Abstract
Dermatophytes are associated with superficial infections in humans worldwide. The aim of the present study was to determine the species distribution and susceptibility patterns of clinical dermatophytes. Samples received for routine mycological processing from 124 suspected cases attending a dermatologic clinic in a tertiary care hospital were included in the study. On direct microscopy, 74.1% (92/124) were positive and 53.2% (66/124) grew on culture. The isolates were comprised of Trichophyton interdigitale (56%) followed by Trichophyton tonsurans (25.7%), Trichophyton rubrum (7.5%), Trichophyton violaceum (4.5%), Microsporum gypseum (4.5%), and Trichophyton verrucosum (1.5%). Conventional mycological identification was concordant with ITS sequencing except for T. mentagrophytes. High minimum inhibitory concentration (MIC) values (geometric mean, >1 µg/mL) were observed for T. tonsurans and T. rubrum to terbinafine and griseofulvin. This study highlights the shift in epidemiology from T. rubrum to T. interdigitale. It also raises a concern of high MICs of terbinafine and griseofulvin among our isolates. Surveillance of antifungal susceptibility patterns can provide clinicians with local MIC data that can further aid in guiding better management in relapse cases of dermatomycosis.
Keywords: dermatophytes, internal transcribed spacer (ITS) sequencing, antifungal susceptibility, CLSI M38-A2, EUCAST E-Def 9.2 revision, India
1. Introduction
Dermatophytes are a group of closely related species that are keratinophilic and morphologically similar. They have the capacity to invade the keratinized tissue (skin, hair, and nails) of humans and other animals to produce an infection, dermatophytosis, commonly referred to as ringworm [1,2]. The universal occurrence of dermatomycosis as estimated by the World Health Organization is about to be 20% [3].
Ringworm is caused by the members of three genera Microsporum, Trichophyton, and Epidermophyton. These keratinophilic pathogenic organisms are also saprophytic in nature [1,2]. Microsporum and Trichophyton are human and animal pathogens. Epidermophyton is only a human pathogen [2].
These infections are not life-threatening, but they cause physical discomfort to the affected persons, which may even lead to a lower self-esteem. Within the past two decades, the incidence of such infections is on the rise, especially in the immunocompromised patient groups including AIDS, diabetes mellitus, cancer and organ transplantation patients, etc. [2]. These are also associated with secondary bacterial infections that may lead to systemic skin infections [4,5].
Over time, a vast range of antifungals has been used to treat dermatophytosis starting with griseofulvin about six decades ago and the first oral imidazole, ketoconazole, about four decades ago [6]. These drugs were followed by other oral azoles—fluconazole, itraconazole, and efinaconazole—and topical allylamines—terbinafine, butanafine, and naftifine [6]. Nowadays, other antifungal agents including luliconazole, amorolfine, and ciclopirox olamine (pyridine) are also popular in clinical practices [7]. The drugs fluconazole, itraconazole, and terbinafine have shown success when used for systemic treatment [8]. Despite the availability of the wide range of antifungals for therapeutic purposes, the failure in treatment has been extensively reported. This may be multifactorial, and the reasons include the extent of the onychomycosis (total onychomycosis, very thick subungual hyperkeratosis and dermatophytoma), causative agents (high MICs of the dermatophyte causing the infection or the presence of non-dermatophytic species, which do not respond to systemic antifungals, such as Neoscytalidium, Scopulariopsis, and Fusarium sp.), and patient comorbidities (immunosuppressed patients, and some drugs may modify antifungal blood levels), inappropriate/insufficient drug administration, discontinuation of therapy, and noncompliance of the patient [4,8,9].
The exact role of drug resistance in treatment failure is not clearly understood. This prospective study was designed to determine the prevalence of different tinea infections and the species distribution with their susceptibility patterns.
2. Materials and Methods
This was a purely laboratory-based study including consecutive samples received in the mycology laboratory from 124 patients clinically suspected of dermatophytosis from the dermatology outpatient department of AIIMS, New Delhi from June 2014 to July 2015. Repeat samples from patients were excluded. Ethical clearance from the institute was not required, as the study incorporated the samples sent for routine fungal investigations and the brief clinical history (demographic data, clinical presentation, and site of involvement) incorporated in the analysis was provided on the investigation requisition form sent with the sample. No additional clinical history was collected from the patients, and no follow-up was performed.
2.1. Sample Processing
Samples received were subjected to direct microscopic examination using 10% KOH for skin scrapings or hair and 20% KOH for nail samples. For primary isolation, the samples were inoculated in Sabouraud’s dextrose agar (SDA) slopes and were incubated at 25 °C for 30 days before ascribing them as negative for fungi.
