Abstract
Cannon reviews new evidence supporting a key role for anomalous inward currents in the etiology of hypokalemic periodic paralysis.
Hypokalemic periodic paralysis (HypoKPP) is a rare, dominantly inherited disorder of muscle that is characterized by recurrent and prolonged episodes of severe weakness. These episodes are associated with low serum K+ (often <3.0 mM, normally 3.5–5.5 mM), often triggered by events that lower extracellular [K+] such as carbohydrate-rich meals, and result in depolarization-induced loss of excitability. In 1994, genetic linkage and positional cloning established a HypoKPP locus in CACNA1S, which encodes the pore-forming α subunit of the skeletal muscle L-type Ca2+ channel CaV1.1 (Fontaine et al., 1994). The first missense mutations were identified soon after (Jurkat-Rott et al., 1994; Ptáček et al., 1994). Despite the tantalizing observation that the first three mutations were all missense substitutions of arginines in S4 segments, the identification of a functional defect with a plausible mechanistic connection to depolarization-induced loss of excitability and weakness in low K+ did not emerge until some 13 yr later. Two major challenges impeded functional studies of HypoKPP mutant channels. First, expression of CaV1.1 at the plasma membrane is poor in model systems not derived from muscle. Second, the critical channel defect—an anomalous inward current—is of relatively small amplitude and difficult to distinguish from a nonspecific leakage current. In the current issue of the Journal of General Physiology, Fuster et al. use in vivo electroporation into mouse muscle as a system in which to achieve high expression of human CaV1.1. They express CaV1.1 channels carrying an atypical HypoKPP mutation—V876E in S3 of domain III—and demonstrate an anomalous inward current at hyperpolarized potentials. This finding is an important advance in the field because it reveals that the one HypoKPP mutation that departs from the canonical pattern of R/X missense mutations in S4 segments still produces an anomalous inward current. Moreover, unlike all other CaV1.1 HypoKPP mutations studied to date, V876E does not alter voltage-dependent gating of the conventional Ca2+ current, strongly supporting the notion that the anomalous inward current is the critical defect in HypoKPP.
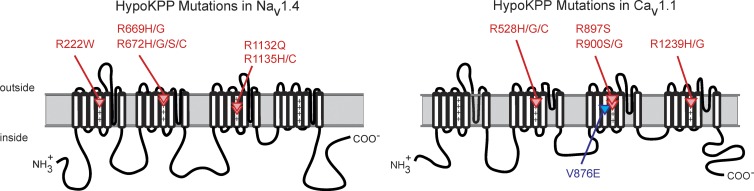
The experimental insight on the source of the anomalous inward current in HypoKPP originated from the remarkable observation that missense mutations of SCN4A, encoding the α subunit of the skeletal muscle sodium channel NaV1.4, are also a cause of HypoKPP. The clinical HypoKPP phenotype is essentially identical, regardless of whether the underlying mutation is in CaV1.1 or NaV1.4. This genetic heterogeneity was initially puzzling, especially because the functional roles for NaV1.4 and CaV1.1 in excitation-contraction coupling are very different. Moreover, the final common pathway for weakness in an episode of HypoKPP is a failure to maintain the resting potential (Vrest) in low K+, and yet studies in human HypoKPP fibers showed this paradoxical depolarization is not prevented by the channel blockers tetrodotoxin or nitrendipine (Ruff, 1999; Jurkat-Rott et al., 2000). The solution to the mystery of the depolarizing current in HypoKPP was gleaned from recent advances in understanding the conformational changes that occur in the voltage sensor domain of KV channels. First, histidine-scanning mutagenesis in Shaker S4, as a tool to discern gating-dependent changes in aqueous accessibility of specific S4 residues to protons, also created a proton conduction pathway (Starace and Bezanilla, 2001). Other R/X substitutions even supported currents carried by Na+ or K+ (Tombola et al., 2005). These were initially called omega currents, to distinguish this conduction mechanism from ion flux through the conventional pore (α current). In subsequent publications, the preferred term became the “gating pore current” to convey the view that the S4 helix undergoes a voltage-dependent translocation through a crevasse in the channel—the gating pore—and furthermore that mutations at this critical interface may permit ion flux as an anomalous gating pore current. Second, a consistent pattern emerged as more HypoKPP mutations were identified (Fig. 1). All 10 HypoKPP missense mutations in NaV1.4 are in the outer arginine residues of S4 (R1 or R2) in domains I, II, or III; and 8 of 9 HypoKPP mutations in CaV1.1 are in R1 or R2 of domains II, III, and IV (Matthews et al., 2009). Only CaV1.1-V876E, the mutant channel now studied functionally for the first time by Fuster et al. (2017b), is an exception to the pattern.
Figure 1.
