Abstract
Purpose
Prenatal exposure to environmental chemicals is a growing concern, because such exposures have been shown to be associated with various diseases. The levels of chemicals and heavy metals in maternal blood, cord blood, maternal urine and amniotic fluid in Japanese pregnant women were investigated.
Methods
A total of 145 women, including 14 fetal growth restriction cases, were included in the present study. The levels of phthalates (di[2‐ethylhexyl]phthalate and mono[2‐ethylhexyl]phthalate), perfluorinated compounds (perfluorooctane sulfonate, perfluorohexanoic acid, perfluorooctanoic acid, and perfluorononanoic acid), pesticides (dimethylphosphate, dimethylthiophosphate, diethylphosphate, diethylthiophosphate, 3‐phenoxybenzoic acid, and octachlorodipropyl ether), bisphenol A, nicotine (nicotine, nornicotine, cotinine, norcotinine, and trans‐3’‐hydroxycotinine), polybrominated diphenyl ethers, and heavy metals were measured. The relationship between fetal growth and the levels of chemicals and heavy metals were investigated.
Results
Phthalates, perfluorinated compounds, pesticides, polybrominated diphenyl ethers, and heavy metals were detected in high frequency, whereas nicotine and bisphenol A were almost negative. Phthalates, perfluorinated compounds, and several heavy metals were transferred to the fetus. High perfluorononanoic acid levels in the maternal blood and cord blood, and low perfluorooctanoic acid level in the cord blood were significantly and negatively associated with fetal growth.
Conclusions
The present study showed that pregnant women in Japan and their fetuses are exposed to a variety of chemicals and heavy metals.
Keywords: chemical exposure, endocrine disrupting chemicals, fetal growth, heavy metals, pregnant women
1. INTRODUCTION
Prenatal stress is recognized as a cause of fetal developmental reprograming, and is associated with various disorders, such as hypertension, coronary heart disease, obesity, and diabetes in adults.1 A number of neurological diseases and disorders, such as vasomotor problems, attention deficit, impaired cognition and reduced brain volume in children, are also thought to be caused by prenatal stress.1 These human diseases have been shown to be closely related with prenatal stress in animal studies with rodents and non‐human primates.1
There is concern that humans are exposed to many toxic chemicals, such as phtalates, perfluorinated compounds (PFCs), pesticides, bisphenol A, nicotine and its related metabolites, polybrominated diphenyl ethers (PBDEs), and heavy metals.2, 3 Such environmental chemicals that alter developmental plasticity often affect the endocrine control of development, and are called endocrine disrupting chemicals. The time when humans are most sensitive to environmental chemical is when organs are developing, especially in utero.1 We previously reported that several environmental chemicals caused aberrant DNA methylation at multiple gene loci at the serum concentrations of maternal blood and cord blood using mouse embryonic stem cells or human induced pluripotent stem cells as an in vitro model for early embryos.2, 3 Thus, environmental chemicals have the potential to cause epigenetic dysfunction in developing embryos.
Chemical exposures during pregnancy are important causes of prenatal stresses. Fetuses are more vulnerable than adults to the harmful effects of chemicals.1 Prenatal exposure to environmental chemicals, such as organophosphate pesticides, tobacco smoke, PFCs and heavy metals, has been shown to be associated with low birthweight of infants and neurobehavioral function.4, 5, 6, 7, 8, 9 Exposure to phthalates is associated with allergic diseases, aberrant neurodevelopment, endocrine disruption, and preterm birth.10, 11 Some previous reports showed that PFCs are associated with fetal growth and pregnancy‐induced hypertension.6, 7 PFCs exposure in early life is also associated with energy metabolic disorders.12 In addition, some animal studies showed that neonatal low‐dose pesticide exposure disrupts energy homeostasis in a persistent manner.13 Bisphenol A exposure during the prenatal period is associated with cryptorchidism.14 Prenatal exposure to PBDEs disrupts thyroid function, and is involved in adverse neurodevelopmental outcomes.15 Octachlorodipropyl ether (S‐421) is a pesticide, and has been used in Japan and East Asia as a synergist in pyrethrum insecticides for mosquitoes, and it remains in human bodies persistently.16 Taken together, it is very important to know the exposure levels of these environmental chemicals in pregnant women.
In the present study, we investigated the levels of phthalates, PFCs, pesticides, bisphenol A, nicotine and its metabolites, PBDEs, and heavy metals in maternal blood, maternal urine, cord blood, amniotic fluid, and maternal breast milk. In addition, we analyzed the transfer of the chemicals from mothers to fetuses, the exposure of infants by breast‐feeding, and the relationship between the chemicals and fetal growth.
2. METHODS
2.1. Ethical approval
The present study was carried out in accordance with the protocol approved by the institutional review board of Yamaguchi University Graduate School of Medicine. Written informed consent was obtained from all study participants.
2.2. Participants
For this study, 145 pregnant women at Yamaguchi University Hospital during 2008‐2010 were included in the study. A total of 70 cases were nulliparous and 75 cases were multiparous. Of the 145 cases, 14 women showed fetal growth restriction (FGR). FGR was defined as lower than −1.5 SD compared with expected fetal weights at delivery shown by The Japan Society of Obstetrics and Gynecology. Eight of the women had a history of tobacco smoking. Maternal blood and urine samples were obtained at admission at the start of labor (at 37‐42 weeks‐of‐gestation). Amniotic fluids were obtained after rupture of the membrane. Cord blood samples were obtained immediately after delivery. Breast milk samples were obtained after they started breast‐feeding. The numbers of the samples were as follows: maternal blood, 115; maternal urine, 42; maternal breast milk, 46; cord blood, 112; and amniotic fluid, 52. After blood sampling, serum fractions were separated by centrifugation and stored at −80°C until analysis. Other samples were stored at −80°C until analysis. The chemicals measured in this study are shown in Table 1.
Table 1.
