Abstract
Penicillium is a large genus of common molds with over 400 described species; however, identification of individual species is difficult, including for those species that cause postharvest rots. In this study, blue rot fungi from stored apples and pears were isolated from a variety of hosts, locations, and years. Based on morphological and cultural characteristics and partial amplification of the β-tubulin locus, the isolates were provisionally identified as several different species of Penicillium. These isolates were investigated further using a suite of molecular DNA markers and compared to sequences of the ex-type for cognate species in GenBank, and were identified as P. expansum (3 isolates), P. solitum (3 isolates), P. carneum (1 isolate), and P. paneum (1 isolate). Three of the markers we used (ITS, internal transcribed spacer rDNA sequence; benA, β-tubulin; CaM, calmodulin) were suitable for distinguishing most of our isolates from one another at the species level. In contrast, we were unable to amplify RPB2 sequences from four of the isolates. Comparison of our sequences with cognate sequences in GenBank from isolates with the same species names did not always give coherent data, reinforcing earlier studies that have shown large intraspecific variability in many Penicillium species, as well as possible errors in some sequence data deposited in GenBank.
Keywords: blue molds, stored fruits, Penicillium spp., ITS, benA, CaM
1. Introduction
“Blue mold” is a common term used to describe several species of Penicillium that cause postharvest decay of important fruit crops because visible sporulation on infected fruits is blue-green in color [1,2]. Penicillium expansum [3,4], Penicillium digitatum [5], and Penicillium italicum [6] not only cause fruit decay and economic losses of apple and citrus fruits in the United States, but also produce extrolites (secondary metabolites) that may be harmful to humans.
Penicillium is one of the largest and most important genera of microscopic fungi, with over 400 described species distributed worldwide [7]. Its name comes from the Latin “penicillus”, which refers to the brush-like appearance of the conidiophores that resemble a painter’s brush. The type species for the genus, P. expansum, is primarily responsible for postharvest decay of pome fruits [8]. Penicillium species are difficult to distinguish from each other (even to expert taxonomists), and many species display a great deal of intraspecific variability [8,9,10,11].
Traditional identification of Penicillium species focuses on the color and texture of colonies; the growth rate and size of colonies on standardized media; conidiophore morphology, including branching patterns and shapes, dimensions, and ornamentations of the different parts of the conidiophore; and the production of certain extrolites. However, these morphological and biochemical characteristics may be influenced or changed by various environmental factors, confounding both identification and taxonomic classification [12,13]. Molecular methods are required for unambiguous identification of Penicillium species, but the selection of appropriate markers for use in the genus is challenging [14]. The internal transcribed spacer rDNA sequence (ITS) is the most widely sequenced marker for fungi. Universal primers are available, and it is the official sequence for barcoding [15]. Unfortunately, the ITS sequence is not diagnostic enough in Penicillium species for distinguishing all closely-related species [15,16]. Furthermore, GenBank contains many misidentified sequences, further complicating Penicillium species identification [17]. Because of the limitations associated with the ITS, several additional gene regions including β-tubulin (benA), calmodulin (CaM), and RNA polymerase II (RPBs) have been used to distinguish closely-related Penicillium species [7,10,11,18].
Ease, practicality, and accuracy are essential for the identification of Penicillium species in agricultural settings in order to predict patterns of virulence, mycotoxin production, and fungicide resistance. Thus, it is important to determine which molecular markers should be used for blue mold verification. The immediate objective of this study was to use molecular markers to verify species identification of blue molds accomplished via traditional approaches, and to compare our sequence data to those published for cognate species in GenBank. The overarching goal of our work is to find a rapid, convenient, and accurate way to identify Penicillium species involved in blue mold so as to tailor appropriate strategies for the control of postharvest fruit decay.