2.2. Identification of Isolates
Identification of the isolates was done by standard mycological laboratory procedures including morphology on SDA and potato dextrose agar (PDA). Slide culture or microculture was done to study the morphology of microconidia and macroconidia, the nature of the sporulation, the formation of chlamydospores, or the special structures such as spirals, pectinate, the racquet hyphae on corn meal agar (CMA) and PDA. Other special tests were performed where necessary including hair perforation test and growth on rice grain medium. Biochemical tests with urea hydrolysis, 1% peptone agar, and SDA with 5% NaCl were used for species identification.
2.3. ITS DNA Sequencing
The DNA was extracted using liquid nitrogen freezing with mortar pestle grinding followed by phenol chloroform extraction [10]. Segments of DNA comprising the internal transcribed region (ITS) were amplified with Primers ITS1 and ITS4 [11]. The samples were amplified by using the following cycling parameters: one initial cycle of 2 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 50 s at 56 °C, and 2 min at 72 °C, with one final cycle of 7 min at 72 °C. Sequencing reactions were done with 4 μL of a sequencing kit (BigDye Terminator v3.1 cycle sequencing ready reaction; Applied Biosystems, Carlsbad, CA, USA), 1 µM of the primers (ITS1, ITS4), and 3 µL of the PCR product in a final volume of 10 µL. Sequence analysis was performed by comparison of the test nucleotide sequences with the dermatophyte reference nucleotide sequences obtained from the GenBank database (Available online: http://www.ncbi.nih.gov/GenBank/) and were speciated as the reference ITS sequence with a similarity of >99%. The representative sequences obtained were submitted to the GenBank database: T. interdigitale (Genbank accession no. KY427899–KY427905); T. tonsurans (GenBank accession no. KY427906–KY427910); T. rubrum (Genbank accession no. KY427911–KY427912); M. gypseum (Genbank accession no. KY427913).
2.4. Antifungal Susceptibility Testing
Antifungal susceptibility was performed using the broth microdilution assay according to Clinical Laboratory Standards Institute (CLSI) approved standard M38-A2 and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (EDef 9.2 Revision) guidelines suggested for molds [12,13]. Quality control isolates (Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258) were included for both methodologies. The antifungal drugs tested were terbinafine, amphotericin B, itraconazole, and griseofulvin (Sigma Chemical Corporation, St. Louis, MO, USA). The medium used was RPMI-1640 with l-glutamine, without bicarbonate, buffered at pH 7.0 with 0.165 M morpholine propane sulfonic acid (MOPS buffer).
The dermatophyte inoculum suspension was prepared using a spectrophotometer to match the optical density with that of 70% transmittance at a 530 nm wavelength. The final inoculum concentration was from 1 × 103 to 3 × 103 CFU/mL. The assays were incubated at 28–30 °C.
2.5. Statistical Analysis
Statistical analysis was done to assess the correlation between the two antifungal susceptibility testing methods used. The on-scale results were included as obtained, and the high off-scale MICs were converted to the next highest concentration to be included in the analysis. Agreement was evaluated by concordance between the MICs obtained by the two different susceptibility testing methods and was defined as a difference of no more than 2 log2 dilutions in the MIC values. In addition, to calculate the intraclass correlation coefficients (ICCs) for the MICs, the values were transformed on log2 data and were expressed over a maximum value of 1 with a confidence interval of 95% [14]. All statistical analysis was performed with Statistical Package for the Social Sciences software (version 16.0; SPSS S.L., Madrid, Spain).
3. Results
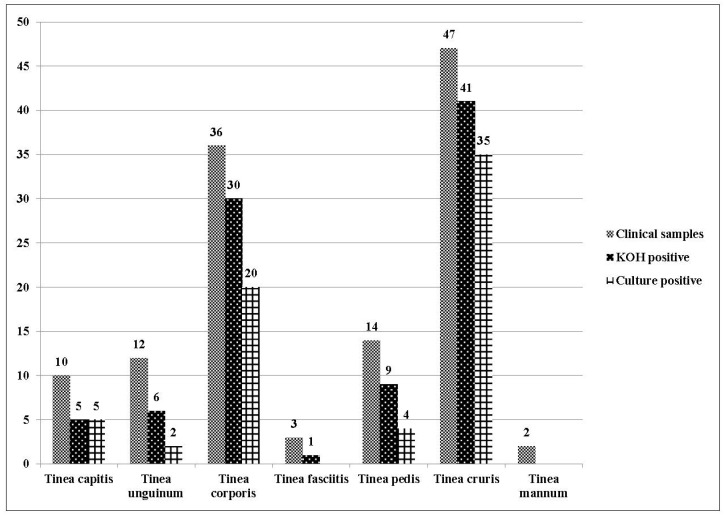
Out of 124 suspected cases of dermatophytosis, 53.2% (66/124) were positive for thin septate hyphae on direct microscopy and grew on culture, whereas 20.9% (26/124) were positive only on direct microscopy with sterile cultures, making a total of 74.1% (92/124) cases in which direct microscopy revealed thin septate hyphae. Clinical manifestation of the patients and the mycological findings are shown in Figure 1.
