Abstract
Background
Following promising results to increase survival and reduce treatment burden in intracranial non-germinomatous germ cell tumors (NGGCTs), we conducted a European study using dose-intense chemotherapy followed by risk-adapted radiotherapy.
Methods
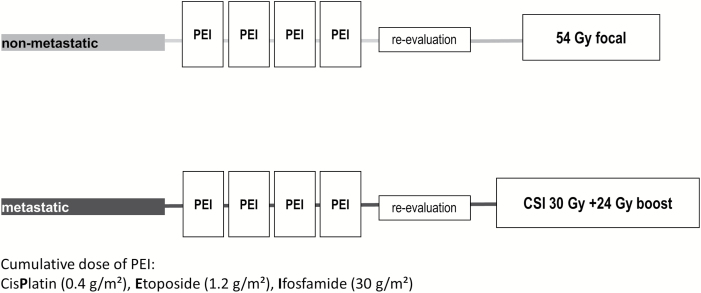
All patients received 4 courses of cisplatin/etoposide/ifosfamide. Non-metastatic patients then received focal radiotherapy only (54 Gy); metastatic patients received 30 Gy craniospinal radiotherapy with 24 Gy boost to primary tumor and macroscopic metastatic sites.
Results
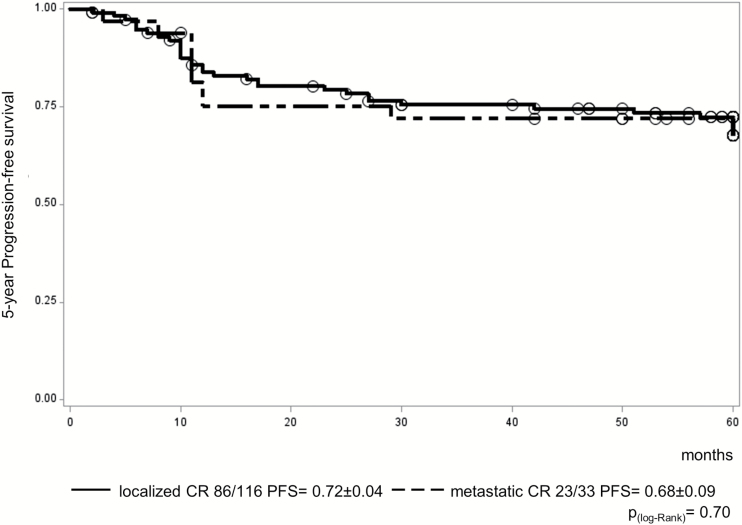
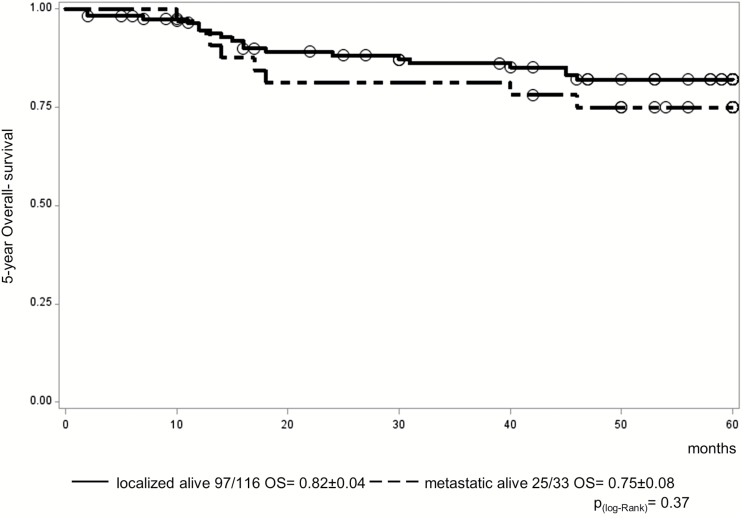
Patients with localized malignant NGGCT (n = 116) demonstrated 5-year progression-free survival (PFS) and overall survival (OS) of 0.72 ± 0.04 and 0.82 ± 0.04, respectively. Primary tumor sites were: 67 pineal, 35 suprasellar, 5 bifocal, 9 others. One patient died postsurgery in clinical remission; 3 patients progressed during treatment and 27 (23%) relapsed afterward. Fourteen were local, 6 combined, and 7 distant relapses (outside radiation field). Seventeen of the 27 relapsed patients died of disease. Patients with metastatic disease (n = 33) demonstrated 5-year PFS and OS of 0.68 ± 0.09 and 0.75 ± 0.08, respectively; 1 patient died following progression on treatment and 9 (27%) relapsed afterward (5 local, 1 combined, 3 distant). Only one metastatic patient with recurrence was salvaged. Multivariate analysis identified diagnostic alpha-fetoprotein level (serum and/or cerebrospinal fluid level >1000 ng/mL, 19/149 patients, of whom 11 relapsed; P < 0.0003) and residual disease following treatment, including after second-look surgery (n = 52/145 evaluable patients, 26 relapsed; P = 0.0002) as significant prognostic indicators in this cohort.
Conclusion
In localized malignant NGGCT, craniospinal radiotherapy could be avoided without increased relapses outside the radiotherapy field. Chemotherapy and craniospinal radiotherapy remain the gold standard for metastatic disease.
Keywords: chemotherapy, intracranial non-germinoma, radiotherapy, relapse, toxicity
Importance of the study
We report the results of the European SIOP-CNS-GCT-96 trial for patients with intracranial NGGCT, using cisplatin-based chemotherapy followed by risk-adapted radiotherapy (54 Gy focal for localized disease; 30 Gy craniospinal plus 24 Gy tumor boost to primary and metastatic sites).
The study confirms that focal radiotherapy fields are sufficient for disease control in localized intracranial NGGCT patients (5-year PFS, 72%) in the context of dose-intense chemotherapy; for metastatic cases, use of craniospinal radiotherapy results in similar outcomes (5-year PFS, 68%).
Diagnostic serum or cerebrospinal fluid alpha-fetoprotein >1000 ng/mL is identified as a risk factor for subsequent relapse and allows upfront identification of patients who should receive treatment intensification.
Residual disease not resected at the end of treatment is associated with an increased relapse risk; surgical resection of residuals is therefore advocated.