2. Materials and Methods
2.1. Penicillium Isolation, Morphological Identification, and Mycotoxin Detection
Eight Penicillium strains were isolated from “Golden Delicious” and “Red Delicious” apples with blue mold in 2011 and 2012 from commercial storage facilities. For comparison, one P. solitum strain (NJ1) originally obtained as a subculture of a sector of strain 2159A from the Northern Regional Research Laboratory culture collection [19], and one strain of P. sclerotiorum (113) collected from a flooded home in Manasquan, New Jersey were included [20,21]. Isolates were purified by culturing from single spores, and a preliminary characterization was conducted using macro- and microscopic observations and measurements, including colony color, diameter and texture, conidiospores and conidiophore morphology, and mycotoxin production. If a culture contained more than one type of spore or colony morphology, isolates were sub-cultured on 2% malt extract agar until the characteristics were consistent. Using identification keys from previous studies [11,22], most of the isolates were tentatively identified to the species level. Penicillium isolates that could not be identified microscopically were grouped into morphotypes by their growth characteristics on three differential media: malt extract agar (MEA), Czapek yeast agar (CYA), and corn meal agar (CMA), yeast extract sucrose agar (YES), and creatine sucrose agar (CREA) [7,20,23,24,25]. To obtain a preliminary analysis of mycotoxins (patulin and citrinin), the isolates were cultured in potato dextrose broth at 25 °C for seven days and extracted using an organic solvent extraction method [26,27]. A total of 20 mL potato dextrose broth from growing cultures was extracted three times with 25 mL of ethyl acetate by shaking vigorously for 1 min each time. Then, the organic phases were combined. Five drops of glacial acetic acid were added to the combined organic phase solution, and the solution was evaporated to dryness in a 40 °C water bath under a gentle stream of nitrogen gas. The dried residue was immediately dissolved in 1 mL of acetic acid buffer solution. Acetate buffer was prepared by adding 0.45 mL acetic acid glacial and 0.245 g of sodium acetate trihydrate to 40 mL of ddH2O. The pH was adjusted to 4.0 with acetic acid glacial. The volume was then adjusted to 50 mL with ddH2O. The toxins in the extracted sample were subjected to thin layer chromatography [26,27].
2.2. DNA Extraction and PCR Amplification
For each strain, a culture was grown on potato dextrose agar at 25 °C for 3 days before subculturing to a 250 mL Erlenmeyer flask containing 100 mL liquid potato dextrose medium for 3–4 days with shaking (180–200 rpm) at 25 °C. All the mycelia were separated from the media by filtration through sterile No. 5 Whatman filter paper and transferred to Eppendorf 1.5 mL tubes. These mycelial samples were stored at −70 °C before use. Fungal mycelia were disrupted using a TissueLyser II (Qiagen Inc, West Chester, PA, USA) with beads (5 mm diameter) set at a shaking frequency of 20 Hz per second for 25 min. Then, DNA was extracted using a DNeasy Plant Mini Kit following the manufacturer’s instructions (Qiagen Inc). The purity and concentration of fungal genomic DNA (including mitochondrial DNA) was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc, Wilmington, DE, USA). The extracted DNA samples had A260/280 and A260/230 values greater than 1.8.
For molecular identification of Penicillium species, primers specific for ITS, benA, CaM and RPB2 loci were selected for PCR amplification (Table 1) [7]. PCR was performed in a 20 µL reaction system including 1 µL gDNA template (about 100 ng), 0.2 µL Pfu DNA polymerase (2.5 U/µL) (Stratagene), 0.5 µL each forward and reverse primers (10 µM), 1 µL dNTPs (2.5 mM), and added sterile ddH2O to a final volume of 20 µL. The thermocycler was programed as follows: pre-heated at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C (ITS; benA, β-tubulin; and CaM, calmodulin) for 45 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min. For RPB2 amplification, we used a touch-up PCR according to the method of Visagie and his colleagues [7]. Eight microliters of PCR product was mixed with 1 µL GoldView dye for electrophoresis on a 1.5% agarose gel at 120 V for 15 min. PCR products were cleaned using ExoSAP-IT (Affymetrix, Santa Clara, CA, USA), followed by sequencing using the BigDye sequencing protocol (Applied Biosystems, Inc., Foster City, CA, USA). DNA sequencing was performed at Major Biosystem (Shanghai, China).
Table 1.
Primers used for Penicillium species identification.
| Gene | Primer | Sequence (5′→3′) | Length (bp) | Reference |
|---|---|---|---|---|
| Internal transcribed spacer (ITS) | ITS1F | CTTGGTCATTTAGAGGAAGTAA | ~600 | [28] |
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| β-tubulin (benA) | Bt2a | GGTAACCAAATCGGTGCTGCTTTC | ~550 | [29] |
| Bt2b | ACCCTCAGTGTAGTGACCCTTGGC | |||
| Calmodulin (CaM) | CMD5 | CCGAGTACAAGGARGCCTTC | ~580 | [30] |
| CMD6 | CCGATRGAGGTCATRACGTGG | |||
| CF1 | GCCGACTCTTTGACYGARGAR | ~750 | [31] | |
| CF4 | TTTYTGCATCATRAGYTGGAC | |||
| RNA polymerase II second largest subunit (RPB2-1) | 5F | GAYGAYMGWGATCAYTTYGG | ~1000 | [32] |
| 7CR | CCCATRGCTTGYTTRCCCAT | |||
| RNA polymerase II second largest subunit (RPB2-2) | 5Feur | GAYGAYCGKGAYCAYTTCGG | ~1000 | [33] |
| 7CReur | CCCATRGCYTGYTTRCCCAT |
2.3. Sequencing and Phylogenetic Analysis
Each isolate was identified by DNA sequencing according to a standard protocol [34]. Final sequences were aligned and analyzed using BioEdit 7.2.5 software (http://www.mbio.ncsu.edu/bioedit/bioedit.html) and Clustal Omega [35]. The corresponding fungal isolates were assigned species names after comparison with representative ex-type sequences which were available in NCBI GenBank. All the sequences from this study were deposited in GenBank.