Chemicals and samples measured in the study
| Group | Chemicals | Chemicals (abbeviation) | Measured samples |
|---|---|---|---|
| Phthalates | Mono(2‐ethylhexyl)phthalate | MEHP | Maternal blood/cord blood/amniotic fluid |
| Di(2‐ethylhexyl)phthalate | DEHP | Maternal blood/cord blood/amniotic fluid | |
| Perfluorinated compounds (PFCs) | Perfluorohexanoic acid | PFHxS | Maternal blood/cord blood |
| Perfluorooctane sulfonate | PFOS | Maternal blood/cord blood | |
| Perfluorononanoic acid | PFNA | Maternal blood/cord blood | |
| Perfluorooctanoic acid | PFOA | Maternal blood/cord blood | |
| Pesticides | Dimethylphosphate | DMP | Maternal blood/cord blood/maternal urine |
| Dimethylthiophosphate | DMTP | Maternal blood/cord blood/maternal urine | |
| Diethylphosphate | DEP | Maternal blood/cord blood/maternal urine | |
| Diethylthiophosphate | DETP | Maternal blood/cord blood/maternal urine | |
| 3‐Phenoxybenzoic acid | 3‐PBA | Maternal blood/cord blood/maternal urine | |
| 3,5,6‐Trichloro‐2‐pyridinol | TCP | Maternal blood/cord blood/maternal urine | |
| Octachlorodipropyl ether | S‐421 | Breast milk/milk fat | |
| Plastics/epoxy resins | Bisphenol A | BPA | Maternal blood/cord blood/maternal urine |
| Nicotine and metablites | Nicotine | NIC | Maternal blood/cord blood |
| Nornicotine | NNIC | Maternal blood/cord blood | |
| Cotinine | COT | Maternal blood/cord blood | |
| Norcotinine | NCOT | Maternal blood/cord blood | |
| Trans‐3’‐hydroxycotinine | HCOT | Maternal blood/cord blood | |
| PBDEs | Polybrominated diphenyl ethers | BDE‐28, −47, −99, −100, −153, −154, −183, −197, −207, and −209 | Breast milk/milk fat |
| Heavy metals | Li, B, Mg, Al, Mn, Ca, Fe, Co, Cu, Ni, An, Se, Sr, Rb, Mo, Cd, Hg, Sb, and Pb | Maternal blood/amniotic fluid |
2.3. Sample preparation and chromatography conditions
Abbreviations of the chemicals are shown in Table 1. Concentrations of phthalates (MEHP and DEHP), PFCs (PFHxS, PFOS, PFNA, and PFOA), bisphenol A, nicotines (NIC, NNIC, COT, NCOT, and HCOT), and PBDEs were determined as we previously reported.17, 18, 19, 20, 21, 22
In pesticides, 3‐PBA and TCP were determined with a modified version of our previous method with liquid chromatography–tandem mass spectrometry (Alliance 2695 and Quattro micro; Waters, Milford, MA, USA) equipped with an electrospray ionization probe.23, 24 After addition of beta‐glucronidase for derivatization, samples were purified with an Oasis HLB extraction column (Waters), and then fractionated by reverse‐phase chromatography on a symmetric C18 column (50 mm × 2.1 mm i.d., 3.0 μm film thickness; Waters) with 35% acetonitrile and 0.1% acetic acid. After sample loading, tandem mass spectrometry was carried out in negative ion mode. For S‐421, human milk samples were prepared according to the method of Kakimoto et al with a slight modification, and the concentration of S‐421 was measured by gas chromatography/mass spectrometry.25 Dialkylphosphate metabolites, such as DEP, DETP, DMP, and DMTP, were determined using the method reported by Ueyama et al with some modifications.26 Samples were purified on a C18 cartridge column (Aisti Science, Wakayama, Japan) and then deproteinized with acetonitrile. After derivatization with pentafluorobenzyl bromide, samples were purified using a three‐layer column of Florisil, PSA and anhydrous sodium sulfate. The concentrations of DMP, DEP, DMTP, and DETP were measured using gas chromatography with a flame photometric detector (Agilent Technologies, Tokyo, Japan) equipped with an LVI‐S200 injection port (Aisti Science) on a DB‐1701 or DB‐5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies).
Heavy metals were measured as we previously reported with a slight modification.27 Serum and urine samples were mixed with nitric acid in a Nano‐Band MV (GL Science, Tokyo, Japan) overnight. After microwave digestion, samples were mixed with internal standards (scandium, yttrium, iridium) and analyzed by inductively coupled plasma‐mass spectrometry (Agilent Technologies 7500i).
2.4. Statistical analysis
The significance of differences between groups was analyzed by the Wilcoxon rank sum test, Fisher's exact test, Kruskal–Wallis test and Tukey–Kramer test. All statistical analyses were carried out with R (The R Foundation for Statistical Computing, version 3.2.4, https://www.r-project.org/). Differences were considered significant at P < .05.
3. RESULTS
3.1. Phthalates
The detection rates of DEHP and MEHP in the maternal blood, cord blood, and amniotic fluid ranged from 14.3% to 65.9% (Table 2). The detection rates and concentrations of DEHP and MEHP were higher in the amniotic fluid than in the maternal blood and cord blood, whereas they did not differ between the maternal blood and cord blood (Table 2). Then, correlation analyses were carried out using 36 paired samples of maternal blood, cord blood, and amniotic fluid. The levels of DEHP and MEHP in the maternal blood, cord blood, and amniotic fluid were not significantly correlated.
Table 2.
