Abstract
Purpose
To assess the efficacy of fertility preservation (FP) and the impact of chemotherapy on the reproductive potential of Japanese patients with breast cancer.
Methods
Sixty‐two patients with breast cancer visited the authors’ centers from October, 2003 to June, 2015. They were divided into two groups according to the treatment: oocyte or embryo vitrification for FP before cancer treatment (group A) or infertility treatment after cancer treatment (group B). Group B was divided into two subgroups, B1 (no chemotherapy) and B2 (postchemotherapy), in order to analyze the effect of anticancer drugs on ovarian reserves and assisted reproductive technology outcomes. The number of retrieved oocytes, vitrified oocytes or embryos, and pregnancy rates were analyzed and compared: group A compared to group B1 compared to group B2.
Results
The patients in groups A and B1 underwent egg collection without any chemotherapy. The numbers of collected oocytes and vitrified embryos were significantly higher in groups A and B1 than in group B2. Nearly 50% of the in vitro fertilization patients who underwent an embryo transfer (ET) became pregnant, including two patients in group A who underwent a vitrified‐warmed ET. Among the pregnant women, 70% did not have chemotherapy.
Conclusion
For patients with breast cancer, FP with unfertilized oocytes or embryos before chemotherapy seems to be promising for achieving higher pregnancy rates, with no risk of minimal residual disease.
Keywords: breast cancer, chemotherapy, embryo cryopreservation, fertility preservation, oocyte cryopreservation
1. INTRODUCTION
Breast cancer is one of the most common malignant diseases that is diagnosed in reproductive‐aged women. According to the cancer statistics in Japan, 113.0 women per 100,000 were diagnosed with breast cancer in 2012 and one out of 11 women were estimated to be diagnosed with breast cancer in their lifetime, which is the highest of all cancers; furthermore, the incidence increases from the age of 30 years.1 The 5 year survival rate of breast cancer in women is 91.1%,1 which enables reproductive‐aged survivors of cancer to hope for childbearing; such cases also have been gradually increasing in Japan. In contrast, breast cancer in young women often presents with ductal invasion and most of these patients are likely to undergo adjuvant chemotherapy, with well‐known gonadotoxic effects.2 Anticancer drugs, such as alkylating agents, suppress ovarian function and frequently induce premature ovarian failure (POF).3 In addition, when treatment requires anti‐estrogenic agents, such as tamoxifen, for adjuvant treatment, the treatment can last for 5 years, resulting in severe infertility due to aging.4 The survivors of cancer of reproductive age have high risks of infertility, making it very difficult to have a biological child after they have recovered from their cancer.
In the authors’ centers, 140 patients with various forms of cancer have undergone fertility treatment and fertility preservation (FP) since 2003. In this study, the focus was on patients with breast cancer in order to assess the efficacy of FP, as well as the impact of chemotherapy on the reproductive potential of patients with breast cancer in the Japanese population.
2. MATERIALS AND METHODS
A total of 62 patients with breast cancer visited the authors’ centers from October, 2003 to June, 2015. Among them, 53 patients underwent some kind of fertility treatment, including 46 patients who underwent in vitro fertilization (IVF) (Figure 1). Four patients (three patients who developed breast cancer during fertility treatment and one patient with an unknown past chemotherapeutic status) were excluded from this study. The rest of them (who had no previous IVF) (n = 42) were divided into two groups, according to their treatment: group A (patients with cancer who cryopreserved their oocytes and/or embryos before cancer treatment for FP) and group B (patients with cancer who underwent infertility treatment after cancer treatment). All the group B patients were approved for infertility treatment by their oncologists. Group B was further divided into two subgroups: group B1 (no chemotherapy for cancer treatment) and group B2 (postchemotherapy) in order to assess the effect of anticancer drugs on their ovarian reserve and IVF outcome. In this study, there was no patient with POF due to chemotherapy.
Figure 1.