The DNA sequences of the ITS, benA, and CaM genes from nine of our Penicillium isolates and nine other ex-type Penicillium isolates (P. griseofulvum CBS185.27, P. carneum CBS468.95, P. paneum CBS303.97, P. sclerotiorum CV0934, P. expansum CV2861, P. solitum FS06278, P. solitum BT-18-1, P. paneum CBS464.95, P. sclerotiorum FS50, and two Aspergillus species (A. flavus PW2962 and A. niger 13L06I1) in GenBank were chosen for phylogenetic analysis. All ambiguous positions were removed. Sequence similarities were inferred using the neighbor-joining method [36]. The percentage of replicate trees in which the associated taxa clustered in the bootstrap test (500 replicates) are shown next to the nodes [37]. The evolutionary distances were computed using the Kimura 2-paramenter method [38] and expressed in units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated. Analyses were conducted using MEGA6 [39].
3. Results
3.1. The Biological Characteristics of Penicillium spp.
Eight blue mold Penicillium spp. isolates from stored fruits were selected based on their morphology in culture, their abilities to produce different toxins, and diversity with respect to host and location from which the isolates were obtained. The isolate number, source, toxin production, and culture characteristics are listed in Table 2. Using traditional means, the eight strains were identified tentatively as belonging to four different Penicillium species: P. expansum (three isolates; R19, R21, and R27), Penicillium solitum (three isolates; RS1, SA and NJ1), Penicillium carneum (one isolate; G2), and Penicillium paneum (one isolate; G9). In our preliminary mycotoxin analysis, P. expansum isolates R19 and R27 produced patulin and citrinin, while R21 produced only patulin. For P. solitum RS1, SA, and NJ1, none of the isolates produced detectable mycotoxin. One isolate of P. sclerotiorum isolated from an indoor environment source was included for comparison.
Table 2.
Cultural characterization and mycotoxin production of nine blue mold Penicillium isolates.
| Species | Host | Source (Location) | Year | Patulin 1 | Citrinin | Back Color |
|---|---|---|---|---|---|---|
| P. carneum G2 | Golden delicious | Pennsylvania | 2011 | +/− | +/− | tan |
| P. paneum G9 | Golden delicious | Pennsylvania | 2011 | +/− | +/− | yellow |
| P. expansum R19 | Red delicious | Pennsylvania | 2011 | + | + | tan |
| P. expansum R21 | Red delicious | Pennsylvania | 2011 | + | − | green |
| P. expansum R27 | Red delicious | Pennsylvania | 2011 | + | + | green |
| P. solitum RS1 | Apple | Oregon | 2011 | − | − | tan |
| P. solitum SA | Peach seed | West Virginia | 2011 | − | − | tan |
| P. solitum NJ1 | - | NRRL, Illinois | 2012 | − | − | tan |
| P. sclerotiorum 113 | Obtained from home living room | New Jersey | 2013 | +/− | +/− | orange |
1 The mycotoxins patulin and citrinin were detected by organic solvent extraction followed by thin layer chromatography (TLC) separation and visualization under UV light at 365 nm wave length; +/−: low level faint band on TLC plate and it is unclear whether the mycotoxin is produced or not; +: mycotoxin present; −: mycotoxin not detected; NRRL = culture collection at National Center for Agricultural Utilization Research located in Peoria, Illinois, USA.
3.2. Sequencing Analysis of Penicillium spp. and Phylogenetic Analysis
Six sets of primers (Table 1) [7] were chosen to amplify five marker genes from the nine Penicillium isolates. Using these primers, not all strains yielded successful PCR products. For example, no sequence from P. expansum R19 was amplified by the ITS primers; P. expansum R21 and P. solitum SA were not amplified by the benA primers. P. solitum SA and RS1 were not amplified by RPB2-1 and RPB2-2 primers, nor were P. expansum R19 and R27. All successfully amplified sequences were submitted to GenBank, and their accession numbers are listed in Table 3. The initial species identifications based on traditional criteria were largely confirmed by sequence analysis.
Table 3.