Detection rates and concentrations of phthalates, perfluorinated compounds pesticides in maternal blood, cord blood, maternal urine, and amniotic fluid
| Maternal blood | Cord blood | Maternal urine | Amniotic fluid | P‐valuec | |||||
|---|---|---|---|---|---|---|---|---|---|
| Detection rate | Concentrations | Detection rate | Concentrations | Detection rate | Concentrations | Detection rate | Concentrations | ||
| Phthalate | n = 95 | ng/mLa | n = 98 | ng/mLa | n=41 | ng/mLa | |||
| DEHP | 33/95 (34.7%) | 13.2 (10.2‐460.1) | 20/98 (20.4%) | 12.8 (10.1‐37.4) | 21/41 (51.2%) | 26.6 (10.2‐376.2) | |||
| MEHP | 20/95 (21.1%) | 2.4 (2.1‐14.7) | 14/98 (14.3%) | 2.4 (2.1‐3.9) | 27/41 (65.9%) | 7.7 (2.1‐28.2) | |||
| PFCs (n=40) | ng/mL | ng/mL | |||||||
| PFHxS | 39/40 (97.5%) | 0.285 (0‐0.94) | 38/40 (95%) | 0.18 (0‐0.57) | |||||
| PFOS | 40/40 (100%) | 3.595 (1.7‐9.33) | 39/40 (97.5%) | 1.23 (0‐3.04) | |||||
| PFNA | 40/40 (100%) | 1.31 (0.27‐7.35) | 40/40 (100%) | 0.775 (0.22‐2.76) | |||||
| PFOA | 40/40 (100%) | 1.38 (0.76‐3.65) | 40/40 (100%) | 1.465 (0.73‐3.09) | |||||
| Pesticides (n=42) | ppba | ppba | ppba | ||||||
| DMP | 23/42 (54.8%) | 7.8 (1.0‐18.0) | 18/42 (42.9%) | 2.5 (0.9‐14.0) | 29/42 (69.0%) | 14.6 (0.9‐57.2) | |||
| DMTP | 21/42 (50.0%) | 10.3 (0.6‐22.2) | 7/42 (16.7%) | 0.6 (0.3‐3.6) | 33/42 (78.6%) | 3.4 (0.4‐92.3) | |||
| DEP | 14/42 (33.3%) | 0.5 (0.3‐7.6) | 7/42 (16.7%) | 0.4 (0.3‐2.0) | 30/42 (71.4%) | 5.1 (0.4‐25.9) | |||
| DETP | 35/42 (83.3%) | 5.9 (0.4‐14.5) | 28/42 (66.7%) | 1.7 (0.3‐6.8) | 19/42 (45.2%) | 1.4 (0.3‐12.7) | |||
| 3‐PBA | 1/42 (2.4%) | 0.3 (0.3‐0.3) | 0/42 (0%) | – | 11/42 (26.2%) | 0.9 (0.4‐2.6) | |||
| TCP | 0/42 (0%) | – | 0/42 (0%) | – | 2/42 (4.8%) | 0.9 (0.6‐1.2) | |||
| Nicotines (n=40) | ppb | ppb | |||||||
| NIC | 0/40 (0%) | 1/40 (2.5%) | 1.7c | ||||||
| NNIC | 0/40 (0%) | 2/40 (5%) | 0.2b, 0.7b | ||||||
| COT | 9/40 (22.5%) | 0.1, 0.8, 0.8, 0.9, 1.0, 1.0b, 1.3, 14.3b, 378.8b | 7/40 (17.5%) | 1.0, 1.0, 1.0b, 1.1b, 4.4, 4.8b, 176.5b | |||||
| NCOT | 1/40 (2.5%) | 4.9c | 1/40 (2.5%) | 4.1c | |||||
| HCOT | 2/40 (5%) | 3.7, 114.2b | 2/40 (5%) | 1.8, 74.8c | |||||
| Heavy metals (n = 32) | ng/mL | ng/mL | |||||||
| Li | 0.514 (0.209‐1.09) | 0.544 (0.151‐1.22) | .4321567 | ||||||
| B | 12.15 (2.79‐20.3) | 18.4 (5.43‐53.9) | .000204928 | ||||||
| Mg | 18500 (14 900‐21 300) | 12900 (9650‐22 900) | 8.70E‐10 | ||||||
| Al | 7.825 (0.24‐19.3) | 4.33 (1.04‐13) | .1209367 | ||||||
| Mn | 1.085 (0.324‐2.26) | 3.155 (0.263‐13.9) | 2.54E‐06 | ||||||
| Ca | 82250 (76 800‐93 900) | 59500 (42 222‐74 600) | 6.46E‐12 | ||||||
| Fe | 954.5 (382‐2970) | 619 (49.7‐3700) | .04689312 | ||||||
| Co | 0.2055 (0.0485‐0.593) | 0.094 (0.00179‐0.872) | .01130088 | ||||||
| Cu | 2030 (1430‐2540) | 100.05 (22.5‐379) | 6.49E‐12 | ||||||
| Ni | 0.374 (0.217‐0.744) | 1.43 (0.139‐9.82) | 3.32E‐09 | ||||||
| Zn | 555.5 (398‐739) | 540 (56.4‐3140) | .7986313 | ||||||
| Se | 114.5 (82.2‐162) | 11.8 (6.57‐22.8) | 1.42E‐11 | ||||||
| Sr | 33.3 (20.4‐68) | 26.15 (15.5‐64) | .03114184 | ||||||
| Rb | 149.5 (72.6‐440) | 104.5 (65.9‐190) | .000868071 | ||||||
| Mo | 1.125 (0.334‐4.14) | 2.135 (0.535‐5.78) | .000338897 | ||||||
| Cd | 0.03695 (0.019‐0.0827) | 0.0296 (0.00575‐0.142) | .6109451 | ||||||
| Hg | 0.605 (0.177‐2.1) | 0.1325 (0.0194‐0.511) | 1.57E‐08 | ||||||
| Sb | 0.1375 (0.0139‐0.2) | 0.272 (0.0437‐0.488) | 3.74E‐05 | ||||||
| Pb | 0.178 (0.114‐0.964) | 0.147 (0.0226‐0.315) | .009441496 | ||||||
The values of concentrations were median (range).
The detection limits are di(2‐ethylhexyl)phthalate (DEHP; 10 ng/mL), mono(2‐ethylhexyl)phthalate (MEHP; 2 ng/mL), dimethylphosphate (DMP; 0.5 ppb), dimethylthiophosphate (DMTP; 0.2 ppb), diethylphosphate (DEP; 0.2 ppb), diethylthiophosphate (dETP; 0.2 ppb), 3‐phenoxybenzoic acid (3‐PBA; 0.2 ppb), and 3,5,6‐trichloro‐2‐pyridinol (TCP; 0.5 ppb).
COT, cotinine; HCOT, trans‐3’‐hydroxycotinine; NCOT, norcotinine; NIC, nicotine; NNIC, nornicotine.
The median (range) was calculated in the detected samples.
Cases with a history of smoking.
Wilcoxon rank sum test was carried out between the maternal blood and the amniotic fluid.
3.2. PFCs
PFCs were detected in the maternal blood and cord blood in >95% of the cases (Table 2). As shown in Fig. 1a, PFOS, PFHxS, and PFNA levels were significantly lower in the cord blood compared with the maternal blood, whereas PFOA showed no significant difference between the maternal blood and the cord blood. Then, correlation analyses were carried out using 40 paired samples of maternal blood and cord blood. The levels of PFOS, PFHxS, PFOA, and PFNA in the maternal blood were significantly correlated with their levels in the cord blood (Fig. 1b). As multiparous women tend to have lower levels of PFCs than nulliparous women, we investigated the differences between multiparous cases and nulliparous cases. In the maternal blood, PFOS and PFHxS in the multiparous cases were significantly lower than those of the nulliparous cases (Table 3).28 In cord blood, PFHxS and PFNA in the multiparous cases were significantly lower than those of the nulliparous cases (Table 3).