Intracranial malignant non-germinomatous germ cell tumors (NGGCTs) are a heterogeneous group of neoplasms mostly located in pineal and/or suprasellar regions. They include yolk sac tumor (YST), embryonal carcinoma (EC), choriocarcinoma (CHC), and mixed malignant tumors in various combinations, often together with teratoma or germinoma components. YST and CHC can be diagnosed based on serum and/or cerebrospinal fluid (CSF) elevation of tumor markers, namely alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG), respectively, with/without histological confirmation.
Compared with pure germinoma, malignant NGGCTs have poor prognoses. Long-term disease-free survival of <10% was reported in the 1970s, mostly based on treatment with radiotherapy following biopsy or small resection.1 Since then, platinum-based chemotherapy has proven highly effective in disseminated extracranial malignant germ cell tumors (GCTs)2–4 and has been administered for intracranial GCTs.5–9 Different approaches, such as neoadjuvant or preoperative chemotherapy, were subsequently added to conventional surgery and radiotherapy to increase remission rates. Published results for intracranial malignant NGGCT are mostly based on single institution studies with small patient numbers with limited long-term follow-up data.
In Germany, patients with malignant NGGCT were treated with a combined strategy from 1986 onward, employing platinum-based chemotherapy (bleomycin/etoposide/cisplatin [BEP] or vinblastine/ifosfamide/cisplatin [VIP]) followed by 30 Gy craniospinal radiotherapy and a 24 Gy boost2,3; 5-year relapse-free survival was 67% with 110 months median follow-up.10 In 1993, another European pilot study tested a 4-course cisplatin-containing regimen (cisplatin/etoposide/ifosfamide [PEI]) followed by 30 Gy craniospinal irradiation and 24 Gy boost, leading to an event-free survival (EFS) of 81% (median follow-up 11 mo).8
The Japanese group published data on patients treated in 1983–1995 according to risk groups.11 In the intermediate-risk group, a total tumor-free rate of 55.6% was achieved with carboplatin/etoposide or cisplatin/etoposide followed by local radiotherapy of 30 Gy and additional maintenance chemotherapy. For poor-risk patients, treatment with ifosfamide/cisplatin/etoposide chemotherapy followed by 30 Gy craniospinal radiotherapy and 30 Gy tumor boost followed by further adjuvant chemotherapy failed to control disease. Chemotherapy-only approaches were considered inadequate for disease control, as half of patients relapsed.6,12 The recent North American trial ACNS0122 reported 102 NGGCT patients diagnosed by tumor markers, histology, or both.13 Induction chemotherapy comprised 6 cycles of carboplatin/etoposide alternating with ifosfamide/etoposide, and second-look surgery for incomplete response. Patients who did not achieve complete remission (CR) or partial remission after chemotherapy (with/without second-look surgery) received high-dose chemotherapy and stem cell rescue. All patients received craniospinal irradiation (36 Gy spinal with 18 Gy tumor boost, 45 Gy to metastases) after chemotherapy. Five-year EFS was 0.84 ± 0.04 and overall survival (OS) 0.93 ± 0.03. Relapse occurred mainly at the primary site.13
The European SIOP-CNS-GCT-96 trial opened in 1996, evaluating treatment in a large cohort, defining standardized approaches for diagnosis/staging. Patients with intracranial malignant NGGCTs received a combination of 4 courses of dose-intense cisplatin-based chemotherapy, followed by focal radiotherapy in non-metastatic cases or craniospinal if metastatic.
Methods
Patients
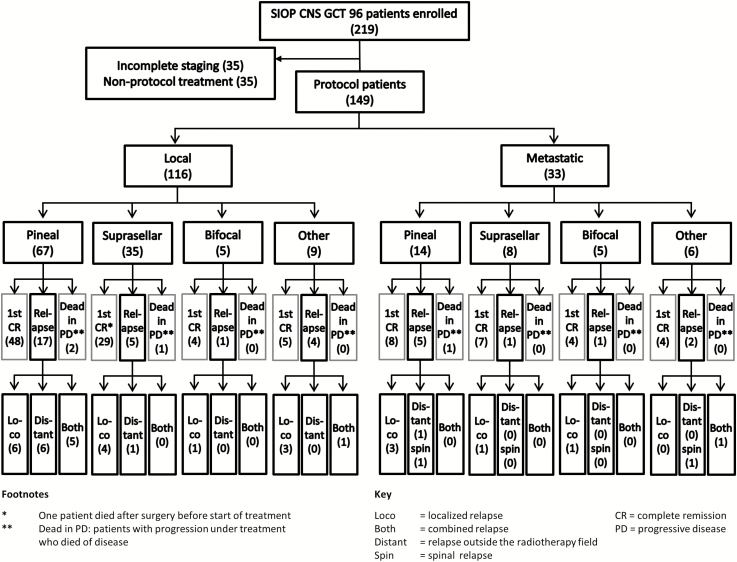
A total of 219 patients were enrolled into the SIOP-CNS-GCT-96 trial, of whom 35 were withdrawn because of incomplete staging and 35 because they did not receive treatment according to protocol (Fig. 1). A total of 149 patients (116 males, 33 females) with malignant NGGCT, confirmed by histology and/or tumor markers and complete workup, were enrolled by December 31, 2005 and followed to December 1, 2014. The median age at diagnosis was 12 years (range 4–30 y). Thirty-eight (26%) were younger than 10 years and 27 (18%) older than 16 years at diagnosis; most of these were 16–23 years; one was 30 years. Patients were registered from Austria, Belgium, France, Germany, Italy, the Netherlands, Poland, Sweden, Switzerland, Spain, and the UK. Approval was gained from national ethics committees and all patients/parents gave written consent.
Fig. 1.
CONSORT diagram of the 219 patients enrolled on the SIOP-CNS-GCT-96 trial; 149 patients were eligible.
