Abstract
In agriculture, there is an urgent need for alternate ecofriendly products to control plant diseases. These alternate products must possess preferable characteristics such as new modes of action, cost effectiveness, biodegradability, and target specificity. In the current scenario, studies on macrofungi have been an area of importance for scientists. Macrofungi grow prolifically and are found in many parts of the world. Basidiomycetes (mushrooms) flourish ubiquitously under warm and humid climates. Basidiomycetes are rich sources of natural antibiotics. The secondary metabolites produced by them possess antimicrobial, antitumor, and antioxidant properties. The present review discusses the potential role of Basidiomycetes as anti-phytofungal, anti-phytobacterial, anti-phytoviral, mosquito larvicidal, and nematicidal agents.
Keywords: biocontrol properties, basidiomycetes, anti-phytofungal activity, anti-phytobacterial activity, anti-phytoviral activity, phytonematicidal activity, mosquito larvicidal activity
1. Introduction
Synthetic chemicals are extensively used in all countries for controlling agricultural pests and plant pathogens [1]. Currently 15% of global crop production is lost due to crop pests [2]. To counteract this, agrochemicals are used in excessive quantities/volumes; it has become apparent that these chemicals are responsible for causing environmental pollution. They leave their residues in food [3]. Unrestrained application of synthetic chemicals causes pesticide resistance, toxicity to humans, plants, and animals, and therefore they are regarded as ecologically unacceptable [4].
Mosquitoes are the most well-known vectors of disease causing pathogens which affect millions of people every year [5]. In India, major diseases are caused by mainly three types of mosquitoes [6], namely Aedes aegypti L., Anopheles stephensi (Liston), and Culex quinquefasciatus (Say). Ae. aegypti is a known vector for dengue and chikungunya virus. The malarial parasite is transmitted by An. stephensi and the filarial nematode is transmitted by Cx. quinquefasciatus. Prevention of mosquito borne diseases is important in order to improve public health, and it is primarily achieved by controlling the vector mosquito population. In recent years, mosquito control programs have encountered failures due to the rapid development of pesticide resistance in mosquitoes [7].
Nematodes have been present for nearly a billion years and are known to cause severe losses to farmers [8]. They feed on many, if not all plants [9]. This pathogen is predominantly part of class Chromodorea, order Rhabdihida [10]. Due to chemical contamination of soil caused by commercial nematicides, newer sources of eco-friendly biomolecules are required in the future.
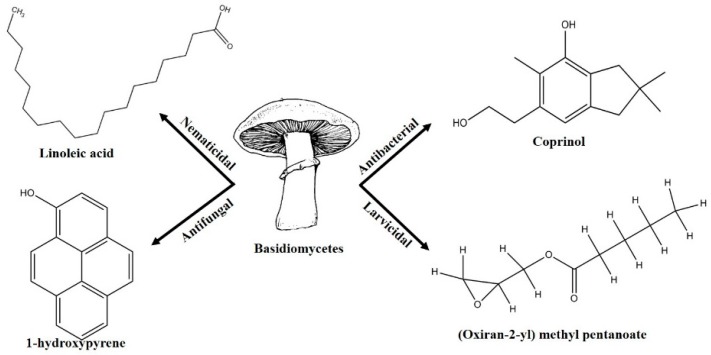
Pest and pathogen diversities are continuously expanding and new strains are continuously evolving over time [11]. Scientists are looking for safe and more potent alternate products for controlling plant pathogens and pests. The use of natural products for pest control is ideal for sustainable agricultural production with minimum damage to the environment [12]. Basidiomycetes are the fruiting bodies of higher fungi [13]. Several compounds have been isolated from wild Basidiomycetes (Figure 1) which showed growth inhibition of bacteria, virus, and fungi (Table 1) and recorded nematicidal and insecticidal properties [14,15,16,17,18,19,20,21,22,23,24,25,26,27]. In the last few years, Basidiomycetes have received great attention due to their medicinal values, easy availability, and lower side effects and toxicity on non-target organisms [28]. There are nearly 140,000 Basidiomycetes species reported among which about 660 species possess medicinal properties [29]. A number of pharmaceutical substances with potent and unique characteristics have been extracted from Basidiomycetes [30]. Higher Basidiomycetes contain active polysaccharides in their fruiting bodies, cultured mycelia, and cultured broth [31]. The present review focused on anti-phytofungal, anti-phytobacterial, anti-phytoviral, phytonematicidal, and mosquito larvicidal activity of Basidiomycetes.
Figure 1.
Some of the biocontrol properties of Basidiomycetes compounds.
Table 1.
Active compounds isolated from different mushroom species.
| Active Compound | Compound Structure | Target Pathogen | Reference |
|---|---|---|---|
| Ganodermin (Ganoderma lucidum) Antifungal protein | NA | Physalospora piricola; Botytis cinerea | [19] |
| Pleurostrin (Pleurotus ostreatus) Antifungal protein | NA | Botryosphaeria berengeriana | [20] |
| Eryngin (Pleurotus eryngii) Antifungal protein | NA | Mycosphaerella arachidicola | [20,23] |
| Antifungal protein mLAP (Lyophyllum shimeji) | NA | Physalospora piricola | [22] |
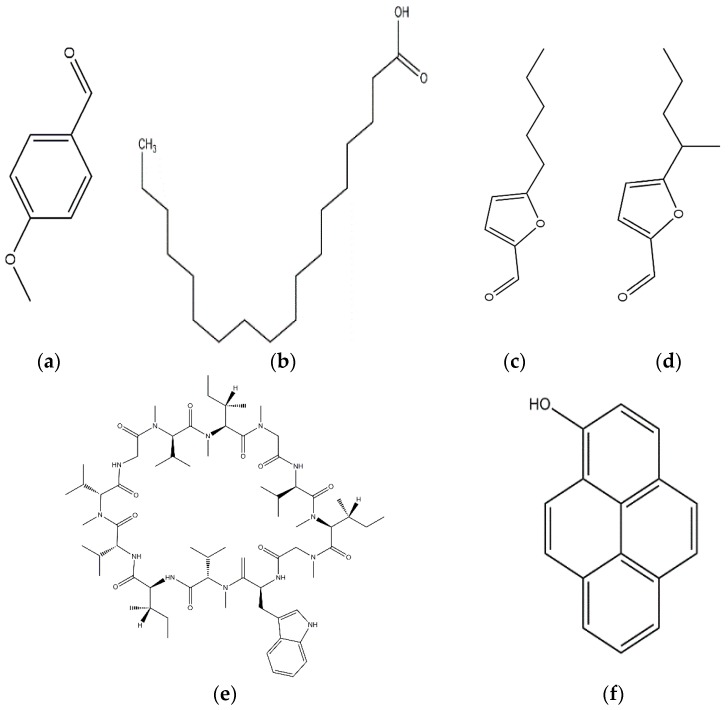
| Lyophyllin (Lyophyllus shimeji) | 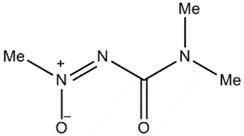 |
Physalospora piricola | [22] |
| Grifoline (Albatrellus dispansus) | 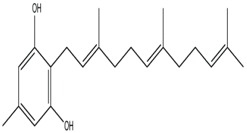 |
Alternaria alternate; Pyricularia oryzae; Rhizoctonia solani; Sclerotina sclerotiorum; Fusarium graminearum; Botytis cinerea; Gaeumannomyces graminis; Gloesporium fructigenum | [46] |
| Hypsin (Hypsizigus marmoreus) Antifungal protein | NA | Botryosphaeria berengeriana; Botytis cinerea; Mycosphaerella arachidicola | [47] |
| Rufuslactone (Lactarius rufus) | 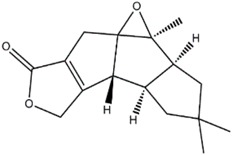 |