Figure 1.
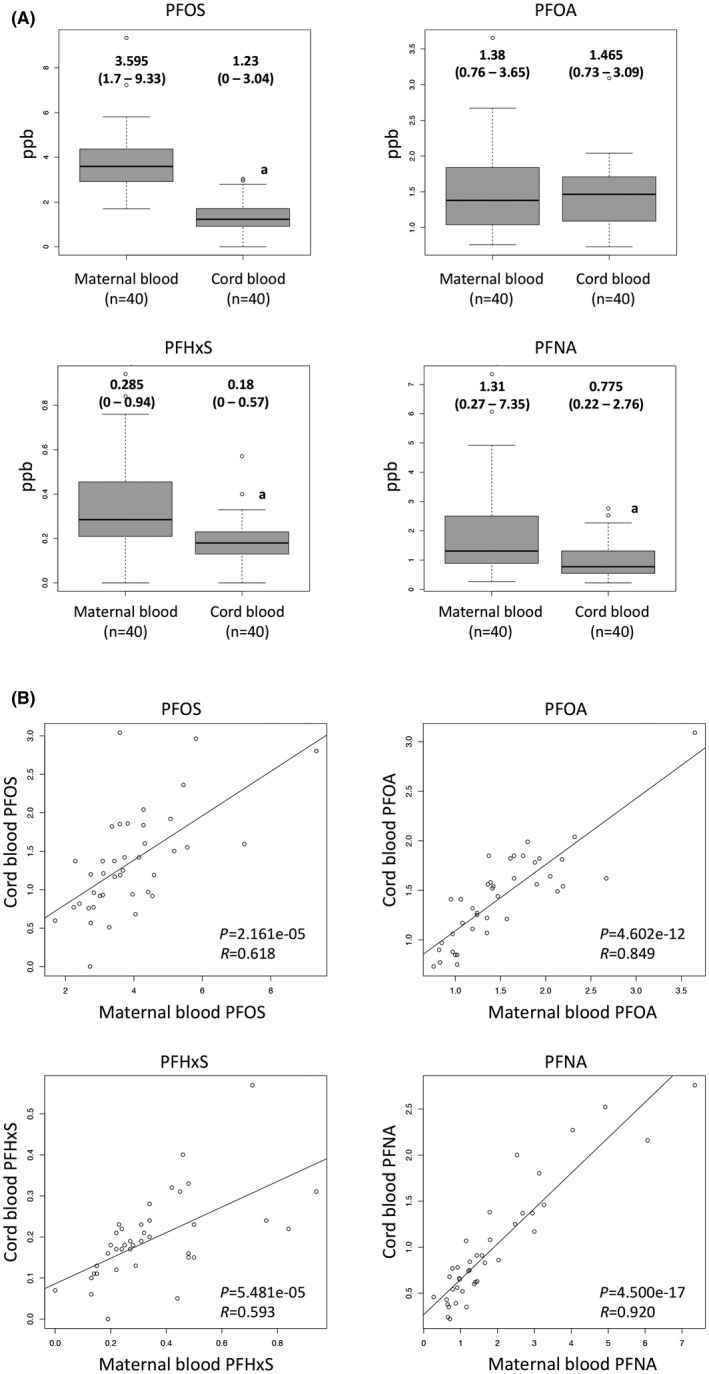
Perfluorinated compounds (PFCs) levels in maternal blood and cord blood. A, Perfluorooctane sulfonate (PFOS), perfluorohexanoic acid (PFHxS), perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA) levels. The y‐axis indicates the concentrations (ppb). Values are median (range). For statistical analysis, the Wilcoxon rank sum test were used. *P < .01. B, Correlation analysis of the levels of PFCs between the maternal blood and the cord blood using 40 paired samples of maternal blood and cord blood. P‐value and R‐value of correlation analysis are shown in each graph. Open circles indicate each case
Table 3.
Levels of perfluorinated compounds in nulliparous and multiparous cases
| Nulliparous (n = 17) | Multiparous (n = 23) | P‐valuea | |
|---|---|---|---|
| Maternal blood (ppb) | |||
| PFOS | 4.05 (2.29‐9.33) | 3.43 (1.70‐5.45) | .04881 |
| PFHxS | 0.46 (0.19‐0.94) | 0.240 (0‐0.50) | .0008759 |
| PFOA | 1.41 (0.83‐3.65) | 1.35 (0.76‐2.18) | .2737 |
| PFNA | 1.79 (0.62‐7.35) | 1.24 (0.27‐6.07) | .1324 |
| Cord blood (ppb) | |||
| PFOS | 1.42 (0.57‐2.96) | 1.19 (0‐3.04) | .1547 |
| PFHxS | 0.20 (0‐0.57) | 0.17 (0‐0.28) | .03997 |
| PFOA | 1.56 (0.75‐3.09) | 1.41 (0.73‐1.85) | .292 |
| PFNA | 1.08 (0.35‐2.76) | 0.74 (0.22‐2.16) | .04149 |
Concentrations indicate median (range).
PFHxS, perfluorohexanoic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
Wilcoxon rank sum test was carried out.
3.3. Pesticides
In approximately half of the cases, the maternal blood was positive for DMP, DMTP, DEP, and DETP, but the detection rates of 3‐PBA and TCP were very low (Table 2). All the detection rates in the cord blood were lower than those of the maternal blood. All pesticide metabolites except DETP in the maternal urine showed higher detection rates compared with the maternal blood and the cord blood (Table 2).
3.4. Bisphenol A
Bisphenol A levels in the maternal blood (n = 42), cord blood (n = 42), and maternal urine (n = 42) were all below the detection limit (0.5 ppb).
3.5. Nicotine and its metabolites
The detection rates of COT were 22.5% (9/40) and 17.5% (7/40) in the maternal blood and cord blood, respectively (Table 2). Of the detected cases, six and three did not have any histories of smoking, respectively. The levels of COT varied among the cases with 0.1‐378.8 ppb in the maternal blood and 1.0‐176.5 ppb in the cord blood. The detection rates of the others were low in both the maternal blood and the cord blood (Table 2). Eight patients who had histories of smoking showed high levels of COT, NCOT, and HCOT in the maternal and the cord blood (Table 2).
