Abstract
Advances in molecular profiling and the application of advanced imaging techniques are currently refreshing diagnostic considerations in meningioma patients. Not only technical refinements but also sophisticated histopathological and molecular studies have the potential to overcome some of the challenges during meningioma management. Exact tumor delineation, assessment of tumor growth, and pathophysiological parameters were recently addressed by “advanced” MRI and PET. In the field of neuropathology, high-throughput sequencing and DNA methylation analysis of meningioma tissue has greatly advanced the knowledge of molecular aberrations in meningioma patients. These techniques allow for more reliable prediction of the biological behavior and clinical course of meningiomas and subsequently have the potential to guide individualized meningioma therapy. However, higher costs and longer duration of full molecular work-up compared with histological assessment may delay the implementation into clinical routine.
This review highlights the diagnostic challenges of meningiomas from both the neuroimaging as well as the neuropathological side and presents the latest scientific achievements and studies potentially helping in overcoming these challenges. It complements the recently proposed European Association of Neuro-Oncology guidelines on treatment and diagnosis of meningiomas by integrating data on nonstandard imaging and molecular assessments most likely impacting the future.
Keywords: diagnosis, meningioma, molecular markers, MRI, neuropathology, PET
Meningiomas are the most frequently reported primary CNS tumors, comprising ~36.1% of all CNS tumors, with an incidence of 7.61/100000.1 They originate from arachnoid meningothelial cells and therefore belong to the group of intracranial extra-axial neoplasms. Just as in other neuro-oncologic entities (eg, gliomas), efforts are under way to incorporate molecular profiling into the diagnostic work-up to allow for a better characterization of the biological behavior of meningiomas and subsequently guide individualized therapy.2,3 In the updated World Health Organization (WHO) classification of 2016, the grading and classification of meningiomas did not undergo major revisions, except for the introduction of brain invasion as a criterion for the diagnosis of an atypical meningioma.4
Recently, guidelines for diagnosis and treatment of meningiomas have been published by the European Association of Neurooncology (EANO).2 If treatment is required, surgery is the first option. However, there is a significant subset of patients (especially with higher WHO grades) who are not successfully managed by surgery alone or in whom a complete resection is not possible due to the location of the tumor to eloquent brain areas. In these patients, adjuvant therapy regimens mainly in the form of radiotherapy need to be applied. Various treatment concepts combining surgery and radiosurgery or fractionated radiotherapy with different radiation schedules as well as pharmacological approaches are being developed and prospective randomized trials are currently ongoing.2 Importantly, according to the EANO guidelines, incidentally diagnosed and radiologically presumed meningiomas (usually asymptomatic) may be managed with observation only. In these patients, treatment may be withhold until symptoms develop, sustained growth occurs, or concerns of entrapment on sensitive structures arises.2,5 In these cases, definitive diagnosis including histological classification and grading is lacking without tissue diagnosis; risk assessment for tumor growth and progression is then exclusively based on clinical and standard MRI findings. Given the number of tumors managed without invasive diagnosis, meningiomas are one of the most important neuro-oncologic entities requiring highly precise and reliable non-invasive diagnostic modalities.
This review highlights the daily diagnostic challenges that arise during clinical management of meningioma patients from both the neuroimaging and the neuropathology point of view and complements the current guidelines by integrating nonstandard imaging and molecular assessments most likely impacting the future.