Accession numbers of amplified nucleotide sequences from Penicillium spp. isolates.
| Penicillium spp. | ITS | benA | CaM a | CaM b | RPB2-1 | RPB2-2 |
|---|---|---|---|---|---|---|
| P. carneum G2 | KX243324 | KX243333 | KX243341 | - | KX243353 | KX243356 |
| P. paneum G9 | KX243325 | KX243334 | KX243342 | - | KX243354 | KX243357 |
| P. expansum R19 | - | KX243337 | - | - | - | - |
| P. expansum R21 | KX243328 | - | KX243345 | KX243351 | KX243355 | KX243358 |
| P. expansum R27 | KX243329 | KX243338 | KX243346 | - | - | - |
| P. solitum SA | KX243330 | - | KX243347 | - | - | - |
| P. solitum RS1 | KX243331 | KX243339 | KX243348 | - | - | - |
| P. solitum NJ1 | KX243323 | KX243332 | KX243340 | - | KX243352 | - |
| P. sclerotiorum 113 | KX365203 | KX365204 | KX365205 | - | KX365206 | - |
“-” denotes no clear PCR products were obtained using primers from Table 1. P. sclerotiorum 113 was added for comparison; a CaM: amplified using CMD5 and CMD6 primers; b CaM: amplified using CF1 and CF4 primers, RPB2-1 amplified using 5F and 7CR primers; RPB2-2 using 5Feur and 7CReur primers. See Table 1.
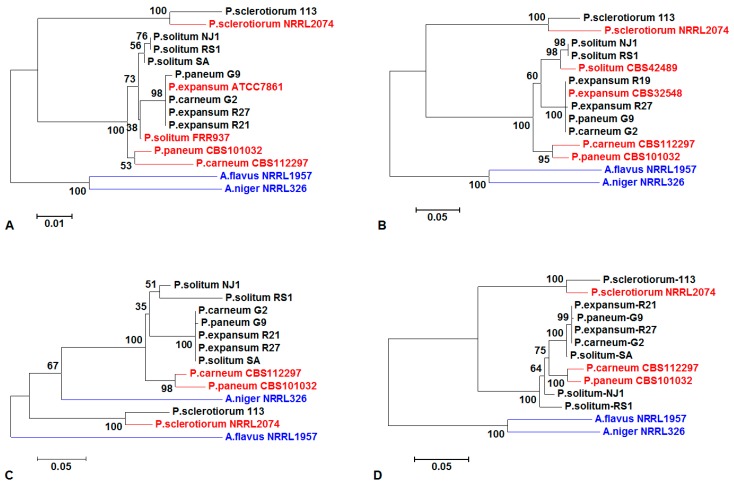
Phylogenetic trees were made with each individual gene: ITS (Figure 1A), benA (Figure 1B), and CaM (Figure 1C), and where successful amplification product has been achieved by all three combined (Figure 1D) [40,41]. Because so few strains were amplified by the RPB2-1 and RPB2-2 primers, these sequences were not used in the phylogenetic analysis. In building the phylogenetic tree, the equivalent sequences for ITS of the ex-type of P. solitum and P. expansum in GenBank have the culture collection numbers FRR937 and ATCC7861, respectively; however, the benA sequences of P. solitum and P. expansum are found to have the culture collection numbers CBS42489 and CBS32548, respectively, which are transfers of the same strains. There were no available CaM sequences of the ex-types of P. expansum and P. solitum in GenBank. All the P. expansum and P. solitum species were grouped in one clade; however, not all isolates with the same tentative species identification could be grouped together in a phylogenetic tree. The strains tentatively identified as P. carneum G2 and P. paneum G9 grouped with P. expansum when analyzed by ITS, benA, and CaM (Figure 1). Penicillium sclerotiorum isolates were consistently grouped together using all three markers. Aspergillus flavus and A. niger were used as outgroups, and were also grouped together using all markers except for CaM (Figure 1). When a multi-gene (ITS, benA, and CaM) phylogeny was constructed based on all three genes, the topologies for strains supposedly in the same species did align and form the same clades, except P. carneum G2 and P. paneum G9 (Figure 1D).
Figure 1.
Penicillium spp. phylogenetic trees constructed using markers (A) ITS; (B) benA; (C) CaM; and (D) combinations of the two or three genes. Nine of our isolates and nine ex-types from NCBI GenBank were phylogenetically arranged using available marker sequences. The nine strains obtained from NCBI were P. expansum ATCC7861 = P. expansum CBS32548, P. carneum CBS112297, P. paneum CBS101032, P. sclerotiorum NRRL2074, P. solitum CBS42489 = P. solitum FRR937, A. flavus NRRL1957, and A. niger NRRL326. All the Penicillium species from GenBank are shown in red; the two Aspergillus species are shown in blue; our own sequenced Penicillium species are shown in black. Three genes were used to perform sequences analysis in Figure 1D, except only two genes (ITS and CaM) were used in P. expansum R21 and P. solitum SA analysis.