3.6. PBDEs and S‐421
PBDEs and S‐421 were detected in the breast milk and the milk fat in high frequency (Table S1). In all the examined cases (n = 45), at least one or more PBDEs were detected in the breast milk and the milk fat.
3.7. Heavy metals
All heavy metals were detected in all the samples of the maternal blood and the amniotic fluid (Table 2). The concentrations of B, Mn, Ni, Mo, and Sb were significantly higher in the amniotic fluid compared with the maternal blood, whereas Mg, Ca, Fe, Co, Cu, Se, Sr, Rb, Hg, and Pb were significantly lower in the amniotic fluid. Then, correlation analyses were carried out using 32 paired samples of maternal blood and amniotic fluid. As shown in Fig. 2, the levels of Li, Ca, Mo, Mg, and Sr in the maternal blood were significantly correlated with their levels in the amniotic fluid.
Figure 2.
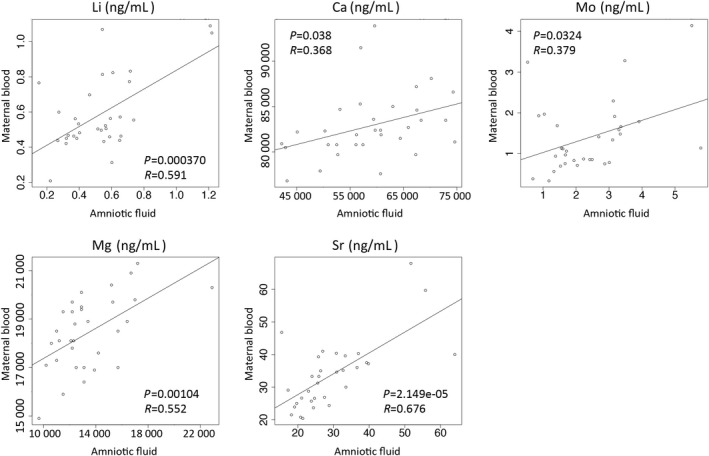
Correlation analysis of the levels of heavy metals between the maternal blood and the amniotic fluid using 32 paired samples of maternal blood and amniotic fluid. Li, Mg, Ca, Sr, and Mo levels were analyzed. P‐value and R‐value of correlation analysis are shown in each graph. Open circles indicate each case
3.8. Comparison of FGR and non‐FGR cases
The present study included 14 FGR cases out of 145 cases. Therefore, we compared the levels of chemicals and heavy metals between FGR cases (n = 14) and non‐FGR cases (n = 131). The birthweights and placental weights of the FGR cases were approximately 20% less than those of non‐FGR cases, whereas the maternal age, past history of pregnancy, and delivery weeks did not differ between the two groups (Table S2).
The PFNA levels of the maternal blood and the cord blood were significantly higher in the FGR cases than in the non‐FGR cases (Fig. 3a). PFOS, PFHxS, and PFOA levels in the maternal blood and the cord blood did not differ between the two groups (Fig. 3a). We next investigated whether there is a correlation between birthweights or placental weights and PFCs levels in the maternal blood and the cord blood. Birthweight was significantly and negatively correlated with the PFNA levels in the maternal blood (Fig. 3b, left panel) and cord blood (Fig. 3b, middle panel), but positively correlated with the PFOA level in the cord blood (Fig. 3b, right panel). Placental weight was significantly and inversely correlated with the PFNA levels in the maternal blood and cord blood (Fig. 3c).
Figure 3.
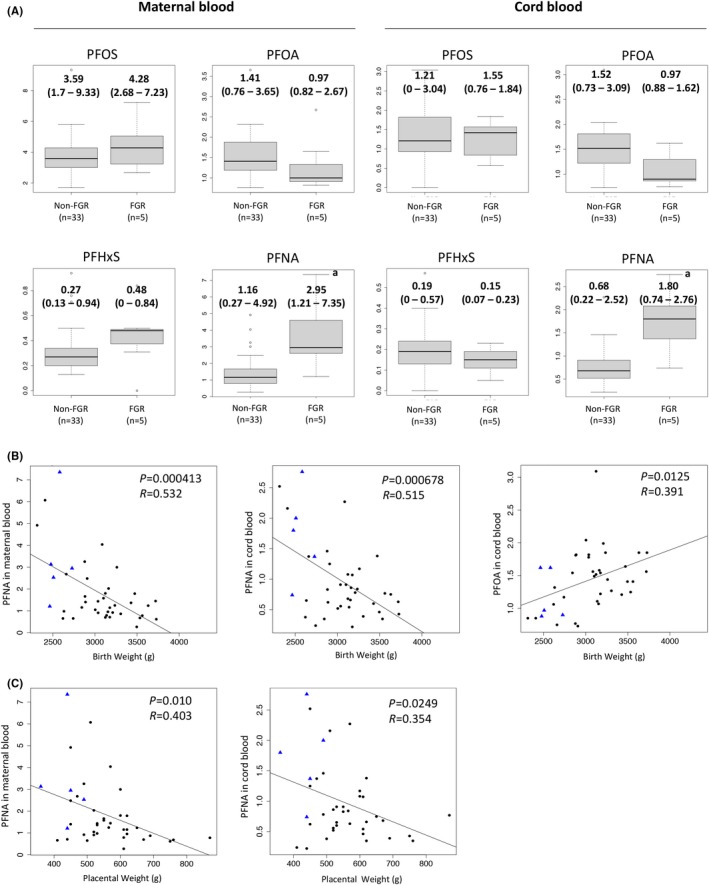
Levels of perfluorinated compounds (PFCs) in fetal growth restriction (FGR) and non‐FGR cases. A, The levels of PFCs (perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorohexanoic acid (PFHxS), and perfluorononanoic acid (PFNA) levels of the maternal blood (left) and the cord blood (right) are shown by boxplot. The y‐axis shows concentrations (ppb). For statistical analysis, the Wilcoxon rank sum test was used. *P < .01. B, Correlation analyses between the PFNA levels in the maternal blood or the cord blood and the birthweight. C, Correlation analyses between the PFNA levels in the maternal blood or the cord blood and the placental weight. Closed circles indicate non‐FGR cases and closed triangles indicate FGR cases
In terms of heavy metals, Rb levels in the FGR cases were significantly lower in the maternal blood (median 81 ng/mL, range 75.6‐88.3 ng/mL) than in the non‐FGR cases (median 165 ng/mL, range 72.6‐440 ng/mL; P = .023), whereas Cd levels in the amniotic fluid were significantly higher in the FGR cases (median 0.0502 ng/mL, range 0.0496‐0.112 ng/mL) than in the non‐FGR cases (median 0.0296 ng/mL, range 0.00575‐0.142 ng/mL; P = .021).
