Abstract
Background
The overall incidence of Pneumocystis jirovecii pneumonia (PJP) in solid organ transplant recipients is 5–15%. A timely diagnosis of PJP is difficult and relies on imaging and detection of the organism.
Methods
We present a case series of four patients displaying hypercalcaemia with an eventual diagnosis of PJP and document the management of the outbreak with a multidisciplinary team approach. We discuss the underlying pathophysiology and previous reports of hypercalcaemia preceding a diagnosis of PJP. We also reviewed the evidence concerning PJP diagnosis and treatment.
Results
Within our renal transplant cohort, four patients presented within 7 months with hypercalcaemia followed by an eventual diagnosis of PJP. We measured their corrected calcium, parathyroid hormone (PTH), 1,25-dihydroxycholecalciferol [1,25-(OH)2D3] and 25-hydroxycholecalciferol [25(OH)D] levels at admission and following treatment of PJP. All four patients diagnosed with PJP were 4–20 years post-transplantation. Three of the four patients demonstrated PTH-independent hypercalcaemia (corrected calcium >3.0 mmol/L). The presence of high 1,25(OH)2D3 and low 25(OH)D levels suggest negation of the negative feedback mechanism possibly due to an extrarenal source; in this case, the alveolar macrophages. All four patients had resolution of their hypercalcaemia after treatment of PJP.
Conclusions
Given the outbreak of PJP in our renal transplant cohort, and based on previous experience from other units nationally, we implemented cohort-wide prophylaxis with trimethoprim–sulphamethoxazole for 12 months in consultation with our local infectious diseases unit. Within this period there have been no further local cases of PJP.
Keywords: hypercalcaemia, immunosuppression, kidney transplantation, Pneumocystis jirovecii, transplant, trimethoprim-sulphamethoxazole
Background
Pneumocystis jirovecii is a human-specific ascomycetous fungal organism discovered in the 1900s [1]. Pneumocystis infection is thought to involve aerosolized particle transmission [1]. Pneumocystis colonization may not manifest clinically in immunocompetent humans [1, 2], yet in an immunocompromised patient, Pneumocystis has lung tropism and can cause opportunistic pneumonia [1, 2].
In renal transplant recipients (RTRs), the incidence of P.jirovecii pneumonia (PJP) is 5–15% in patients without prophylaxis, with a greater relative risk up to 6 months post-transplant [3, 4]. Mortality rates are 13–38% in this population [5]. Cytomegalovirus (CMV) infection, glucocorticoid use and recurrent rejection are independent risk factors for PJP [6, 7]. Trimethoprim–sulphamethoxazole (TMP-SMX) prophylaxis [8] is recommended for 3–6 months post-transplant [9–11], which is when most infections occur [3, 10]. Beyond the first year post-transplant, risk factors for PJP include increased levels of immunosuppression [12], allograft rejection and abnormal renal function [13].
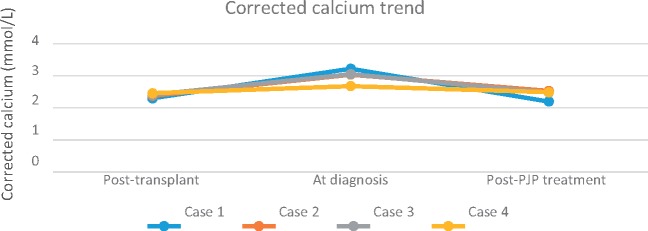
While PJP in RTRs is relatively uncommon, recent PJP outbreaks in Australian renal transplant units [9, 14] have refocused attention on the current evidence behind PJP prophylaxis. An uncommon feature of PJP is hypercalcaemia, often preceding radiographic findings [15–21]. We present four RTRs with hypercalcaemia refractory to medical management preceding the diagnosis of PJP. We hypothesize that PJP contributed to the preceding hypercalcaemia in three of the cases. Biochemical and haematological results are summarized in Table 1. Immunosuppression post-transplant and at diagnosis are recorded in Table 2. The trend of hypercalcaemia is depicted in Figure 1.
Table 1.
Serum creatinine, corrected calcium, PTH, 25-(OH)D and 1,25(OH)2D pre-transplant, at diagnosis and post-treatment and any calcium/vitamin D supplementation (in bold) at diagnosis
| Case | Corrected calcium post- transplant (2.1–2.6 mmol/L) | PTH post- transplant (1.6– 6.9 pmol/L) | Serum creatinine post- transplant (µmol/L) | Vitamin D or calcium supplements at diagnosis | Corrected calcium at diagnosis (2.1–2.6 mmol/L) | 25-(OH)D at diagnosis (>50 nmol/L) | 1,25-(OH)2D at diagnosis (72– 225 pmol/L) | PTH at diagnosis (1.6–6.9 pmol/L) | Serum creatinine at diagnosis (µmol/L) | Corrected calcium post-treatment (2.1–2.6 mmol/L) | PTH post- treatment (1.6– 6.9 pmol/L) | Serum creatinine post-treatment (µmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.3 | 7.52 | 175 | Nil | 3.43 | 28 | 450 | <0.32 | 290 | 2.20 | 11 | 350 |
| 2 | 2.38 | N/A | 160 | Nil | 3.04 | 83 | 588 | <0.32 | 208 | 2.53 | 5.6 | 180 |
| 3 | 2.41 | N/A | 170 | Vitamin D 2 tabs daily + calcitriol 0.25mcg daily | 3.05 | 116 | N/A | 2.38 | 455 | 2.48 | 2.38 | Dialysis dependent |
| 4 | 2.46 | 12.8 | 145 | Calcitriol 0.25mcg alternate days at diagnosis | 2.68 | 28 | 75 | 18.8 | 159 | 2.49 | 18.8 | 170 |
N/A, not available (sample not collected).
