Abstract
Purpose
To assess the kisspeptin concentrations in follicular fluid and their relationship with clinical outcomes during assisted reproductive technology.
Methods
Thirty‐nine patients who were aged 24‐40 years and underwent oocyte retrieval for in vitro fertilization/intracytoplasmic sperm injection participated in this study. In 65 follicular fluid samples that had been obtained from 30 patients and their blood samples, the kisspeptin levels were measured in order to investigate the correlations with their gonadal hormone levels. Venous blood samples were collected from 14 patients to investigate their plasma kisspeptin levels across different phases of assisted reproductive technology.
Results
The follicular fluid kisspeptin level was significantly higher than that of the plasma level and was positively associated with the follicular fluid estradiol concentration and with the serum estradiol and number of mature oocytes. In the plasma, the maximum concentration of kisspeptin was observed on the day of ovum pick‐up and on the day of embryo transfer during ovarian stimulation for assisted reproductive technology.
Conclusion
Kisspeptin was present in the follicular fluid and the plasma kisspeptin concentration was affected by ovarian stimulation. Kisspeptin appears to affect oocyte maturation and ovulation.
Keywords: assisted reproductive technology, estradiol, follicular fluid, kisspeptin, mature oocyte
1. INTRODUCTION
Kisspeptin is a bioactive peptide that was identified in 20011, 2, 3 and that is localized in the hypothalamic arcuate nucleus and the anteroventral periventricular nucleus in the brain.4, 5 Estrogen receptors are present in kisspeptin neurons and are thought to play a role in mediating the positive and negative feedback of estrogen and in regulating gonadotropin‐releasing hormone secretion.6, 7
In contrast, recently it has been reported that kisspeptin is expressed in granulosa‐lutein cells and theca cells in the ovary.8, 9, 10 There are reports that, if a kisspeptin antagonist is locally administered to the ovary, delayed vaginal opening and a disorder of the estrous cycle occur,11 and that mice heterozygous for kisspeptin receptors are in the same state as premature ovarian failure.12 Additionally, it has been reported that an increase in the corpus luteum is observed when kisspeptin is administered to rat ovaries.13 These results suggest that kisspeptin is involved in follicular development and ovulation, but its mechanism is still unclear.
It is known that kisspeptin in the periphery is localized in the pancreas, small intestine, and placenta,1 besides the ovary, and research on it as a biomarker of various diseases is proceeding.14, 15, 16 In the reproductive field, it has been reported that plasma kisspeptin levels are increased in adolescent patients with polycystic ovary syndrome17 and they rise during the pre‐ovulatory phase and the luteal phase across the menstrual cycle.18, 19 However, there has been no report of the plasma kisspeptin levels during the ovarian stimulation that is associated with in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI).
In contrast, follicular fluid contains various hormones and cytokines and the relationships with egg maturity and IVF results have been studied.20, 21, 22, 23, 24 There are reports that egg maturity correlates significantly with growth hormone, insulin‐like growth factor‐I, and estradiol (E2) concentrations in the follicular fluid20 and that E2 and progesterone (P4) concentrations in the follicular fluid are significantly higher when mature eggs are present21; the relationship between the E2 concentration in the follicular fluid and egg maturity is well known. However, there has not been any report on the concentration of kisspeptin in the follicular fluid and it is not known whether kisspeptin is present in the follicular fluid and whether it is related to various clinical events or egg maturity.
The purpose of this study was to measure the kisspeptin concentrations in the follicular fluid for the first time and to examine their relationship with patients’ clinical characteristics, such as their age, number of follicles, number of eggs collected, and serum sex hormone levels. As the sex hormone's level in the blood is greatly affected by the number of growing follicles25, 26 and there is a possibility that the association with the kisspeptin concentration could be masked, the associations between the serum E2 levels and number of growing follicles, E2 and number of retrieved oocytes, and E2 and number of matured eggs and the follicular fluid kisspeptin levels also were investigated. The correlation with the E2 concentration in the follicular fluid additionally was examined and the possibility that the concentration of kisspeptin in the follicular fluid might be an indicator of egg maturity was evaluated. Finally, how the plasma kisspeptin levels fluctuate across the ovarian stimulation that is associated with IVF/ICSI was assessed.