Mutations of NaV1.4 and CaV1.1 associated with hypokalemic periodic paralysis occur predominantly at arginine residues in S4 transmembrane segments of the voltage sensor domains. The V876E mutation in S3 of domain III in CaV1.1 is the only exception (blue highlight).
The existence of gating pore currents caused by HypoKPP mutations was first established for NaV1.4 channels expressed in Xenopus oocytes. Compared with CaV1.1, mutations of NaV1.4 are a less frequent cause of HypoKPP (∼20% of families), and the discovery of SCN4A mutations lagged the identification of calcium channel mutations by five years. Nevertheless, it was the ability to achieve high expression of NaV1.4 in oocytes that provided the first experimental insights on the defect responsible for HypoKPP. In the presence of TTX to block the conventional pore, an inward rectifying current was detected for all 8 NaV1.4 HypoKPP mutant channels tested, but not wild-type channels (Sokolov et al., 2007; Struyk and Cannon, 2007; Struyk et al., 2008). The voltage-dependent translocation of the S4 helix is the basis for this rectification. In HypoKPP channels, the gating pore is permissive for ion conduction at hyperpolarized potentials because the S4 helix is in the inward conformation, which creates misalignment between the missense mutation of R1 or R2 and the narrow hydrophobic waist of the gating charge transfer center that normally occludes ion conduction. With depolarization, the S4 helix moves outward, taking the mutant R1 or R2 out of the hydrophobic waist and thereby shutting the gating pore leak. An anomalous inward current, consistent with a gating pore leak, has also been detected in muscle fibers from a HypoKPP knock-in mutant mouse model harboring NaV1.4-R669H at R1 in domain II (Wu et al., 2011). The mouse model confirmed that an S4 R/X mutation for NaV1.4 expressed in a native context of skeletal muscle does conduct a gating pore current and also established the magnitude of this anomalous conductance as 7 µS/cm2 or ∼1% of the total resting conductance.
Once a gating pore current was identified in NaV1.4 HypoKPP mutant channels, the key question in the field was whether HypoKPP mutations in CaV1.1 would also support a gating pore current. If this were true, then it could explain why mutations of either CaV1.1 or NaV1.4 might produce the same clinical phenotype. This hypothesis was plausible because S4 mutations cause gating pore currents in both Kv and NaV channels, whose voltage sensor domains are highly conserved with CaV channels. The initial test of this hypothesis was performed on fibers biopsied from the quadriceps of HypoKPP patients. A larger slope conductance, by ∼10–20 µS/cm2, was observed at negative potentials for HypoKPP fibers compared with wild type, consistent with a gating pore leak (Jurkat-Rott et al., 2009). In retrospect, a larger “depolarizing inward current” was observed in R528H fibers compared with wild type from the steady-state I-V relation eight years before the gating pore hypothesis (Ruff, 1999). It is remarkable that evidence of a gating pore conductance was detectable in these heroic studies on scarcely available human HypoKPP fibers because the anomalous increase in conductance was only ∼10% of the total (in Cl−-free solution to reduce GCl and 1 mM K+ to reduce GKir) for these three-electrode voltage-clamp studies on 3.5-cm-long resealed cut fibers. The creation of an R528H knock-in mutant mouse model of HypoKPP provided an opportunity for more in-depth studies to confirm that a mutation of R1 in DIIS4 supports a gating pore current (Wu et al., 2012). An anomalous inward current was indeed detected in R528H mouse fibers, but even with a cocktail of blockers to reduce endogenous currents (TTX, TEA, 4-AP, Co2+, Ba2+, and 9-AC), the increased conductance attributable to the mutation was only ∼10% of the total residual conductance.
Upon this background of technical challenges to ascertain the presence of gating pore currents in mutant CaV1.1 channels, Fuster et al. (2017b) has demonstrated that expression of exogenous human CaV1.1 by in vivo electroporation in mouse muscle has sufficiently robust expression to screen for gating pore currents. This approach markedly increases the throughput of screening CaV1.1 HypoKPP mutations as sufficient expression occurs by 30 d rather than the time-consuming and expensive process of creating a knock-in mouse. Another improvement from their approach is the use of the silicone whole-cell clamp, which achieves a much more efficient dialysis of the myoplasm from a broken pipette tip and thereby enables better control of the intracellular ionic composition to reduce endogenous currents. Their initial study with this technique demonstrated a gating pore current for CaV1.1-R1239H at R2 of DIVS4 (Fuster et al., 2017a). The sensitivity for discerning a gating pore current distinct from the nonspecific background leak was improved, now being about a 35% increase above wild type, but a large number of fibers must still be sampled (50 in their case) to deal with the high variance of residual background currents in this preparation. Having established that this technique is able to confirm a gating pore current at a canonical R/X mutation in DIVS4 previously identified in human HypoKPP fibers, they now use this transient expression system in mouse muscle to test whether the atypical HypoKPP mutation V876E in S3 of DIII also supports a gating pore current. On average, the inward current was ∼25% larger at hyperpolarized potentials for V786E compared with wild type. This important result convincingly shows that V786E shares a common functional defect with all other HypoKPP mutant channels studied to date, for both CaV1.1 and NaV1.4. Moreover, the voltage dependence of activation and amplitude of the Ca2+ current for V786E-expressing fibers were indistinguishable from wild type, whereas the R/X HypoKPP mutations in S4 have reduced current density and altered activation. This further supports the notion that the essential defect that causes susceptibility to HypoKPP is the anomalous inward current that is active at the resting potential. An alternative technical advance provides an opportunity to characterize HypoKPP mutant CaV1.1 channels in a membrane environment with much less interference from endogenous channels. Co-expression of Stac3 along with CaV1.1, α2δ and β1a in Xenopus oocytes markedly enhances channel expression at the plasma membrane where gating pore currents have been observed for R528H and R528G (Wu and Cannon, 2017).