Diagnostic and Staging Procedures
Cranial and spinal MRI was mandatory for diagnosis and staging. For cytological detection of microdissemination, CSF sampling was required, obtained by lumbar puncture or, in cases of raised intracranial pressure, from the ventricles. Localized disease was defined as NGGCT without evidence of dissemination (negative CSF cytology and no metastatic disease on cranial/spinal imaging). Definition of bifocal disease required radiological detection of tumor in both pineal and suprasellar regions and was treated as localized disease. Metastatic NGGCT was defined as the presence of more than one intracranial focus (except bifocal disease), spinal metastases, metastases outside the CNS, or positive CSF cytology. Out of 149 patients, 116 (78%) had localized tumors. The most frequent primary tumor site was pineal (n = 67), then suprasellar (n = 35). Five patients had bifocal disease. Other intracranial primary sites (n = 9) included the ventricular system (n = 3), diencephalon (n = 3), frontal/parietal lobes (n = 2), and internal capsule (n = 1). Thirty-three patients (22% of the total) with metastatic disease presented with primary tumors located in the pineal (n = 14) and suprasellar regions (n = 8) and as bifocal (n = 5). Other intracranial sites (n = 6) included the ventricular system (n = 3), diencephalon (n = 1), and frontal/parietal lobes (n = 2). Of the 33 patients with metastatic disease, 17 (52%) showed macroscopic metastases on cranial and/or spinal MRI with negative cytology, 3 (9%) showed macroscopic metastases and positive CSF cytology, and 13 (39%) had positive CSF cytology alone.
Tumor symptoms, neurological status, endocrine function, and hearing and visual status of patients were recorded at diagnosis. Fifty-nine patients (40% of total cohort) presented with diabetes insipidus at diagnosis (36 suprasellar site, 10 bifocal, 7 pineal, 6 other site). Isolated pineal tumors with diabetes insipidus but no radiological evidence of neurohypophyseal/suprasellar involvement were not considered bifocal in this study. Twenty-nine patients (19%) presented with panhypopituitarism (23 suprasellar, 2 bifocal, 1 pineal, 3 other site). Forty-five (30%) presented with visual disturbances, including oculomotor cranial nerve palsies (40 pineal, 2 suprasellar, 3 other site). In total, 89 patients (60%) presented with abnormal ophthalmological examination (52 pineal, 23 suprasellar, 5 bifocal, 9 other). Thirteen had hearing abnormalities at diagnosis (10 pineal).
Assessment of markers (AFP and HCG) in both serum and CSF was mandatory, but the site of CSF sampling (ventricular/lumbar) was not routinely recorded. A malignant intracranial NGGCT was diagnosed without need for biopsy confirmation if AFP was >25 ng/mL and/or HCG >50 IU/L in at least one serum/CSF compartment. The AFP and HCG cutoffs were pragmatic thresholds designed to avoid morbidity/mortality associated with upfront neurosurgical intervention. It is recognized that some HCG-secreting germinomas and AFP-secreting teratomas might consequently have been included. Histological verification was only required for markers below these thresholds. In cases with elevated tumor markers and biopsy showing pure germinoma or teratoma, mixed-malignant GCT was diagnosed and treated as malignant NGGCT. Tumor markers were measured in all 149 patients at diagnosis. In 146, results of both markers in both compartments were available. In 3 patients, either AFP or HCG was measured and was elevated. AFP values were available in 148 patients (maximum 27100 ng/mL) and HCG in 147 (maximum 60600 IU/L). Patients with isolated elevated markers above threshold numbered 54 (36%) with AFP and 53 (36%) with HCG. Elevation of both markers was recorded in 32 patients (21%). Ten patients (7%) showed no marker elevation.
Correlation of Pathological Analysis with Tumor Markers
Pretreatment surgical diagnosis was undertaken in 76 of 149 patients (51%). Fifty-five had a tumor resection and 21 underwent biopsy. In 6 of these 76, histology was either unavailable (n = 3) or inconclusive (n = 3) but all 6 had elevated tumor markers and were therefore diagnosed as malignant NGGCTs. Histology reports (local and/or reference pathology) with detection of GCTs were available for 70 patients; in 2 of these, the diagnosis was “malignant GCT”/“teratoma with malignant transformation” without a more precise histological classification. Among the remaining 68 patients with full histological classification, 28 showed mixed malignant GCTs (including more than one malignant component of YST, CHC, EC, and germinoma). Twenty-five patients presented with germinoma, either pure or mixed with YST (n = 6), EC (n = 3), or CHC (n = 1). Five patients showed pure teratoma at biopsy; AFP levels in all of these 5 patients were, however, elevated (range 115–430 ng/mL), suggestive of a malignant NGGCT. Four of these 5 remained in CR, and 1 relapsed (initial suprasellar tumor with AFP of 430 ng/mL).
Ten of 68 patients, with full pathology results of pretreatment surgery available, showed no marker elevation at diagnosis. Centrally reviewed histology showed a YST component in 5 (all remained in CR), YST and EC components in 1 (subsequent spinal relapse), and pure EC in 4 (three CR, one combined ventricular/spinal relapse).
AFP elevation only, or together with HCG elevation, was seen in 37 of 68 patients with upfront surgical intervention and full histology; in 19 of these, YST was also detected histologically; in the 12 patients with combined AFP/HCG elevation, only 2 showed CHC histologically. Of the 21 with only HCG elevation, YST was diagnosed histologically in 1 and CHC components were present in 4.
Shunt Procedures
For patients who had symptomatic hydrocephalus (81 of 149; 54%), CSF diversion was performed before chemotherapy; 25 had third ventriculostomies, 15 short-term external ventricular drains, and 41 ventriculo-peritoneal shunts.
Treatment
After diagnosis, patients received 4 cycles of dose-intense chemotherapy, comprising cisplatin/etoposide/ifosfamide (Fig. 2). Body surface area cutoff was 2 m2. Response was evaluated with MRI head/spine and serum markers after 2/4 courses of chemotherapy and again at end of treatment. Patients in whom tumor markers had not returned to normal by the end of chemotherapy (n = 4) were regarded as having an event, and treated off-protocol; all died of progressive disease. Patients were considered for surgery, and resection attempted, if there was evidence of growing tumor with normalized markers after the second chemotherapy cycle (usually growing-teratoma syndrome; not classed as an event) or residual disease after chemotherapy.
Fig. 2.
Overview of the treatment regimen for the treatment of patients with intracranial malignant NGGCTs on the SIOP-CNS-GCT-96 trial protocol.