2. MATERIALS AND METHODS
2.1. Patients
A total of 39 patients, aged 24‐40 years and who were undergoing oocyte retrieval for IVF/ICSI, participated in this study between October, 2012 and July, 2016. The stimulation was performed with recombinant follicle‐stimulating hormone (FOLLISTIM®; MSD Company, Ltd., Tokyo, Japan) and human menopausal gonadotrophin (HMG; Ferring Pharmaceuticals Company, Ltd., Tokyo, Japan). The total doses of the administered gonadotrophins were individualized according to the transvaginal ultrasound measurements of the developing follicles. For pituitary suppression, 33 patients were treated with buserelin acetate (Suprecur®; MOCHIDA Pharmaceutical Company, Ltd., Tokyo, Japan) and six patients were treated with cetrorelix (Cetrotide®; Merck Serono Company, Ltd., Tokyo, Japan). In all cases, induction of ovulation was performed with 10,000 IU human chorionic gonadotrophin (hCG) (GONATROPIN®; ASKA Pharmaceutical Company, Ltd., Tokyo, Japan) when the leading follicle reached a diameter of 18‐20 mm, as measured by the transvaginal ultrasound. The ovum pick‐up (OPU) was scheduled 35 hours after hCG injection.
Then, 2‐6 days after IVF/ICSI, one (n = 19) or two (n = 2) embryos were transferred in each cycle. In the patients with moderate‐to‐severe ovarian hyperstimulation syndrome (n = 10) or a suspicion of endometrial abnormality (n = 1), all the blastocysts were cryopreserved by vitrification. Seven patients could not undergo an embryo transfer (ET) because the embryos did not become blastocysts. All the patients who underwent an ET received the administration of hCG every 2‐3 days or daily progesterone by i.m. injection (PROGEHORMON; MOCHIDA Pharmaceutical Company, Ltd.) or transvaginally (Lutinus®; Ferring Pharmaceuticals Company, Ltd.) for luteal phase support.
2.2. Sample collection
In total, 65 samples of follicular fluid were obtained from 30 patients. During the oocyte retrieval, each follicle was punctured and the follicular fluid was aspirated and collected individually. Only blood‐free, clear‐yellow follicular fluid aspirates were included in this study. Follicular fluid from follicles that contained no egg was not included in this study. The samples were centrifuged 200xg and frozen at −80°C. The patients’ venous blood samples also were collected on the day of the OPU to investigate the relationship between the plasma kisspeptin levels and the follicular fluid kisspeptin levels.
In order to investigate the plasma kisspeptin levels across different phases of assisted reproductive technology, venous blood samples were collected from 14 patients (including five patients whose follicular fluid samples were collected for this study) who had been treated with buserelin acetate at the beginning of stimulation (phase I), on the day the schedule for the OPU was decided (2‐6 days before OPU) (phase II), and on the days of the OPU (phase III) and ET (phase IV).
In addition, venous blood samples were collected in phase II from all the patients as a routine for hormonal analysis. After collecting the blood, the serum fraction and the plasma fraction were separated rapidly by a centrifuge and frozen at −80°C until assayed. The plasma and follicular fluid samples’ characteristics are summarized in Table 1.
Table 1.