Meningioma Diagnosis
Imaging Techniques and Findings
The tentative diagnosis of meningioma can be made by contrast-enhanced MRI,2 which is also used for long-term follow-up because of the superior soft-tissue capabilities and absence of radiation exposure. In case of contraindications (eg, pacemaker), contrast-enhanced CT can be applied as an alternative cross-sectional technique.6 Intra-arterial cerebral angiography has no routine role in the diagnostic workup of meningiomas but can be used as an adjunct to treatment planning or preoperative devascularization in selected cases.2 As meningioma cells strongly express somatostatin-receptor subtype 2 (SSTR2),7 PET-based imaging using SSTR ligands such as 68Ga-DOTATOC (DOTA-(Tyr3)-octreotide) and 68Ga DOTATATE (DOTA-D-Phe1-Tyr3-octreotate) has shown to be a helpful additional diagnostic tool.8–10
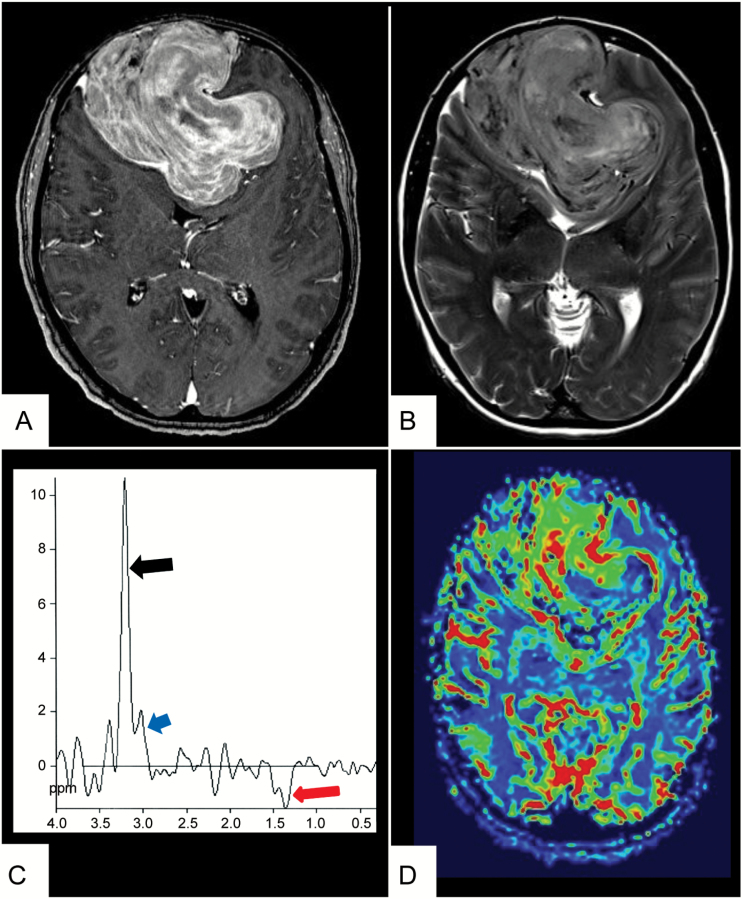
Meningiomas appear as broad-based dural hemispheric or oval lesions, attached to the dura mater. They most frequently occur supratentorially at the calvaria or the skull base meninges, along the falx and in the parafalcine location, but they can also be found attached to the tentorium, in the cerebello-pontine angle, within the optic nerve sheath, or occurring intraventricularly.11 On CT, meningiomas usually appear isodense but can occasionally be hyperdense or slightly hypodense compared with brain tissue.12 CT is more sensitive than MRI in detecting psammomatous calcifications in the tumor, which is seen in approximately 25% of meningiomas. Best demonstrated on CT are osseous destructions which were shown to be indicative of atypical or malignant meningioma. In contrast, hyperostosis of adjacent skull bone, radiologically characterized by cortical thickening and hyperdensity, is highly suggestive of benign meningioma.6 Hyperostosis associated with meningiomas has shown to be caused by tumor invasion of the bone.12 On MRI, meningiomas present isointense to the cortex on T1- and T2-weighted sequences and with typically a strong homogeneous enhancement following administration of gadolinium contrast. An enhancing “dural tail” adjacent to the tumor, which in histopathological correlations has occasionally shown a tumor invasion into the dura mater, represents a hypervascular, nonneoplastic reaction in the majority of cases.13 Benign meningiomas typically derive their main blood supply from the external carotid via dural branches. These vessels do not contain a blood–brain barrier and are thus permeable to gadolinium, generating typical time–intensity curves in T2*-weighted MRI, with little or no return to baseline following contrast agent application.14 However, as the meningioma enlarges, it may recruit pial branches at the periphery of the tumor from the brain parenchyma, which do contain a blood–brain barrier, showing an elevated relative cerebral blood volume (rCBV) with time–intensity curves that return to baseline signal levels.15 MR spectroscopy with the ability to evaluate metabolite concentrations within a given region of interest shows a characteristic alanin peak at 1.3–1.5 ppm16 (Fig. 1). These pathophysiologic backgrounds allow functional imaging techniques to assess tumor aggressiveness and solve differential diagnostic problems as stated below.11,15,17
Fig. 1.