3.3. Mycotoxin
We also did a preliminary screen for the production of patulin and/or citrinin in some Penicillium isolates. Two of the three identified P. expansum strains produced both toxins. P. expansum R21 produced patulin, but did not produce a detectable amount of citrinin. None of the P. solitum strains (RS1, SA, and NJ1) appeared to produce either of the toxins. If P. carneum G2, P. paneum G9, or P. sclerotiorum 113 produce mycotoxins, they may produce toxin in trace amounts (Table 2).
4. Discussion and Conclusions
In storage, harvested crops and fruits are subjected to various attacks from insects and microbial plant pathogens. Penicillium species are a major cause of storage decays in pome and citrus fruits, as well as other postharvest crops [6,11,42,43,44]. It is often difficult to identify and distinguish different Penicillium species, as the genus is large and many of the common species look similar to each other. However, accurate identification is necessary for effective decay and toxin control, to estimate fungicide resistance, and to tailor control strategies. For example, P. expansum is the most virulent species and produces more toxins on apples and pears than P. solitum [45], making it important to have a fast and reliable method for distinguishing between the two species. Considering the frequently ambiguous identification of blue mold fungi, molecular tools are needed for the correct identification of closely-related Penicillium species [7,18]. In this study, we identified eight Penicillium isolates collected from fruits with blue mold by traditional means. We then used molecular markers to compare our sequences to sequences of the ex-type strains to confirm the species identifications and further to characterize the isolates isolated from stored fruits.
The ITS regions of ribosomal DNA have been widely applied in phylogenetic studies of fungal genomes because these regions are highly conserved and can be easily investigated using PCR amplification [46]. However, the ITS sequence information alone cannot be used to place a fungus at the species level. In this study, using the ITS1F-ITS4 primer set, we could easily amplify divergent ITS regions among morphologically distinct fungal species, but we could not rely on these sequences alone to differentiate closely-related Penicillium species. Other markers are needed in combination with the ITS markers for effective fungal identification.
We attempted to employ other common molecular markers to more precisely distinguish Penicillium isolates [47]. These included the genes of β-tubulin, calmodulin, and RPB2, all of which have been previously shown to be effective markers for species identification in other fungal genera such as Alternaria [48], Aspergillus [49,50], Botrytis and Fusarium [51], and Ramularia and Wallemia [52]. The β-tubulin marker locus includes about 550 bp of the 5′ end of the gene with introns and exons 3, 4, 5, and partial exon 6. The calmodulin marker is about 580 bp long, containing introns and exons 2, 3, 4, and partial exon 5. The RPB2 markers involve about 1,000 bp of the gene for RNA polymerase II second largest subunit [33]. At least with respect to the primers we used, these markers were not applicable to all of the tested Penicillium isolates from stored fruits. The primers for RPB2 only facilitated PCR amplification for half of the isolates.
One goal of studying conserved sequences within the genus Penicillium is the possibility of developing a DNA array representing all possible blue mold pathogens. Such an array would be useful in the rapid diagnoses of strains that have developed resistance to benzimidazole fungicides [53]. Another goal is to improve our understanding of how mycotoxin production is related to the pathogenicity and virulence of different strains and species of Penicillium. Some Penicillium mycotoxins—especially patulin—are toxic to humans and animals, and contaminate food products [54,55]. In this study, we are not sure if P. carneum G2, P. paneum G9, or P. sclerotiorum 113 could produce mycotoxins or not, and recommend that different agar media should be used to detect their production. In future work with Penicillium species identification and genome sequencing, we are developing other more effective markers, with an emphasis on distinguishing the most virulent and toxigenic blue molds that cause postharvest decay.
Finally, high genome variation and the existence of two different mating type genes were recently shown in P. expansum—a species previously considered to lack a sexual stage [56]. Future studies on Penicillium strains involved in blue mold decays need to consider the fact that phenotypic variability in virulence, mycotoxin production, and fungicide resistance in the P. expansum species complex may be due to occult sexual recombination [56].
Acknowledgments
This work was funded by the Special Fund for Agro-scientific Research in the Public Interest of China (Grant No. 201403075) and the USDA-ARS Cooperative Agreement (Grant No. 2-47012).