Table 2.
Demographics of cases, time from transplantation, immunosuppression doses after renal transplant and at time of diagnosis and white cell, lymphocyte and neutrophil counts at diagnosis
| Case | Age (years) and gender | Time from transplant (years) | Immunosuppression post-transplant |
Immunosuppression at diagnosis |
White cell count at diagnosis | Lymphocyte count at diagnosis | Neutrophil count at diagnosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcineurin inhibitors | Antiproliferative agents | Prednisolone | Calcineurin inhibitors | Antiproliferative agents | Prednisolone | ||||||
| 1 | 45 F | 4 | Tac 4 mg twice daily | Myfortic 720 mg twice daily | 20 mg daily | Tac 3.5 mg once daily (trough level 3.2 ng/ml) | Myfortic 360 mg twice daily | 5 mg once daily | 7.5 | 0.9 | 6.6 |
| 2 | 57 M | 11 years | CYC 200 mg twice daily | Cellcept 1 g twice daily | 20 mg daily | None | Cellcept 1 g twice daily | 6 mg daily | 8.9 | 1.1 | 6.7 |
| 3 | 45 F | 30 years | CYC 450 mg twice daily | Azathioprine50mg daily | 5 mg daily | None | Cellcept 250 mg twice daily | 5 mg daily | 12.3 | 0.9 | 11.0 |
| 4 | 52 M | 6 years | Tac 5.5 mg twice daily | Cellcept 1 g three times daily | 25 mg daily | Tac 0.5 mg twice daily (trough level 2.5 ng/ml) | Cellcept 750 mg twice daily | 5 mg daily | 8.9 | 1.4 | 5.9 |
Cellcept, mycophenolate mofetil; Myfortic, mycophenolate sodium; CYC, cyclosporin; Tac, tacrolimus.
Fig. 1.
Trend of serum corrected calcium post-transplant, at diagnosis and post-PJP treatment.
Case 1 (January 2014)
A 45-year-old restaurant worker with a living-related ABO-incompatible renal transplant 4 years prior presented with exertional dyspnoea and hypercalcaemia of 3.43 mmol/L. She had 6 months of TMP-SMX prophylaxis post-transplant. She was on tacrolimus, mycophenolate and prednisolone without vitamin D or calcium supplementation at presentation (Tables 1 and 2). She previously had CMV viraemia (treated with currently undetectable levels) and cellular-mediated graft rejection (diagnosed November 2011). This was treated with pulsed methylprednisolone and increased immunosuppression. A biopsy post-treatment showed no further rejection. Bactrim was not restarted during treatment for graft rejection.
Auscultation revealed fine crackles bilaterally and she was afebrile. Her parathyroid hormone (PTH)-independent hypercalcaemia (PTH <0.32 pmol/L) remained high after intravenous crystalloids and calcitonin. Bisphosphonates were avoided due to impaired renal function (creatinine 258 µmol/L). Other tests showed elevated 1,25-dihydroxycholecalciferol [1,25(OH)2D3] 450 pmol/L, low 25-hydroxycholecalciferol [25(OH)D] 28 nmol/L, serum angiotensin-converting enzyme (ACE) 37.7 U/L and PTH-related protein of 2.7 pmol/L. She was not leucopenic on admission.
Chest x-Ray (CXR) showed interstitial infiltrates. A high-resolution CT scan of the chest (HRCT) showed ground-glass opacities bilaterally. TMP-SMX was commenced at the treatment dose for presumed PJP. Bronchoalveolar lavage (BAL) revealed P.jirovecii on polymerase chain reaction (PCR) testing. Following PJP treatment, she recovered and her serum corrected calcium and PTH improved to 2.2 mmol/L and 11 pmol/L, respectively, on discharge (Table 1, Figure 1).
Case 2 (March 2014)
A 57-year-old policeman with a deceased-donor renal transplant 11 years before with no previous allograft rejection presented with exertional dyspnoea over the preceding month. He was on mycophenolate and prednisolone without vitamin D or calcium supplementation (Tables 1 and 2). Auscultation revealed bilateral fine crackles and he was afebrile.
