wallin A, Pellicer A, Brännström M. First report on fertility after allogeneic uterus transplantation. Acta Obstet Gynecol Scand. 2010;89:1491‐1494. [DOI] [PubMed] [Google Scholar]
- 64. Hanafy A, Diaz‐Garcia C, Olausson M, Brännström M. Uterine transplantation: one human case followed by a decade of experimental research in animal models. Aust N Z J Obstet Gynaecol. 2011;51:199‐203. [DOI] [PubMed] [Google Scholar]
- 65. Johannesson L, Diaz‐Garcia C, Leonhardt H, et al. Vascular pedicle lengths after hysterectomy: toward future human uterus transplantation. Obstet Gynecol. 2012;119:1219‐1225. [DOI] [PubMed] [Google Scholar]
- 66. Saso S, Ghaem‐Maghami S, Chatterjee J, et al. Immunology of uterine transplantation: a review. Reprod Sci. 2012;19:123‐134. [DOI] [PubMed] [Google Scholar]
- 67. Saso S, Logan K, Abdallah Y, et al. Use of cyclosporine in uterine transplantation. J Transplant. 2012;2012:134936 https://doi.org/10.1155/2012/134936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saso S, Hamed AH, Doctor C, et al. Is there a role for transplantation in gynecologic oncology? Autotransplantation and other lessons. Int J Gynecol Cancer. 2013;23:413‐416. [DOI] [PubMed] [Google Scholar]
- 69. Gauthier T, Piver P, Pichon N, et al. Uterus retrieval process from brain dead donors. Fertil Steril. 2014;102:476‐482. [DOI] [PubMed] [Google Scholar]
- 70. Mölne J, Broecker V, Ekberg J, Nilsson O, Dahm‐Kähler P, Brännström M. Monitoring of human uterus transplantation with cervical biopsies: a provisional scoring system for rejection. Am J Transplant. 2017;17:1628‐1636. [DOI] [PubMed] [Google Scholar]
- 71. Ozkan O, Akar ME, Ozkan O, et al. Preliminary results of the first human uterus transplantation from a multiorgan donor. Fertil Steril. 2013;99:470‐476. [DOI] [PubMed] [Google Scholar]
- 72. Ozkan O, Akar ME, Erdogan O, Ozkan O, Hadimioglu N. Uterus transplantation from a deceased donor. Fertil Steril. 2013;100:e41. [DOI] [PubMed] [Google Scholar]
- 73. Erman Akar M, Ozkan O, Aydinuraz B, et al. Clinical pregnancy after uterus transplantation. Fertil Steril. 2013;100:1358‐1363. [DOI] [PubMed] [Google Scholar]
- 74. Akar ME, Ozkan O, Ozekinci M, Sindel M, Yildirim F, Oguz N. Uterus retrieval in cadaver: technical aspects. Clin Exp Obstet Gynecol. 2014;41:293‐295. [PubMed] [Google Scholar]
- 75. Hansen A. Swedish surgeons report world's first uterus transplantations from mother to daughter. BMJ. 2012;345:e6357. [DOI] [PubMed] [Google Scholar]
- 76. Brännström M, Johannesson L, Dahm‐Kähler P, et al. First clinical uterus transplantation trial: a six‐month report. Fertil Steril. 2014;101:1228‐1236. [DOI] [PubMed] [Google Scholar]
- 77. Johannesson L, Kvarnström N, Mölne J, et al. Uterus transplantation trial: 1‐year outcome. Fertil Steril. 2015;103:199‐204. [DOI] [PubMed] [Google Scholar]
- 78. Brännström M. The Swedish uterus transplantation project: the story behind the Swedish uterus transplantation project. Acta Obstet Gynecol Scand. 2015;94:675‐679. [DOI] [PubMed] [Google Scholar]
- 79. Serour GI, Dickens BM. Assisted reproduction developments in the Islamic world. Int J Gynaecol Obstet. 2001;74:187‐193. [DOI] [PubMed] [Google Scholar]
- 80. Hellström M, El‐Akouri RR, Sihlbom C, et al. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014;10:5034‐5042. [DOI] [PubMed] [Google Scholar]
- 81. Hellström M, Moreno‐Moya JM, Bandstein S, et al. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil Steril. 2016;106:487‐496; e1. [DOI] [PubMed] [Google Scholar]
- 82. Hellström M, Bandstein S, Brännström M. Uterine tissue engineering and the future of uterus transplantation. Ann Biomed Eng. 2017;45:1718‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nair A, Stega J, Smith JR, Del Priore G. Uterus transplant: evidence and ethics. Ann N Y Acad Sci. 2008;1127:83‐91. [DOI] [PubMed] [Google Scholar]
- 84. Catsanos R, Rogers W, Lotz M. The ethics of uterus transplantation. Bioethics. 2013;27:65‐73. [DOI] [PubMed] [Google Scholar]
- 85. Olausson M, Johannesson L, Brattgård D, et al. Ethics of uterus transplantation with live donors. Fertil Steril. 2014;102:40‐43. [DOI] [PubMed] [Google Scholar]