Alternaria alternata; Fusarium graminearum; Botytis cinerea; Alternaria brassicae | [48] |
| Cordymin (Cordyceps militaris) Antifungal protein | NA | Rhizoctonia solani; Mycosphaerella arachidicola; Bipolaris maydis | [49] |
| p-Hydroxybenzoic and Cinnamic acids (Ganoderma lucidum) | 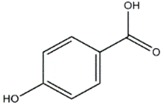 |
Trichoderma viride; Penicillium ochrochloron; P. funiculosum | [50] |
| p-Hydroxybenzoic | |||
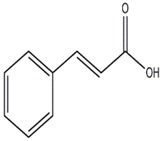 | |||
| Cinnamic acids | |||
| Chrysotrione A and B (Hygrophorus chrysodon) |
|
Fusarium verticillioides | [50] |
| Chrysotrione A | |||
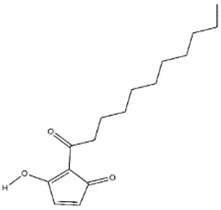 | |||
| Chrysotrione B | |||
| Agrocybin (Agrocybe cylindracea) | 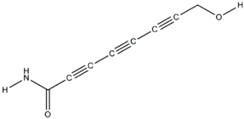 |
Mycosphaerella arachidicola | [51] |
| Lentin (Lentinus edodes) Antifungal protein | NA | Mycosphaerella arachidicola | [52] |
| 1-hydroxypyrene (Cordyceps militaris) | 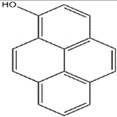 |
Fusarium oxysporum | [53] |
| Phellinsin A (Phellinus sp.) | 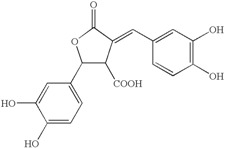 |
Gloeosporium orbiculare; Pyricularia grisea | [54] |
Note: NA—not available.
Plant Fungal Diseases
Fungi may cause catastrophic plant diseases, because many fungi sporulate prolifically and the spores provide copious inoculums which may infect further plants. The time between infection and the production of further infectious propagules (usually spores) may be only a few days [32]. The spores, if they are wettable, may be spread as high-density inoculums in surface water or in droplets by rain-splash [33]. They may produce phytotoxic compounds [34]. Pathogens may draw nutrients away from the plant by the production or induction of growth regulators [32]. Colletotrichum gloeosporioides (anamorph) or Glomerella cingulata (teleomorph) causes anthracnoses in many tropical and subtropical crops [35]. Considerable variation occurs in culture and host range, with some strains able to attack many host species whereas others, such as those infecting mango, are confined to a single species [36]. Molecular approaches demonstrated that C. gloeosporioides infecting Stylosanthes in Australia consisted of two clone populations that did not combine readily in the field which had resulted from two separate introductions into the country [37]. Some strains of C. gloeosporioides present a considerable threat to crops growing in countries where there is no fallow period corresponding to the winter of temperate climates [38]. Rice is second only to maize in global production [39]. It is more important since it is the staple food for about half of the world’s populations. It is attacked by the Ascomycete fungus Pyricularia oryzae (teleomorph), Magnaporthe grisea, causing rice blast, resulting in 10%–30% crop loss every year [40]. More than 700 ha of rice of diverse genotypes with varying levels of resistance in Bhutan were affected in 1995 resulting in losses of 1090 tonnes [40]. Other cereals are also affected by P. oryzae or similar species [41]. These include finger millet, Eleusine coracana, which, when attacked before grain formation, can suffer complete loss of yield [40]. P. oryzae caused yield losses up to 40% in Tanzania [42]. Several fungi produce powerful mycotoxins. For example, the Fumonisin toxins were discovered in the Transkei region of South Africa. They were isolated from cultures of Gibberella fujikuroi (anamorph) and Fusarium moniliforme grown on maize, and the most active compound was designated as fumonisin B1 (FB1) [43]. FB1 is a sphinganine analogue that, in both plant and animal cells, competitively inhibits sphingolipid biosynthesis causing sphingoid bases to accumulate [44]. High levels of virulence for maize were always associated with strains of the fungus that produce fumonisins [45].
2. Biocontrol Properties of Basidiomycetes
2.1. Anti-Phytofungal Activity (Table 1)
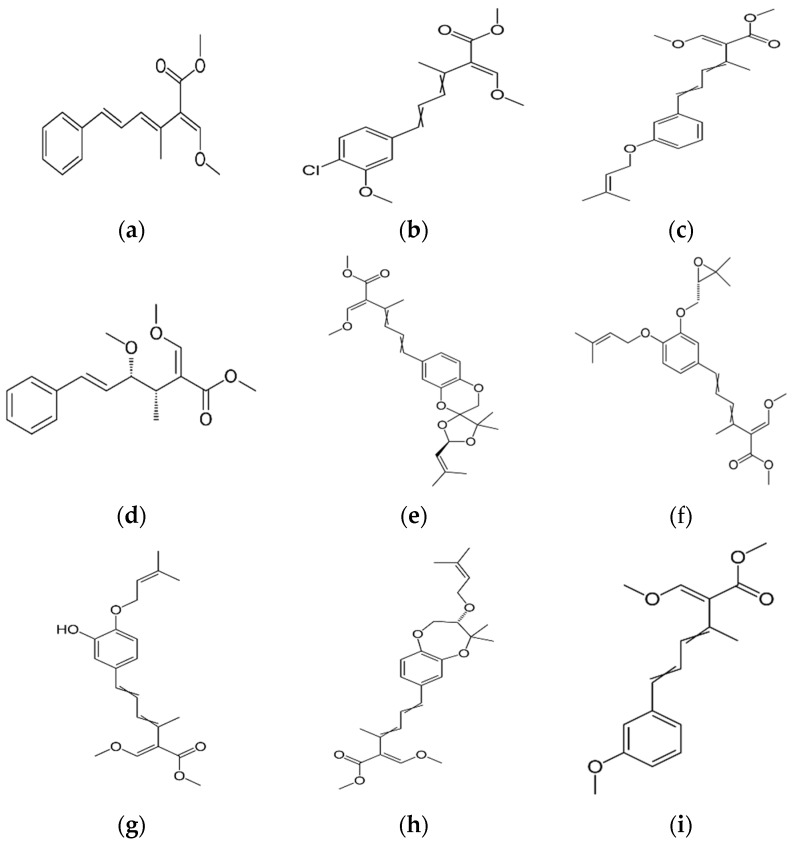
The antifungal agent Grifoline isolated from Albatrellus dispansus was effective against several plant fungi in in vitro studies [46]. Phellinsin A was isolated from Phellinus sp. It was capable of inhibiting the growth of fungi such as Gloeosporium orbiculare, Pyricularia grisea, Thanatephorus cucumeris, Aspergillus fumigatus, and Trichophyton mentagrophytes [54]. A major compound in agricultural chemistry was derived from the pine cone fungus, Strobilurus tenacellus [55]. Strobilurins are a class of fungicidal compounds, which are extracted from mycelia of the S. tenacellus. Strobilurins A (Figure 2a) and B (Figure 2b), that are highly active by inhibiting respiration of yeast and other filamentous fungi [56]. The biochemical activities of strobilurins involve ubihydroquinone cytochrome reductase, which plays a crucial role in respiration [57]. Their activity, however, depends on the presence of (E)-β-methoxyacrylate moiety [58]. Strobilurin fungicides have become valuable tools for managing plant diseases [59]. These strobilurins are site specific (inhibition of mitochondrial respiration) and translaminar (systemic) compounds that provide control of Oomycota, Ascomycota, Basidiomycota, and Deuteromycota fungi. (E)-β-methoxyacrylates of strobilurin C (Figure 2c) and Oudemansin B (Figure 2d) from cultures of Xerula pudens inhibit many phytopathogenic fungi. Like the strobilurins A and B, they have also been shown to inhibit fungal respiration [60]. Strobilurin E (Figure 2e) is another antifungal compound of the (E)-β-methoxyacrylate class extracted from mycelial cultures of Crepidotus fulvotomentosus. In addition to inhibiting fungal respiration, it has been shown to induce cell deformations [57]. Strobilurins D (Figure 2f) and F (Figure 2g) are other strobilurins, extracted from mycelial cultures of the Basidiomycete Merismodes anomala; they have cytostatic and antifungal antibiotics of the (E)-β-methoxyacrylate class. These strobilurins inhibit many fungi, and like strobilurins A and B, they also are potent inhibitors of respiration [57,61]. Strobilurin M which was isolated from the Mycena sp. showed antifungal and cytostatic activities [61]. Other strobilurins F (Figure 2g), G (Figure 2h), and H (Figure 2i) extracted from culture fluids of Bolinea lutea inhibited Aspergillus fumigatus, Botrytis cinerea, Microsporum canis, and Sporothrix schenckii. These compounds reduced fungal respiration. However, they might be different from the analogs previously described [58]. Wang et al. reported that 15 kDa antifungal protein designated as Ganodermin, was isolated from Ganoderma lucidum. It inhibited mycelial growth of Botrytis cinerea, Fusarium oxyporum, and Botryosphaeria berengeriana. The IC50 (concentration which inhibits 50% growth) values of the ganodermin against B. cinerea, Fusarium oxysporum, and B. berengeriana were 15.2–0.7 µM, 12.4–0.3 µM, and 18.1–0.5 µM, respectively. Pleurostrin, an antifungal peptide with about half the size of ganodermin, has been isolated from the oyster Basidiomycetes Pleurotus ostreatus. Ganodermin inhibited mycelial growth in the phytopathogenic fungi Botrytis cinerea, F. oxyporum, and Peyronellaea arachidicolla. Very few bioactive proteins, such as a lectin and a ribonuclease, have been isolated from G. lucidum [19]. The antifungal activity of culture filtrates, methanol, and water extracts of Stereum ostrea, an inedible Basidiomycetes, was tested against three plant fungal pathogens namely Botrytis cinerea, Colletotrichum miyabeanus, and Colletotrichum gloeosporioides [61].
Figure 2.
Strobilurin A (a); Strobilurin B (b); Strobilurin C (c); Oudemansin B (d); Strobilurin E (e); Strobilurin D (f); Strobilurin F (g); (h); Strobilurin H (i).
2.2. Anti-Phytobacterial Activities
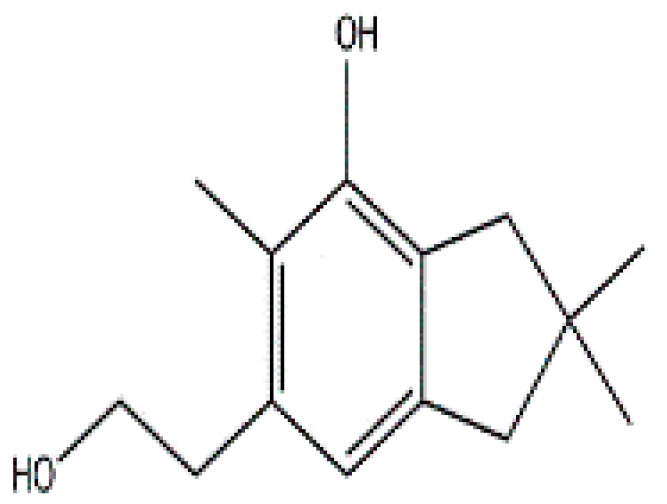
Secondary metabolites isolated from various Basidiomycetes have been known to show antibacterial properties [62,63,64,65]. Basidiomycetes provide effective and low-cost products for human and plant disease control. Members of Ganodermatales, Poriales, Agaricales, and Stereales show potential antibacterial activity and these may become substitutes for developing new antibiotics [66]. The effect of secondary metabolites of Basidiomycetes has been investigated mainly on human and animal pathogens. Erjave et al. reported that 15 Basidiomycetes extracts showed moderate to high antibacterial activities and three extracts regressed the disease as well as reduced the severity in vitro and in vivo against bacterial wilt disease caused by Ralstonia solanacearum [67]. Extracts from Clytocybe geotropa showed broad range of inhibition against R. solanacearum, Erwinia carotovora subsp. carotovora, P. syringae pv. syringae, X. campestris pv. Vesicatoria, and Clavibacter michiganensis subsp. sepedonicus. Purified protein, Clitocypin, from C. geotropa showed effective inhibition against C. michiganensis subsp. Sepedonicus [68]. The fungicide strobilurin F 500 enhanced resistance of tobacco to the wild fire pathogen Pseudomonas syringae pv. tabaci [69]. Coprinol (Figure 3), isolated from Coprinus sp., showed inhibitory activity against most of the plant pathogens [70].
Figure 3.
Coprinol.
2.3. Anti-Phytoviral Activity
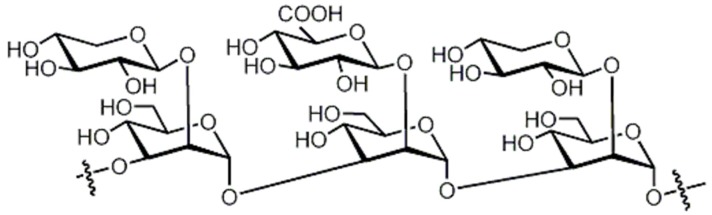
Brandt and Piraino (2000) divided the antiviral compounds from fungi into two major classes: (i) biological response modifiers and (ii) viral inhibitors [71]. The effect of Basidiomycete polysaccharides has been investigated using human and animal virus models [72]. Ganoderma lucidum and G. applanatum strains were able to inhibit the tobacco mosaic virus (TMV) at a concentration of 1000 µg/mL [73]. The filtrate from cultured biomass of the polypore Fomes fomentarius was effective against the mechanical transmission of TMV [74]. A new lectin, named AAL (Agrocybe aegerita lectin), had been purified from the fruiting bodies of the edible Basidiomycetes A. aegerita [26]. Aqueous extracts from Agaricus brasiliensis and Lentinula edodes fruiting bodies showed antiviral activity against the aphid-borne mosaic virus [75]. The neutral and acid polysaccharides have different characteristics of anti-phytoviral activity. Glucuronoxylomannan (GXM) was considerably less active; in this case, the total preparation occupied an intermediate position, revealing evidence that activity of the total preparation relative to the infectivity of TMV was induced to a greater extent [76]. The effect of Basidiomycete metabolites on plant pathogenic viruses has been poorly studied [75]. A pink quinone, tentatively identified as β-l-glutaminyl-3,4-benzoquinone, present in sporophores of Agaricus bisporus is a potent inhibitor of plant virus infections [77]. It showed inhibitory activity to infection of TMV on Nicotiana glutinosa. In particular, the acid polysaccharide of GXM (Figure 4) produced by Tremella mesenterica consisted of a linear backbone of β-(1→2) (1→4)-linked oligosaccharides of xylose and glucuronic acid [78,79,80].