At presentation, he had PTH-independent hypercalcaemia (corrected calcium 3.04 mmol/L, PTH <0.32 pmol/L) with acute-on-chronic renal failure (creatinine 208 µmol/L from a baseline of 170 µmol/L in 2013). Other tests showed 1,25(OH)2D3 of 588 pmol/L, PTH-related protein <2.0 pmol/L and serum ACE <5.0 U/L. Serum protein electrophoresis and urine electrophoresis were normal. An HRCT showed diffuse ground-glass changes. BAL was negative for P.jirovecii oocysts and other infections.
Management with intravenous fluids, increased prednisolone dose and intravenous bisphosphonate was commenced. His dyspnoea improved and his corrected calcium improved to 2.89 mmol/L and PTH to 4.04 pmol/L at discharge. He was readmitted the following week with worsening dyspnoea and persisting hypercalcaemia of 3.19 mmol/L. He had a normal transthoracic echocardiogram and a negative septic screen, but a repeat HRCT showed worsening ground-glass infiltrates. A repeat bronchoscopy revealed P.jirovecii PCR positive on BAL. TMP-SMX was commenced; this was briefly changed to atovaquone while his renal function recovered. At discharge, his corrected calcium and PTH were 2.53 mmol/L and 5.6 pmol/L, respectively (Table 1, Figure 1).
Case 3 (May 2014)
A 45-year-old woman with a living-related renal transplant 30 years before presented with worsening exertional dyspnoea. Her comorbidities included ischaemic heart disease and no previous allograft rejection. She was on mycophenolate and prednisolone as well as vitamin D and calcitriol (Tables 1 and 2). Auscultation revealed fine crackles, worse on the left, and she was afebrile.
A CXR was clear. Empirical treatment for presumed atypical pneumonia with ceftriaxone and azithromycin was started. An HRCT showed ground-glass opacities bilaterally. She was hypercalcaemic (corrected calcium 3.05 pmol/L) and had renal failure (creatinine 455 µmol/L). Other tests showed serum ACE <5.0 U/L, serum PTH of 2.38 pmol/L and a normal serum/urine electrophoresis and autoimmune screen. Viral PCR on blood was positive for CMV. Induced sputum was tested for PJP PCR. Calcitriol and vitamin D were ceased.
Septic shock necessitated intensive care unit (ICU) admission and intubation while awaiting a bronchoscopy. A broad-spectrum antibiotic (piperacillin-tazobactam) was started. PJP PCR from admission was positive and intravenous TMP-SMX was commenced. Intravenous ganciclovir for CMV viraemia was withheld, as she clinically improved. She was extubated and discharged from the ICU after 3 days; however, her renal function continued to deteriorate, necessitating dialysis.
Respiratory distress prompted a return to the ICU for re-intubation. Antibiotics were changed to meropenem and doxycycline, while intravenous TMP-SMX was continued. Bronchoscopy was negative for P.jirovecii but positive for CMV PCR (titre 9471 copies/mL). Intravenous ganciclovir was added. Meropenem and doxycycline were ceased after 8 days while ganciclovir and TMP-SMX were continued (14 and 21 days, respectively). She returned to the ward after an 18-day ICU stay. Prophylactic oral valganciclovir and oral TMP-SMX were commenced after her initial treatment. Her corrected calcium was 2.48mmol/L on discharge (Table 1, Figure 1). She remained dialysis dependent.
Case 4 (July 2014)
A 52-year-old salesman with a deceased-donor renal transplant 6 years before with no previous allograft rejection was admitted with worsening exertional dyspnoea. He had pre-existing primary hyperparathyroidism with a subtotal parathyroidectomy in 2004. Subsequent PTH levels were 10–44 pmol/L. He was on tacrolimus, mycophenolate and prednisolone as well as calcitriol (Tables 1 and 2). Respiratory examination was unremarkable and he was afebrile.
Treatment for presumed atypical pneumonia with ceftriaxone and azithromycin was commenced after a normal CXR and septic screen. His corrected calcium was 2.68 mmol/L. Other tests showed PTH of 18.8 pmol/L, 1,25(OH)2D3 of 75 pmol/L, serum creatinine of 159 µmol/L and serum ACE of 29.4 U/L. An HRCT showed small patchy areas of ground-glass opacity. Other viral PCRs, including CMV, were negative. Calcitriol was ceased.
Given our prior cases, we started TMP-SMX at a lower dose in consultation with the infectious diseases unit. A bronchoscopy was done 3 days after starting TMP-SMX, as he failed to improve. This was negative for fungi, tuberculosis, bacteria and PJP PCR; however, PJP PCR from an induced sputum sample on admission returned positive. He was commenced on TMP-SMX at the treatment dose with a brief change to atovaquone for renal impairment. Interestingly, his hypercalcaemia had peaked and returned to normal (corrected calcium 2.49 mmol/L) pre-bronchoscopy (Table 1, Figure 1).