- 86. Arora KS, Blake V. Uterus transplantation: the ethics of moving the womb. Obstet Gynecol. 2015;125:971‐974. [DOI] [PubMed] [Google Scholar]
- 87. Farrell RM, Falcone T. Uterine transplant: new medical and ethical considerations. Lancet. 2015;385:581‐582. [DOI] [PubMed] [Google Scholar]
- 88. Dickens BM. Legal and ethical issues of uterus transplantation. Int J Gynaecol Obstet. 2016;133:125‐128. [DOI] [PubMed] [Google Scholar]
- 89. Bayefsky MJ, Berkman BE. The ethics of allocating uterine transplants. Camb Q Healthc Ethics. 2016;25:350‐365. [DOI] [PubMed] [Google Scholar]
- 90. Wilkinson S, Williams NJ. Should uterus transplants be publicly funded? J Med Ethics. 2016;42:559‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Semba Y, Chang C, Hong H, Kamisato A, Kokado M, Muto K. Surrogacy: donor conception regulation in Japan. Bioethics. 2010;24:348‐357. [DOI] [PubMed] [Google Scholar]
- 92. Grynberg M, Ayoubi JM, Bulletti C, Frydman R, Fanchin R. Uterine transplantation: a promising surrogate to surrogacy? Ann N Y Acad Sci. 2011;1221:47‐53. [DOI] [PubMed] [Google Scholar]
- 93. Robertson JA. Other women's wombs: uterus transplants and gestational surrogacy. J Law Biosci. 2016;3:68‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Testa G, Koon EC, Johannesson L. Living donor uterus transplant and surrogacy: ethical analysis according to the principle of equipoise. Am J Transplant. 2017;17:912‐916. [DOI] [PubMed] [Google Scholar]
- 95. Hayashi A, Hirai S, Tsutsumishita Y, Kisu I, Mihara M, Suganuma N. Awareness Survey on Clinical Application of Uterus Transplantation among General Public [Abstract]. Paper presented at the American Society for Reproductive Medicine 2014 Annual Meeting; October 18–22, 2014; Honolulu, HI.
- 96. Hayashi A, Tsutsumishita Y, Kisu I, Mihara M, Suganuma N. Sequential Awareness Surveys on Clinical Application of Uterine Transplantation Among Japanese General Public [Abstract]. Paper presented at the 31st Annual Meeting of the European Society of Human Reproduction and Embryology; June 14–17, 2015; Lisbon, Portugal.
- 97. Kisu I, Banno K, Soeda E, et al. Survey of attitudes toward uterus transplantation among Japanese women of reproductive age: a cross‐sectional study. PLoS ONE. 2016;11:e0156179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wennberg AL, Rodriguez‐Wallberg KA, Milsom I, Brännström M. Attitudes towards new assisted reproductive technologies in Sweden: a survey in women 30–39 years of age. Acta Obstet Gynecol Scand. 2016;95:38‐44. [DOI] [PubMed] [Google Scholar]
- 99. Saso S, Clarke A, Bracewell‐Milnes T, et al. Survey of perceptions of health care professionals in the United Kingdom toward uterine transplant. Prog Transplant. 2015;25:56‐63. [DOI] [PubMed] [Google Scholar]
- 100. Milliez J. Uterine transplantation FIGO Committee for the Ethical Aspects of Human Reproduction and Women's Health. Int J Gynaecol Obstet. 2009;106:270.19501356 [Google Scholar]
- 101. Saso S, Ghaem‐Maghami S, Louis LS, Ungar L, Del Priore G, Smith JR. Uterine transplantation: what else needs to be done before it can become a reality? The Indianapolis consensus J Obstet Gynaecol. 2013;33:232‐238. [DOI] [PubMed] [Google Scholar]
- 102. Del Priore G, Saso S, Meslin EM, et al. Uterine transplantation – a real possibility? The Indianapolis consensus Hum Reprod. 2013;28:288‐291. [DOI] [PubMed] [Google Scholar]
- 103. Balayla J, Dahdouh EM, Lefkowitz A. The Montreal Criteria for the Ethical Feasibility of Uterine Transplantation Research Group. Livebirth after uterus transplantation. Lancet. 2015;385:2351‐2352. [DOI] [PubMed] [Google Scholar]
- 104. Kisu I, Mihara M, Banno K, et al. Risks for donors in uterus transplantation. Reprod Sci. 2013;20:1406‐1415. [DOI] [PubMed] [Google Scholar]
- 105. Kisu I, Banno K, Mihara M, Suganuma N, Aoki D. Uterine transplantation: towards clinical application. Gynecol Obstet Invest. 2013;76:74. [DOI] [PubMed] [Google Scholar]
- 106. Womb Transplant UK . http://wombtransplantuk.org/. Accessed May 20, 2017.