An important advantage of in vivo mouse model systems is that the HypoKPP CaV1.1 channels are expressed in their native environment of fully differentiated and innervated adult skeletal muscle. In this context, it is reasonable to assume that the full complement of posttranslational modification, accessory subunits, protein–protein interactions at the triad, and signaling events are intact, which strengthens the confidence and relevance of identified functional defects for discovering disease mechanisms. For example, the specific conductance of the gating pore leak is a critical parameter for simulating the defect of Vrest stability in HypoKPP (Struyk and Cannon, 2008). The knock-in mouse is widely considered to be the best standard because transcript levels and trafficking to the membrane are under control of endogenous systems. Moreover, the R528H mice survive as homozygous mutants (Wu et al., 2012), from which we know the specific conductance of the gating pore current is 28 µs/cm2 (in 100 mM Na+). Assuming equal abundance of wild-type and R528H CaV1.1 subunits at the membrane in the heterozygous state, as occurs in humans with HypoKPP, the predicted specific conductance is 14 µS/cm2, which is remarkably consistent with the value of 12 µS/cm2 estimated from limited studies on human HypoKPP R528H fibers (Jurkat-Rott et al., 2009).
In the transient expression system used by Fuster et al. (2017b), the specific conductance of the anomalous inward current was estimated to be 19.6 µS/cm2 for R1239H (carried by protons in a TEA external solution) and 68 µS/cm2 for the atypical HypoKPP mutation V786E (in 140 mM Na+). An interesting question is how these values translate to the heterozygous condition in HypoKPP patients. The endogenous CaV1.1 was not ablated in the electroporation experiments, and remarkably, the peak Ca2+ current density (∼5 A/F) was comparable with that in naive wild-type fibers. This implies that the total density of CaV1.1 subunits (endogenous and transfected) at the membrane was comparable with naive fibers. Moreover, electroporation studies with a mutated CaV1.1 to reduce isradipine sensitivity showed that up to 70% of the current was insensitive to block (DiFranco et al., 2011). This suggests that the majority of CaV1.1 subunits at the membrane are from the transfected construct. If a similar ratio of V786E to wild-type channels occurred in Fuster’s preparation, then the equivalent specific conductance in a heterozygous patient would be 49 µS/cm2 = (68 * 0.5/0.7), which is notably higher than for the other HypoKPP mutant channels and may account for the more severe clinical phenotype associated with this particular mutation.
More studies are needed to determine whether the inward current for V786E actually passes through the same gating pore conduction pathway as the one created by R/X mutations in S4. For example, the gating pore currents from R/X mutations in S4 of NaV or KV channels, as well as I → H mutations in S1 or S2 of Shaker K channels, all have strong rectification caused by movement of the voltage sensor (Campos et al., 2007). Conversely, V786E has minimal rectification over −120 to 0 mV in the presence of a symmetrical gradient for the permeant ion. This discordance invites alternative models of the abnormal current demonstrated in this valuable new study.
Acknowledgments
This work was supported by National Institute of Arthritis, Musculoskeletal, and Skin Disease of the National Institutes of Health grants AR42703 and AR063182.
The author declares no competing financial interests.
Eduardo Ríos served as editor.