PJP outbreak
At the time of the outbreak, our transplant cohort consisted of 146 RTRs (80 public RTRs and 66 RTRs with private follow-up). With no previous cases in our renal transplant cohort, the case series represented an outbreak of PJP as defined by the World Health Organization (WHO) [22]. After Cases 1 and 2 were identified, a working group (comprising the nephrology, infectious diseases, respiratory and physiotherapy departments) was formed with the aims of early recognition of at-risk patients, institution of universal prophylaxis and minimizing patient-to-patient transmission. Outbreak management was developed according to Phipps et al. [14].
Contact tracing identified our outpatient waiting area for public RTRs as a source of transmission. A protocol (Figure 2) involving induced sputum testing, CXR and HRCT was designed to screen all 80 public RTRs for signs and symptoms of PJP. To minimize further transmission, public RTRs were designated their own waiting room and N95 masks were provided to patients with respiratory symptoms. An isolation room staffed by physiotherapists was designated for induced sputum testing, which was fast-tracked by the pathology laboratory. RTRs were invited to appointments where they were given information about the PJP outbreak. RTRs with respiratory symptoms received a CXR and induced sputum testing, with progression to HRCT if any infiltrates were present.
Fig. 2.
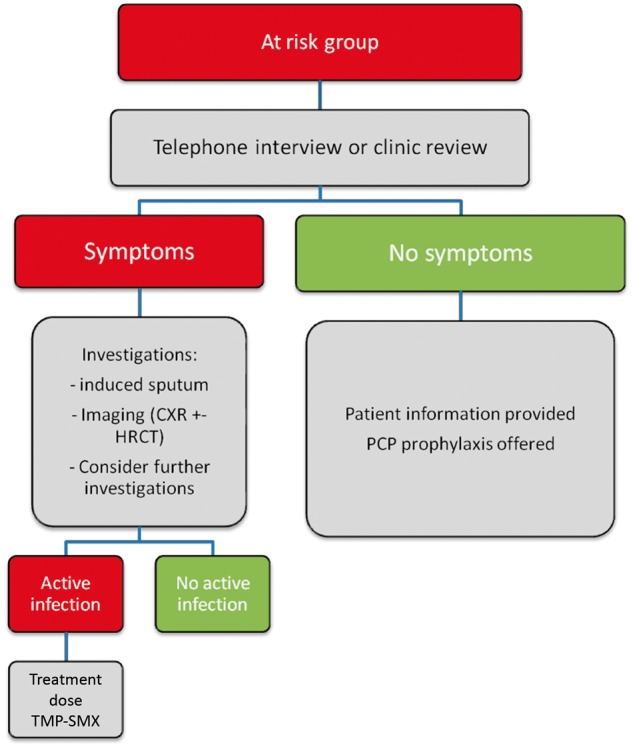
Pathway used to contain outbreak in our renal transplant cohort.
All public RTRs were commenced on a 6-month course of TMP-SMX at a prophylactic dose of 160/800 mg half-tablet daily and adjusted for renal function. This was empirically extended to 1 year based on previous experience [14] of new infections within a 6-month prophylaxis period. Throughout this period, eight patients were intolerant of TMP-SMX and received alternative agents. The major side effect of TMP-SMX in our cohort was a transient increase in serum creatinine that resolved on cessation of TMP-SMX. Cases 3 and 4 presented to the hospital before starting prophylaxis. In all four cases, treatment of PJP with TMP-SMX was dosed at TMP 15–20 mg/kg/day and SMX 75–100 mg/kg/day in three divided doses for 3 weeks with dose adjustment for renal impairment. Private RTRs were not exposed (private rooms located in another building) and were therefore excluded from cohort prophylaxis. None of these patients were ultimately infected with PJP.
Discussion
Hypercalcaemia and PJP
PJP was originally described as a reactivation of latent infection acquired during childhood; however, current evidence points towards de novo infection from environmental sources and infected individuals as other methods of transmission [23, 24]. Five case reports [15–20] and one case series [21] describe similar presentations of hypercalcaemia in RTRs eventually leading to a diagnosis of PJP. The underlying pathophysiology has been linked with alveolar macrophage activity in PJP clearance [25, 26].