Standard plus advanced MRI in a large bifrontal meningioma. MR images of a 67-year-old man with a 4-month history of progressive frontal headaches associated with decreased motivation and personality changes. (A) T1-weighted contrast-enhanced MR image shows a large meningioma bifrontally with intense, relatively inhomogeneous enhancement. (B) Axial T2-weighted sequence shows high signal intensity of the tumor with very little peritumoral edema. (C) MR spectroscopy shows a prominent resonance from choline (black arrow) and creatine (blue arrow) and an inverted doublet peak at 1.45 ppm, corresponding to alanin as a typical marker for meningiomas (red arrow). (D) In MR perfusion there is increased blood volume at the periphery of the lesion due to pial blood supply and heterogeneous blood volume in the tumor center.
Histological Features
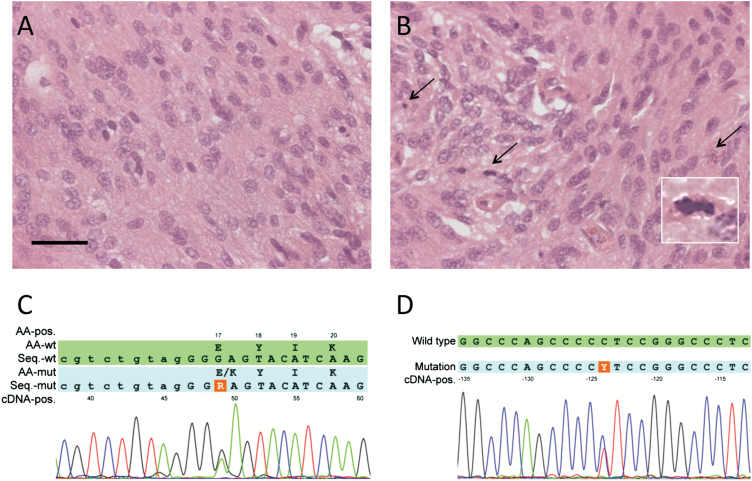
The majority of cases display a histology that allows identification of the entity meningioma upon inspection of hematoxylin/eosin staining. Typical features include formation of whorls of tumor cells, nuclear pseudo-inclusions, pseudo-syncytial growth, and formation of concentric calcifications, called “psammoma bodies.”4 Immunohistochemistry for epithelial membrane antigen or SSTR2a,18 which are usually positive in meningioma, support the histological diagnosis (Fig. 2).
Fig. 2.
Molecular versus histological diagnosis in meningioma. (A) A meningioma with widely inconspicuous histology and low mitotic activity may harbor (B) a small area of elevated proliferation. If this area is included in the 10 HPF for assessment of mitotic activity, it might surpass the threshold for WHO grade II. However, if this area is regarded as nonrepresentative and excluded from assessment of mitotic activity, or not even present in the section due to sampling bias, the identical tumor might be assigned WHO grade I. (C) Detection of an AKT1 mutation associated with WHO grade I and slow or no progression, or (D) of a TERT promoter mutation, indicating a higher risk of recurrence, gives additional objective information on the tumor biology. Scale bar represents 100 µm.
Challenge I: Differential Diagnoses
Radiological Aspects
A variety of intracranial lesions may radiologically mimic meningioma. Tumor location and growth pattern, however, might be helpful for differential diagnostic considerations (Table 1). If located in the cerebral hemispheres, meningiomas can be difficult to distinguish from dural metastases especially from prostate, lung, kidney, or breast cancers,19,20 primary glial tumors that extend into the subarachnoid space,21 and hematopoietic neoplasms such as extra-axial non-Hodgkin lymphoma.22 Characteristic imaging changes, suggestive of a meningioma, along the optic nerve sheath or the cavernous sinus may represent glioma or even inflammatory (rheumatoid arthritis, Wegener’s granulomatosis, extra-axial neurosarcoidosis) and infectious diseases (tuberculosis, syphilitic gumma).13,23,24 Pituitary neoplasms like adenomas or craniopharyngiomas may also mimic meningiomas. If located at the skull base and especially at the cerebello-pontine angle, meningiomas have to be distinguished from vestibular schwannomas and neoplastic meningitis; if located within ventricles, they need to be differentiated from other ventricle tumors such as choroid plexus papillomas/carcinomas, ependymomas or metastases,11 and solitary fibrous tumors/hemangiopericytomas.25 As differential diagnoses along the spinal cord, metastases, subependymoma, and ependymoma have to be considered.