Figure 4.
Polysaccharide glucuronoxylomannan (GXM).
2.4. Phytonematicidal Activity
Researchers throughout the world have become increasingly interested in diminishing the use of chemical strategies against parasites [81,82,83]. A recent study has demonstrated that some species of edible Basidiomycetes such as Pleurotus species possess nematicidal activity through the production of a nematode-toxin, which is able to inhibit the nematode movement, allowing hyphal penetration, and finally digesting the body by enzymatic action [84]. Such biological activity could be the result of a self-defense mechanism in Basidiomycetes which acts against the attack of myceliophagous nematodes [85]. Other studies have shown that P. ostreatus produces a nematode-toxin similar to peroxides which inhibits the movement of nematodes and subsequently degrades them, reaching a mortality of 95% in the free-living nematode Panagrellus redivivus (adults) and the phytopathogenic nematode Bursaphelenchus xylophilus [86]. Another study conducted by Palizi et al. showed that P. eryngii caused 50% mortality against the phytopathogenic nematode Heterodera schantii which caused wilting in sugarcane and other crops. In general, nematicidal activity shown by the different strains of P. eryngii ranged between 4.8%–99.6%. The nematicidal effect of specific edible Basidiomycetes could be influenced by a number of factors like temperature, incubation time of the confrontation, inner genetic characteristics of each of the strains, and differences between nematode species used [87]. Some dead larvae observed with mycelium inside their bodies suggested that the larval death was a consequence of body rupture and invasion by fungal mycelia [87]. Mamiy reported that a strain of Coprinus comatus immobilized, killed, and consumed the free-living nematode Panagrellus redivivus and the root-knot nematode Meloidogyne arenaria [88]. The author reported mechanical damage in the free-living nematode P. redivivus, 8 h post-confrontation with C. comatus mycelia, resulting in 90% nematode immobilization and subsequent degradation, at 24 °C incubation. The strains of the edible Pleurotus ostreatus ECS-1123 and ECS-0152, P. eryngii ECS-1290 and ECS -1291, P. cornucopiae ECS-1328 and ECS-1330, and Lentinula edodes ECS-0401 displayed high nematicidal activity with a range of 82% to 99% mortality [84]. Bua-art et al. reported the potential use of bioactive compounds from luminescent Basidiomycete (Neonothopanus nambi) for control of plant parasitic root-knot nematode Meloidogyne incognita. The results revealed that concentrations of 500 mg/L were highly toxic to M. incognita causing 100% mortality within 30 min [89].
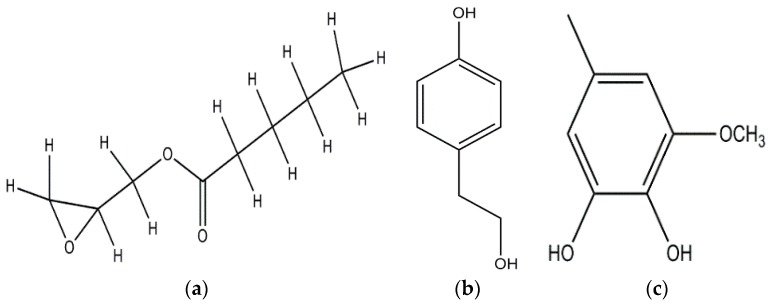
Anisaldehyde, 3-chloro-anisaldehyde, and (4-methoxyphenyl)-1,2-propandiol were isolated from several common wood and forest-litter degrading fungi (e.g., Pleurotus pulmonarius, Bjerkandera adusta, Hypholoma fasciculare, and Pholiota squarrosa) [90]. Weak antifungal and nematicidal properties have been described for p-anisaldehyde (Figure 5a) and (4-methoxyphenyl)-1,2-propandiol. Fatty acids like S-coriolic acid or linoleic acid (Figure 5b) isolated from P. pulmonarius exhibited nematicidal effects against the saprophytic nematode Caenorhabditis elegans, with LD50 (Median lethal dosage) values of 10 and 5 µg/mL, respectively [91]. These nematicidal effects depended on the degree of unsaturation and the length of the fatty acid. A nematicidal monoterpene, 1,2-dihydroxymintlactone, was isolated from Cheimonophyllum candidissimum. It recorded LD50 value of 25 µg/mL against C. elegans and herbicidal effects against Setaria italica and Lepidium sativum at concentrations starting from 50 µg/mL [92]. Cheimonophyllons and cheimonophyllal were isolated from the wood-inhabiting Basidiomycete Cheimonophyllum candidissimum and they exhibited nematicidal activities against nematode C. elegans [92]. The Furaldehydes, 5-pentyl-2-furaldehyde (Figure 5c) and 5(4-penteny)-2-furaldehyde (Figure 5d) isolated from Irpex lacteus, exhibited nematicidal activity against Aphelencoides besseyi [93]. A nematicidal cyclic peptide omphalotin (Figure 5e) was isolated from biomass after fermentation of Omphalotus olearius [94]. 1-Hydroxypyrene (Figure 5f) derived from Crinipellis stipitaria showed very strong nematicidal activity against C. elegans [53]. The cultural filtrates from Amauroderma macer, Laccaria tortilis, and Tylopilus striatulus showed high nematicidal activity against the pine wood nematode Bursaphelenchus xylophilus [95].
Figure 5.
p-anisaldehyde (a); Linoleic acid (b); 5-pentyl-2-furaldehyde (c); 5(4-penteny)-2-furaldehyde (d); Omphalotin (e); 1-Hydroxypyrene (f).
2.5. Mosquito Larvicidal Activity
Few studies have been done to find out the mosquito larvicidal activity of Basidiomycetes extracts. The (Oxiran-2-yl) methylpentanoate (Figure 6a) isolated from submerged culture of Cyptotrama asprata showed 100% larvicidal activity at 1.25 ppm (parts per million) concentration against Aedes aegypti after 8 h [96]. High larvicidal activities against Ae. aegypti and Anopheles nuneztovari were observed using the crude extracts of Pycnoporus sanguineus and Pestalotiopsis virgulata [97]. Crude ethanolic extract of Lactarius gymnocarpoides showed maximum larvicidal activity against Ae. aegypti [98]. Ethanol extracts of Amanita phalloides, Russula cellulata, Lactarius gymnocarpoides, and L. densifolius exhibited weak activity against Cx. quinquefasciatus and Ae. aegypti [99]. The larvicidal activity of methanolic extract of G. lucidum against fourth instar larvae of Cx. pipiens was tested post 24 h exposure and it ranged from 18.25% at 0.5 mg·L−1 concentration to 100% at 5 mg·L−1 concentration [100]. 4-(2-hydroxyethyl) phenol (Figure 6b) and 3-methoxy-5-methyl-1,2-benzenediol (Figure 6c) with LC50 values of 231 and 237 ppm, respectively, were isolated from Basidiomycete JO5289, and were found to be active against Ae. aegypti larvae after 24 h [101]. Thaeogyroporus porentosus, Xylaria nigripes, Chlorophyllum sp., and Steccherinum species had good larvicidal activities against Ae. aegypti with mortality ranging from 10%–70% and 18%–90% for 24 and 48 h exposure times, respectively [102].