Characteristics of the plasma samples in each phase of assisted reproductive technology and of the follicular fluid samples
| Total | Plasma samples in each phase | Follicular fluid samples | |
|---|---|---|---|
| Number of cycles | 39 | 14 | 30 |
| Age (y) | 34.3 ± 4.0 | 33.7 ± 4.6 | 34.7 ± 3.8 |
| Sterility | |||
| Primary (N) | 23 | 9 | 17 |
| Secondary (N) | 16 | 5 | 13 |
| Number of ovum pick‐ups | 1.8 ± 1.4 | 1.6 ± 0.9 | 1.9 ± 1.6 |
| Stimulation protocol | |||
| GnRH agonist (long) (N) | 33 | 14 | 24 |
| GnRH antagonist (N) | 6 | 0 | 6 |
| Number of retrieved oocytes | 12.2 ± 5.6 | 12.9 ± 5.0 | 11.5 ± 5.7 |
| Outcome | |||
| Pregnancy | |||
| Delivered (N) | 3 | 1 | 3 |
| Ongoing (N) | 4 | 2 | 2 |
| Spontaneous abortion (N) | 2 | 1 | 1 |
| No pregnancy (N) | 19 | 7 | 10 |
| All blastocysts were cryopreserved (N) | 11 | 3 | 8 |
| Blastocysts were not obtained and embryo transfer was impossible (N) | 7 | 1 | 6 |
| Pregnancy rate or transfer (%) | 42.9 (9/21) | 40 (4/10) | 37.5 (6/16) |
GnRH, gonadotropin‐releasing hormone.
2.3. Hormonal analysis
In all the follicular fluid samples, the E2 levels were measured by chemiluminescence immunoassay. In the blood samples of phase II, the serum E2, luteinizing hormone (LH), and follicle‐stimulating hormone (FSH) also were measured by chemiluminescence and P4 was measured by enzyme immunoassay. The E2 levels on the days prior to the OPU were revised as the rate of the exponential rise of serum E2 to 0.50.27
In all of the follicular fluid samples and blood samples, the kisspeptin levels were measured with a Kisspeptin‐10/Metastin (45‐54)‐Amide (Human) EIA Kit (Catalog# EK‐048‐56; Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) after extraction with Phoenix Peptide sep‐columns (Catalog# RK‐Sepcol‐1; Phoenix Pharmaceuticals, Inc.).28, 29 The minimal detectable concentration was 0.06 ng/mL. The intra‐assay variation and interassay variation were <10% and <15%, respectively. The linear range was 0.06‐0.9 ng/mL.
The principle of the enzyme immunoassay with this kit is as follows. The immunoplate is precoated with a secondary antibody and non‐specific binding sites are blocked. The secondary antibody can bind to the Fc fragment of the primary antibody (peptide antibody), whose Fab fragment will be competitively bound by both biotinylated peptide and peptide standard or targeted peptide in the samples. The biotinylated peptide interacts with streptavidin‐horseradish peroxidase (SA‐HRP), which catalyzes the substrate solution. The intensity of the yellow is directly proportional to the amount of biotinylated peptide–SA‐HRP complex, but is inversely proportional to the amount of the peptide in standard solutions or samples. This is due to the competitive binding of the biotinylated peptide with the standard peptide or samples to the peptide antibody (primary antibody). A standard curve of known concentration can be established accordingly. The unknown concentration in the samples can be determined by extrapolation to this standard curve. The absorbances were read at 450 nm.
2.4. Statistical analysis
The normality of all the studied parameters was checked with the chi‐square test of goodness‐of‐fit. Comparisons of the means between each phase were performed with a repeated‐measures single‐factor ANOVA. In order to identify which specific means differed, the paired t‐test was used. Comparisons between the mean plasma kisspeptin and the follicular fluid kisspeptin concentrations were performed with Welch's t test. Spearman's or Pearson's coefficients were used to assess the correlation of kisspeptin with each parameter. The level of significance was set at P < .05. The software that was used for statistical analysis was Statcel (v.4; OMS Publishing, Inc., Tokorozawa, Japan).
3. RESULTS
With 39 patients, there was a total of 476 oocytes, 264 of which were fertilized (55.5%). Nine out of 21 patients who underwent ET were pregnant and the pregnancy rate per ET was 42.9% (Table 1).