Table 1.
Differential diagnoses11 ,13 ,21 ,25 ,89–94
| Localization | Differential Diagnoses |
|---|---|
| Cerebral hemispheres | -Dural metastases (prostate, lung, kidney, breast cancer, neuroblastoma) -Glioma -Non Hodgkin lymphoma -Primary Hodgkin disease -Solitary fibrous tumor/hemangiopericytoma -Pleomorphic xanthoastrocytoma -Hemangioblastoma -Cavernoma -Mucosa-associated lymphoid tissue lymphoma (MALT) -Histiocytosis |
| Optic nerve sheath | -Optic glioma -Optic neuritis |
| Cavernous sinus | -Glioma -Inflammatory (rheumatoid arthritis, Wegener granulomatosis, neurosarcoidosis) -Infectious disease (tuberculosis, syphilitic gumma) -Erdheim–Chester disease and eosinophilic granuloma |
| Nasal cavity | -Adenoid cystic carcinoma -Chloroma -Esthesioneuroblastoma |
| Spinal cord | -Glioma -Ependymoma -Subependymoma -Schwannoma -Embryonal tumor -Meningeal melanoma |
| Cerebello- pontine angle | -Schwannoma -Neoplastic meningitis -Papillary middle-ear tumor -Metastases -Plasmacytoma -Teflon granuloma following microvascular decompression |
| Ventricles | -Choroid plexus papillomas/carcinomas -Ependymomas -Metastases |
| Pituitary gland | -Pituitary adenoma -Adenohypophysitis -Pituitary apoplexy -MALT95 -Craniopharyngioma |
| Arterial malformations | -Arterial aneurysm -Dural cavernous angioma |
Functional imaging techniques including MR perfusion may differentiate between meningioma and dural metastases from different entities (breast, colon, and prostate carcinoma),26 with the exception of metastases from Merkel cell carcinoma, renal carcinoma, or melanoma, which also represent hypervascular lesions with elevated CBV values.26,27 High-grade glioma invading the dura mater may also be difficult to distinguish from meningioma, as both lesions show high rCBV values in perfusion MRI.28,29 In these cases, the evaluation of the time–intensity curve was shown to be a helpful approach14 (Table 2). In MR spectroscopy, an elevated distinct metabolite peak at 3.8 ppm may allow a differentiation between meningiomas, high-grade gliomas, and intracranial metastases.30
Table 2.