References
- Campos F.V., Chanda B., Roux B., and Bezanilla F.. 2007. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl. Acad. Sci. USA. 104:7904–7909. 10.1073/pnas.0702638104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco M., Tran P., Quiñonez M., and Vergara J.L.. 2011. Functional expression of transgenic α1sDHPR channels in adult mammalian skeletal muscle fibres. J. Physiol. 589:1421–1442. 10.1113/jphysiol.2010.202804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Vale-Santos J., Jurkat-Rott K., Reboul J., Plassart E., Rime C.S., Elbaz A., Heine R., Guimarães J., Weissenbach J., et al. 1994. Mapping of the hypokalaemic periodic paralysis (HypoPP) locus to chromosome 1q31-32 in three European families. Nat. Genet. 6:267–272. 10.1038/ng0394-267 [DOI] [PubMed] [Google Scholar]
- Fuster C., Perrot J., Berthier C., Jacquemond V., and Allard B.. 2017a Elevated resting H(+) current in the R1239H type 1 hypokalaemic periodic paralysis mutated Ca(2+) channel. J. Physiol. 595:6417–6428. 10.1113/JP274638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster C., Perrot J., Berthier C., Jacquemond V., Charnet P., and Allard B.. 2017b Na leak with gating pore properties in hypokalemic periodic paralysis V876E mutant muscle Ca channel. J. Gen. Physiol. 149 10.1085/jgp.201711834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K., Lehmann-Horn F., Elbaz A., Heine R., Gregg R.G., Hogan K., Powers P.A., Lapie P., Vale-Santos J.E., Weissenbach J., et al. 1994. A calcium channel mutation causing hypokalemic periodic paralysis. Hum. Mol. Genet. 3:1415–1419. 10.1093/hmg/3.8.1415 [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K., Mitrovic N., Hang C., Kouzmekine A., Iaizzo P., Herzog J., Lerche H., Nicole S., Vale-Santos J., Chauveau D., et al. 2000. Voltage-sensor sodium channel mutations cause hypokalemic periodic paralysis type 2 by enhanced inactivation and reduced current. Proc. Natl. Acad. Sci. USA. 97:9549–9554. 10.1073/pnas.97.17.9549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkat-Rott K., Weber M.A., Fauler M., Guo X.H., Holzherr B.D., Paczulla A., Nordsborg N., Joechle W., and Lehmann-Horn F.. 2009. K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proc. Natl. Acad. Sci. USA. 106:4036–4041. 10.1073/pnas.0811277106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E., Labrum R., Sweeney M.G., Sud R., Haworth A., Chinnery P.F., Meola G., Schorge S., Kullmann D.M., Davis M.B., and Hanna M.G.. 2009. Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology. 72:1544–1547. 10.1212/01.wnl.0000342387.65477.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptáček L.J., Tawil R., Griggs R.C., Engel A.G., Layzer R.B., Kwieciński H., McManis P.G., Santiago L., Moore M., Fouad G., et al. 1994. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 77:863–868. 10.1016/0092-8674(94)90135-X [DOI] [PubMed] [Google Scholar]
- Ruff R.L. 1999. Insulin acts in hypokalemic periodic paralysis by reducing inward rectifier K+ current. Neurology. 53:1556–1563. 10.1212/WNL.53.7.1556 [DOI] [PubMed] [Google Scholar]
- Sokolov S., Scheuer T., and Catterall W.A.. 2007. Gating pore current in an inherited ion channelopathy. Nature. 446:76–78. 10.1038/nature05598 [DOI] [PubMed] [Google Scholar]
- Starace D.M., and Bezanilla F.. 2001. Histidine scanning mutagenesis of basic residues of the S4 segment of the shaker k+ channel. J. Gen. Physiol. 117:469–490. 10.1085/jgp.117.5.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyk A.F., and Cannon S.C.. 2007. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J. Gen. Physiol. 130:11–20. 10.1085/jgp.200709755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyk A.F., and Cannon S.C.. 2008. Paradoxical depolarization of BA2+-treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle Nerve. 37:326–337. 10.1002/mus.20928 [DOI] [PubMed] [Google Scholar]
- Struyk A.F., Markin V.S., Francis D., and Cannon S.C.. 2008. Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis. J. Gen. Physiol. 132:447–464. 10.1085/jgp.200809967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F., Pathak M.M., and Isacoff E.Y.. 2005. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 45:379–388. 10.1016/j.neuron.2004.12.047 [DOI] [PubMed] [Google Scholar]
- Wu F., and Cannon S.C.. 2017. Stac3 facilitated expression of CaV1.1 in Xenopus oocytes to assess functional consequences of HypoPP mutant CaV1.1-R528H. Biophys. J. 112:245a 10.1016/j.bpj.2016.11.1341 [DOI] [Google Scholar]
- Wu F., Mi W., Burns D.K., Fu Y., Gray H.F., Struyk A.F., and Cannon S.C.. 2011. A sodium channel knockin mutant (NaV1.4-R669H) mouse model of hypokalemic periodic paralysis. J. Clin. Invest. 121:4082–4094. 10.1172/JCI57398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Mi W., Hernández-Ochoa E.O., Burns D.K., Fu Y., Gray H.F., Struyk A.F., Schneider M.F., and Cannon S.C.. 2012. A calcium channel mutant mouse model of hypokalemic periodic paralysis. J. Clin. Invest. 122:4580–4591. 10.1172/JCI66091 [DOI] [PMC free article] [PubMed] [Google Scholar]