Radiotherapy was delivered according to initial dissemination and commenced after recovery from the fourth chemotherapy course and after second-look surgery, if undertaken. Patients without dissemination received 54 Gy focal radiotherapy (30 fractions; 6 wk). Bifocal disease was treated with focal radiotherapy, including both primary sites. In patients with metastases, craniospinal radiotherapy was delivered to a total dose of 30 Gy (19 fractions; 7 wk) followed by a 24 Gy boost to the primary tumor and macroscopic metastatic sites (Fig. 2). Central review of radiotherapy plans was not part of this trial.
End-of-Treatment Evaluation
Follow-up tumor markers were obtained in serum only, unless clinical concern. End-of-treatment MRI was classified as radiological CR or residual disease (defined as any persistent enhancing visible lesions at the tumor site, regardless of treatment modality). Patients with residuals who underwent further surgery and in whom postoperative imaging confirmed disease clearance were also classified as CR.
Follow-up
Evaluation was performed regularly, combined with full clinical examination (including neurological status and ophthalmological, audiological, and endocrine assessments), laboratory studies, measurements of serum tumor markers, and imaging. MRI with contrast of brain (and spine if metastatic at diagnosis) was performed at least every 6 months for 2 years and annually thereafter. Median follow-up was 65 months (range 1 mo–16 y) for all patients (mean 63 mo). Long-term assessment via follow-up questionnaires included the patient’s general condition, any relapse, second malignancy, and persistent side effects of therapy. Patients were followed up until December 1, 2014.
Statistical Analysis
The survival probability was estimated according to the Kaplan–Meier method. Five-year OS was calculated from date of diagnosis to date of last follow-up or death. Progression-free survival (PFS) measured the proportion of patients among those treated for intracranial malignant NGGCT whose disease remained stable (without progression) for ≥5 years after treatment. EFS was calculated to measure the proportion who remain free of an event (relapse or death from any cause). The log-rank test was used to compare survival distributions (P < 0.05 significant). For multivariate analysis, the variables age (<10 y, ≥10 y but ≤16 y, and >16 y), sex, primary tumor site (pineal/suprasellar/bifocal/other), metastatic status, AFP levels (≤1000 ng/mL, >1000 ng/mL), and end-of-treatment residual disease (including after any additional surgery) were examined (P < 0.05 significant). Data were recorded and monitored at University Hospital of Muenster. SAS v9.2 for Windows was used for statistical analysis.
Results
Localized Malignant NGGCT
One patient with a suprasellar tumor died from surgical complications before the start of treatment but was in CR. In 30 of 116 patients (26%) with localized malignant NGGCT, progression/relapse occurred during (n = 3; all died) or after treatment (n = 27), giving a 5-year PFS of 0.72 ± 0.04 (Fig. 3). Five-year OS was 0.82 ± 0.04 (Fig. 4). For those relapsing after the end of treatment, the most common site was local (locoregional, including ventricular; n = 14), with 6 patients having a combined relapse (locoregional and distant) and 7 patients relapsing only outside the locoregional tumor area (distant). Isolated ventricular relapses occurred in only 2 patients. The primary tumor sites of the 14 locoregional relapses were pineal (n = 6), suprasellar (n = 4), bifocal (n = 1), left frontomedial lobe (n = 1), internal capsule (n = 1), and inferior frontal lobe (n = 1). Five of 6 patients with combined relapses outside the radiotherapy field had pineal disease at presentation (Table 1).
Fig. 3.
PFS for the 149 eligible patients on the SIOP-CNS-GCT-96 protocol (116 localized vs 33 metastatic patients).
Fig. 4.
OS for the 149 eligible patients on the SIOP-CNS-GCT-96 protocol (116 localized vs 33 metastatic patients).
Table 1.
Pattern of relapses following treatment for the 27 localized patients and 9 metastatic patients on the SIOP-CNS-GCT-96 trial
| Localized Malignant NGGCT (n = 27) | |||
|---|---|---|---|
| Case | Primary Tumor Site | Tumor Site at Relapse | Relapse Category* |
| 1 | Pineal | Pineal | Locoregional |
| 2 | Pineal | Pineal | Locoregional |
| 3 | Pineal | Pineal | Locoregional |
| 4 | Pineal | Pineal | Locoregional |
| 5 | Pineal | Pineal | Locoregional |
| 6 | Pineal | Pineal + chiasm/suprasellar | Locoregional and distant |
| 7 | Pineal | Pineal + spinal | Locoregional and distant |
| 8 | Pineal | Pineal + suboccipital + spinal | Locoregional and distant |
| 9 | Pineal | Bifocal + spinal | Locoregional and distant |
| 10 | Pineal | Pineal + parietal + spinal | Locoregional and distant |
| 11 | Pineal | Spinal | Distant |
| 12 | Pineal | Leptomeningeal + cranial + spinal | Distant |
| 13 | Pineal | Cranial + spinal | Distant |
| 14 | Pineal | Optic nerve + subependymal frontal horns + globus pallidus | Distant |
| 15 | Pineal | Third ventricle + suprasellar + frontal horns of ventricle + spinal | Distant |
| 16 | Pineal | Floor of third ventricle, chiasm | Distant |
| 17 | Pineal | Cranial | Suspected local relapse, only tumor marker elevation |
| 18 | Suprasellar | Suprasellar | Locoregional |
| 19 | Suprasellar | Suprasellar | Locoregional |
| 20 | Suprasellar | Hypothalamic | Locoregional |
| 21 | Suprasellar | Fourth ventricle | Locoregional |
| 22 | Suprasellar | Subependymal | Distant |
| 23 | Bifocal | Pineal | Locoregional |
| 24 | Frontomedial lobe | Right frontal lobe | Locoregional |
| 25 | Inferior frontal lobe (left) | Left occipital lobe | Locoregional |
| 26 | Internal capsule | Ventricles | Locoregional |
| 27 | Left ventricle | Cranial + ventricle + spinal | Locoregional and distant |
| Metastatic Malignant NGGCT (n = 9) | |||
| Case | Primary Tumor Site and Metastatic Group | Tumor Site at Relapse | Relapse Category* |
| 1 | Pineal micrometastatic |
Spinal | Distant |
| 2 | Pineal macrometastatic |
Extracranial (abdominal) | Distant (outside radiotherapy field) |
| 3 | Pineal macrometastatic |
Cranial | Locoregional |
| 4 | Pineal micrometastatic |
Pineal | Locoregional |
| 5 | Pineal macrometastatic |
Pineal | Locoregional |
| 6 | Suprasellar macrometastatic |
Suprasellar | Locoregional (raised tumor marker) |
| 7 | Bifocal macrometastatic |
Subarachnoid space | Locoregional |
| 8 | Right frontal inferior dura macrometastatic |
Left frontal lobe + spinal | Locoregional and distant |
| 9 | Ventricle micrometastatic |
Spinal | Distant |
Key: micrometastatic = positive CSF cytology only; macrometastatic = radiological metastases present on full neuroaxis imaging (MRI head and spine); localized relapse = locoregional; combined relapse = locoregional and distant. ‘Distant’ here refers to relapses distant from the primary tumor site.