Challenges during menigioma management and recent study results on how to overcome them
| CHALLENGE | Diagnostic Approach | |
|---|---|---|
| Differential diagnosis | MRI |
Standard MRI—tumor location might be helpful (Table 1) MR perfusion14 ,26 to differentiate between meningioma and dural metastases, CAVE— tumors with elevated blood perfusion MRS peak at 3.8 ppm in meningiomas.30 |
| PET | - | |
| Neuropathology | STAT6 staining 31–34 to differentiate between meningioma and solitary fibrous tumor/ hemangiopericytoma | |
| Subtyping and grading | MRI |
Anatomical features
41
to distinguish between WHO grades Contradictory results on DW-MRI42 ,43 and perfusion MRI17 for meningioma subtyping |
| PET | Dynamic 18 F-FET PET 44 to distinguish between high-grade and low-grade meningiomas | |
| Neuropathology |
Histological features
4
: brain invasion as new additional criterion to diagnose atypical meningioma WHO grade II Molecular pathology62–67 : -AKT1/TRAF7 and SMO mutations mostly in basal meningioma with meningothelial histology -KLF4/TRAF7 mutation in secretory meningiomas WHO grade I |
|
| Assessment of tumor growth and risk of recurrence | MRI |
Follow-up imaging according to WHO grade
2
-WHO grade I: 1x/year for 5 years, afterward every 2 years -WHO grade II: every 6 months for 5 years, afterward 1x/year -WHO grade III: every 3–6 months indefinitely |
| PET |
68
Ga-DOTATATE PET
75
: SUVmax predicts faster growth in WHO grade I and II meningioma, not in WHO grade III 11C-MET PET76 ,77 : contradictory results on predicting tumor growth |
|
| Neuropathology | TERT promoter region mutation 3 , 68 , 69 is associated with increased risk of recurrence and shorter progression-free survival, present in only 6%–8% of meningiomas | |
| Delineation of tumor extent | MRI | Standard MRI has difficulties in differentiating meningioma from adjacent anatomical structures (skull base) and postoperative changes. |
| PET | 68 Ga-DOTATATE PET 10 to discriminate meningioma from tumor-free tissue uptake (SUVmax threshold, 2.3) and to improve target volume definition in radiation planning8 ,82–84 in the vicinity of bony skull base or after complex surgical procedures | |
| Neuropathology | Histological assessment of dura invasion and of other surrounding structures. | |
Abbreviations: ADC = apparent diffusion coefficient, MRS = magnetic resonance spectroscopy, ppm = parts per million, STAT6 = signal transducer and activator of transcription 6, 18F-FET = O-(2-[18F]-fluoro-ethyl)-L-tyrosine, 11C-MET = [11C]Methionine,68 Ga-DOTATATE = DOTA-(Tyr3)-octreotide, TERT = telomerase reverse transcriptase. SUV = standardized uptake value.
Histological Aspects
Compared with imaging, differential diagnostic problems play only a minor role during neuropathological assessment. If necessary, the most common differential diagnoses such as solitary fibrous tumor/hemangiopericytoma can be ruled out by staining for signal transducer and activator of transcription 6, which is strongly positive in the nucleus of solitary fibrous tumor/hemangiopericytoma but confined to the cytoplasm in meningioma31–34 (Table 2). Once the entity is identified, the meningioma has to be assigned to one of 15 meningioma subtypes which have evolved over decades. This dates back to the 1920s when Bailey and Cushing proposed the first classification schemes of meningioma, establishing the still recognized fibroblastic, meningothelial, and angiomatous subtypes.35,36 Importantly, subsequent studies added to the spectrum of subtypes and reported different propensities for recurrence.37–40
Challenge II: Meningioma Subtyping and Grading
Imaging Features
Up to now, the value of meningioma grading on the basis of neuroimaging has been low. In a retrospective study of 120 meningioma patients, distinct MRI features (such as indistinct tumor–brain interface, positive capsular enhancement, and heterogeneous tumor enhancement) were shown to correlate with a higher WHO grade.41 Contradictory results on tumor grading were reported for diffusion-weighted (DW) MRI.42,43 In a retrospective study of 177 meningioma patients, DW-MRI had no value in both determining the histological behavior and differentiating between histopathological subtypes of meningiomas.42 In contrast, the preoperative assessment of the apparent diffusion coefficient (ADC) from DW-MRI showed to be inversely correlated with the histological grade of 77 meningioma patients. Studies on MR perfusion for meningioma subtyping are scarce and existing evidence is likewise contradictory.17,43 In a small pilot study, 24 meningioma patients were examined using dynamic O-(2-[18F]-fluoro-ethyl)-L-tyrosine (18F-FET) PET and it could be observed that different patterns of time–activity curves in combination with tumor-to-brain ratios are able to differentiate between high-grade and low-grade meningiomas44 (Table 2).