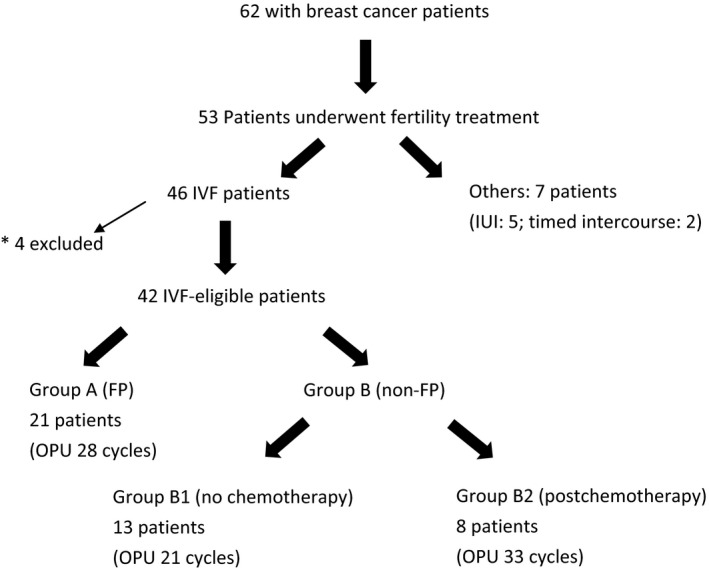
Details of the 62 patients with breast cancer who were included in this study from October, 2003 to June, 2015. *Three patients who developed breast cancer during fertility treatment and one patient with an unknown past chemotherapeutic status were excluded from this study. FP, fertility preservation; IUI, intrauterine insemination; IVF, in vitro fertilization; OPU, oocyte pick‐up
The modes of ovarian stimulation are listed in Table 1. Controlled ovarian stimulation (COS) for IVF was performed with a letrozole‐antagonist protocol5 or without letrozole (Table 1). Letrozole was used mainly in the estrogen receptor‐positive patients with breast cancer: 20 out of 28 cycles in group A (Table 1). The doses of follicle‐stimulating hormone (FSH) product or human menopausal gonadotropin (HMG) were determined according to the ovarian reserve (anti‐Müllerian hormone [AMH], basal FSH, antral follicular count, weight, and age). The cryopreservation method was vitrification by using cryotop (Kitazato Biopharma, Ltd., Fuji, Japan). Mainly, the blastocysts were vitrified and the early‐stage embryos were vitrified in the cases of few oocytes from poor responders.
Table 1.
Patient profile and modes of treatment
| Variable | Group A (FP) | Group B1 (no FP/no chemotherapy) | Group B2 (no FP/postchemotherapy) |
|---|---|---|---|
| No. of patients | 21 | 13 | 8 |
| No. of multigravida patients | 4 | 3 | 0 |
| No. of multipara patients | 1 | 1 | 0 |
| No. (%) of patients without a partner | 7 (33.3) | 0 | 0 |
| No. of cancer deaths | 1 (oocyte cryopreservation) | 0 | 0 |
| Oocyte vitrification | 8 patients (10 cycles) | 0 | 0 |
| Embryo vitrification | 11 patients (11 cycles) | 10 patients (12 cycles) | 5 patients (7 cycles) |
| No embryo vitrificationa | 2 patients | 3 patients | 3 patients |
| Ovarian stimulation protocolb | |||
| With letrozole | 20 cycles | 8 cycles | 7 cycles |
| Without letrozole | 8 cycles | 13 cycles | 26 cycles |
FP, fertility preservation. aPatients who did not vitrify embryos due to no fertilization or embryo development arrest; bLetrozole was used in the estrogen receptor‐positive patients in Group A. Letrozole was used in the patients who were estrogen receptor‐positive and/or had a low ovarian reserve in groups B1 and B2.
The numbers of retrieved oocytes, vitrified oocytes or embryos, and pregnancy rates were compared in order to assess the effect of FP on the subsequent reproductive performance of patients with breast cancer. A statistical analysis (using software StatMate V, Tokyo, Japan) was performed by using the Kruskal‐Wallis test for multiple comparison and the Mann‐Whitney U‐test, chi‐square analysis, or Fisher's exact test where appropriate. A P‐value of <.05 was considered to be statistically significant.
This study was approved by the institutional review boards of Kyono ART Clinic, Sendai, and Kyono ART Clinic Takanawa, Tokyo, Japan. All the patients who were involved in this study allowed the researchers to use their medical record data for research in an unidentifiable manner. Written, informed consent was obtained from all the patients prior to assisted reproductive technology treatment in the two centers.