Metastatic Malignant NGGCT
One patient of 33 with metastatic disease progressed on treatment (rising markers) and died; 9 relapsed after treatment, resulting in 5-year PFS of 0.68 ± 0.09 (Fig. 3). Five-year OS was 0.75 ± 0.08 (Fig. 4). Seven progressions/relapses occurred in the 20 patients (35%) with macroscopic metastatic disease and 3 in the 13 (23%) with micrometastatic (cytological) disease only. There was no significant difference in relapse rates between these 2 groups (P = 0.51). The primary tumor sites of metastatic patients were 14 pineal, 8 suprasellar, 5 bifocal, and 6 other (3 ventricles, 2 frontoparietal, 1 diencephalon). Of the 9 patients relapsing following treatment, 5 had local relapses, 1 combined, and 3 distant (of which only one was outside the radiation field). Only one of the 10 patients with progression/relapse could be salvaged (Fig. 3). The primary tumor sites of these 5 local relapse patients were pineal (n = 3), suprasellar (n = 1), and bifocal (n = 1) (Table 1).
Prognostic Factors
Diagnostic tumor markers
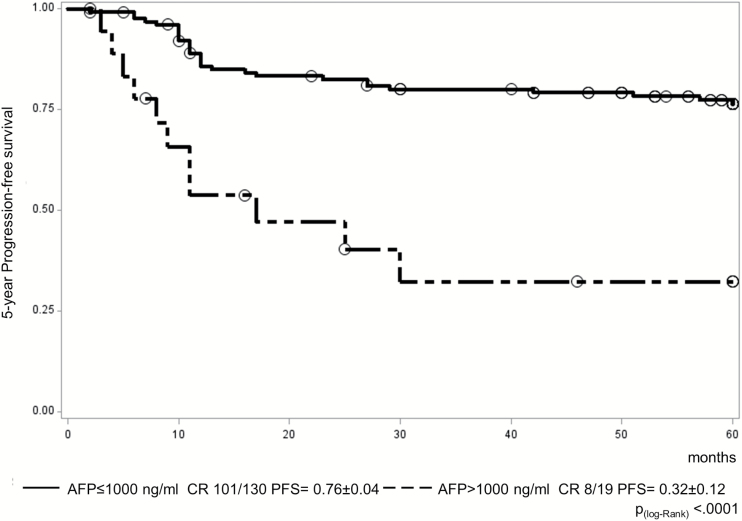
Markers were below the defined thresholds in only 10 patients (7%), and 2 of these patients relapsed. HCG levels in HCG marker–elevated patients were 51–32500 IU/L (median 321) in serum and 53–60600 IU/L (median 306) in CSF. AFP levels in marker-positive patients were 29–27100 ng/mL (median 314) in serum and 26–15700 ng/mL (median 97) in CSF. Isolated elevation of HCG did not affect prognosis (7/53 patients relapsed; 13%), whereas 31 of 86 (36%) with isolated AFP elevation or combined elevation with HCG relapsed. These figures compare with 40 progression/relapses from the total cohort of 149 patients (27%). AFP levels were divided into 4 groups for initial comparison (ng/mL; ≤25, “low” group; >25 but ≤100, “mild” elevation group; >100 but ≤1000, “moderate” group; and >1000, “high” group). There was a significantly worse PFS for patients in the high AFP group (>1000 ng/mL) compared with each of the 3 other groups individually by log-rank test (P < 0.0001 vs low AFP group; P = 0.04 vs mild AFP group; P = 0.0015 vs moderate AFP group). None of the other AFP group comparisons were significant. Having identified an AFP value of >1000 ng/mL as a risk factor for worse PFS, we undertook a comparison of just 2 groups using this cutoff. This confirmed a worse PFS for the high AFP group (>1000 ng/mL), where 11/19 patients relapsed (5-y PFS, 0.32 ± 0.12), compared with all other AFP levels (≤1000 ng/mL), where only 29/130 patients relapsed (5-y PFS, 0.76 ± 0.04) (P < 0.0001) (Fig. 5). Analysis of HCG levels did not reveal any additional adverse prognostic information.
Fig. 5.
PFS for the 149 eligible patients on the SIOP-CNS-GCT-96 protocol by diagnostic AFP level (130 patients with AFP ≤1000 ng/mL vs 19 patients with AFP >1000 ng/mL).