There is a high prevalence of hypercalcaemia after renal transplant (up to 25% in the first year post-transplant [27]), which was reflected in our cohort (21.6% at the time of the outbreak). Our cases had relatively normal corrected calcium levels post-transplant (Table 1). At presentation, only Cases 3 and 4 were on calcium and/or vitamin D supplementation, which may have potentiated hypercalcaemia. Cases 1, 2 and 3 had significant PTH-independent hypercalcaemia (corrected calcium >3.0 mmol/L) that was resistant to medical management. Case 4 technically had primary hyperparathyroidism causing hypercalcaemia, however, he was included as part of the PJP outbreak. With increased 1,25-(OH)2D3 levels, we postulate increased hydroxylation via 25-hydroxyvitamin D 1-alpha hydroxylase from alveolar macrophages could be responsible for the hypercalcaemia [28]. While negative feedback of this enzyme usually occurs in hypercalcaemia, extra-renal synthesis and metabolism of 1,25-(OH)2D3 is less tightly regulated by PTH and calcium, and calcium intake does not lower serum 1,25-(OH)2D3 in such patients [29]. Increased 1,25-(OH)2D3 production by alveolar macrophages occurs in sarcoidosis [28–31], and a similar process has been demonstrated post-nephrectomy [32]. The high 1,25-(OH)2D3 levels were present in Cases 1, 2 and 4 (no results were recorded for Case 3). Low 25-(OH)D levels were present in Cases 1 and 4 [Cases 2 and 3 were taking cholecalciferol at the time of diagnosis, contributing to a high 25-(OH)D level]. The low 25-(OH)D levels may have been caused by ongoing 1-alpha hydroxylation with consumption of 25-(OH)D. The eventual normalization of 1,25-(OH)2D3 could correlate with decreased alveolar macrophage activity with treatment of PJP. However, low 25-(OH)D levels are also common post-transplantation [33] for various reasons [34] including renal impairment and increased levels of fibroblast growth factor 23.
1,25-(OH)2D3 production invitro has been demonstrated in pulmonary alveolar macrophages (PAMs) from patients with sarcoidosis [30]. A similar response occurs with normal PAMs incubated with recombinant human interferon-gamma; however, exogenous 1,25-(OH)2D3 was able to decrease endogenous hormone production in normal PAMs to a greater degree than in sarcoid PAMs [31]. The increased ability of sarcoid PAMs to produce 1,25-(OH)2D3 compared with normal PAMs has been documented in other granulomatous diseases [35] but has not been studied at a molecular level in infections such as PJP. Hypercalcaemia associated with elevated 1,25(OH)2D3 has also been documented in fungal infections in immunocompromised patients (Cryptococcus and Coccidioides) [36, 37].
PJP prophylaxis regimens, risk factors and difficulties in diagnosis
Our case series illustrates that PJP can present late post-transplant. It also raises the question of the optimal duration of PJP prophylaxis post-transplant and the efficacy of TMP-SMX as prophylaxis. Prevention is important, as PJP presentations are difficult to differentiate from other respiratory infections [1], as seen in our series. Cases 3 and 4 in particular highlight how PJP can be misdiagnosed as atypical pneumonia. Further diagnostic issues include direct microscopy being less sensitive in HIV-negative patients [38] and PCR being positive in patients colonized with P.jirovecii [39]. While new methods of diagnosing PJP are being developed [39, 40], PJP diagnosis remains reliant on the correlation of clinical and radiological findings with microbiological investigations [1].
While PJP usually occurs within the first year post-transplant [3, 4, 15, 17, 19, 21], presentations up to 17–40 months post-transplant have been noted (5, 41–45). In these groups, risk factors of PJP included early discontinuation of TMP-SMX prophylaxis within the first year post-transplant (or complete lack of prophylaxis), number and type of rejection treatments, age ≥65 years, lymphocyte count <750/mm3 and CMV infection. The use of mycophenolate mofetil was also a risk factor [46]. In our cohort, only Case 1 had previous allograft rejection preceding PJP diagnosis. Our unit policy at the time did not include restarting PJP prophylaxis during rejection treatment. Regarding age as a risk, all our patients were <65 years [45]. Cases 1–3 had varying levels of renal impairment at diagnosis; a risk factor identified by Eitner et al. [13]. All cases had a minimum of 6 months TMP-SMX prophylaxis post-transplantation. Case 1 had prior CMV viraemia that was treated (viral load <400 copies/mL at PJP outbreak). None of our cases were lymphopaenic at presentation (Table 2).
The dosage and type of immunosuppression may also influence the risk of PJP [13, 47–49]. Increased immunosuppression during rejection treatment increases PJP risk [13]. Retrospective studies highlight high-dose corticosteroids over a prolonged period [47] and tacrolimus-based regimens [48] (compared with cyclosporin) as causing a higher PJP incidence. The US Renal Data System (USRDS) cohort showed no association between induction immunosuppression levels and PJP risk, but did show that regimens containing sirolimus were associated with PJP infection [49]. None of our cases were on sirolimus. At transplantation, Cases 1, 2 and 4 had standard immunosuppression doses that were decreased to appropriately lower levels at the time of diagnosis. Case 3 had a lower immunosuppression dose at transplantation and Cases 2 and 3 had ceased tacrolimus and were only on mycophenolate and prednisolone at presentation (Table 2).