- 107. Huet S, Tardieu A, Filloux M, et al. Uterus transplantation in France: for which patients? Eur J Obstet Gynecol Reprod Biol. 2016;205:7‐10. [DOI] [PubMed] [Google Scholar]
- 108. Flyckt R, Kotlyar A, Arian S, Eghtesad B, Falcone T, Tzakis A. Deceased donor uterine transplantation. Fertil Steril. 2017;107:e13. [DOI] [PubMed] [Google Scholar]
- 109. Ma L, Yao Y. China's first successful womb transplant brings woman's baby dream closer. China Daily. November 26, 2015. http://www.chinadaily.com.cn/china/2015-11/26/content_22521266.htm. Accessed May 20, 2017. [Google Scholar]
- 110. Testa G, Koon EC, Johannesson L, et al. Living donor uterus transplantation: a single center's observations and lessons learned from early setbacks to technical success. Am J Transplant 2017; [Epub ahead of print]. https://doi.org/10.1111/ajt.14326 [DOI] [PubMed] [Google Scholar]
- 111. Chmel R, Nováčková M, Pastor Z, Matěcha J, Čekal M, Froněk J. Possibility of uterus transplantation trial in Czech Republic – indications, research and clinical experience. Cas Lek Cesk. 2017;156:28‐35. (in Czech). [PubMed] [Google Scholar]
- 112. Anonymous . In Germany performed the first successful transplant of a uterus. Ukropnews24. July 5, 2017. http://ukropnews24.com/in-germany-performed-the-first-successful-transplant-of-a-uterus. Accessed May 20, 2017. [Google Scholar]
- 113. Arora M. Mom donates womb to daughter in India's first uterus transplant. CNN. May 19, 2017. http://edition.cnn.com/2017/05/19/health/india-uterus-womb-transplant. Accessed May 20, 2017. [Google Scholar]
- 114. Järvholm S, Johannesson L, Clarke A, Brännström M. Uterus transplantation trial: psychological evaluation of recipients and partners during the post‐transplantation year. Fertil Steril 2015;104:1010‐1015. [DOI] [PubMed] [Google Scholar]
- 115. Järvholm S, Johannesson L, Brännström M. Psychological aspects in pre‐transplantation assessments of patients prior to entering the first uterus transplantation trial. Acta Obstet Gynecol Scand. 2015;94:1035‐1038. [DOI] [PubMed] [Google Scholar]
- 116. Kvarnström N, Järvholm S, Johannesson L, Dahm‐Kähler P, Olausson M, Brännström M. Live donors of the initial observational study of uterus transplantation – psychological and medical follow‐up until 1 year after surgery in the 9 cases. Transplantation. 2017;101:664‐670. [DOI] [PubMed] [Google Scholar]
- 117. Saso S, Bracewell‐Milnes T, Ismail L, et al. Psychological assessment tool for patients diagnosed with absolute uterine factor infertility and planning to undergo uterine transplantation. J Obstet Gynaecol. 2014;34:504‐507. [DOI] [PubMed] [Google Scholar]
- 118. Saso S, Clarke A, Bracewell‐Milnes T, et al. Psychological issues associated with absolute uterine factor infertility and attitudes of patients toward uterine transplantation. Prog Transplant. 2016;26:28‐39. [DOI] [PubMed] [Google Scholar]
- 119. Del Priore G, Schlatt S, Wagner R, Reynoso E, Malanowska‐Stega J. Uterus transplantation: on the edge. Semin Reprod Med. 2011;29:55‐60. [DOI] [PubMed] [Google Scholar]
- 120. Caplan AL. Ensuring the future of uterine transplantation. Fertil Steril. 2013;99:682‐683. [DOI] [PubMed] [Google Scholar]
- 121. Johannesson L, Dahm‐Kähler P, Eklind S, Brännström M. The future of human uterus transplantation. Womens Health (Lond). 2014;10:455‐467. [DOI] [PubMed] [Google Scholar]
- 122. Johannesson L, Enskog A. Experimental uterus transplantation. Best Pract Res Clin Obstet Gynaecol. 2014;28:1198‐1210. [DOI] [PubMed] [Google Scholar]
- 123. Brännström M. Uterus transplantation. Curr Opin Organ Transplant. 2015;20:621‐628. [DOI] [PubMed] [Google Scholar]
- 124. Dahm‐Kähler P, Diaz‐Garcia C, Brännström M. Human uterus transplantation in focus. Br Med Bull. 2016;117:69‐78. [DOI] [PubMed] [Google Scholar]
- 125. Brännström M, Bokström H, Dahm‐Kähler P, et al. One uterus bridging three generations: first live birth after mother‐to‐daughter uterus transplantation. Fertil Steril. 2016;106:261‐266. [DOI] [PubMed] [Google Scholar]
- 126. Jones BP, Saso S, Yazbek J, Smith JR. Uterine transplantation: past, present and future. BJOG. 2016;123:1434‐1438. [DOI] [PubMed] [Google Scholar]