Impact of residual disease
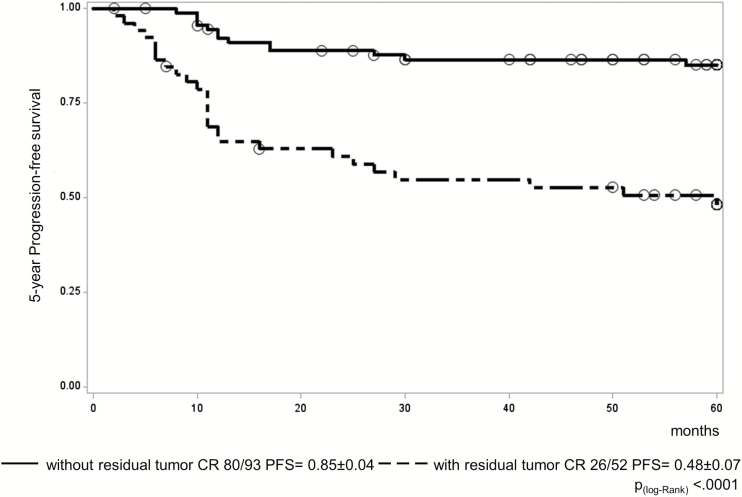
The presence of residual disease on imaging at the end of treatment was also assessed as a potential risk factor for relapse. Of 145 evaluable patients (end-of-treatment MRI centrally reviewed), 52 had residual disease and 93 were in CR. Of these latter, 41 were completely resected. Consequently, there were 93 patients (65%) in CR with 52 remaining patients (35%) with residual. Only 13 of 93 CR patients relapsed compared with 26 of 52 with residual, resulting in 5-year PFS of 0.85 ± 0.04 and 0.48 ± 0.07, respectively (P < 0.0001) (Fig. 6). Pathological findings at resection after chemotherapy could not be correlated with survival, as they were not collected in case report forms, although those with information (n = 12) had either teratoma (n = 5) or necrotic/scar tissue (n = 7).
Fig. 6.
PFS for the 145 evaluable patients on the SIOP-CNS-GCT-96 protocol by end-of-treatment status (93 patients without residual tumor vs 52 with residual).
Multivariate analysis
Multivariate analysis identified high diagnostic serum or CSF AFP (>1000 ng/mL) and residual disease (after treatment and any second-look surgery) as conferring a worse PFS. Frequency of relapse differed significantly between patients with and without high AFP level at diagnosis (58% vs 22%, respectively; P = 0.0003) as well as with and without residual disease at the end of treatment (50% vs 14%, respectively; P = 0.0002). No significant differences in outcome were identified by patient age at diagnosis, sex, primary tumor site, or metastatic status.
Acute Side Effects of Treatment
Acute Common Terminology Criteria for Adverse Events grade 3 or 4 toxicities occurred in 93/116 (80%) patients with localized disease and in 28/33 (85%) with metastatic disease; as toxicity rates were similar in both groups, they were evaluated together. Grades 3 and 4 chemotherapy toxicities consisted predominantly of leukopenia (n = 109), thrombocytopenia (n = 62), and vomiting/nausea (n = 45). Nineteen patients developed grade 3 or 4 infection. Renal function was impaired in 16 patients (low glomerular filtration rate in 13 and elevated serum creatinine in 3). Fourteen patients experienced metabolic disturbances with severe fluid imbalance, all attributed to diabetes insipidus. Nine patients developed central neurotoxicity and 9 subjective or objective hearing disturbance. In another 10 patients, liver enzyme disturbances were seen. Other reported grade 3 or 4 toxicities were rare and included oral toxicity (n = 4), diarrhea (n = 5), elevated bilirubin (n = 3), and peripheral neurotoxicity (n = 2). One patient had an asystolic arrest after the first course of PEI requiring cardiopulmonary resuscitation and survived, but the relationship to chemotherapy remained unclear. All reported acute toxicities related to chemotherapy resolved.
Grade 3 or 4 toxicities related to radiotherapy were rarely reported in localized and metastatic disease and included, mainly in metastatic disease, nausea/vomiting/diarrhea (n = 9), myelosuppression (leukopenia, n = 5; thrombocytopenia, n = 3), and skin toxicity (n = 1). One patient died of surgical complications. Other toxicities after surgery (initial or during treatment) were confined to central neurotoxicity and infection. Four developed neurological deficits (hemiplegia, 2 third cranial nerve palsy, disturbance of sensation, and gait disorder of left leg). Infections were observed in 3 patients (meningitis, 2 with osteomyelitis of bone flap).
Discussion
We describe the largest prospective series of patients with intracranial malignant NGGCT, treated in the multinational European protocol SIOP-CNS-GCT-96, with follow-up outcome data. Previously, our group reported excellent outcomes for patients with pure germinoma treated on this study, using a combined chemoradiation approach in localized cases to reduce radiotherapy volumes.14
Intracranial malignant NGGCT diagnosis on this protocol was based on typical radiology and then predominantly on AFP and HCG tumor markers in both serum and CSF. Biopsy was only mandated when both markers were below threshold values in both body fluid compartments,15 in particular to distinguish germinoma from EC cases, as treatment schedules were different for these 2 malignant subtypes. This approach was recommended to minimize avoidable morbidity and mortality from unnecessary neurosurgical interventions.15 Consequently, tumor tissue collection on this trial for molecular studies was limited.16 Patients were only included in the trial if they had full diagnostic workup, including craniospinal MRI, marker analysis, and CSF cytological assessment.
Our trial approach was to optimize overall outcomes, given the relatively poor prognosis for malignant NGGCTs compared with their pure germinoma counterparts.8,16 Previous studies had confirmed the inadequacy of radiotherapy-only1 and chemotherapy-only strategies6,12,17,18 for intracranial NGGCTs. Other reports showed early promise for a combined chemo/radiation strategy for intracranial NGGCTs, albeit in single institution studies and/or with small cohorts and limited follow-up. These included the combined BEP/VIP,10 pilot-PEI,8 and VP16/cisplatin19,20 studies. The pilot-PEI study was important in showing that the proposed strategy for SIOP-CNS-GCT-96 was both safe and well tolerated,8 and the trial itself contained detailed guidance for managing complex patients (eg, those with diabetes insipidus) to minimize potential acute treatment-related toxicities.
The SIOP-CNS-GCT-96 study reported here shows that combination chemotherapy and radiotherapy for all intracranial NGGCT patients, with risk-adapted radiotherapy tailored according to initial dissemination (focal for those with localized disease and craniospinal plus focal boost for metastatic cases), was effective at producing long-term durable treatment responses. Five-year PFS of 72% and 68% for localized and metastatic disease, respectively, reflect this achievement. The results from this large multinational study were similar to previous and more limited reports on chemoradiation delivery.