Figure 1.
Clinical manifestations, direct microscopy, and culture positivity for all patients enrolled in the study (n = 124).
The mean age of the patients enrolled was 31.2 years. A preponderance of males (79/124, 64%) over females (45/124, 36%) was observed in the selected cohort. The most common clinical manifestation was Tinea cruris (47/124, 37.9%) followed by Tinea corporis (29%), and similar results were obtained for the culture positive cases Tinea cruris (35/66; 53%) followed by Tinea corporis (20/66; 30.3%).
3.1. Identification of Dermatophytes
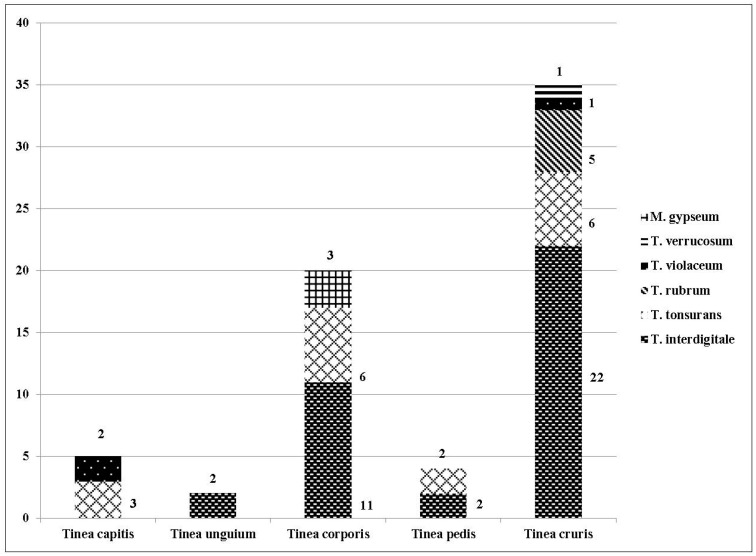
The most common dermatophytes implicated were Trichophyton species in 95.4% (63/66) cases while Microsporum species were detected only in (3/66) 4.5% cases. In the present study, no case of dermatophytosis due to Epidermophyton species was observed. The most common clinical manifestation with maximum multiple species (5 of 6 different dermatophyte species isolated in the study, 83.3%) involved was Tinea cruris (35/66, 53.03%) followed by Tinea corporis (20/66, 30.03%) with the isolation of three different species. T. rubrum and T. verrucosum were two species that were recovered only from Tinea cruris cases, whereas M. gypseum was isolated only from Tinea corporis cases. The detailed species distribution in different clinical manifestations is shown in Figure 2.
Figure 2.
Different species distribution in culture positive patients (n = 66).
The conventionally identified T. mentagrophytes were identified as T. interdigitale (GenBank accession no. KY427899–KY427905) when subjected to ITS sequencing. The identification for other isolates was concordant for both methods (Out of the total 66 strains, 15 were submitted to the GenBank; T. tonsurans (GenBank accession no. KY427906–KY427910); T. rubrum (GenBank accession no. KY427911–KY427912); M. gypseum (GenBank accession no. KY427913)). According to the species distribution, T. interdigitale was the predominant organism (56% cases) followed by T. tonsurans (25.7% cases).
3.2. Antifungal Susceptibility Testing
The MIC values of quality control strains were reproducible, fell within the established ranges published by CLSI and EUCAST methodologies. Table 1 summarizes the in vitro susceptibility value ranges of all the isolates to the antifungals tested by the two methodologies followed.
Table 1.
MIC value range with geometric mean, mode, and MIC50 and MIC90 values for the different dermatophytic species by the CLSI M38-A2 and EUCAST methodologies.