Histological Features
As stated above, the challenge in the neuropathological work-up of meningioma is usually not the identification of the entity, but subtyping and grading. The WHO classification recognizes 15 subtypes of meningioma4: 9 are allotted to WHO grade I, and 3 each to WHO grades II and III.4 The criteria for consecutive subtyping and grading are based purely on histology. Among grade I meningiomas, the 9 variants are defined by cytological or histoarchitectonic features. Examples are presence of secretory granula, yielding the diagnosis of secretory meningioma WHO grade I, or spindle cells in a collagen-rich matrix in fibrous meningioma WHO grade I. Yet, several of the various histological patterns can occur in the same tumor to varying extent, making subtyping prone to a considerable interobserver and sampling bias.45 The lack of exact delineation of subtypes might be of limited clinical relevance among WHO grade I tumors. However, the diagnosis of a higher grade is based on similar, purely histological criteria. Atypical meningioma WHO grade II is diagnosed if increased mitotic activity is detected, independently of the presence of any of the histological patterns found in grade I tumors. As a threshold for increased mitotic activity, the current classification recommends using either ≥4 mitotic figures in 10 microscopic high-power fields (HPF) (ie, about 0.16 mm2 each) or ≥5 mitotic figures in 10 HPF if the tumor has markedly increased cellularity. Moreover, a catalogue of alternative criteria exists: high cell density, pleomorphism, necrosis, “sheeting”-like growth, high core/nucleus ratio. If 3 of these are present, atypical meningioma can be diagnosed in the absence of increased mitotic activity. Finally, histological evidence for brain invasion is another independent criterion for atypical meningioma WHO grade II, newly introduced in the recent update of the WHO classification.4
Besides these manifold criteria for atypical meningioma WHO grade II, clear cell histology and cord-like growth pattern in a mucoid matrix also render a grade II diagnosis, namely clear cell or chordoid meningioma WHO grade II. Observations that meningioma with these features tend to have more aggressive behavior lead to their general designation as WHO grade II.4,37,40 However, these variants are so rare that studies investigating the prognostic relevance had to rely on limited case numbers. Similarly, grade III meningioma is defined by “overtly malignant histology” and “markedly elevated mitotic activity,” usually regarded as ≥20 mitotic figures per 10 HPF, then termed anaplastic meningioma WHO grade III. Formation of perivascular pseudopapillary growth or rhabdoid cells can also qualify for grade III, termed papillary or rhabdoid meningioma WHO grade III.4,38,39,46,47
Recent studies have questioned the reliability of several of these criteria. Brain invasion, a criterion for atypical meningioma WHO grade II, was not associated with increased risk of recurrence in 3 recent reports.48–50 In another recent study, rhabdoid cytology was not indicative of increased risk of recurrence when other criteria of malignancy were lacking.51 Accordingly, the updated WHO classification recommends to diagnose rhabdoid meningioma WHO grade III only if these cytological features are accompanied by increased proliferation. Although the concordance for histological grading was up to 87.2% in a comparative Radiation Therapy Oncology Group study,45 the high subjectivity of evaluation criteria is a matter of ongoing debates. Risk stratification based on biomarkers is anticipated to increase the prediction power of the classification (Table 2).
Molecular Profiles
Meningiomas were among the first tumors in which cytogenetic aberrations were identified: loss of a copy of 22q52 frequently accompanied by mutations of the remaining NF2 allele affects 60%–80% of meningiomas.53–57 Subsequent studies identified that an accumulation of cytogenetic aberrations, most frequently losses of 1p, 10, and 14q, is associated with malignancy and risk of recurrence.58–60 Thus, novel grading algorithms were proposed that take chromosomal aberrations into account.61 However, their advantage over the WHO classification in prognostic accuracy and the cost of cytogenetic analyses rendered this approach unfeasible for routine diagnostic application.
High-throughput sequencing of meningiomas have significantly advanced the knowledge of molecular aberrations in meningioma during the past 4 years. Besides NF2 mutations, recurrent mutations in AKT1, SMO, PIK3CA, KLF4, TRAF7, POLR2A, PRKAR1A, and SUFU were detected.62–67 Except for SUFU, they occur mutually exclusively to NF2 mutations in most cases. Moreover, activating hotspot mutations in AKT1 and KLF4 usually coincide with mutations in TRAF7. No exact hotspot was detected for TRAF7. Instead, the mutations are distributed throughout the sequence that codes for the WD40 domain of the TRAF7 protein. Intriguingly, several of these mutations or combinations of mutations are associated with distinct tumor localizations or histological subtypes. AKT1/TRAF7 and SMO mutations mostly affect basal meningioma with meningothelial histology. KLF4/TRAF7 mutant meningiomas are virtually always associated with occurrence of secretory granula in the tumor and, therefore, molecularly define secretory meningiomas of WHO grade I. These findings might have both a diagnostic as well as a therapeutic impact. With inhibitors of mutant AKT, SMO, and PIK3CA available, this novel insight might soon be applied for novel therapy approaches in meningioma. First clinical trials have already been initiated (eg, NCT02523014).