No significant differences were observed in the levels of DEHP and MEHP, DMT, DMTP, DEP, DETP, 3‐PBA, TCP, NIC, NNIC, COT, NCOT, and HCOT between FGR and non‐FGR cases.
4. DISCUSSION
The present study shows the levels of chemicals in the maternal blood, cord blood, amniotic fluid, and maternal breast milk in Japanese women. Some chemicals, such as NIC, NIC‐metabolites, and bisphenol A, were almost negative, but phthalates, PFCs, pesticides, PBDEs, and heavy metals were considerably positive in Japanese pregnant women. Furthermore, the present results provide evidence that fetuses are also exposed to various chemicals in the uterus.
Phthalates are used in many consumer products including plastics and cosmetics. Most pregnant women were found to be positive for phthalates or phthalate metabolites, and phthalates are transferred to fetuses.10, 11 Early‐life exposure to phthalates has been associated with allergic diseases, altered neurodevelopment, endocrine disruption, and preterm birth.10, 11 Although the use of DEHP has been strictly restricted in Japan since 2002, phthalates were still positive in pregnant Japanese women in the present study. The amniotic fluid had higher detection rates and concentrations of DEHP and MEHP compared with the maternal blood and cord blood. Amniotic fluid consists of fetal urine and is taken into the fetus orally, and then fetal urine is excreted as amniotic fluid again. Phthalates are mainly excreted to urine.11 Therefore, it is likely that DEHP and MEHP transfer to the fetus and are accumulated in the amniotic fluid. This indicates that fetuses are continuously exposed to DEHP and MEHP during pregnancy. Regarding the phthalates levels in the amniotic fluid, there are several reports showing that there is a strong geographical difference in phthalates levels of the amniotic fluid, and that phthalates levels in amniotic fluid differ among the gestational weeks.10 Further studies with large and global scales are required.
PFCs are persistent industrial chemicals used for imparting water and stain resistance to consumer products. Elevated concentrations of PFCs have been associated with the consumption of seafood, meat, and a high intake of salty snacks.28 Exposure to PFCs is associated with reduced fetal growth6, 7 and endocrine disruption in females.29 PFCs remain in the human body for a long time after exposure because of their highly stable chemical structure.10 The present study showed almost 100% of pregnant women were positive for PFCs, in agreement with previous reports.10, 28
The present study showed that the levels of PFOS, PFHxS, PFOA, and PFNA in the maternal blood were significantly correlated with those in the cord blood, indicating that PFCs transfer to fetuses, which is consistent with a recent report.30 The levels of PFOS, PFHxS, and PFNA in the cord blood were significantly lower than those in the maternal blood, whereas PFOA levels in the cord blood were similar to those in the maternal blood. The result is also consistent with previous reports showing that PFOA was abundantly detected in cord blood compared with other PFCs.30 Gutzkow et al reported that the transfer of PFCs from mothers to fetuses depends on two factors ‐ the types of PFCs and the length of the C (carbon)‐chain.31 There are two major types of PFCs; PFCAs (PFOA and PFNA) and PFASs (PFOS and PFHxS). PFCAs easily pass through the placenta compared with PFASs.31 PFOA has shorter C‐chains (eight Cs) compared with PFNA (nine Cs). Therefore, it is likely that PFOA highly transfers to fetuses, resulting in high levels of PFOA in the cord blood.31
Pesticides are widely used for agricultural and landscape pest control, and are in the majority of commercial household insecticides. The primary route of exposure is the oral intake of contaminated food and inhalation of sprayed insecticides. Pesticides are associated with impaired glucose tolerance,32 neuronal and renal toxicity,33 inhibited fetal growth,9 and neurobehavioral deficits34 The present study showed that 33.3%‐54.8% of the patients were positive for DMP, DMTP, DEP, and DETP in the maternal blood, and 45.2%‐78.6% were positive in the maternal urine. These results are consistent with the previous reports, in which pesticides were detected in the maternal blood of most women.32 We also detected DMP, DMTP, DEP, and DETP in the cord blood, indicating the transfer of pesticides to fetuses. The detection rate of pesticides was higher in the maternal urine than in the maternal blood, suggesting that the pesticides in the maternal blood are excreted into the maternal urine. In contrast, the detection rate of DETP was lowest in the maternal urine. This, together with the fact that DETP was the most common pesticide in the maternal blood and the cord blood suggests that DETP remains in the maternal blood and is highly transferred to fetuses.
PBDEs are used as flame retardants in furniture, electronics, and textile products. Prenatal exposure to PBDEs has been shown to disrupt thyroid function and cause neurodevelopmental disorders.15 Exposure to lactational PBDEs has been associated with anxiety and withdrawal.35 In the present study, the detection rates of PBDEs were extremely high, in agreement with a previous report.36 The production of most PBDEs is prohibited in many countries, including Japan. However, because PBDEs are lipophilic persistent chemicals, they accumulate in the human body.36 There are growing concerns about the effect of BDEs on children.36 S‐421 is often used as an insecticide synergist in mosquito coils in East Asia.16 As the degradation products of S‐421 includes bis (chloromethyl) ether, which is a lung carcinogen, mosquito coils are used to a limited extent in other parts of the world.16 In the present study, the detection rate of S‐421 in breast milk was extremely high, in agreement with the previous report.16 This is also a growing concern, although the effect of S‐421 on children is still unknown.
Heavy metals, such as Cd, Pb, Hg, and Ni, are widely distributed environmental pollutants. They are found in batteries, fish, and drinking water, and can cause cancer, renal failure, and neurological symptoms.37 In the present study, the levels of several heavy metals in the maternal blood were significantly correlated with their levels in the amniotic fluid, showing that heavy metals in the amniotic fluid are derived from maternal blood.