The recommended duration of PJP prophylaxis is 3–12 months after solid organ transplantation [9]. The Kidney Disease: Improving Global Outcomes (KDIGO) 2009 clinical practice guidelines recommend 3–6 months post-transplant prophylaxis, and at least 6 weeks during and after treatment for acute rejection [50]. The European Best Practice guidelines recommend at least 4 months of prophylaxis post-transplant, with a further 3–4 months of prophylaxis during rejection [11]. There is worldwide variation in the use of TMP-SMX prophylaxis [5, 41] with PJP outbreaks in Japan, the UK and Switzerland occurring in units that did not offer routine PJP prophylaxis [51].
No prophylaxis following heart, liver and kidney–pancreas transplant was associated with a higher incidence of PJP compared with either a low-dose or high-dose TMP-SMX prophylaxis regimen [52].
There was no difference in PJP incidence between the low-dose TMP-SMX (80/400 mg/day or 160/800 mg/alternate days) and high-dose TMP-SMX (160/800 mg/day) groups. Second-line prophylaxis options include dapsone, atovaquone and pentamidine. Dapsone [53] and atovaquone [54] have been compared with TMP-SMX in two separate retrospective case-matched studies of RTRs, with no PJP cases documented in either arm over a 12-month period. In the dapsone study, a higher rate of infections occurred in the dapsone group, while in the atovaquone study, patients on TMP-SMX prophylaxis had a higher rate of treatment discontinuation due to adverse events.
Six months has been suggested as the optimal duration of PJP prophylaxis post-transplant, as it correlates with the estimated median incubation period of PJP (53 days with a range of 7–188 days) [55]. Without randomized controlled trial evidence for both PJP prophylaxis and treatment in RTRs [24], current prophylaxis guidelines such as from KDIGO are the best guide and are certainly preferable to no prophylaxis [5]. While previous reviews mention treatment of PJP outbreaks with TMP-SMX and instituting universal prophylaxis in RTRs, prophylaxis duration from that point on is unclear [41–42, 44]. Post-PJP outbreak, Phipps et al. [14] and Mitsides et al. [56] instituted a 12-month TMP-SMX prophylaxis regimen across their transplant cohorts, with no further cases diagnosed apart from patients who were not compliant with prophylaxis.
In total, 83 cases of PJP occurred in Australia from 2010 to 2012, comprising a total of 14 outbreaks, with the majority being in RTRs [9]. Our case series adds to the literature of hypercalcaemia presenting as a feature of PJP in the renal transplant population and demonstrates the need for ongoing vigilance against post-transplant infections and minimizing the risk factors thereof. We highlight the importance of a multidisciplinary approach in managing an outbreak. Measures such as contact tracing and a 12-month prophylaxis strategy were important in limiting further infections. To date, there have been no further PJP cases in this cohort. We have also instituted PJP prophylaxis in patients treated for graft rejection as part of our unit policy.
Conclusion
In conclusion, we submit that hypercalcaemia of an unclear aetiology and refractory to treatment in a renal transplant recipient can be a feature of impending PJP. An early bronchoscopy is essential to aid the diagnosis. Early recognition and cohort prophylaxis for 12 months is a proven strategy that can limit PJP progression, with a multidisciplinary approach representing a crucial component of any outbreak containment strategy.
Conflict of interest statement
None declared.
References
- 1. Carmona EM, Limper AH.. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther Adv Respir Dis 2011; 5: 41–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morris A, Wei K, Afshar K. et al. Epidemiology and clinical significance of Pneumocystis colonization. J Infect Dis 2008; 197: 10–17 [DOI] [PubMed] [Google Scholar]
- 3. Gordon SM, LaRosa SP, Kalmadi S. et al. Should prophylaxis for Pneumocystis carinii pneumonia in solid organ transplant recipients ever be discontinued? Clin Infect Dis 1999; 28: 240–246 [DOI] [PubMed] [Google Scholar]
- 4. Fishman JA. Prevention of infection due to Pneumocystis carinii. Antimicrob Agents Chemother 1998; 42: 995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Boer MGJ, de Fijter JW, Kroon FP.. Outbreaks and clustering of Pneumocystis pneumonia in kidney transplant patients: a systematic review. Med Mycol 2011; 49: 673–680 [DOI] [PubMed] [Google Scholar]
- 6. de Boer MGJ, Kroon FP, Le Cessie S. et al. Risk factors for Pneumocystis jiroveci pneumonia in kidney transplant recipients and appraisal of strategies for selective use of chemoprophylaxis. Transpl Infect Dis 2011; 13: 559–569 [DOI] [PubMed] [Google Scholar]