Risk Factors
AFP
For many years, serum AFP levels have been used for risk stratifying both pediatric21 and adult22 extracranial GCT management. In the former study, serum AFP >10000 ng/mL identified patients at greater risk of relapse, and thus many classification systems have resulted in such patients receiving additional chemotherapy cycles. For adults, the International Germ Cell Consensus Classification system used serum AFP to assist classification in good-, intermediate-, and poor-risk groups, which influenced prognosis as well as the number of chemotherapy cycles delivered. Previous studies of intracranial disease involved too few patients to reliably identify high-risk groups warranting treatment intensification. In this study, 11 of 19 patients (58%) with diagnostic serum or CSF AFP levels >1000 ng/mL relapsed, identifying them as a group with significantly worse outcome. Diagnostic HCG levels were, however, not associated with increased relapse risk; indeed only 13% of patients with an isolated elevation of HCG relapsed, compared with 36% of cases with an AFP increase or an elevation of both markers. This may reflect the fact that the HCG-only group may have included some HCG-secreting germinomas.
Residual disease
The frequency of surgery to resect residual disease either after the end of induction chemotherapy or at the end of treatment was variable in patients treated on the SIOP-CNS-GCT-96 study. However, this allowed the assessment of the presence of residual disease on the likelihood of relapse. We previously demonstrated that residual disease in intracranial pure germinoma was not associated with inferior outcomes.14 In contrast, here we identified that survival for patients with intracranial malignant NGGCTs who had end-of-treatment residual disease (including after second-look surgery) was significantly worse. Based on these results, we now recommend surgical resection of any residual lesions following completion of chemotherapy, to maximize the chance of achieving local tumor control.
Relapses
Twenty-seven of 116 localized NGGCT patients (23%) experienced a relapse following treatment, although importantly, only 7 of these 27 (26%) experienced an isolated distant relapse. It should also be noted that only 1 of 13 patients with diabetes insipidus and non-suprasellar primary tumors relapsed in the suprasellar region, outside the radiotherapy field. These data support the use of focal radiotherapy fields for this population, particularly in the context of a dose-intense chemotherapy induction regimen. Nine of 33 metastatic patients (27%) had a relapse of their disease following treatment. These events were comparable between those with macroscopic and those with microscopic (cytological) metastatic disease. The similar rates of relapse between patients with localized and metastatic disease treated on the SIOP-CNS-GCT-96 protocol underscore the benefit of the risk-adapted radiotherapy strategy. These data also serve to further emphasize the importance of full diagnostic workup for all intracranial GCT patients, including assessment of tumor marker levels in both serum and CSF compartments, whole neuroaxis MRI, and CSF cytology, to allow appropriate risk-adapted treatment to be delivered. Outcomes of a subgroup of patients who relapsed following treatment according to the SIOP-CNS-GCT-96 protocol are described elsewhere.23
Radiotherapy fields for localized NGGCT
It should be noted that for patients with localized NGGCTs, the volumes and doses of radiotherapy have historically varied. Prior to SIOP-CNS-GCT-96, the French group reported their chemoradiation approach for NGGCT cases with focal fields, with EFS of 67%.24 Other national groups have used wider radiotherapy fields.13,25 The North American study reported excellent results using 6 courses of chemotherapy (alternating carboplatin/etoposide with ifosfamide/etoposide), prior to craniospinal irradiation.13 The most recent North American trial (ACNS1123) asked whether in localized intracranial NGGCT cases radiotherapy volumes can be reduced to include just the whole ventricular system, and results of this trial are awaited. As noted above, however, the focal radiotherapy fields employed for such cases in the SIOP-CNS-GCT-96 study were not associated with an excess of relapses in the ventricles or at distant intracranial sites, likely due to the dose-intense chemotherapy regimen and short time to radiotherapy.
Late effects
With improving survival for patients with intracranial NGGCT, minimizing potential long-term treatment-related effects assumes greater importance. SIOP-CNS-GCT-96 late-effects data will be reported separately.
Limitations
The SIOP-CNS-GCT-96 trial had a number of limitations. Firstly, it was not randomized, but merely recommended risk-adapted treatment based on extent of disease. Secondly, there was no real-time central review to confirm eligibility for trial inclusion based on adequate diagnostic and staging workup, and consequently, a substantial number of enrolled patients had to be excluded from formal trial analysis due to missing data. Furthermore, reviewed pathology reports from second-look surgery at the end of treatment were not routinely available. That notwithstanding, this description of the largest prospective series of intracranial NGGCT patients treated in a uniform manner has allowed key risk factors for recurrence to be identified.
Future directions and biology
In future trials, incorporation of prospective biological studies will be important. Much recent progress has been made in understanding the molecular changes underlying intracranial GCT pathogenesis (eg, the GCT mutational landscape through whole-exome sequencing).26,27 Given the limited intracranial NGGCT tissue specimens available to study in Europe and North America, however, collection of serum/plasma and CSF may in future allow both non-invasive diagnosis using microRNA expression levels28 and the identification of the presence of tumor mutations through circulating tumor DNA analysis,29 which may inform novel treatment strategies.
Conclusion
In summary, we report the outcomes of the largest prospective series of patients with intracranial malignant NGGCT, treated in a multinational European protocol with a successful combined, risk-adapted, chemoradiation approach. Following dose-intense chemotherapy, focal radiotherapy fields were sufficient for the treatment of localized disease, whereas patients with metastatic disease had similar outcomes using craniospinal radiotherapy. The trial identified two key risk factors for recurrence. Firstly, patients with diagnostic serum or CSF AFP levels >1000 ng/mL warrant treatment intensification. Secondly, those with end-of-treatment residual disease, including after second-look surgery, have worse outcomes. Resection of residual disease is therefore strongly recommended. These additional refinements aim to further improve OS for this patient cohort while continuing to spare patients with localized disease the long-term sequelae of craniospinal irradiation, through the successful use of focal radiotherapy.
Funding
The work was supported in part by Deutsche Krebshilfe e. V., Bonn, Germany and Elisabeth Dreves Stiftung, Germany.
Acknowledgments
We thank all medical centers that contributed to SIOP-CNS-GCT-96. The authors gratefully acknowledge Catherine Patte and Marie Christine Baranzelli for their valuable contributions and Carmen Teske for her secretarial support.
The supporting association of the study (German Cancer Aid and Elisabeth Dreves Stiftung) had no role in study design, data collection, analysis and interpretation, or report writing. All authors had access to national-level raw data. The corresponding author (G.C.) had full access to all study data and had final responsibility for publication.
Conflict of interest statement. We have no conflicts of interest.