3.1. Follicular fluid kisspeptin levels and plasma kisspeptin levels
In the 65 follicular fluid samples, the kisspeptin concentration averaged 15.090 ± 4.357 pg/mL and ranged from 7.564 to 27.901 pg/mL. Compared with the plasma kisspeptin concentration on the day of the OPU (average: 9.453 ± 2.347 pg/mL; range: 5.429‐14.172 pg/mL), the follicular fluid kisspeptin level was significantly higher (P < .001).
The follicular fluid E2 concentration (average: 607 677 ±183 364 pg/mL) was positively associated with the follicular fluid kisspeptin concentration (r = .249, P < .05) (Figure 1). No correlation was found between the patients’ age, serum hormone levels (E2, FSH, and LH), number of growing follicles, number of retrieved oocytes, and the follicular fluid kisspeptin concentration or the plasma kisspeptin concentration.
Figure 1.
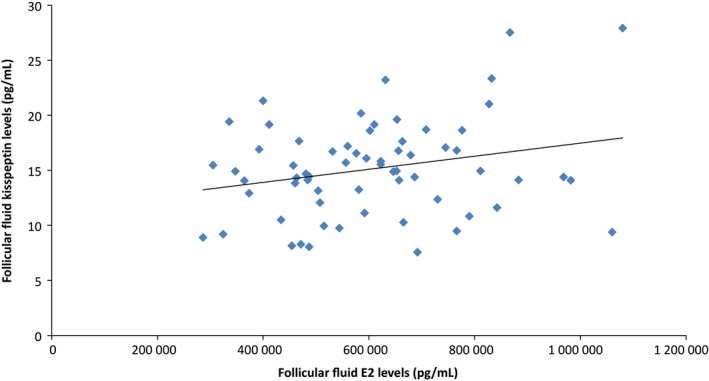
Follicular fluid estradiol (E2) concentration is positively associated with the follicular fluid kisspeptin concentration (r = .249, P < .05)
3.2. Correlation between the follicular fluid kisspeptin level and the serum estradiol and number of oocytes
The level of follicular fluid kisspeptin had a positive correlation with the serum E2 and number of mature oocytes (r = .374, P < .05) (Figure 2). No correlation was found between the serum E2 and number of retrieved oocytes or the serum E2 and number of growing follicles and follicular fluid kisspeptin.
Figure 2.
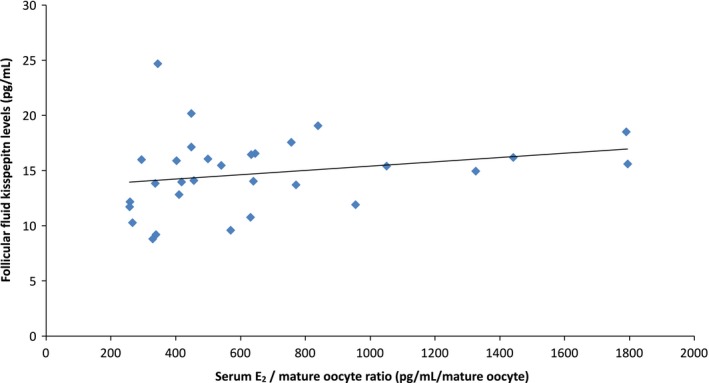
Follicular fluid kisspeptin concentration has a positive correlation with the serum estradiol (E2)/number of mature oocyte ratio (r = .374, P < .05). The revised serum E2's exponential rise rate is 0.50
3.3. Plasma kisspeptin levels during controlled ovarian stimulation in in vitro fertilization/intracytoplasmic sperm injection
In the 14 of those who were patients being treated with buserelin acetate, the plasma kisspeptin levels were different in the different phases of the IVF/ICSI program. The maximum concentrations of kisspeptin were observed on the day of the OPU (phase III) and on the day of the ET (phase IV). There was a significant increase (P < .05) in the plasma kisspeptin levels from the beginning of stimulation (phase I) to phase III (5.620 ± 2.285 pg/mL to 7.754 ± 2.104 pg/mL) and from phase I to phase IV (5.620 ± 2.285 pg/mL to 7.212 ± 2.831 pg/mL) (Table 2).