3. RESULTS
All the patients had breast cancer surgery. The patients agreed to preserve their fertility (group A) or to undergo fertility interventions (group B). All the group A patients underwent egg collection for FP before receiving any chemotherapy. In group A, the interval between the cancer operation and chemotherapy was 2.1 ± .7 months (1‐3 months). The average age at first admission was significantly lower in group A than in group B2 (35.19 ± 3.14 vs 40.50 ± 2.56 years old, P < .001), although the serum AMH levels were similar among the three groups (Table 2). The numbers of collected oocytes and vitrified embryos were significantly larger in groups A and B1 than in group B2 (group A vs group B2: collected oocytes, 6.86 ± 5.62 vs 2.42 ± 2.54, P < .001; vitrified embryos, 2.24 ± 2.11 vs .24 ± .50, P < .002) (group B1 vs B2: collected oocytes, 5.76 ± 4.95 vs 2.42 ± 2.54, P < .01; vitrified embryos, 2.00 ± 2.83 vs 0.24 ± 0.50, P < .01) (Table 2). There was a statistically significant difference in the serum peak estradiol (E2) levels between groups B1 and B2 (1211.10 ± 1017.97 vs 504.82 ± 423.82 pg/mL, P < .001) (Table 2), possibly derived from the ovarian reserve of the patients, and the presence or absence of letrozole between the groups (Table 1). The peak serum E2 levels tended to be lower in the cycles with letrozole than in the cycles without letrozole, although this was not statistically significant (Table 3). The ratio of patients with a past history of irradiation and hormonal therapy for breast cancer was similar between groups B1 and B2 (Table 2).
Table 2.
Clinical outcomes of in vitro fertilization
| Variable | Group A (FP) | Group B1 (no FP/no chemotherapy) | Group B2 (no FP/postchemotherapy) |
|---|---|---|---|
| No. of patients | 21 | 13 | 8 |
| No. of OPU cycles | 28 | 21 | 33 |
| Age at first admission (years) | 35.19 ± 3.14a | 38.69 ± 5.39 | 40.50 ± 2.56a |
| Age at cancer diagnosis (years) | 34.81 ± 3.27 | 35.82 ± 6.79 | 33.00 ± 4.75 |
| Body mass index (kg/m2) | 20.00 ± 1.70 | 21.50 ± 3.10 | 20.20 ± 3.30 |
| Serum AMH level (ng/mL) | 3.56 ± 4.01 | 3.48 ± 2.66 | 1.34 ± 1.09 |
| Peak serum E2 level(pg/mL) | 887.93 ± 800.03 | 1211.08 ± 1017.97a | 504.82 ± 423.82a |
| No. of collected oocytes | 6.86 ± 5.62a | 5.76 ± 4.95b | 2.42 ± 2.54a , b |
| No. of MII oocytes | 4.89 ± 3.96a | 5.14 ± 4.21c | 1.97 ± 2.27a , c |
| No. of vitrified oocytes | 5.44 ± 4.20 | N/A | N/A |
| No. of fertilized oocytes | 2.95 ± 2.95 | 3.86 ± 3.68b | 1.39 ± 1.64b |
| No. of cryopreserved embryos | 2.24 ± 2.11d | 2.00 ± 2.83b | 0.24 ± 0.50b , d |
| No. (%) of patients with past radiation therapy | N/A | 11 (84.6)e | 5 (62.5)e |
| No. (%) of patients with past hormonal therapy | N/A | 4 (30.8)f | 6 (75.0)f |
AMH, anti‐Müllerian hormone; E2, estradiol; FP, fertility preservation; MII, metaphase II; N/A, not applicable; OPU, oocyte pick‐up. a P < .001; b P < .01; c P < .001; d P < .002, according to the Mann‐Whitney U‐test; e P = .33; f P = .08, according to Fisher's exact test.
Table 3.
Peak estradiol (E2) levels, number of retrieved oocytes, and metaphase (M)II oocytes between letrozole (+) and letrozole (−) in group A
| Variable | Letrozole (+) | Letrozole (–) | P‐value |
|---|---|---|---|
| Peak serum E2 level (pg/mL) | 675.96 ± 592.31 | 1391.35 ± 1031.29 | NS |
| No. of retrieved oocytes | 6.58 ± 5.37 | 7.44 ± 6.42 | NS |
| No. of MII oocytes | 4.89 ± 4.19 | 4.89 ± 3.66 | NS |
NS, not significant, according to the Mann–Whitney U‐test.