- 7. Kubak BM. Fungal infection in lung transplantation. Transpl Infect Dis 2002; 4(Suppl 3): 24–31 [DOI] [PubMed] [Google Scholar]
- 8. Green H, Paul M, Vidal L. et al. Prophylaxis for PCP in non-HIV immunocompromised patients (Review). The Cochrane Library. 2007; 3: 1–47 [DOI] [PubMed] [Google Scholar]
- 9. Chapman JR, Marriott DJ, Chen SCA. et al. Post-transplant Pneumocystis jiroveci pneumonia – a re-emerged public health problem? Kidney Int 2013; 84: 240–243 [DOI] [PubMed] [Google Scholar]
- 10. Kasiske BL, Zeier MG, Chapman JR. et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int 2010; 77: 299–311 [DOI] [PubMed] [Google Scholar]
- 11. EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.7.1 Late infections. Pneumocystis carinii pneumonia. Nephrol Dial Transplant 2002; 17(Suppl 4): 36–39 [PubMed] [Google Scholar]
- 12. Shelton E, Yong M, Cohney S.. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology 2009; 14: 696–699 [DOI] [PubMed] [Google Scholar]
- 13. Eitner F, Hauser IA, Rettkowski O. et al. Risk factors for Pneumocystis jiroveci pneumonia (PcP) in renal transplant recipients. Nephrol Dial Transplant 2011; 26: 2013–2017 [DOI] [PubMed] [Google Scholar]
- 14. Phipps LM, Chen SCA, Kable K. et al. Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients. Transplantation 2011; 92: 1327–1334 [DOI] [PubMed] [Google Scholar]
- 15. Chen WC, Chang SC, Wu TH. et al. Hypercalcemia in a renal transplant recipient suffering with Pneumocystis carinii pneumonia. Am J Kidney Dis 2002; 39: E8. [DOI] [PubMed] [Google Scholar]
- 16. Chatzikyrkou C, Clajus C, Haubitz M. et al. Hypercalcemia and Pneumocystis pneumonia after kidney transplantation: report of an exceptional case and literature review. Transpl Infect Dis 2011; 13: 496–500 [DOI] [PubMed] [Google Scholar]
- 17. Aguirre AR, Balbo BEP, Ianhez LE. et al. Hypercalcemia and suppressed PTH levels in a renal transplant patient infected with pneumocystis carinii. Ren Fail 2007; 29: 513–516 [DOI] [PubMed] [Google Scholar]
- 18. Ramalho J, Marques IDB, Aguirre AR. et al. Pneumocystis jirovecii pneumonia with an atypical granulomatous response after kidney transplantation. Transpl Infect Dis 2014; 16: 315–319 [DOI] [PubMed] [Google Scholar]
- 19. Hung YM. Pneumocystis carinii pneumonia with hypercalcemia and suppressed parathyroid hormone levels in a renal transplant patient. Transplantation 2006; 81: 639. [DOI] [PubMed] [Google Scholar]
- 20. Bency R, Roger SD, Elder GJ.. Hypercalcemia as a prodromal feature of indolent Pneumocystis jiroveci after renal transplantation. Nephrol Dial Transplant 2011; 26: 1740–1742 [DOI] [PubMed] [Google Scholar]
- 21. Hajji K, Dalle F, Harzallah A. et al. Vitamin D metabolite-mediated hypercalcemia with suppressed parathormone concentration in Pneumocystis jiroveci pneumonia after kidney transplantation. Transplant Proc 2009; 41: 3320–3322 [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization. Disease Outbreaks http//www.who.int/topics/disease_outbreaks/en/ (2 February 2017, date last accessed)
- 23. Morris A, Norris KA.. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 2012; 25: 297–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goto N, Futamura K, Okada M. et al. Management of Pneumocystis jirovecii pneumonia in kidney transplantation to prevent further outbreak. Clin Med Insights Circ Respir Pulm Med 2015; 9(Suppl 1): 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de la Rua NM, Samuelson DR, Charles TP. et al. CD4+ T-cell-independent secondary immune responses to Pneumocystis pneumonia. Front Immunol 2016; 7: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Limper AH, Hoyte JS, Standing JE.. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Invest 1997; 99: 2110–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muirhead N, Zaltman JS, Gill JS. et al. Hypercalcemia in renal transplant patients: prevalence and management in Canadian transplant service. Clin Transplant 2014; 28: 161–165 [DOI] [PubMed] [Google Scholar]
- 28. Reichel H, Koeffler HP, Barbers R. et al. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65: 1201–1209 [DOI] [PubMed] [Google Scholar]
- 29. Bell NH. Renal and nonrenal 25-hydroxyvitamin D-1alpha-hydroxylases and their clinical significance. J Bone Miner Res 1998; 13: 350–353 [DOI] [PubMed] [Google Scholar]
- 30. Adams JS, Sharma OP, Gacad MA. et al. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest 1983; 72: 1856–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma OP. Vitamin D, calcium and sarcoidosis. Chest 1996; 109: 535–539 [DOI] [PubMed] [Google Scholar]
- 32. Barbour GL, Coburn JW, Slatopolsky E. et al. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med 1981; 305: 440–443 [DOI] [PubMed] [Google Scholar]