Table 2.
Changes in the plasma kisspeptin levels in each phase of assisted reproductive technology (ART)
| Phase I | Phase II | Phase III | Phase IV | P‐value | P‐value | ||
|---|---|---|---|---|---|---|---|
| Phase I vs phase III | Phase I vs phase IV | ||||||
|
Kisspeptin level (pg/mL) (mean ± SD) |
5.62 ± 2.29 | 6.85 ± 3.24 | 7.75 ± 2.10 | 7.21 ± 2.83 | .008 | .003 | .002 |
Values are the mean ± SD of the plasma kisspeptin levels in each phase of ART. Phases III and IV were significantly higher than phase I. Phase I, the beginning of stimulation (after menstruation); Phase II, the day the schedule for the ovum pick‐up (OPU) was decided (2‐6 days before the OPU); Phase III, the day of the OPU (35 h after the 10 000 IU human chorionic gonadotrophin injection); Phase IV, the day of the embryo transfer (2‐6 days after the OPU).
4. DISCUSSION
For the first time, the kisspeptin concentrations were measured in follicular fluid and it was found that kisspeptin was present in the follicular fluid. The follicular fluid kisspeptin levels were significantly higher than the plasma kisspeptin levels and this is considered to reflect the expression of kisspeptin in the ovary. In addition, a significant positive correlation was confirmed between the kisspeptin concentration and the E2 concentration in the follicular fluid. The correlation between the E2 concentration in the follicular fluid and egg maturity is known from previous reports,20, 21 suggesting a relationship between the concentration of kisspeptin in the follicular fluid and egg maturity. In addition, there has been a report that the higher that the serum estradiol : oocyte ratio is, the higher the maturity of the egg becomes,30 and the correlation between the serum E2 level and number of matured eggs and the concentration of kisspeptin in the follicular fluid also supported the association between egg maturity and follicular fluid kisspeptin.
This experiment showed that the plasma kisspeptin levels in IVF/ICSI increased significantly with ovarian stimulation. This is consistent with previous reports that the concentration of kisspeptin is seen to increase during the pre‐ovulatory period and the luteal phase during the menstrual cycle.18, 19 It is known that kisspeptin is expressed in the ovarian granulosa‐lutein cells and theca cells of growing follicles8, 9, 10 and the rise in plasma kisspeptin concentration coincides with the time when the expression of kisspeptin increases in the ovary. Although there are multiple expression sites of kisspeptin in the peripheral organs,1 the concentrations show a constant fluctuation in the short period of ovarian stimulation; thus, an increase in kisspeptin in the ovary accompanying follicular growth might contribute to an increase in plasma concentrations. In contrast, despite the plasma kisspeptin concentration being increased at the time of the OPU, a correlation between the kisspeptin concentration and the number of oocytes that were collected was not confirmed. In this study, the number of specimens was small and the relatively low kisspeptin concentration in the plasma could be the reasons for the results that were obtained. It is planned in the future to further increase the number of specimens and to examine them.
In rat studies, it is known that the expression level of KiSS‐1 messenger RNA in the immature ovary is low and it is increased with pregnant‐mare serum gonadotropin–hCG stimulation.8 In addition, it has been reported that the signal of neurotrophin via the NTRK2 receptor, which is indispensable for the maturation of oocytes, depends on the signal of the kisspeptin receptor.31 In addition to the results of this experiment, it can be said that these are all related to egg maturation and kisspeptin. Many of the mechanisms are not yet understood, but now that kisspeptin is known to be present in the follicular fluid, it will be possible to investigate in detail the kisspeptin concentrations in the follicular fluid and egg maturity in the future, which could help to elucidate the function of kisspeptin in the ovary.