As stated above, cytogenetic aberrations have shown to be associated with malignancy and risk of recurrence.58–60 However, none of these markers has been demonstrated to be of prognostic relevance. In contrast, mutations in the promoter region of TERT are strongly associated with increased risk of recurrence and shorter progression-free survival compared with meningiomas with identical histology but TERT wild-type status.3,68,69 Thus, TERT might emerge as the first molecular biomarker of more aggressive meningioma. While TERT mutations affect only about 6%–8% of meningiomas, further studies and trials are warranted to identify reliable markers for the full range of meningioma subtypes (Table 2).
Recent studies on genome-wide DNA methylation profiling distinguished 6 distinct molecular classes associated with a more homogeneous clinical course. The methylation-based classification system allows prognostication with higher power than the WHO classification, which is morphology based. Specifically, patients with WHO grade I histology but a high risk of recurrence and patients at lower risk of recurrence among WHO grade II tumors are better differentiated. In addition, the number of relevant subtypes may be reduced from the currently recognized 15 histological variants to 6 clinically relevant molecular classes, each with a characteristic molecular profile70 (Fig. 2).
Challenge III: Assessment of Tumor Growth
Imaging Features
A major challenge in meningioma management is the early prediction of tumor recurrence or progression (ie, the identification of meningioma patients with progressive tumor growth). Given the generally slow growth rate of benign meningiomas, the tumor must reach a substantial size before regrowth and progression can be diagnosed, and it is critical to select an appropriate time point for therapy initiation, especially in regions difficult to access, such as the skull base. Recommended intervals of follow-up imaging by standard MRI currently depend on the WHO grade.2 WHO I grade tumors require annual MRI assessments for 5 years followed by biannual follow-ups. Follow-up imaging of WHO grade II meningiomas should be done every 6 months, then annually after 5 years, and WHO grade III tumors require follow-up every 3–6 months indefinitely. The most important factor to estimate tumor recurrence is the grade of surgical resection as defined by Simpson.71,72 Further risk factors for progression and recurrence of meningiomas are not fully understood and it therefore is of great interest to find methods that can predict tumor progression earlier than solely when there is a radiological increase in size. In this context, the natural history of 273 intracranial meningiomas being managed purely conservatively was studied, and factors associated with a higher annual growth rate were male sex, initial tumor diameter greater than 25 mm, T2 signal hyperintensity and edema on MRI, and the presence of symptoms.73
An imaging-based approach in 144 meningioma patients following surgery showed that DW-MRI including ADC maps outperformed WHO grading for the prediction of progression after initial treatment.74 In this retrospective study the authors were able to stratify the patients into 3 risk groups and showed that patients with non-Simpson grade I resection and low ADC values have a significant risk of progression or recurrence and may benefit from adjuvant radiotherapy and/or additional surgery.
Promising results for the identification of an increased growth rate were recently reported by Sommerauer et al,75 who showed that a high expression of SSTR2 in 64 meningioma patients as measured by maximum standardized uptake value (SUVmax) in 68Ga-DOTATATE PET predicts faster growth in WHO grades I and II meningioma, whereas WHO grade III meningioma did not show an association of tumor growth rate with tracer binding. The authors stated that these data may help in planning the optimal time point for surgery and for follow-up imaging and may especially be valuable for newly diagnosed meningioma in critical locations such as the skull base.75 Contradictory findings on the assessment of the tumor growth rate were reported for the radiolabeled amino acid 11C-methyl-L-methionine (11C-MET).76,77 However, a recently reported 10-year follow-up evaluation of proton beam irradiated meningioma patients using 11C-MET PET78 showed that in cases where tumor remnants showed progression (n = 2), the 11C-MET uptake ratio increased considerably earlier than the volume increase on MRI.78
Challenge IV: Delineation of Tumor Extent
Following surgery, conventional neuroimaging with CT or MRI has limitations in distinguishing between tumor remnants and adjacent anatomical structures, postoperative changes (eg, scars),79 or bone involvement.80 This constitutes a major challenge in delineating tumor borders, which is particularly important for subsequent treatment planning such as (re-)resection or radiation therapy (ie, definition of the target volume).