PFNA levels in the maternal blood and cord blood were significantly higher in FGR cases than in non‐FGR cases (Fig. 3a), and PFNA was inversely correlated with birthweight (Fig. 3b). These results are consistent with several epidemiological studies that have shown a negative association between PFC levels in cord blood and birthweight.6, 7, 10 PFCs, PFOS, and PFOA levels in human serum have been decreasing as a result of efforts to reduce the use of these chemicals beginning in the year 2000.38 However, because of the continued production, exposure to PFNA has recently been increasing in many countries, including Japan, Korea, Vietnam,39 and Norway.38 More effort is required to reduce the exposure of pregnant women to PFNA.
It is unclear why only PFOA is positively correlated with birthweight. Interestingly, PFOA increases the risk of diabetes by interfering with glucose metabolism.12 Because gestational diabetes is associated with increased birthweight, PFOA might also increase birthweight.
Levels of PFCs in the maternal blood and cord blood were significantly lower in multiparous cases than in nulliparous cases (Table 3), in agreement with previous reports that showed PFCs levels in maternal blood decreased with increasing parity.10, 28 Elimination through breast milk is believed to be involved in the decrease of PFCs in maternal blood.10, 28 Indeed, PFCs in infant blood have been shown to increase during breast‐feeding after birth, and become higher than maternal serum levels.40 Because PFCs might retard postnatal growth, we should also be concerned by postnatal exposure by breast‐feeding.7
Some heavy metals, such as Pb, Cd, Hg, and Mn, have been associated with FGR and pediatric diseases.4, 5, 41 Our finding that Cd levels in the amniotic fluid were higher in FGR cases is consistent with the finding that Cd levels in maternal urine were negatively associated with birthweight.41 Cd is chemically similar to Zn (an essential element), so that high maternal Cd levels might impair the transport of Zn to the placenta, resulting in FGR.41 However, Zn levels were not significantly different between FGR and non‐FGR cases in the present study. Although Rb levels in the maternal blood were lower in the FGR cases than in non‐FGR cases, the reason is unclear. Regarding the influence of heavy metals on fetal growth, further studies are required, because little information is available.
In conclusion, the present study shows that pregnant women in Japan, and their fetuses, are exposed to a variety of chemicals and heavy metals. We found possible links between several chemicals and fetal growth, although the number of FGR cases was small. Furthermore, the exposure levels of chemicals and heavy metals shown in the present study would be very useful for researchers to investigate the effects of the chemicals and heavy metals in vitro and in vivo in future studies. The present results should be of interest to public health agencies and other investigators, as they will help them focus on which measures would be of greatest value in protecting the health of newborn children.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human rights statement and informed consent statement: The study protocol was reviewed and approved by the institutional review board of Yamaguchi University Graduate School of Medicine. Informed consent was obtained from the participants before the collection of any samples. All experiments involving the handling of human tissues were performed in accordance with the tenets of the Declaration of Helsinki. Animal studies: This article does not contain any study with animal participants that has been performed by any of the authors.
Supporting information
ACKNOWLEDGEMENTS
This work was supported by a Health Science Research Grant from the Ministry of Health, Labor and Welfare, Japan. This article is dedicated to the memory of Emeritus Professor Hiroyuki Nakazawa of Hoshi University. Ryo Maekawa is the first/corresponding author, and participated in the study design, supervision, analysis, and interpretation of the measured data of chemicals and heavy metals and drafting of the manuscript. Rie Ito, Yusuke Iwasaki, and Koichi Saito participated in the measurement of perfluorinated compounds, and nicotine and nicotine metabolites, and drafting of the manuscript. Kazuhiko Akutsu and Satoshi Takatori participated in the measurement of polybrominated diphenyl ethers and phthalates, and drafting of the manuscript. Rie Ishii participated in the measurement of pesticides and bisphenol A, and drafting of the manuscript. Fumio Kondo participated in the measurement of heavy metals and phthalates, and drafting of the manuscript. Yoshikazu Arai, Jun Ohgane, and Kunio Shiota participated in the statistical analysis and interpretation of the measured data of chemicals and heavy metals, and drafting of the manuscript. Norihiro Sugino, Kunio Shiota, and Tsunehisa Makino conceived, designed, and surpervised the study, and participated in drafting the manuscript.
Maekawa R, Ito R, Iwasaki Y, et al. Evidence of exposure to chemicals and heavy metals during pregnancy in Japanese women. Reprod Med Biol. 2017;16:337‐348. https://doi.org/10.1002/rmb2.12049
REFERENCES
- 1. Heindel JJ, Balbus J, Birnbaum L, et al. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156:3416‐3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arai Y, Ohgane J, Yagi S, et al. Epigenetic assessment of environmental chemicals detected in maternal peripheral and cord blood samples. J Reprod Dev. 2011;57:507‐517. [DOI] [PubMed] [Google Scholar]
- 3. Arai Y, Hayakawa K, Arai D, et al. Putative epimutagens in maternal peripheral and cord blood samples identified using human induced pluripotent stem cells. Biomed Res Int. 2015;2015:876047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung SE, Cheong HK, Ha EH, et al. Maternal blood manganese and early neurodevelopment: the Mothers and Children's Environmental Health (MOCEH) Study. Environ Health Perspect. 2015;123:717‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caserta D, Graziano A, Lo Monte G, Bordi G, Moscarini M. Heavy metals and placental fetal‐maternal barrier: a mini‐review on the major concerns. Eur Rev Med Pharmacol Sci. 2013;17:2198‐2206. [PubMed] [Google Scholar]
- 6. Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid‐Ohio Valley, 2005‐2010. Environ Health Perspect. 2013;121:1207‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Adgent M, Su PH, et al. Prenatal exposure to perfluorocarboxylic acids (PFCAs) and Fetal and Postnatal Growth in the Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2016;124:1794‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jaddoe VW, Troe EJ, Hofman A, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatr Perinat Epidemiol. 2008;22:162‐171. [DOI] [PubMed] [Google Scholar]