Figure 6.
(Oxiran-2-yl) methylpentanoate (a); 4-(2-hydroxyethyl) phenol (b); 3-methoxy-5-methyl-1,2-benzenediol (c).
3. Conclusions
Basidiomycetes occupy a prominent position in medical and biological research fields because of their antimicrobial, insecticidal, and nematicidal properties. They are easily available or cultivable and economic. As such, their secondary metabolites with active principles can be produced cost-effectively. The present review clearly shows the various biological properties of Basidiomycetes compounds against plant pathogenic microbes, vector mosquitoes, and nematodes. Discovery of more novel natural products from Basidiomycetes for the control of plant diseases, vector mosquitoes, and nematodes will lead to ecofriendly crop protection methods. The interactions between the bioactive metabolites of Basidiomycetes and host cells should be studied to understand the mechanisms behind their antibacterial, larvicidal, nematicidal, and other activities. The potential bioactive metabolites obtained from Basidiomycetes should be urgently commercialized to reduce the unwanted effects of synthetic chemicals.
Acknowledgments
The authors are thankful to Entomology Research Institute, Loyola College, Chennai for financial assistance and facilities. This was also partially financially supported by King Saud University through vice Deanship of Research Chairs. We thank Stephen Nehru, G. for reading through the manuscript.
Author Contributions
The authors Subramaniyan Sivanandhan, Ameer Khusro and Michael Gabriel Paulraj collected the data and wrote the manuscript. The authors Savarimuthu Ignacimuthu, and Naif Abdullah AL-Dhabi designed the outline and assisted in manuscript preparation and correction.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Whipps J.M., Lumsden R.D. Commercial use of fungi as plant disease biological control agents: Status and prospects. In: Butt T.M., Jackson C., editors. Fungal Biocontrol Agents: Progress, Problems and Potential. Volume 9. CABI Publishing; Oxfordshire, UK: 2001. pp. 9–22. [Google Scholar]
- 2.Maxmen A. Crop pests: Under attack. Nature. 2013;501:S15–S17. doi: 10.1038/501S15a. [DOI] [PubMed] [Google Scholar]
- 3.Montesinos E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003;6:245–252. doi: 10.1007/s10123-003-0144-x. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee R., Dey U. An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. Afr. J. Microbiol. Res. 2014;8:1749–1762. [Google Scholar]
- 5.Reegan A.D., Gandhi M.R., Paulraj M.G., Ignacimuthu S. Ovicidal and oviposition deterrent activities of medicinal plant extracts against Aedes aegypti L. and Culex quinquefasciatus Say mosquitoes (Diptera: Culicidae) Osong Public Health Res. Perspect. 2015;6:64–69. doi: 10.1016/j.phrp.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhiman R.C., Pahwa S., Dhillon G.P., Dash A.P. Climate change and threat of vector-borne diseases in India: Are we prepared? Parasitol. Res. 2010;106:763–773. doi: 10.1007/s00436-010-1767-4. [DOI] [PubMed] [Google Scholar]
- 7.Benelli G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015;114:2801–2805. doi: 10.1007/s00436-015-4586-9. [DOI] [PubMed] [Google Scholar]
- 8.Williamson V.M., Gleason C.A. Plant-nematode interactions. Curr. Opin. Plant Biol. 2003;6:327–333. doi: 10.1016/S1369-5266(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 9.Dropkin V.H. Introduction to Plant Nematology. 2nd ed. John Wiley and Sons Inc.; Columbia, SC, USA: 1989. pp. 1–304. [Google Scholar]
- 10.Campbell J.F., Kaya H.K. How and why a parasitic nematode jumps. Nature. 1999;397:485–486. doi: 10.1038/17254. [DOI] [Google Scholar]
- 11.Spread of Crop Pest Threatens Global Food Security as Earth Warms. [(accessed on 1 September 2013)]. Available online: http://www.exeter.ac.uk/research/inspiring/keythemes/science/news/archive/title_316965_en.html.
- 12.Gillespie A. Conservation, Biodiversity and International Law. Edward Elgar Publishing; Chelteham, New Zealand: 2013. pp. 1–579. [Google Scholar]
- 13.Wasser S.P., Weis A.L. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: Current perspectives (review) Int. J. Med. Mushrooms. 1999;1:31–62. doi: 10.1615/IntJMedMushrooms.v1.i1.30. [DOI] [PubMed] [Google Scholar]
- 14.Anke T. Basidiomycetes: A source for new bioactive secondary metabolites. Prog. Ind. Microbiol. 1989;27:51–66. [Google Scholar]
- 15.Gowrie S.U., Chathurdevi G., Rani K. Evaluation of bioactive potential of basidiocarp extracts of Ganoderma lucidum. Int. J. Pharm. Res. Allied Sci. 2014;3:36–46. [Google Scholar]
- 16.Kolundžić M., Grozdanić N.Đ., Dodevska M., Milenković M., Sisto F., Miani A., Farronato G., Kundaković T. Antibacterial and cytotoxic activities of wild mushroom Fomes fomentarius (L.) Fr., Polyporaceae. Ind. Crops. Prod. 2016;79:110–115. [Google Scholar]
- 17.Ren L., Hemar Y., Perera C.O., Lewis G., Krissansen G.W., Buchanan P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre. 2014;3:41–51. doi: 10.1016/j.bcdf.2014.01.003. [DOI] [Google Scholar]
- 18.Santos D.N., de Souza L.L., de Oliveira C.A., Silva E.R., de Oliveira A.L. Arginase inhibition, antibacterial and antioxidant activities of Pitanga seed (Eugenia uniflora L.) extracts from sustainable technologies of high pressure extraction. Food Biosci. 2015;12:93–99. doi: 10.1016/j.fbio.2015.09.001. [DOI] [Google Scholar]
- 19.Wang H., Ng T. Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom Ganoderma lucidum. Peptides. 2006;27:27–30. doi: 10.1016/j.peptides.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Chu K., Xia L., Ng T. Pleurostrin, an antifungal peptide from the oyster mushroom. Peptides. 2005;26:2098–2103. doi: 10.1016/j.peptides.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Sajeena A., Marimuthu T. Efficacy, stability and persistence of Ganosol, a Ganoderma based fungicide against plant pathogens. J. Plant Prot. Sci. 2013;5:17–25. [Google Scholar]
- 22.Lam S., Ng T. First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeji) together with evidence for synergism of their antifungal effects. Arch. Biochem. Biophys. 2001;393:271–280. doi: 10.1006/abbi.2001.2506. [DOI] [PubMed] [Google Scholar]