Also analyzed was the background of the pregnant patients. Nearly half (10/21) of the IVF patients who underwent an embryo transfer (ET) achieved a pregnancy, including the two patients in group A who underwent a vitrified‐warmed ET (Table 4). All the pregnant women were nulligravida. It was of note that 70% (7/10) of those pregnant patients were pre‐ or without chemotherapy with the vitrified‐warmed ET (six patients) or fresh ET (one patient). Among the pregnant women, the serum AMH was measured in seven cases: the average serum AMH level was 3.00 ± 2.41 ng/mL. There was no statistically significant difference in the AMH levels between the pregnant women without chemotherapy (five patients) and those after chemotherapy (two patients) (3.35 ± 2.70 vs 2.13 ± 1.92 ng/mL, P = .59). The miscarriage rate was 20% (2/10), two patients have an ongoing pregnancy, and six patients delivered healthy babies without complications (average birthweight: 3182 ± 345 g; birth height: 49.5 ± 2.1 cm).
Table 4.
Pregnancy outcome after in vitro fertilization (IVF)
| Group A (FP) | Group B1 (no FP/no chemotherapy) | Group B2 (no FP/postchemotherapy) | |
|---|---|---|---|
| No. of patients (ET cycles) | 2 (3) | 11 (24) | 8 (28) |
| No. of pregnancies | 2 | 5 | 3 |
| IVF pregnancy rate (per patient who underwent ET) | 100% (2/2) | 45.5% (5/11) | 37.5% (3/8) |
| IVF pregnancy rate (per ET) | 66.7% (2/3) | 20.8% (5/24) | 10.7% (3/28) |
| No. of miscarriages | 1 | 0 | 1 |
| No of ongoing pregnancies or deliveries | 1 | 5 | 2 |
ET, embryo transfer; FP, fertility preservation.
Those patients who underwent oocyte cryopreservation have not yet returned to use their oocytes.
4. DISCUSSION
To the best of the authors’ knowledge, there has been no other study of the Japanese population that focuses on FP among the survivors of breast cancer and reports their outcomes of fertility interventions and pregnancy rates. Although the authors were unable to identify the precise cancer stage, regimen, or anticancer agent that was used for the patients in this study, the significance of preserving their fertility before chemotherapy appears to be remarkable, as chemotherapy significantly compromised the ovarian reserve, leading to poorer IVF outcomes (Table 2). Advanced cancer stages also might have a negative impact on patients’ reproductive outcomes due to the inevitability of thorough adjuvant cancer treatment. Despite the similar ovarian reserves, the numbers of collected oocytes, metaphase II oocytes, and vitrified embryos were significantly larger in groups A and B1 than in group B2 (Table 2). This might be related in part to the younger age at admission of the FP patients (Table 2) and an absence of the possible detrimental effects of chemotherapy on the oocytes because both the group A and group B1 patients were never exposed to gonadotoxic agents at the time of egg collection. The patients who underwent FP might not have been infertile at the time of cryopreservation. For ovarian stimulation, letrozole was used for the patients with breast cancer who were estrogen receptor‐positive and/or for the poor responders. Their serum peak E2 levels were lower in the letrozole cycles than in the no‐letrozole cycles; however, this was not statistically significant. This might have been related to the small number of patients.
Although the number of studied women was limited, their pregnancy rate was relatively good, especially with FP (Table 4). That might be related in part to the fact that 70% of the pregnant women had their embryos cryopreserved or had a fresh ET pre‐ or without chemotherapy. This indicates that, in patients with breast cancer, it is important to preserve their fertility before chemotherapy and then relatively good pregnancy rates are expected.