- 33. Bienaime F, Girard D, Anglicheau D. et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol 2013; 24: 831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGregor R, Li G, Penny H, et al. Vitamin D in renal transplantation –from biological mechanisms to clinical benefits. Am J Transplant 2014; 14: 1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma OP. Hypercalcaemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med 2000; 6: 442–447 [DOI] [PubMed] [Google Scholar]
- 36. Spindel SJ, Hamill R, Georghiou PR. et al. Case report: vitamin D-mediated hypercalcemia in fungal infections. Am J Med Sci 1995; 310: 71–76 [DOI] [PubMed] [Google Scholar]
- 37. Ali MY, Gopal KV, LLerena LA. et al. Hypercalcemia associated with infection by Cryptococcus neoformans and Coccidioides immitis. Am J Med Sci 1999; 318: 419. [DOI] [PubMed] [Google Scholar]
- 38. Limper AH, Offord KP, Smith TF. et al. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 1989; 140: 1204–1209 [DOI] [PubMed] [Google Scholar]
- 39. Robert-Gangneux F, Belaz S, Revest M. et al. Diagnosis of Pneumocystis jirovecii pneumonia in immunocompromised patients by real-time PCR: a 4-year prospective study. J Clin Microbiol 2014; 52: 3370–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Onishi A, Sugiyama D, Kogata Y. et al. Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 2012; 50: 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borstnar S, Lindic J, Tomazic J. et al. Pneumocystis jirovecii pneumonia in renal transplant recipients: a national centre experience. Transplant Proc 2013; 45: 1614–1617 [DOI] [PubMed] [Google Scholar]
- 42. Jairam A, Dassi M, Chandola P. et al. Pneumocystis jirovecii outbreak in a renal transplant centre: lessons learnt. Indian J Nephrol 2014; 24: 276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radisic M, Lattes R, Chapman JF. et al. Risk factors for Pneumocystis carinii pneumonia in kidney transplant recipients: a case-control study. Transpl Infect Dis 2003; 5: 84–93 [DOI] [PubMed] [Google Scholar]
- 44. Iriart X, Challan Belval T, Fillaux J. et al. Risk factors of Pneumocystis pneumonia in solid organ recipients in the era of the common use of posttransplantation prophylaxis. Am J Transplant 2015; 15: 190–199 [DOI] [PubMed] [Google Scholar]
- 45. Brakemeier S, Durr M, Bachmann F. et al. Risk evaluation and outcome of Pneumocystis jirovecii pneumonia in kidney transplant patients. Transplant Proc 2016; 48: 2924–2930 [DOI] [PubMed] [Google Scholar]
- 46. Arichi N, Kishikawa H, Mitsui Y. et al. Cluster outbreak of Pneumocystis pneumonia among kidney transplant patients within a single centre. Transplant Proc 2009; 41: 170–172 [DOI] [PubMed] [Google Scholar]
- 47. Yale SH, Limper AH.. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc 1996; 71: 5–13 [DOI] [PubMed] [Google Scholar]
- 48. Lufft V, Kliem V, Behrend M. et al. Incidence of Pneumocystis carinii pneumonia after renal transplantation. Impact of immunosuppression. Transplantation 1996; 62: 421–423 [DOI] [PubMed] [Google Scholar]
- 49. Neff RT, Jindal RM, Yoo DY. et al. Analysis of USRDS: incidence and risk factors for Pneumocystis jiroveci pneumonia. Transplantation 2009; 88: 135–141 [DOI] [PubMed] [Google Scholar]
- 50. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3): S1–S155. [DOI] [PubMed] [Google Scholar]
- 51. McCaughan JA, Courtney AE.. Pneumocystis jiroveci pneumonia in renal transplantation: time to review our practice? Nephrol Dial Transplant 2012; 27: 13–15 [DOI] [PubMed] [Google Scholar]
- 52. Di Cocco P, Orlando G, Bonanni L. et al. A systematic review of two different trimethoprim-sulfamethoxazole regimens used to prevent Pneumocystis jirovecii and no prophylaxis at all in transplant recipients: appraising the evidence. Transplant Proc 2009; 41: 1201–1203 [DOI] [PubMed] [Google Scholar]
- 53. Evans RA, Clifford TM, Tang S. et al. Efficacy of once-weekly dapsone dosing for Pneumocystis jirovecii pneumonia prophylaxis post transplantation. Transpl Infect Dis 2015; 17: 816–821 [DOI] [PubMed] [Google Scholar]
- 54. Gabardi S, Millen P, Hurwitz S. et al. Atovaquone versus trimethoprim-sulfamethoxazole as Pneumocystis jirovecii pneumonia prophylaxis following renal transplantation. Clin Transplant 2012; 26: 184–190 [DOI] [PubMed] [Google Scholar]
- 55. Yazaki H, Goto N, Uchida K. et al. Outbreak of PJP in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation 2009; 88: 380–385 [DOI] [PubMed] [Google Scholar]
- 56. Mitsides N, Greenan K, Green D. et al. Complications and outcomes of trimethoprim-sulphamethoxazole as chemoprophylaxis for pneumocystis pneumonia in renal transplant recipients. Nephrology 2014; 19: 157–163 [DOI] [PubMed] [Google Scholar]