- 23.Wang H., Ng T. Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii. Peptides. 2004;25:1–5. doi: 10.1016/j.peptides.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Wasser S.P., Weis A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999;19:65–96. [PubMed] [Google Scholar]
- 25.Faccin L.C., Benati F., Rincão V.P., Mantovani M.S., Soares S.A., Gonzaga M.L., Nozawa C., Carvalho L.R. Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Lett. Appl. Microbiol. 2007;45:24–28. doi: 10.1111/j.1472-765X.2007.02153.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun H., Zhao C.G., Tong X., Qi Y.P. A lectin with mycelia differentiation and antiphytovirus activities from the edible mushroom Agrocybe aegerita. J. Biochem. Mol. Biol. 2003;36:214–222. doi: 10.5483/BMBRep.2003.36.2.214. [DOI] [PubMed] [Google Scholar]
- 27.Wang M., Triguéros V., Paquereau L., Chavant L., Fournier D. Proteins as active compounds involved in insecticidal activity of mushroom fruitbodies. J. Econ. Entomol. 2002;95:603–607. doi: 10.1603/0022-0493-95.3.603. [DOI] [PubMed] [Google Scholar]
- 28.Jo W.S., Hossain M.A., Park S.C. Toxicological profiles of poisonous, edible, and medicinal mushrooms. Mycobiology. 2014;42:215–220. doi: 10.5941/MYCO.2014.42.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasser S.P. A Book Review: The Fungal Pharmacy: Medicinal Mushrooms of Western Canada (Robert Rogers, 2006, Prairie Deva Press, Edmonton Alberta, 234 pp., $39.95 CDN) Int. J. Med. Mushrooms. 2008;10:97–100. doi: 10.1615/IntJMedMushr.v10.i1.130. [DOI] [Google Scholar]
- 30.Valverde M.E., Hernández-Pérez T., Paredes-López O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015;2015 doi: 10.1155/2015/376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasser S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Antimicrob. Agents Chemother. 2011;89:1323–1332. doi: 10.1007/s00253-010-3067-4. [DOI] [PubMed] [Google Scholar]
- 32.Richard N.S. Introduction to Plant Pathology. John Wiley & Sons; New York, NY, USA: 2006. pp. 1–50. [Google Scholar]
- 33.Knogge W. Fungal infection of plants. Plant Cell. 1996;8:1711–1722. doi: 10.1105/tpc.8.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronson C.R. The genetics of phytotoxin production by plant pathogenic fungi. Experientia. 1991;47:771–776. doi: 10.1007/BF01922456. [DOI] [Google Scholar]
- 35.Than P.P., Prihastuti H., Phoulivong S., Taylor P.W., Hyde K.D. Chilli anthracnose disease caused by Colletotrichum species. J. Zhejiang Univ. Sci. B. 2008;9:764–778. doi: 10.1631/jzus.B0860007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayden H.P.K., Aitken E., Irwin J. Genetic-relationships as assessed by molecular markers and cross-infection among strains of Colletotrichum gloeosporioides. Aust. J. Bot. 1994;42:9–18. doi: 10.1071/BT9940009. [DOI] [Google Scholar]
- 37.Braithwaite K.S., Irwin J.A., Manners J.M. Restriction fragment length polymorphisms in Colletotrichum gloeosporioides infecting Stylosanthes spp. in Australia. Mycol. Res. 1990;94:1129–1137. doi: 10.1016/S0953-7562(09)81345-2. [DOI] [Google Scholar]
- 38.Talbot N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003;57:177–202. doi: 10.1146/annurev.micro.57.030502.090957. [DOI] [PubMed] [Google Scholar]
- 39.Hafner S. Trends in maize, rice, and wheat yields for 188 nations over the past 40 years: A prevalence of linear growth. Agric. Ecosyst. Environ. 2003;97:275–283. doi: 10.1016/S0167-8809(03)00019-7. [DOI] [Google Scholar]
- 40.Thinlay X., Finckh M.R., Bordeos A.C., Zeigler R.S. Effects and possible causes of an unprecedented rice blast epidemic on the traditional farming system of Bhutan. Agric. Ecosyst. Environ. 2000;78:237–248. doi: 10.1016/S0167-8809(99)00129-2. [DOI] [Google Scholar]
- 41.Prabhu A.S., Filippi M.C., Castro N. Pathogenic variation among isolates of Pyricularia oryzae affecting rice, wheat, and grasses in Brazil. Int. J. Pest Manag. 1992;38:367–371. [Google Scholar]
- 42.Hubert J., Mabagala R.B., Mamiro D.P. Efficacy of selected plant extracts against Pyricularia grisea, causal agent of rice blast disease. Am. J. Plant Sci. 2015;6:602–611. doi: 10.4236/ajps.2015.65065. [DOI] [Google Scholar]
- 43.Gelderblom W.C., Jaskiewicz K., Marasas W.F., Thiel P.G., Horak R.M., Vleggaar R., Kriek N.P. Fumonisins—Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988;54:1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madden L., Nutter J.F. Modeling crop losses at the field scale. Can. J. Plant Pathol. 1995;17:124–137. doi: 10.1080/07060669509500703. [DOI] [Google Scholar]
- 45.Desjardins A.E., Plattner R.D., Nelsen T.C., Leslie J.F. Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microbiol. 1995;61:79–86. doi: 10.1128/aem.61.1.79-86.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo D.Q., Shao H.J., Zhu H.J., Liu J.K. Activity in vitro and in vivo against plant pathogenic fungi of grifolin isolated from the basidiomycete Albatrellus dispansus. Z. Naturforsch. C J. Biosci. 2005;60:50–56. doi: 10.1515/znc-2005-1-210. [DOI] [PubMed] [Google Scholar]
- 47.Lam S., Ng T. Hypsin, a novel thermostable ribosome-inactivating protein with antifungal and antiproliferative activities from fruiting bodies of the edible mushroom Hypsizigus marmoreus. Biochem. Biophys. Res. Commun. 2001;285:1071–1075. doi: 10.1006/bbrc.2001.5279. [DOI] [PubMed] [Google Scholar]
- 48.Luo D.Q., Wang F., Bian X.Y., Liu J.K. Rufuslactone, a new antifungal sesquiterpene from the fruiting bodies of the basidiomycete Lactarius rufus. J. Antibiot. 2005;58:456–459. doi: 10.1038/ja.2005.60. [DOI] [PubMed] [Google Scholar]
- 49.Wong J.H., Ng T.B., Wang H., Sze S.C., Zhang K.Y., Li Q., Lu X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine. 2011;18:387–392. doi: 10.1016/j.phymed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Heleno S.A., Ferreira I.C., Esteves A.P., Ćirić A., Glamočlija J., Martins A., Soković M., Queiroz M.J. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013;58:95–100. doi: 10.1016/j.fct.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Ngai P.H., Zhao Z., Ng T. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea. Peptides. 2005;26:191–196. doi: 10.1016/j.peptides.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Ngai P.H., Ng T. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003;73:3363–3374. doi: 10.1016/j.lfs.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Lambert M., Kremer S., Anke H. Antimicrobial, phytotoxic, nematicidal, cytotoxic, and mutagenic activities of 1-hydroxypyrene, the initial metabolite in pyrene metabolism by the basidiomycete Crinipellis stipitaria. Bull. Environ. Contam. Toxicol. 1995;55:251–257. doi: 10.1007/BF00203017. [DOI] [PubMed] [Google Scholar]