Author Contributions
Guohua Yin and Anping Guo conceived and designed the experiments, Guohua Yin and Yuliang Zhang performed the experiments and analyzed the data; Guohua Yin and Joan W. Bennett wrote the paper, Wayne M. Jurick II isolated and provided Penicillium spp. strains for analysis, Kayla K. Pennerman, Guangxi Wu, Sui Sheng T. Hua, Jiujiang Yu contributed to reagents/materials/analysis tools and the revisions of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jurick W.M., II, Yu J., Bennett J.W. Blue Mould to Genomics and Beyond: Insights into the Biology and Virulence of Phytopathogenic Penicillium Species. In: de Vries R.P., Gelber I.B., Anderson M.R., editors. Aspergillus and Penicillium in the Post-Genmic Era. Caister Academic Press; Norfolk, UK: 2016. p. 210. [Google Scholar]
- 2.Kim W.K., Sang H.K., Woo S.K., Park M.S., Paul N.C., Yu S.H. Six species of Penicillium associated with blue mold of grape. Mycobiology. 2007;35:180–185. doi: 10.4489/MYCO.2007.35.4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neri F., Mari M., Brigati S. Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 2006;55:100–105. doi: 10.1111/j.1365-3059.2005.01312.x. [DOI] [Google Scholar]
- 4.Jurick W.M., II, Vico I., McEvoy J.L., Whitaker B.D., Janisiewicz W., Conway W.S. Isolation, purification, and characterization of a polygalacturonase produced in Penicillium solitum-decayed "golden delicious" apple fruit. Phytopathology. 2009;99:636–641. doi: 10.1094/PHYTO-99-6-0636. [DOI] [PubMed] [Google Scholar]
- 5.Eckert J., Sievert J., Ratnayake M. Reduction of imazalil effectiveness against citrus green mold in california packinghouses by resistant biotypes of Penicillium digitatum. Plant Dis. 1994;78:971–974. doi: 10.1094/PD-78-0971. [DOI] [Google Scholar]
- 6.Chalutz E., Wilson C. Postharvest biocontrol of green and blue mold and sour rot of citrus fruit by Debaryomyces hansenii. Plant Dis. 1990;74:134–137. doi: 10.1094/PD-74-0134. [DOI] [Google Scholar]
- 7.Visagie C., Houbraken J., Frisvad J.C., Hong S.-B., Klaassen C., Perrone G., Seifert K., Varga J., Yaguchi T., Samson R. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawksworth D.L. Problems and prospects for improving the stability of names in Aspergillus and Penicillium. In: Samson R.A., Pitt J.I., editors. Modern Concepts in Penicillium and Aspergillus Classification. Plenum Press; New York, NY, USA: London, UK: 1990. pp. 75–82. [Google Scholar]
- 9.Pitt J.I. The Genus Penicillium and its Teleomorphic States Eupenicillium and Talaromyces. Academic Press Inc., Ltd.; London, UK: 1979. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces. [Google Scholar]
- 10.Seifert K., Louis-Seize G. Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification. Harwood Academic Publishers; Amsterdam, The Netherlands: 2000. Phylogeny and Species Concepts in the Penicillium aurantiogriseum Complex as Inferred from Partial β-Tubulin gene DNA Sequences; pp. 189–198. [Google Scholar]
- 11.Samson R.A., Hoekstra E.S., Frisvad J.C. Introduction to Food-and Airborne Fungi. Centraalbureau voor Schimmelcultures (CBS); Utrecht, The Netherlands: 2004. [Google Scholar]
- 12.Tiwari K., Jadhav S., Kumar A. Morphological and molecular study of different Penicillium species. Middle-East J. Sci. Res. 2011;7:203–210. [Google Scholar]
- 13.Pianzzola M., Moscatelli M., Vero S. Characterization of Penicillium isolates associated with blue mold on apple in Uruguay. Plant Dis. 2004;88:23–28. doi: 10.1094/PDIS.2004.88.1.23. [DOI] [PubMed] [Google Scholar]
- 14.La Guerche S., Garcia C., Darriet P., Dubourdieu D., Labarère J. Characterization of Penicillium species isolated from grape berries by their internal transcribed spacer (ITS1) sequences and by gas chromatography–mass spectrometry analysis of geosmin production. Curr. Microbiol. 2004;48:405–411. doi: 10.1007/s00284-003-4176-4. [DOI] [PubMed] [Google Scholar]