| Antifungals, Dermatophyte Species, and the Methodologies Followed | MIC Distribution (μg/mL) (No. of Isolates) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | +GM | MIC50 | MIC90 | |
| Itraconazole | |||||||||||||
| T. interdigitale (n = 37) | |||||||||||||
| CLSI | 21 | 13 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.042 | 0.03 | 0.06 |
| EUCAST | 7 | 6 | 11 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0.077 | 0.06 | 0.125 |
| T. tonsurans (n = 17) | |||||||||||||
| CLSI | 15 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.033 | 0.03 | 0.06 |
| EUCAST | 0 | 15 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0.06 | 0.125 |
| T. rubrum (n = 5) | |||||||||||||
| CLSI | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.039 | 0.03 | 0.06 |
| EUCAST | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0.06 | 0.125 |
| T. violaceum (n = 3) | |||||||||||||
| CLSI | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.037 | 0.03 | 0.06 |
| EUCAST | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.037 | 0.03 | 0.06 |
| M. gypseum (n = 3) | |||||||||||||
| CLSI | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.037 | 0.03 | 0.06 |
| EUCAST | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.037 | 0.03 | 0.06 |
| T. verrucosum (n = 1) | |||||||||||||
| CLSI | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | *NA | *NA | *NA |
| EUCAST | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | *NA | *NA | *NA |
| Terbinafine | |||||||||||||
| T. interdigitale (n = 37) | |||||||||||||
| CLSI | 3 | 2 | 5 | 5 | 14 | 5 | 0 | 0 | 2 | 1 | 0.375 | 0.5 | 1 |
| EUCAST | 0 | 5 | 0 | 4 | 6 | 15 | 4 | 0 | 2 | 1 | 0.683 | 1 | 2 |
| T. tonsurans (n = 17) | |||||||||||||
| CLSI | 3 | 0 | 0 | 2 | 2 | 1 | 4 | 2 | 2 | 1 | 0.878 | 2 | 8 |
| EUCAST | 0 | 2 | 1 | 0 | 2 | 2 | 4 | 2 | 3 | 1 | 1.379 | 2 | 8 |
| T. rubrum (n = 5) | |||||||||||||
| CLSI | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0.863 | 0.5 | 8 |
| EUCAST | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0.991 | 0.5 | 8 |
| T. violaceum (n = 3) | |||||||||||||
| CLSI | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.048 | 0.03 | 0.125 |
| EUCAST | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.076 | 0.06 | 0.125 |
| M. gypseum (n = 3) | |||||||||||||
| CLSI | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.048 | 0.03 | 0.125 |
| EUCAST | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.076 | 0.06 | 0.125 |
| T. verrucosum (n = 1) | |||||||||||||
| CLSI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | *NA | *NA | *NA |
| EUCAST | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | *NA | *NA | *NA |
| Griseofulvin | |||||||||||||
| T. interdigitale (n = 37) | |||||||||||||
| CLSI | 3 | 2 | 5 | 5 | 14 | 5 | 0 | 0 | 2 | 1 | 0.375 | 0.5 | 1 |
| EUCAST | 0 | 5 | 0 | 5 | 11 | 13 | 0 | 0 | 0 | 3 | 0.577 | 0.5 | 1 |
| T. tonsurans (n = 17) | |||||||||||||
| CLSI | 1 | 2 | 0 | 0 | 4 | 3 | 5 | 1 | 0 | 1 | 0.777 | 1 | 4 |
| EUCAST | 0 | 1 | 1 | 1 | 0 | 2 | 4 | 3 | 4 | 1 | 1.995 | 2 | 8 |
| T. rubrum (n = 5) | |||||||||||||
| CLSI | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 3.031 | 4 | 8 |
| EUCAST | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 5.278 | 8 | 8 |
| T. violaceum (n = 3) | |||||||||||||
| CLSI | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.037 | 0.03 | 0.06 |
| EUCAST | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.037 | 0.03 | 0.06 |
| M. gypseum (n = 3) | |||||||||||||
| CLSI | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.076 | 0.06 | 0.125 |
| EUCAST | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.076 | 0.06 | 0.125 |
| T. verrucosum (n = 1) | |||||||||||||
| CLSI | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | *NA | *NA | *NA |
| EUCAST | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | *NA | *NA | *NA |
| Amphotericin B | |||||||||||||
| T. interdigitale (n = 37) | |||||||||||||
| CLSI | 13 | 4 | 18 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0.072 | 0.125 | 0.125 |
| EUCAST | 13 | 4 | 18 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0.072 | 0.125 | 0.125 |
| T. tonsurans (n = 17) | |||||||||||||
| CLSI | 4 | 3 | 5 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 0.133 | 0.125 | 1 |
| EUCAST | 0 | 7 | 5 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0.177 | 0.125 | 1 |
| T. rubrum (n = 5) | |||||||||||||
| CLSI | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0.162 | 0.25 | 1 |
| EUCAST | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0.245 | 0.25 | 2 |
| T. violaceum (n = 3) | |||||||||||||
| CLSI | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.125 | 0.125 | 0.125 |
| EUCAST | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.157 | 0.125 | 0.25 |
| M. gypseum (n = 3) | |||||||||||||
| CLSI | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.25 | 0.25 | 0.25 |
| EUCAST | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.25 | 0.25 | 0.25 |
| T. verrucosum (n = 1) | |||||||||||||
| CLSI | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | *NA | *NA | *NA |
| EUCAST | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | *NA | *NA | *NA |
(Note: the underlined values denote the modal MICs; *NA: not applicable; +GM: geometric mean).