- 54.Hwang E.I., Yun B.S., Kim Y.K., Kwon B.M., Kim H.G., Lee H.B., Jeong W.J., Kim S.U. Phellinsin A, a novel chitin synthases inhibitor produced by Phellinus sp. PL3. J. Antibiot. 2000;53:903–911. doi: 10.7164/antibiotics.53.903. [DOI] [PubMed] [Google Scholar]
- 55.Robles-Hernández L., Gonzalez-Franco A.C., Soto-Parra J.M., Montes-Domínguez F. Review of agricultural and medicinal applications of basidiomycete mushrooms. Tecnociencia Chihuah. 2008;2:95–107. [Google Scholar]
- 56.Anke T., Oberwinkler F., Steglich W., Schramm G. The strobilurins-new antifungal antibiotics from the basidiomycete Strobilurus tenacellus. J. Antibiot. 1977;30:806–810. doi: 10.7164/antibiotics.30.806. [DOI] [PubMed] [Google Scholar]
- 57.Weber W., Anke T., Steffan B., Steglich W. Antibiotics from basidiomycetes. XXXII. Strobilurin E: A new cytostatic and antifungal (E)-BETA-methoxyacrylate antibiotic from Crepidotus fulvotomentosus Peck. J. Antibiot. 1990;43:207–212. doi: 10.7164/antibiotics.43.207. [DOI] [PubMed] [Google Scholar]
- 58.Fredenhagen A., Kuhn A., Peter H.H., Cuomo V., Giuliano U. Strobilurins F, G and H, three new antifungal metabolites from Bolinea lutea. I. Fermentation, isolation and biological activity. J. Antibiot. 1990;43:655–660. doi: 10.7164/antibiotics.43.655. [DOI] [PubMed] [Google Scholar]
- 59.Gullino M.L., Leroux P., Smith C.M. Uses and challenges of novel compounds for plant disease control. Crop Prot. 2000;19:1–11. doi: 10.1016/S0261-2194(99)00095-2. [DOI] [Google Scholar]
- 60.Anke T., Besl H., Mocek U., Steglich W. Antibiotics from basidiomycetes. XVIII. Strobilurin C and Oudemansin B, two new antifungal metabolites from Xerula species (Agaricales) J. Antibiot. 1983;36:661–666. doi: 10.7164/antibiotics.36.661. [DOI] [PubMed] [Google Scholar]
- 61.Imtiaj A., Jayasinghe C., Lee G.W., Lee T.S. Antibacterial and antifungal activities of Stereum ostrea, an inedible wild mushroom. Mycobiology. 2007;35:210–214. doi: 10.4489/MYCO.2007.35.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Bao L., Yang X., Li L., Li S., Gao H., Yao X.S., Wen H., Liu H.W. Bioactive sesquiterpenoids from the solid culture of the edible mushroom Flammulina velutipes growing on cooked rice. Food Chem. 2012;132:1346–1353. doi: 10.1016/j.foodchem.2011.11.117. [DOI] [PubMed] [Google Scholar]
- 63.Fu L.Q., Guo X.S., Liu X., He H.L., Wang Y.L., Yang Y.S. Synthesis and antibacterial activity of C-2 (S)-substituted pleuromutilin derivatives. Chin. Chem. Lett. 2010;21:507–510. doi: 10.1016/j.cclet.2010.01.001. [DOI] [Google Scholar]
- 64.Saddiqe Z., Naeem I., Maimoona A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010;131:511–521. doi: 10.1016/j.jep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 65.Barros L., Baptista P., Estevinho L.M., Ferreira I.C. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food. Chem. 2007;55:8766–8771. doi: 10.1021/jf071435+. [DOI] [PubMed] [Google Scholar]
- 66.Alves M.J., Ferreira I.C., Dias J., Teixeira V., Martins A., Pintado M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012;78:1707–1718. doi: 10.1055/s-0032-1315370. [DOI] [PubMed] [Google Scholar]
- 67.Erjavec J., Ravnikar M., Brzin J., Grebenc T., Blejec A., Gosak M.Ž., Sabotič J., Kos J., Dreo T. Antibacterial activity of wild mushroom extracts on bacterial wilt pathogen Ralstonia solanacearum. Plant Dis. 2016;100:453–464. doi: 10.1094/PDIS-08-14-0812-RE. [DOI] [PubMed] [Google Scholar]
- 68.Dreo T., Zelko M., Skubic J., Brzin J., Ravnikar M. Antibacterial activity of proteinaceous extracts of higher basidiomycetes mushrooms against plant pathogenic bacteria. Int. J. Med. Mushrooms. 2007;9:226–237. [Google Scholar]
- 69.Herms S., Seehaus K., Koehle H., Conrath U. A strobilurin fungicide enhances the resistance of tobacco against tobacco mosaic virus and Pseudomonas syringae pvtabaci. Plant Physiol. 2002;130:120–127. doi: 10.1104/pp.004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johansson M., Sterner O., Labischinski H., Anke T. Coprinol, a new antibiotic cuparane from a Coprinus species. Z. Naturforsch. C J. Biosci. 2001;56:31–34. doi: 10.1515/znc-2001-1-205. [DOI] [PubMed] [Google Scholar]
- 71.Brandt C.R., Piraino F. Mushroom antivirals. Recent Res. Dev. Antimicrob. Agents Chemother. 2000;4:11–26. [Google Scholar]
- 72.Gao Y., Zhou S., Huang M., Xu A. Antibacterial and antiviral value of the genus Ganoderma P. Karst. species (Aphyllophoromycetideae): A review. Int. J. Med. Mushrooms. 2003;5 doi: 10.1615/InterJMedicMush.v5.i3.20. [DOI] [Google Scholar]
- 73.Kovalenko O., Polishchuk O., Krupodorova T. Screening of metabolites produced by strains of Ganoderma lucidum [Curt.: Fr] P. Karst and Ganoderma applanatum [Pirs.: Waller] Pat. for their activity against tobacco mosaic virus. Bull. Tara Shevchenko Nat. Univ. Kyiv. Ser. Biol. 2008;51:32–34. [Google Scholar]
- 74.Zjawiony J.K. Biologically Active Compounds from Aphyllophorales (Polypore) Fungi. J. Nat. Prod. 2004;67:300–310. doi: 10.1021/np030372w. [DOI] [PubMed] [Google Scholar]
- 75.Di Piero R.M., Novaes Q.S., Pascholati S.F. Effect of Agaricus brasiliensis and Lentinula edodes mushrooms on the infection of passionflower with Cowpea aphid-borne mosaic virus. Braz. Arch. Biol. Technol. 2010;53:269–278. doi: 10.1590/S1516-89132010000200004. [DOI] [Google Scholar]
- 76.Kovalenko O.G., Polishchuk O.N., Wasser S.P. Virus Resistance induced by glucuronoxylomannan isolated from submerged cultivated yeast-like cell biomass of medicinal yellow brain mushroom Tremella mesenterica Ritz.: Fr.(Heterobasidiomycetes) in hypersensitive host plants. Int. J. Med. Mushrooms. 2009;11:199–205. doi: 10.1615/IntJMedMushr.v11.i2.90. [DOI] [Google Scholar]