- 15.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Bolchacova E., Voigt K., Crous P.W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc. Natl. Acad. Sci. USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifert K.A., Samson R.A., Houbraken J., Lévesque C.A., Moncalvo J.M., Louis-Seize G., Hebert P.D. Prospects for fungus identification using CO1 DNA barcodes, with Penicillium as a test case. Proc. Natl. Acad. Sci. USA. 2007;104:3901–3906. doi: 10.1073/pnas.0611691104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoch C.L., Robbertse B., Robert V., Vu D., Cardinali G., Irinyi L., Meyer W., Nilsson R.H., Hughes K., Miller A.N. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for fungi. Database. 2014;2014 doi: 10.1093/database/bau061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houbraken J., Visagie C.M., Meijer M., Frisvad J.C., Busby P.E., Pitt J.I., Seifert K.A., Louis-Seize G., Demirel R., Yilmaz N., et al. A taxonomic and phylogenetic revision of Penicillium section Aspergilloides. Stud. Mycol. 2014;78:373–451. doi: 10.1016/j.simyco.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin G., Zhang Y., Pennerman K.K., Hua S.S., Yu J., Guo A., Liu Z., Bennett J.W. Draft genome sequence of the fungus Penicillium solitum NJ1. Genome Announc. 2016;4:e01176-16. doi: 10.1128/genomeA.01176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao G., Yin G., Inamda A.A., Luo J., Zhang N., Yang I., Buckley B., Bennett J.W. Volatile organic compounds emitted by filamentous fungi isolated from flooded homes after hurricane Sandy show toxicity in a Drosophila bioassay. Indoor Air. 2016 doi: 10.1111/ina.12350. [DOI] [PubMed] [Google Scholar]
- 21.Yin G., Zhang Y., Pennerman K.K., Hua S.S.T., Huang Q., Guo A., Liu Z., Bennett J.W. Genome sequencing and analysis of the filamentous fungus Penicillium sclerotiorum 113, isolated after hurricane Sandy. Genome Announc. 2016;4:e01153-16. doi: 10.1128/genomeA.01153-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C.-J.K., Zabel R.A. Identification Manual for Fungi from Utility Poles in the Eastern United States. American Type Culture Collection; Manassas, VA, USA: 1990. [Google Scholar]
- 23.Samson R.A., Hong S., Peterson S., Frisvad J.C., Varga J. Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya. Stud. Mycol. 2007;59:147–203. doi: 10.3114/sim.2007.59.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samson R.A., Noonim P., Meijer M., Houbraken J., Frisvad J.C., Varga J. Diagnostic tools to identify black Aspergilli. Stud. Mycol. 2007;59:129–145. doi: 10.3114/sim.2007.59.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera K., Seifert K. A taxonomic and phylogenetic revision of the Penicillium sclerotiorum complex. Stud. Mycol. 2011;70:139–158. doi: 10.3114/sim.2011.70.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimeno A., Martins M.L. Rapid thin layer chromatographic determination of patulin, citrinin, and aflatoxin in apples and pears, and their juices and jams. J. Assoc. Off. Anal. Chem. 1983;66:85–91. [PubMed] [Google Scholar]
- 27.Frisvad J.C., Filtenborg O., Thrane U. Analysis and screening for mycotoxins and other secondary metabolites in fungal cultures by thin-layer chromatography and high-performance liquid chromatography. Arch. Environ. Contam. Toxicol. 1989;18:331–335. doi: 10.1007/BF01062357. [DOI] [PubMed] [Google Scholar]
- 28.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols Guide Methods Appl. 1990;18:315–322. [Google Scholar]
- 29.Glass N.L., Donaldson G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S.B., Cho H.S., Shin H.D., Frisvad J.C., Samson R.A. Novel Neosartorya species isolated from soil in korea. Int. J. Syst. Evolut. Microbiol. 2006;56:477–486. doi: 10.1099/ijs.0.63980-0. [DOI] [PubMed] [Google Scholar]
- 31.Peterson S.W., Vega F.E., Posada F., Nagai C. Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia. 2005;97:659–666. doi: 10.3852/mycologia.97.3.659. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 33.Houbraken J., Spierenburg H., Frisvad J.C. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie Leeuwenhoek. 2012;101:403–421. doi: 10.1007/s10482-011-9647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner D.L., Banik M.T. Molecular phylogeny of Laetiporus and other brown rot polypore genera in North America. Mycologia. 2008;100:417–430. doi: 10.3852/07-124R2. [DOI] [PubMed] [Google Scholar]