In conclusion, kisspeptin was found to be present in the follicular fluid. In addition, the concentration of kisspeptin in the follicular fluid was significantly correlated with the E2 concentration in the follicular fluid and the E2 concentration in the serum per mature egg. The plasma kisspeptin concentration was shown to fluctuate with ovarian stimulation. It is thought that kisspeptin is involved in egg maturation and ovulation in the ovary and it appears that the measurement of kisspeptin concentrations in the follicular fluid is useful for the elucidation of its mechanism.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This study was approved by the Institutional Ethical Committee of Tokushima University, Tokushima, Japan. All the procedures were followed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the patients to be included in the study. This article does not contain any study with animal participants that has been performed by any of the authors.
Taniguchi Y, Kuwahara A, Tachibana A, et al. Intra‐follicular kisspeptin levels are related to oocyte maturation and gonadal hormones in patients who are undergoing assisted reproductive technology. Reprod Med Biol. 2017;16:380‐385. https://doi.org/10.1002/rmb2.12056
REFERENCES
- 1. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS‐1 encodes peptide ligand of a G‐protein‐coupled receptor. Nature. 2001;411:613‐617. [DOI] [PubMed] [Google Scholar]
- 2. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein‐coupled receptor, activated by the peptide KiSS‐1. J Biol Chem. 2001;276:28969‐28975. [DOI] [PubMed] [Google Scholar]
- 3. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS‐1 encodes kisspeptins, the natural ligands of the orphan G protein‐coupled receptor GPR54. J Biol Chem. 2001;276:34631‐34636. [DOI] [PubMed] [Google Scholar]
- 4. Roa J, Tena‐Sempere M. KiSS‐1 system and reproduction: comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol. 2007;153:132‐140. [DOI] [PubMed] [Google Scholar]
- 5. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687‐6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Navarro VM, Castellano JM, Fernández‐Fernández R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS‐1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone‐releasing activity of KiSS‐1 peptide. Endocrinology. 2004;145:4565‐4574. [DOI] [PubMed] [Google Scholar]
- 7. Seminara SB. Metastin and its G protein‐coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol. 2005;26:131‐138. [DOI] [PubMed] [Google Scholar]
- 8. Castellano JM, Gaytan M, Roa J, et al. Expression of KiSS‐1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147:4852‐4862. [DOI] [PubMed] [Google Scholar]
- 9. Cejudo Roman A, Pinto FM, Dorta I, et al. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012;97:1213‐1219. [DOI] [PubMed] [Google Scholar]
- 10. Gaytán F, Gaytán M, Castellano JM, et al. KiSS‐1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS‐1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2009;296:E520‐E531. [DOI] [PubMed] [Google Scholar]
- 11. Ricu MA, Ramirez VD, Paredes AH, Lara HE. Evidence for a celiac ganglion–ovarian kisspeptin neural network in the rat: intraovarian anti‐kisspeptin delays vaginal opening and alters estrous cyclicity. Endocrinology. 2012;153:4966‐4977. [DOI] [PubMed] [Google Scholar]
- 12. Gaytan F, García‐Galiano D, Dorfman MD, et al. Kisspeptin receptor haplo‐insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology. 2014;155:3088‐3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandois D, Na E, Cuevas F, Cruz G, Lara HE, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol. 2016;228:161‐170. [DOI] [PubMed] [Google Scholar]