The analysis revealed that high MIC values (geometric mean, >1 µg/mL) were obtained for T. tonsurans to terbinafine and griseofulvin (EUCAST methodology: 1.379 and 1.995 µg/mL, respectively), and for T. rubrum to griseofulvin (CLSI and EUCAST methodologies, 3.031 and 5.278 µg/mL, respectively) (Table 1).
Irrespective of the different species, about 50% of our isolates exhibited high MICs (>1 µg/mL) to terbinafine and griseofulvin (Table 2). The detailed statistical analysis between the two methodologies showed high recorded agreement and ICCs between the CLSI and EUCAST results and were within +1 dilution for the antifungals tested, ranging between 83.6% and 98.3% (ICC, 0.73–0.98).
Table 2.
Susceptibilities of dermatophytes to various antifungals and the concordance and intraclass coefficients (ICC) between CLSI M38-A2 and EUCAST guidelines.
| Antifungal Agents | CLSI (%) (n = 66) | EUCAST (%) (n = 66) | Concordance | ICC (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| <0.5 µg/mL | 0.5–1 µg/mL | ≥2 µg/mL | <0.5 µg/mL | 0.5–1 µg/mL | ≥2 µg/mL | |||
| Itraconazole | 100 | 0 | 0 | 93.9 | 6 | 0 | 83.6 | 0.734 (0.038–0.895) |
| Terbinafine | 40.9 | 36.3 | 22.7 | 28.8 | 40.9 | 30.3 | 98.3 | 0.968 (0.396–0.991) |
| Griseofulvin | 36.3 | 42.4 | 21.2 | 28.8 | 39.4 | 31.8 | 96.7 | 0.957 (0.501–0.987) |
| Amphotericin B | 89.4 | 7.6 | 3 | 87.9 | 7.6 | 4.5 | 97.2 | 0.982 (0.965–0.99) |
4. Discussion
The present study highlights the clinical manifestations of dermatophytosis, dermatophyte species distribution, and their susceptibility patterns. It was done at a dermatologic clinic in a tertiary care center in Northern India. The climatic conditions (hot and humid environment pertaining to tropical and sub-tropical regions) in India are favorable for the development of superficial fungal infections [15]. The other factors that aid these infections include unhygienic living standards, especially prevalent among the low socio-economic strata, and a high population density especially seen in communities like the ones around construction sites.
The study also reconfirms previous worldwide studies highlighting a high prevalence among males (64%) [16,17]. The mean age of patients was 31.2 years, which is in line as per previously published data showcasing its highest prevalence among the 21–30 age group. This may be due to the general characteristic of this age group as they are more involved in outdoor activities involving physical labor [18,19]. The higher prevalence amongst males may also prove to be an occupational hazard. Many studies indicate Tinea corporis as the most common presentation followed by Tinea cruris, but in our study Tinea cruris was found to be the most common presentation [17,20,21].
Our T. mentagrophytes identifications were based on phenotypic methods, and were found to be incorrect by ITS sequencing as T. interdigitale. This was not surprising as this has also been reported worldwide [22,23]. For other isolates, the identification results were concordant with conventional and molecular methods. In a study by Li HC et al., it was found that three of the reference strains (BCRC 32066, CBS 160.66, and CBS 361.62) and all clinical isolates when identified by phenotypic methods were found to belong to T. mentagrophytes, but on ITS sequence analyses, except for a single strain, were identified as T. interdigitale. The authors also suggested that most of the human isolates of T. mentagrophytes complex are actually T. interdigitale, with a few exceptions [24]. These misidentifications are due to morphological identification procedures not keeping pace with phylogenetic studies and nomenclatural changes. Interestingly, by ITS sequencing we found T. interdigitale (56%) as the predominant species followed by T. tonsurans (25.7%). Trichophyton rubrum was seen only in five cases, while it globally causes the maximum tinea infections. This finding is contrary to the observations of others in which an opposite trend has been reported [5,19,24,25].
This change in epidemiology had been previously reported in only three previous studies, by Kaur et al., Adhikari et al., and Yadav et al. from India, where T. interdigitale (79.2%), T. tonsurans (44.4%), and T. interdigitale (61%), respectively, was reported as the most common agents of tinea infections [26,27,28]. Globally, this shift had been reported by Agarwalla et al., Hashemi et al., and Chadeganipour et al., with the most common tinea infections caused by T. interdigitale [29,30,31].