- 77.Tavantzis S., Smith S. Isolation and evaluation of a plant-virus-inhibiting quinone from sporophores of Agaricus bisporus. Phytopathology. 1982;72:619–621. doi: 10.1094/Phyto-72-619. [DOI] [Google Scholar]
- 78.Kakuta M., Sone Y., Umeda T., Misaki A. Comparative structural studies on acidic heteropolysaccharides isolated from “Shirokikurage” fruit body of Tremella fuciformis Berk, and the growing culture of its yeast-like cells. Agric. Biol. Chem. 1979;43:1659–1668. doi: 10.1271/bbb1961.43.1659. [DOI] [Google Scholar]
- 79.Kovalenko A. Protein-carbohydrate interactions in the realization of plant resistance to viruses. Mikrobiol. Zhurnal. 1993;55:74–91. [Google Scholar]
- 80.Vinogradov E., Petersen B.O., Duus J.Ø., Wasser S. The structure of the glucuronoxylomannan produced by culinary-medicinal yellow brain mushroom (Tremella mesenterica Ritz.: Fr., Heterobasidiomycetes) grown as one cell biomass in submerged culture. Carbohydr. Res. 2004;339:1483–1489. doi: 10.1016/j.carres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Haydock P.P., Woods S.R., Grove I.G., Hare M.C., Perry R.N., Moens M. Plant Nematology. CABI Publishing; Oxfordshire, UK: 2006. Chemical control of nematodes; pp. 392–410. [Google Scholar]
- 82.Haydock P.P., Woods S.R., Grove I.G., Hare M.C., Perry R.N., Moens M. Plant Nematology. 2nd ed. CABI Publishing; Oxfordshire, UK: 2013. Chemical control of nematodes; pp. 459–479. [Google Scholar]
- 83.Soltani T., Nejad R.F., Ahmadi A.R., Fayazi F. Chemical control of root-knot nematode (Meloidogyne javanica) on olive in the greenhouse conditions. J. Plant Pathol. Microbiol. 2013;2013 doi: 10.4172/2157-7471.1000183. [DOI] [Google Scholar]
- 84.Comans-Pérez R., Aguilar-Marcelino L., Mendoza de Gives P., Sánchez J., López-Arellano M., Singh M. In vitro lethal capability of ten strains of edible mushrooms against Haemonchus contortus (Nematoda) infective larvae [conference poster]; Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8); New Delhi, India. 19–22 November 2014; pp. 557–562. [Google Scholar]
- 85.Luo H., Li X., Li G., Pan Y., Zhang K. Acanthocytes of Stropharia rugosoannulata function as a nematode-attacking device. Appl. Environ. Microbiol. 2006;72:2982–2987. doi: 10.1128/AEM.72.4.2982-2987.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwok O., Plattner R., Weisleder D., Wicklow D. A nematicidal toxin from Pleurotus ostreatus NRRL 3526. J. Chem. Ecol. 1992;18:127–136. doi: 10.1007/BF00993748. [DOI] [PubMed] [Google Scholar]
- 87.Palizi P., Goltapeh E., Pourjam E., Safaie N. Potential of oyster mushrooms for the biocontrol of sugar beet nematode (Heterodera schachtii) J. Plant Prot. Res. 2009;49:27–34. doi: 10.2478/v10045-009-0004-6. [DOI] [Google Scholar]
- 88.Mamiy Y. Attraction of the pinewood nematode to mycelium of some wood-decay fungi. Jpn. J. Nematol. 2006;36:1–9. doi: 10.3725/jjn.36.1. [DOI] [Google Scholar]
- 89.Bua-art S., Saksirirat W., Hiransalee A., Kanokmedhaku S., Lekphrom R. Effect of bioactive compound from luminescent mushroom (Neonothopanus nambi Speg.) on root-knot nematode (Meloidogyne incognita Chitwood) and non-target organisms. KKU Res. J. 2011;16:331–341. [Google Scholar]
- 90.De Jong E., Cazemier A.E., Field J.A., de Bont J.A. Physiological role of chlorinated aryl alcohols biosynthesized de novo by the white rot fungus Bjerkandera sp. strain BOS55. Appl. Environ. Microbiol. 1994;60:271–277. doi: 10.1128/aem.60.1.271-277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stadler M., Mayer A., Anke H., Sterner O. Fatty acids and other compounds with nematicidal activity from cultures of Basidiomycetes. Planta Med. 1994;60:128–132. doi: 10.1055/s-2006-959433. [DOI] [PubMed] [Google Scholar]
- 92.Lorenzen K., Anke T. Basidiomycetes as source for new bioactive natural products. Curr. Org. Chem. 1998;2:329–364. [Google Scholar]
- 93.Hayashi M., Wada K., Munakata K. New nematicidal metabolites from a fungus, Irpex lacteus. Agric. Biol. Chem. 1981;45:1527–1529. [Google Scholar]
- 94.Mayer A., Anke H., Sterner O. Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius I. Fermentation and biological activity. Nat. Prod. Lett. 1997;10:25–32. doi: 10.1080/10575639708043691. [DOI] [Google Scholar]
- 95.Dong J.Y., Li X.P., Li L., Li G.H., Liu Y.J., Zhang K.Q. Preliminary results on nematicidal activity from culture filtrates of Basidiomycetes against the pine wood nematode, Bursaphelenchus xylophilus (Aphelenchoididae) Ann. Microbiol. 2006;56:163–166. doi: 10.1007/BF03174999. [DOI] [Google Scholar]
- 96.Njogu E.M., Njue A., Omolo J.O., Cheplogoi P.K. Larvicidal activity of (oxiran-2-yl) methylpentanoate extracted from mushroom Cyptotrama asprata against mosquito Aedes aegypti. Int. J. Biol. Chem. Sci. 2009;3 doi: 10.4314/ijbcs.v3i6.53134. [DOI] [Google Scholar]
- 97.Bucker A., Bucker N.C., Souza A.Q., Gama A.M., Rodrigues-Filho E., Costa F.M., Tadei W.P. Larvicidal effects of endophytic and basidiomycete fungus extracts on Aedes and Anopheles larvae (Diptera, Culicidae) Rev. Soc. Bras. Med. Trop. 2013;46:411–419. doi: 10.1590/0037-8682-0063-2013. [DOI] [PubMed] [Google Scholar]
- 98.Chelela B.L., Chacha M., Matemu A. Larvicidal potential of wild mushroom extracts against Culex quinquefasciatus. Am. J. Res. Commun. 2014;2:105–114. [Google Scholar]
- 99.Baraza L., Joseph C., Moshi M., Nkunya M. Chemical constituents and biological activity of three Tanzanian wild mushroom species. Tanzan. J. Sci. 2007;33 doi: 10.4314/tjs.v33i1.44280. [DOI] [Google Scholar]
- 100.Olayemi I.K. Evaluation of Larvicidal efficacy of extract of the fungus Ganoderma lucidum, for the control of the filarial vector mosquito, Culex pipiens pipiens (Diptera: Culicidae) Am. J. Drug Discov. Dev. 2013;3:130–139. doi: 10.3923/ajdd.2013.130.139. [DOI] [Google Scholar]
- 101.Chirchir D.K. Master’s Thesis. Egerton University; Njoro, Kenya: Feb, 2010. Isolation and Purification of Mosquito Larvicidal Compounds from Extracts of a Basidiomycete jo5289. [Google Scholar]
- 102.Thongwat D., Pimolsri U., Somboon P. Screening for mosquito larvicidal activity of thai mushroom extracts with special reference to Steccherinum sp against Aedes aegypti (L.)(Diptera: Culicidae) Southeast Asian J. Trop. Med. Public Health. 2015;46:586–595. [PubMed] [Google Scholar]