In a European registry study, 50% of the patients with cancer eventually had live births from their cryopreserved embryos.3 It was speculated that though it is common to estimate the frozen embryo implantation potential with the use of data from the age‐matched infertile population, it is possible that implantation rates in the cancer population could be higher because they are presumably fertile when their embryos are cryopreserved.3
Meanwhile, another study reported the use of the GnRH agonist, goserelin, for ovarian protection during adjuvant chemotherapy in hormone receptor‐negative patients with breast cancer, leading to a better pregnancy rate, disease‐free rate, and overall survival, compared to the chemotherapy‐alone group, which could be promising for patients with breast cancer of reproductive age.6
There have been limited ways to preserve fertility in women: embryo, oocyte, and ovarian tissue cryopreservation. The first embryo cryopreservation for FP was reported in 1996 for a patient with breast cancer.7 Embryo cryopreservation generally is offered as a first choice of FP for curable patients with cancer who have a partner and whose pregnancy must be postponed until the resolution of the primary disease.8
Oocyte cryopreservation has become a well‐established treatment option in both general IVF and FP, owing largely to the introduction of vitrification. The Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology stated in 2013 that oocyte vitrification and warming should no longer be considered as experimental.9 Patients should be informed that the live birth rate per vitrified oocyte is 5%‐7% in egg donation programs, although those results might not be generalized to cancerous patients.10
Ovarian cortex cryopreservation is still experimental, although >95 babies have been born worldwide.10 The surgical removal of ovarian tissue causes no delay in starting cancer treatment and yields a lot of primordial follicles,3 but one must be aware of the possibility of transferring malignant cells by reimplanting cryopreserved ovarian tissue. One study retrospectively examined the prevalence of ovarian metastasis in 5571 autopsy findings of Japanese female patients with cancer who were aged under 40 years and reported that the percentage of ovarian metastasis was 22.4%.11 A review showed that patients with breast cancer at an advanced stage are at moderate risk of ovarian metastasis12 and it is extremely important to detect minimal residual disease before transplanting ovarian tissue. From that point of view, embryo or oocyte cryopreservation might be a much safer and stable method of FP, especially in patients with cancer who have enough time to undergo ovarian stimulation, followed by egg collection, before gonadotoxic treatments.
Patients with breast cancer are often at a reproductive age with a high necessity of chemotherapy and long‐term hormonal therapy, leading to infertility. Compared to other cancer types, they have relatively sufficient time to undergo COS and egg collection prior to adjuvant cancer treatment. This study revealed that chemotherapy significantly reduced the number of collected oocytes, vitrified embryos, and pregnancy rates. With careful monitoring of the serum E2 level during COS, FP with oocytes or embryos before chemotherapy could provide high pregnancy rates for patients with breast cancer with no risk of minimal residual disease. Further studies on the relationship between the serum E2 level and the recurrence of breast cancer are necessary. Reproductive endocrinologists should inform both their patients with breast cancer and their oncologists of these results, so that the survivors of breast cancer can be provided with better chances of preserving their fertility.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This study was approved by the institutional review boards of Kyono ART Clinic, Sendai, and Kyono ART Clinic Takanawa, Tokyo, Japan. All the procedures were followed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the patients to be included in the study. This article does not contain any study with animal participants that has been performed by any of the authors.
ACKNOWLEDGEMENTS
We thank C. Onuma for her technical assistance.
Hashimoto T, Nakamura Y, Obata R, et al. Effects of fertility preservation in patients with breast cancer: A retrospective two‐centers study. Reprod Med Biol. 2017;16:374‐379. https://doi.org/10.1002/rmb2.12054
REFERENCES
- 1. http://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed August 2, 2016 (in Japanese). [Google Scholar]
- 2. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917‐2931. [DOI] [PubMed] [Google Scholar]
- 3. Chung K, Donnez J, Ginsburg E, Meirow D. Emergency IVF versus ovarian tissue cryopreservation: decision making in fertility preservation for female cancer patients. Fertil Steril. 2013;99:1534‐1542. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg SM, Partridge AH. New insights into nonadherence with adjuvant endocrine therapy among young women with breast cancer. J Natl Cancer Inst 2015;107:djv245. [DOI] [PubMed] [Google Scholar]
- 5. Azim AA, Costantini‐Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630‐2635. [DOI] [PubMed] [Google Scholar]
- 6. Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast‐cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown JR, Modell E, Obasaju M, King YK. Natural cycle in‐vitro fertilization with embryo cryopreservation prior to chemotherapy for carcinoma of the breast. Hum Reprod. 1996;11:197‐199. [DOI] [PubMed] [Google Scholar]
- 8. Bedoschi G, Oktay K. Current approach to fertility preservation by embryo cryopreservation. Fertil Steril. 2013;99:1496‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology . Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37‐43. [DOI] [PubMed] [Google Scholar]
- 10. Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen‐thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kyono K, Doshida M, Toya M, Sato Y, Akahira J, Sasano H. Potential indications for ovarian autotransplantation based on the analysis of 5,571 autopsy findings of females under the age of 40 in Japan. Fertil Steril. 2010;93:2429‐2430. [DOI] [PubMed] [Google Scholar]
- 12. Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen‐thawed ovarian tissue. Fertil Steril. 2013;99:1514‐1522. [DOI] [PubMed] [Google Scholar]