- 14. Canbay E, Ergen A, Bugra D, et al. Kisspeptin‐54 levels are increased in patients with colorectal cancer. World J Surg. 2012;36:2218‐2224. [DOI] [PubMed] [Google Scholar]
- 15. Horikoshi Y, Matsumoto H, Takatsu Y, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta‐derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914‐919. [DOI] [PubMed] [Google Scholar]
- 16. Katagiri F, Nagai K, Kida A, et al. Clinical significance of plasma metastin level in pancreatic cancer patients. Oncol Rep. 2009;21:815‐819. [PubMed] [Google Scholar]
- 17. Chen X, Mo Y, Li L, Chen Y, Li Y, Yang D. Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2010;149:72‐76. [DOI] [PubMed] [Google Scholar]
- 18. Katagiri F, Kotani M, Hirai T, Kagawa J. The relationship between circulating kisspeptin and sexual hormones levels in healthy females. Biochem Biophys Res Commun. 2015;458:663‐666. [DOI] [PubMed] [Google Scholar]
- 19. Latif R, Rafique N. Serum kisspeptin levels across different phases of the menstrual cycle and their correlation with serum oestradiol. Neth J Med. 2015;73:175‐178. [PubMed] [Google Scholar]
- 20. Artini PG, Battaglia C, D'Ambrogio G, et al. Relationship between human oocyte maturity, fertilization and follicular fluid growth factors. Hum Reprod. 1994;9:902‐906. [DOI] [PubMed] [Google Scholar]
- 21. Itskovitz J, Rubattu S, Rosenwaks Z, Liu HC, Sealey JE. Relationship of follicular fluid prorenin to oocyte maturation, steroid levels, and outcome of in vitro fertilization. J Clin Endocrinol Metab. 1991;72:165‐171. [DOI] [PubMed] [Google Scholar]
- 22. Suchanek E, Simunic V, Juretic D, Grizelj V. Follicular fluid contents of hyaluronic acid, follicle‐stimulating hormone and steroids relative to the success of in vitro fertilization of human oocytes. Fertil Steril. 1994;62:347‐352. [DOI] [PubMed] [Google Scholar]
- 23. Anifandis G, Koutselini E, Stefanidis I, et al. Serum and follicular fluid leptin levels are correlated with human embryo quality. Reproduction. 2005;130:917‐921. [DOI] [PubMed] [Google Scholar]
- 24. Asimakopoulos B, Köster F, Felberbaum R, Al‐Hasani S, Diedrich K, Nikolettos N. Cytokine and hormonal profile in blood serum and follicular fluids during ovarian stimulation with the multidose antagonist or the long agonist protocol. Hum Reprod. 2006;21:3091‐3095. [DOI] [PubMed] [Google Scholar]
- 25. Peña JE, Chang PL, Chan LK, Zeitoun K, Thornton MH, Sauer MV. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Hum Reprod. 2002;17:83‐87. [DOI] [PubMed] [Google Scholar]
- 26. Sharara FI, McClamrock HD. High estradiol levels and high oocyte yield are not detrimental to in vitro fertilization outcome. Fertil Steril. 1999;72:401‐405. [PubMed] [Google Scholar]
- 27. Pittaway DE, Wentz AC. Evaluation of the exponential rise of serum estradiol concentrations in human menopausal gonadotropin‐induced cycles. Fertil Steril. 1983;40:763‐767. [DOI] [PubMed] [Google Scholar]
- 28. Aluclu MA, Sen S, Cevik M. Association between plasma kisspeptin levels and adolescent gynecomastia. Afr J Paediatr Surg. 2016;13:136‐139. [DOI] [PubMed] [Google Scholar]
- 29. Hofmann T, Elbelt U, Haas V, et al. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite. 2017;108:141‐150. [DOI] [PubMed] [Google Scholar]
- 30. Vaughan DA, Harrity C, Sills ES, Mocanu EV. Serum estradiol: oocyte ratio as a predictor of reproductive outcome: an analysis of data from >9000 IVF cycles in the Republic of Ireland. J Assist Reprod Genet. 2016;33:481‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dorfman MD, García‐Rudaz C, Alderman Z, et al. Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure. Endocrinology. 2014;155:3098‐3111. [DOI] [PMC free article] [PubMed] [Google Scholar]