In this study, we employed the broth microdilution methodologies using two accepted standards, CLSI M38-A2 and EUCAST Edef 9.2, to determine the MICs of antifungal agents for dermatophytes. Globally, the MIC50 and MIC90 reported for itraconazole, terbinafine, and griseofulvin to dermatophytes were found to be generally low (<1 µg/mL) [32,33,34,35,36,37]. However, there were a few species-specific studies where high MIC values were reported for a few antifungals (>1–32 µg/mL) [36,37]. Our antifungal results showed high MIC values of >2 µg/mL to terbinafine and griseofulvin in about 40% and 20% of our isolates, respectively (Table 2). We also found high MIC50 and MIC90 (1 µg/mL) for T. tonsurans and T. rubrum. However, the number of T. rubrum was too low (n = 5) for a concrete interpretation on the basis of MIC50 and MIC90. Our observations of high MICs to terbinafine in comparison to itraconazole is in concordance with the recently reported figures by Afshari et al. in 2016 [38]. In another recent study, from India’s northern region, T. mentagrophyte was identified by phenotypic methods. The species showed low MICs to itraconazole and ketoconazole in comparison to terbinafine (MIC50: 0.125 µg/mL for itraconazole, 0.0625 µg/mL for ketoconazole, and 0.5 µg/mL for terbinafine) [4], whereas from the eastern region of India, low MICs to terbinafine in comparison with griseofulvin and itraconazole were reported [34]. To summarize, our data is not in agreement with previously published studies, indicating low MICs to terbinafine [32,33,34,35,36,39]. The authors clarify that the clinical significance of these high MICs is unclear, as patient outcomes were not followed up, and there is a lack of studies in general correlating dermatophyte antifungal MICs with treatment outcomes.
Although breakpoints needed to analyze this data for practical clinical application are not available, this data can aid in understanding local susceptibility patterns. The dissimilarity of our results can be due to species-specific susceptibility against antifungal drugs or/and inter-laboratory and inter-method variations.
The results for amphotericin B were also included in the study for the likely benefit they may provide for isolates refractory to the standard treatment for the dermatophytic infections. The amphotericin B gel has shown promising results in refractory cases of cutaneous fungal infections and non-dermatophyte mold onychomycosis [40,41].
We performed antifungal susceptibility tests on our isolates with conidia (classical inoculum preparation) instead of using a modified protocol of fragmented mycelium inoculum as our isolates had sporulated nicely. However, our results show an 83.6–98.3% agreement between the two methodologies, which is in accordance with those reported in a recent study by Risslegger et al., where they found an 88.9–100% agreement between the modified EUCAST (fragmented mycelium inoculum) and classical CLSI methodology [42]. Since we found a high recorded agreement between the two methodologies and ICCs in this study, a fair comparison with other studies conducted using either of the two standard methods is presented.
5. Conclusions
The present study provides useful insights on the reliability of the conventional methods for the identification of dermatophytes. It also provides useful information regarding antifungal susceptibility pattern of dermatophytes and raises important concerns regarding high MIC values of terbinafine and griseofulvin in our isolates. Tinea is not considered a life threatening condition, but it certainly leads to personal discomfort, and antifungal treatment regimens can last for a fairly long duration (3–6 months). To prevent the unnecessary usage of toxic drugs, regular surveillance of antifungal susceptibility patterns in patients should be carried out in their long-term interest.
Author Contributions
Yubhisha Dabas, Immaculata Xess and Gagandeep Singh conceived and designed the experiments; Yubhisha Dabas and Mragnayani Pandey performed the experiments; Yubhisha Dabas and Suneeta Meena analyzed the data; Immaculata Xess and Gagandeep Singh contributed reagents/materials/analysis tools; Yubhisha Dabas and Immaculata Xess wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Weitzman I., Summerbell R.C. The Dermatophytes. Clin. Microbiol. Rev. 1995;8:240–259. doi: 10.1016/S0733-8635(05)70320-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chander J. Test Book of Medical Mycology. 3rd ed. Mehta Publishers; Maharashtra, India: 2009. pp. 122–146. [Google Scholar]
- 3.Sharma V., Kumawat T.K., Sharma A., Seth R., Chandra S. Dermatophytes: Diagnosis of dermatophytosis and its treatment. Afr. J. Microbiol. Res. 2015;9:1286–1293. [Google Scholar]