References
- 1. Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63(2):155–167. [DOI] [PubMed] [Google Scholar]
- 2. Göbel U, Bamberg M, Budach V et al. . Intracranial germ cell tumors: analysis of the therapy study MAKEI 83/86 and changes in protocol for the follow-up study. Klin Padiatr. 1989;201(4):261–268. [DOI] [PubMed] [Google Scholar]
- 3. Göbel U, Bamberg M, Calaminus G et al. . Improved prognosis of intracranial germ cell tumors by intensified therapy: results of the MAKEI 89 therapy protocol. Klin Padiatr. 1993;205(4):217–224. [DOI] [PubMed] [Google Scholar]
- 4. Einhorn LH, Donohue JP. Improved chemotherapy in disseminated testicular cancer. J Urol. 1977;117(1):65–69. [DOI] [PubMed] [Google Scholar]
- 5. Herrmann HD, Westphal M, Winkler K, Laas RW, Schulte FJ. Treatment of nongerminomatous germ-cell tumors of the pineal region. Neurosurgery. 1994;34(3):524–529; discussion 529. [DOI] [PubMed] [Google Scholar]
- 6. Balmaceda C, Heller G, Rosenblum M et al. . Chemotherapy without irradiation—a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. 1996;14(11):2908–2915. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi T, Yoshida J, Ishiyama J, Noda S, Kito A, Kida Y. Combination chemotherapy with cisplatin and etoposide for malignant intracranial germ-cell tumors. An experimental and clinical study. J Neurosurg. 1989;70(5):676–681. [DOI] [PubMed] [Google Scholar]
- 8. Calaminus G, Andreussi L, Garré ML, Kortmann RD, Schober R, Göbel U. Secreting germ cell tumors of the central nervous system (CNS). First results of the cooperative German/Italian pilot study (CNS sGCT). Klin Padiatr. 1997;209(4):222–227. [DOI] [PubMed] [Google Scholar]
- 9. Calaminus G, Bamberg M, Jürgens H et al. . Impact of surgery, chemotherapy and irradiation on long term outcome of intracranial malignant non-germinomatous germ cell tumors: results of the German Cooperative Trial MAKEI 89. Klin Padiatr. 2004;216(3):141–149. [DOI] [PubMed] [Google Scholar]
- 10. Calaminus G, Bamberg M, Harms D et al. . AFP/beta-HCG secreting CNS germ cell tumors: long-term outcome with respect to initial symptoms and primary tumor resection. Results of the cooperative trial MAKEI 89. Neuropediatrics. 2005;36(2):71–77. [DOI] [PubMed] [Google Scholar]
- 11. Matsutani M; Japanese Pediatric Brain Tumor Study Group Combined chemotherapy and radiation therapy for CNS germ cell tumors—the Japanese experience. J Neurooncol. 2001;54(3):311–316. [DOI] [PubMed] [Google Scholar]
- 12. Baranzelli MC, Patte C, Bouffet E et al. . An attempt to treat pediatric intracranial alphaFP and betaHCG secreting germ cell tumors with chemotherapy alone. SFOP experience with 18 cases. Société Française d’Oncologie Pédiatrique. J Neurooncol. 1998;37(3):229–239. [DOI] [PubMed] [Google Scholar]
- 13. Goldman S, Bouffet E, Fisher PG et al. . Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: a Children’s Oncology Group Study. J Clin Oncol. 2015;33(22):2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calaminus G, Kortmann R, Worch J et al. . SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicholson JC, Punt J, Hale J, Saran F, Calaminus G; Germ Cell Tumour Working Groups of the United Kingdom Children’s Cancer Study Group (UKCCSG) and International Society of Paediatric Oncology (SIOP) Neurosurgical management of paediatric germ cell tumours of the central nervous system—a multi-disciplinary team approach for the new millennium. Br J Neurosurg. 2002;16(2):93–95. [DOI] [PubMed] [Google Scholar]
- 16. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 17. Kellie SJ, Boyce H, Dunkel IJ et al. . Primary chemotherapy for intracranial nongerminomatous germ cell tumors: results of the second international CNS germ cell study group protocol. J Clin Oncol. 2004;22(5):846–853. [DOI] [PubMed] [Google Scholar]
- 18. da Silva NS, Cappellano AM, Diez B et al. . Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood Cancer. 2010;54(3):377–383. [DOI] [PubMed] [Google Scholar]
- 19. Buckner JC, Peethambaram PP, Smithson WA et al. . Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol. 1999;17(3):933–940. [DOI] [PubMed] [Google Scholar]
- 20. Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997;32(1):71–80. [DOI] [PubMed] [Google Scholar]
- 21. Baranzelli MC, Kramar A, Bouffet E et al. . Prognostic factors in children with localized malignant nonseminomatous germ cell tumors. J Clin Oncol. 1999;17(4):1212. [DOI] [PubMed] [Google Scholar]
- 22. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15(2):594–603. [DOI] [PubMed] [Google Scholar]
- 23. Murray MJ, Bailey S, Heinemann K et al. . Treatment and outcomes of UK and German patients with relapsed intracranial germ cell tumors following uniform first-line therapy. Int J Cancer. 2017;141(3):621–635. [DOI] [PubMed] [Google Scholar]
- 24. Harnden P, Joffe JK, Jones WG. Germ Cell Tumours V: The Proceedings of the Fifth Germ Cell Tumour Conference Devonshire Hall, University of Leeds, 13th-15th September, 2001.London: Springer London; 2002. [Google Scholar]
- 25. Matsutani M, Sano K, Takakura K et al. . Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86(3):446–455. [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Yamaguchi S, Burstein MD et al. . Novel somatic and germline mutations in intracranial germ cell tumours. Nature. 2014;511(7508):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukushima S, Otsuka A, Suzuki T et al. ; Intracranial Germ Cell Tumor Genome Analysis Consortium (iGCT Consortium). Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 2014;127(6):911–925. [DOI] [PubMed] [Google Scholar]
- 28. Murray MJ, Bell E, Raby KL et al. . A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br J Cancer. 2016;114(2):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Mattos-Arruda L, Mayor R, Ng CKY et al. . Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. [DOI] [PMC free article] [PubMed] [Google Scholar]