- 9. Petit C, Chevrier C, Durand G, et al. Impact on fetal growth of prenatal exposure to pesticides due to agricultural activities: a prospective cohort study in Brittany, France. Environ Health. 2010;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitro SD, Johnson T, Zota AR. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep. 2015;2:367‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marie C, Vendittelli F, Sauvant‐Rochat MP. Obstetrical outcomes and biomarkers to assess exposure to phthalates: a review. Environ Int. 2015;83:116‐136. [DOI] [PubMed] [Google Scholar]
- 12. Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril. 2015;103:184‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lassiter TL, Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol Teratol. 2008;30:125‐130. [DOI] [PubMed] [Google Scholar]
- 14. Chevalier N, Brucker‐Davis F, Lahlou N, et al. A negative correlation between insulin‐like peptide 3 and bisphenol A in human cord blood suggests an effect of endocrine disruptors on testicular descent during fetal development. Hum Reprod. 2015;30:447‐453. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert ME, Rovet J, Chen Z, Koibuchi N. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 2012;33:842‐852. [DOI] [PubMed] [Google Scholar]
- 16. Krieger RI, Dinoff TM, Zhang X. Octachlorodipropyl ether (s‐2) mosquito coils are inadequately studied for residential use in Asia and illegal in the United States. Environ Health Perspect. 2003;111:1439‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akutsu K, Takatori S, Nozawa S, et al. Polybrominated diphenyl ethers in human serum and sperm quality. Bull Environ Contam Toxicol. 2008;80:345‐350. [DOI] [PubMed] [Google Scholar]
- 18. Iwasaki Y, Goto M, Mochizuki K, et al. Development and validation of a hydrophilic interaction chromatography‐tandem mass spectrometry for quantification of nicotine and its metabolites in human maternal and cord sera. Biomed Chromatogr. 2011;25:503‐510. [DOI] [PubMed] [Google Scholar]
- 19. Nakata H, Nakata A, Okada F, et al. Development of online solidphase extraction‐HPLC/MS/MS method for the determination of perfluorochemicals in human plasma. Bunseki Kagaku. 2005;54:877‐884. [Google Scholar]
- 20. Nakazawa H, Iwasaki Y, Ito R. Analytical methods for the quantification of bisphenol A, alkylphenols, phthalate esters, and perfluoronated chemicals in biological samples. Anal Sci. 2014;30:25‐34. [DOI] [PubMed] [Google Scholar]
- 21. Okano K, Hinohara M, Iwasaki Y, et al. Determination of nicotine and cotinine in human serum for ecaluation of tobacco smoke exposure by hydrophilic interaction chromatography/mass spectrometry. Bunseki Kagaku. 2007;56:785‐790. [Google Scholar]
- 22. Takatori S, Akutsu K, Kondo F, Ishii R, Nakazawa H, Makino T. Di(2‐ethylhexyl)phthalate and mono(2‐ethylhexyl)phthalate in media for in vitro fertilization. Chemosphere. 2012;86:454‐459. [DOI] [PubMed] [Google Scholar]
- 23. Baker SE, Olsson AO, Barr DB. Isotope dilution high‐performance liquid chromatography‐tandem mass spectrometry method for quantifying urinary metabolites of synthetic pyrethroid insecticides. Arch Environ Contam Toxicol. 2004;46:281‐288. [DOI] [PubMed] [Google Scholar]
- 24. Olsson AO, Baker SE, Nguyen JV, et al. A liquid chromatography–tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and deet in human urine. Anal Chem. 2004;76:2453‐2461. [DOI] [PubMed] [Google Scholar]
- 25. Kakimoto K, Akutsu K, Konishi Y, Tanaka Y. Time trend of hexabromocyclododecane in the breast milk of Japanese women. Chemosphere. 2008;71:1110‐1114. [DOI] [PubMed] [Google Scholar]
- 26. Ueyama J, Saito I, Kamijima M, et al. Simultaneous determination of urinary dialkylphosphate metabolites of organophosphorus pesticides using gas chromatography‐mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832:58‐66. [DOI] [PubMed] [Google Scholar]
- 27. Razagui IB, Ghribi I. Maternal and neonatal scalp hair concentrations of zinc, copper, cadmium, and lead: relationship to some lifestyle factors. Biol Trace Elem Res. 2005;106:1‐28. [DOI] [PubMed] [Google Scholar]
- 28. Berg V, Nost TH, Huber S, et al. Maternal serum concentrations of per‐ and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int. 2014;69:58‐66. [DOI] [PubMed] [Google Scholar]
- 29. Kristensen SL, Ramlau‐Hansen CH, Ernst E, et al. Long‐term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod. 2013;28:3337‐3348. [DOI] [PubMed] [Google Scholar]
- 30. Manzano‐Salgado CB, Casas M, Lopez‐Espinosa MJ, et al. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res. 2015;142:471‐478. [DOI] [PubMed] [Google Scholar]
- 31. Gutzkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G. Placental transfer of perfluorinated compounds is selective–a Norwegian Mother and Child sub‐cohort study. Int J Hyg Environ Health. 2012;215:216‐219. [DOI] [PubMed] [Google Scholar]
- 32. Shapiro GD, Dodds L, Arbuckle TE, et al. Exposure to organophosphorus and organochlorine pesticides, perfluoroalkyl substances, and polychlorinated biphenyls in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC Study. Environ Res. 2016;147:71‐81. [DOI] [PubMed] [Google Scholar]
- 33. Nasr HM, El‐Demerdash FM, El‐Nagar WA. Neuro and renal toxicity induced by chlorpyrifos and abamectin in rats: toxicity of insecticide mixture. Environ Sci Pollut Res Int. 2016;23:1852‐1859. [DOI] [PubMed] [Google Scholar]
- 34. Magby JP, Richardson JR. Developmental pyrethroid exposure causes long‐term decreases of neuronal sodium channel expression. Neurotoxicology. 2017;60:274‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adgent MA, Hoffman K, Goldman BD, Sjodin A, Daniels JL. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr Perinat Epidemiol. 2014;28:48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs–a review of levels and sources. Int J Hyg Environ Health. 2009;212:109‐134. [DOI] [PubMed] [Google Scholar]
- 37. Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167‐182. [DOI] [PubMed] [Google Scholar]
- 38. Nost TH, Vestergren R, Berg V, Nieboer E, Odland JO, Sandanger TM. Repeated measurements of per‐ and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environ Int. 2014;67:43‐53. [DOI] [PubMed] [Google Scholar]
- 39. Harada KH, Hitomi T, Niisoe T, et al. Odd‐numbered perfluorocarboxylates predominate over perfluorooctanoic acid in serum samples from Japan, Korea and Vietnam. Environ Int. 2011;37:1183‐1189. [DOI] [PubMed] [Google Scholar]
- 40. Fromme H, Mosch C, Morovitz M, et al. Pre‐ and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol. 2010;44:7123‐7129. [DOI] [PubMed] [Google Scholar]
- 41. Kippler M, Tofail F, Gardner R, et al. Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect. 2012;120:284‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