- 4.Bhatia V.K., Sharma P.C. Determination of minimum inhibitory concentrations of itraconazole, terbinafine and ketoconazole against dermatophyte species by broth microdilution method. Indian J. Med. Microbiol. 2015;33:533–537. doi: 10.4103/0255-0857.167341. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia V.K., Sharma P.C. Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134. doi: 10.1186/2193-1801-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elewski B.E. Onychomycosis: Pathogenesis, diagnosis, and management. Clin. Microbiol. Rev. 1998;11:415–429. doi: 10.2165/00128071-200001010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda Y., Sugiura K., Hashimoto T., Ueda A., Konno Y., Tatsumi Y. Efficacy coefficients determined using nail permeability and antifungal activity in keratin-containing media are useful for predicting clinical efficacies of topical drugs for onychomycosis. PLoS ONE. 2016;11:e0159661. doi: 10.1371/journal.pone.0159661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piraccini B.M., Alessandrini A. Onychomycosis: A Review. J. Fun. 2015;1:30–43. doi: 10.3390/jof1010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandeputte P., Ferrari S., Coste A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012;2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakousis A., Tan L., Ellis D., Alexiou H., Wormald P.J. An assessment of the efficiency of fungal DNA extraction methods for maximizing the detection of medically important fungi using PCR. J. Microbiol. Methods. 2006;65:38–48. doi: 10.1016/j.mimet.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.White T.J., Bruns T., Lee S., Taylor J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press, Inc.; New York, NY, USA: 1990. pp. 315–322. [Google Scholar]
- 12.Clinical Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard. 2nd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008. CLSI document M38-A2. [Google Scholar]
- 13.Arendrup M.C., Hope W., Howard S.J. EUCAST Definitive Document E.Def 9.2 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. EUCAST; Basel, Switzerland: 2014. [Google Scholar]
- 14.Cuenca-Estrella M., Gómez-López A., Alastruey-Izquierdo A., Bernal-Martinez L., Cuesta I., Buitrago M.J., Rodriguez-Tudela J.L. Comparison of the Vitek 2 antifungal susceptibility system with the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) broth microdilution reference methods and with the Sensititre YeastOne and Etest techniques for in vitro detection of antifungal resistance in yeast isolates. J. Clin. Microbiol. 2010;48:1782–1786. doi: 10.1128/JCM.02316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshmukh S.K., Verekar S.V., Shrivastav A. The occurrence of keratinophilic fungi in selected soils of Ladakh (India) Nature. 2010;2:1247–1252. doi: 10.4236/ns.2010.211151. [DOI] [Google Scholar]
- 16.Singh S., Beena P.M. Profile of dermatophyte infections in Baroda. Indian J. Dermatol. Venereol. Leprol. 2003;69:281–283. [PubMed] [Google Scholar]
- 17.Singh S., Beena P.M. Comparative study of different microscopic techniques and culture media for the isolation of dermatophytes. Indian J. Med. Microbiol. 2003;21:21–24. [PubMed] [Google Scholar]
- 18.Sarma S., Borthakur A.K.A. Clinico—Epidermatological study of dermatophytoses in Northest India. Indian J. Dermatol. Venereol. Leprol. 2007;73:427–428. doi: 10.4103/0378-6323.37068. [DOI] [PubMed] [Google Scholar]
- 19.Patel P., Mulla S., Patel D., Shrimali G. A study of superficial mycosis in south Gujarat region. Natl. J. Commun. Med. 2010;1:85–88. [Google Scholar]
- 20.Venkatesan G., Singh A.J.A., Murugesan A.G., Janaki C., Shankar S.G. Trichophyton rubrum—The predominant aetiological agent in human dermatophytosis in Chennai, India. Afr. J. Microbiol. Res. 2007;1:9–12. [Google Scholar]
- 21.Ranganathan S., Menon T., Sentamil G.S. Effect of socioeconomical status on the prevalence of dermatophytosis in Madras. Indian J. Dermatol. Venereol. Leprol. 1995;61:16–18. [PubMed] [Google Scholar]
- 22.Dhib I., Khammari I., Yaacoub A., Hadj Slama F., Ben Said M., Zemni R., Fathallah A. Relationship between phenotypic and genotypic characteristics of Trichophyton mentagrophytes strains isolated from patients with dermatophytosis. Mycopathologia. 2017 doi: 10.1007/s11046-017-0110-3. [DOI] [PubMed] [Google Scholar]
- 23.Li H.C., Bouchara J.P., Hsu M.M.L., Barton R., Su S., Chang T.C. Identification of dermatophytes by sequence analysis of the rRNA gene internal transcribed spacer regions. J. Med. Microbiol. 2008;57:592–600. doi: 10.1099/jmm.0.47607-0. [DOI] [PubMed] [Google Scholar]