- 35.Goujon M., McWilliam H., Li W., Valentin F., Squizzato S., Paern J., Lopez R. A new bioinformatics analysis tools framework at EMBL_EBI. Nucleic Acids Res. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 37.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 38.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 39.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., Yu L., Kubatko L., Pearl D.K., Edwards S.V. Coalescent methods for estimating phylogenetic trees. Mol. Phylogenet. Evol. 2009;53:320–328. doi: 10.1016/j.ympev.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 41.Gadagkar S.R., Rosenberg M.S., Kumar S. Inferring species phylogenies from multiple genes: Concatenated sequence tree versus consensus gene tree. J. Exp. Zool. Part B Mol. Dev. Evol. 2005;304:64–74. doi: 10.1002/jez.b.21026. [DOI] [PubMed] [Google Scholar]
- 42.Li B., Zong Y., Du Z., Chen Y., Zhang Z., Qin G., Zhao W., Tian S. Genomic characterization reveals insights into patulin biosynthesis and pathogenicity in Penicillium species. Mol. Plant-Microbe Interact. 2015;28:635–647. doi: 10.1094/MPMI-12-14-0398-FI. [DOI] [PubMed] [Google Scholar]
- 43.Yu J., Jurick W.M., II, Cao H., Yin Y., Gaskins V.L., Losada L., Zafar N., Kim M., Bennett J.W., Nierman W.C. Draft genome sequence of Penicillium expansum strain R19, which causes postharvest decay of apple fruit. Genome Announc. 2014;2:e00635-14. doi: 10.1128/genomeA.00635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu J., Wu G., Jurick II W.M., Gaskins V.L., Yin Y., Yin G., Bennett J.W., Shelton D.R. Genome sequence of Penicillium solitum RS1, which causes postharvest apple decay. Genome Announc. 2016;4:e00363-16. doi: 10.1128/genomeA.00363-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitt J., Spotts R., Holmes R., Cruickshank R. Penicillium solitum revived, and its role as a pathogen of pomaceous fruit. Phytopathology. 1991;81:1108–1112. doi: 10.1094/Phyto-81-1108. [DOI] [Google Scholar]
- 46.Gardes M., Bruns T.D. Its primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 47.Stielow J., Lévesque C., Seifert K., Meyer W., Iriny L., Smits D., Renfurm R., Verkley G., Groenewald M., Chaduli D. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Pers. Mol. Phylogeny Evol. Fungi. 2015;35:242. doi: 10.3767/003158515X689135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woudenberg J., Seidl M., Groenewald J., de Vries M., Stielow J., Thomma B., Crous P. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015;82:1–21. doi: 10.1016/j.simyco.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S., An C., Xu S., Lee S., Yamamoto N. High-throughput sequencing reveals unprecedented diversities of Aspergillus species in outdoor air. Lett. Appl. Microbiol. 2016;63:165–171. doi: 10.1111/lam.12608. [DOI] [PubMed] [Google Scholar]
- 50.Lee S., Yamamoto N. Accuracy of the high-throughput amplicon sequencing to identify species within the genus Aspergillus. Fungal Biol. 2015;119:1311–1321. doi: 10.1016/j.funbio.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Diepeningen A.D., Feng P., Ahmed S., Sudhadham M., Bunyaratavej S., Hoog G.S. Spectrum of Fusarium infections in tropical dermatology evidenced by multilocus sequencing typing diagnostics. Mycoses. 2015;58:48–57. doi: 10.1111/myc.12273. [DOI] [PubMed] [Google Scholar]
- 52.Jančič S., Nguyen H.D., Frisvad J.C., Zalar P., Schroers H.-J., Seifert K.A., Gunde-Cimerman N. A taxonomic revision of the Wallemia sebi species complex. PLoS ONE. 2015;10:e0125933. doi: 10.1371/journal.pone.0125933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sholberg P.L., Harlton C., Haag P., Le´vesque C.A., O’Gorman D., Seifert K. Benzimidazole and diphenylamine sensitivity and identity of Penicillium spp. that cause postharvest blue mould of apples using β-tubulin gene sequences. Postharv. Biol. Technol. 2005;36:41–49. doi: 10.1016/j.postharvbio.2004.07.011. [DOI] [Google Scholar]
- 54.Pitt J. The current role of Aspergillus and Penicillium in human and animal health. J. Med. Vet. Mycol. 1994;32:17–32. doi: 10.1080/02681219480000701. [DOI] [PubMed] [Google Scholar]
- 55.Pitt J. Toxigenic fungi and mycotoxins. Br. Med. Bull. 2000;56:184–192. doi: 10.1258/0007142001902888. [DOI] [PubMed] [Google Scholar]
- 56.Julca I., Droby S., Sela N., Marcet-Houben M., Gabaldón T. Contrasting genomic diversity in two closely related postharvest pathogens: Penicillium digitatum and Penicillium expansum. Genome Biol. Evol. 2016;8:218–227. doi: 10.1093/gbe/evv252. [DOI] [PMC free article] [PubMed] [Google Scholar]