- 24.Balakumar S., Rajan S., Thirunalasundari T., Jeeva S. Epidemiology of dermatophytosis in and around Tiruchirapalli, Tamilnadu, India. Asian Pac. J. Trop. Dis. 2012;2:286–289. doi: 10.1016/S2222-1808(12)60062-0. [DOI] [Google Scholar]
- 25.Pandey A., Pandey M. Isolation and characterization of dermatophytes with tinea infection at Gwalior (M.P.), India. Int. J. Pharm. Sci. Investig. 2013;2:5–8. [Google Scholar]
- 26.Kaur R., Kashyap B., Bhalla P. A five-year survey of onychomycosis in New Delhi, India: Epidemiological and laboratory aspects. Indian J. Dermatol. 2007;52:39–42. [Google Scholar]
- 27.Adhikari L., Gupta A.D., Pal R., Singh T. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J. Pathol. Microbiol. 2009;52:194–197. doi: 10.4103/0377-4929.48915. [DOI] [PubMed] [Google Scholar]
- 28.Yadav P., Singal A., Pandhi D., Das S. Clinicomycological study of dermatophyte toenail onychomycosis in New Delhi, India. Indian J. Dermatol. 2015;60:153–158. doi: 10.4103/0019-5154.152511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwalla A., Agrawal S., Khanal B. Onychomycosis in eastern Nepal. Nepal Med. Coll. J. 2006;8:215–219. [PubMed] [Google Scholar]
- 30.Hashemi S.J., Gerami M., Zibafar E., Daei M., Moazeni M., Nasrollahi A. Onychomycosis in Tehran: Mycological study of 504 patients. Mycoses. 2010;53:251–255. doi: 10.1111/j.1439-0507.2009.01703.x. [DOI] [PubMed] [Google Scholar]
- 31.Chadeganipour M., Nilipour S., Ahmadi G. Study of onychomycosis in Isfahan, Iran. Mycoses. 2010;53:153–157. doi: 10.1111/j.1439-0507.2008.01679.x. [DOI] [PubMed] [Google Scholar]
- 32.Jessup C.J., Warner J., Isham N., Hasan I., Ghannoum M.A. Antifungal susceptibility testing of dermatophytes: Establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J. Clin. Microbiol. 2000;38:341–344. doi: 10.1128/jcm.38.1.341-344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Indira G. In vitro antifungal susceptibility testing of 5 antifungal agents against dermatophytic species by CLSI (M38-A) micro dilution method. Clin. Microbial. 2014;3:145. doi: 10.4172/2327-5073.1000145. [DOI] [Google Scholar]
- 34.Araújo C.R., Miranda K.C., Fernandes O.F.L., Soares A.J., Silva M.R.R. In vitro susceptibility testing of dermatophytes isolated in Goiania, Brazil, against five antifungal agents by broth microdilution method. Rev. Inst. Med. Trop. 2009;51:9–12. doi: 10.1590/S0036-46652009000100002. [DOI] [PubMed] [Google Scholar]
- 35.Ghannoum M.A., Hajjeh R.A., Scher R., Konnikov N., Gupta A.K., Summerbell R., Sullivan S., Daniel R., Krusinski P., Fleckman P., et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 2000;43:641–648. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 36.Da Silva Barros M.E., de Assis S.D., Hamdan J.S. Evaluation of susceptibility of Trichophyton mentagrophytes and Trichophyton rubrum clinical isolates to antifungal drugs using a modified CLSI microdilution method (M38-A) J. Med. Microbiol. 2007;56:514–518. doi: 10.1099/jmm.0.46542-0. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A.K., Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br. J. Dermatol. 2003;149:296–305. doi: 10.1046/j.1365-2133.2003.05418.x. [DOI] [PubMed] [Google Scholar]
- 38.Afshari M.A., Shams-Ghahfarokhi M., Razzaghi-Abyaneh M. Antifungal susceptibility and virulence factors of clinically isolated dermatophytes in Tehran, Iran. Iran J. Microbiol. 2016;8:36–46. [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura T., Asahara M., Yamamoto M., Yamaura M., Matsumura M., Goto K., Rezaei-Matehkolaei A., Mirhendi H., Makimura M., Makimura K. In Vitro susceptibility of dermatomycoses agents to six antifungal drugs and evaluation by fractional inhibitory concentration index of combined effects of amorolfine and itraconazole in dermatophytes. Microbiol. Immunol. 2014;58:1–8. doi: 10.1111/1348-0421.12109. [DOI] [PubMed] [Google Scholar]
- 40.Sheikh S., Ahmad A., Ali S.M., Paithankar M., Raval R.C., Shah K., Bhavsar B.A., KR R., AM J., et al. Topical delivery of lipid based amphotericin B gel in the treatment of fungal infection: A clinical efficacy, safety and tolerability study in patients. J. Clin. Exp. Dermatol. Res. 2014;5:248. [Google Scholar]
- 41.Lurati M., Baudraz-Rosselet F., Vernez M., Spring P., Bontems O., Fratti M., Monod M. Efficacious treatment of non-dermatophyte mould onychomycosis with topical amphotericin B. Dermatology. 2011;223:289–292. doi: 10.1159/000335093. [DOI] [PubMed] [Google Scholar]
- 42.Risslegger B., Lass-Flörl C., Blum G., Lackner M. Evaluation of a modified EUCAST fragmented-mycelium inoculum method for in vitro susceptibility testing of dermatophytes and the activity of novel antifungal agents. Antimicrob. Agents Chemother. 2015;59:3675–3682. doi: 10.1128/AAC.04381-14. [DOI] [PMC free article] [PubMed] [Google Scholar]