Data on ultra-high-field MRI were recently reported for 4 meningioma patients. The advantages of 7.0 Tesla MRI over 1.5 Tesla MRI were a more detailed depiction of the peri- and intratumoral vasculature and a clear delineation of the tumor–brain interface useful for surgical planning. The authors, however, reported an impaired image quality in skull base lesions at 7.0 Tesla due to susceptibility artifacts.81
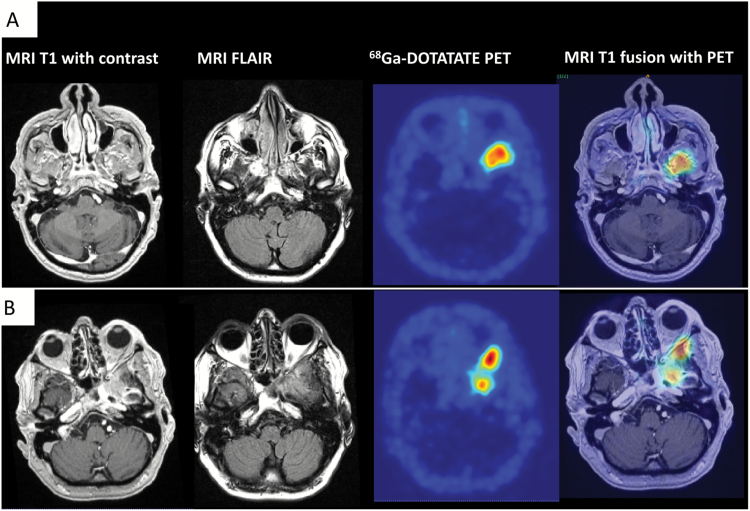
PET studies have shown to be of more value to overcome this challenge. Rachinger and colleagues observed important findings for the understanding of the 68Ga-DOTATATE PET signal.10 In a study with neuronavigated tissue sampling, they found that an increased 68Ga-DOTATATE uptake (SUVmax threshold, 2.3) in PET imaging discriminates meningioma and tumor-free tissue with higher sensitivity than standard MRI (90% vs 79%)10 (Fig. 3).
Fig. 3.
68Ga-DOTATATE PET for the differentiation of meningioma from tumor-free tissue and postoperative changes. (A) Preoperative imaging. MR and PET images of a 55-year-old male patient who presented with therapy-resistant left frontal headache for the past 6 months. T1-weighted MR images show subtle contrast enhancement at the skull base without exact delineation of tumor borders. The fluid attenuated inversion recovery sequence shows diffuse signal changes. In contrast, 68Ga-DOTATATE PET (DOTA-(Tyr3)-octreotide) is able to differentiate between meningioma and tumor-free tissue with an excellent tumor-to-background contrast. Fusion images were used for surgical planning. (B) Imaging at recurrence. Histological grading revealed a WHO grade II meningioma. After subtotal surgical resection (Simpson grade IV), the patient underwent radiation therapy. Follow-up imaging after 2 years revealed further tumor growth/recurrence. The T1-weighted image shows diffuse contrast enhancement at the skull base, difficult to be differentiated from postoperative changes. 68Ga-DOTATATE PET in contrast is consistent with meningioma tissue in this region with additional retrobulbar tumor growth.
A PET-based imaging approach with SSTR2 ligands has therefore been investigated for high-precision radiotherapy planning of subtotally resected or recurring complex skull base meningioma8,82–84 with the goal to spare as much critical tissue as possible without missing tumor. In these studies, radiation planning with 68Ga-DOTATOC-PET showed an improvement of target volume definition by providing additional information in around two thirds of cases,8,82–84 with most adaptations being necessary in the vicinity of bony skull base or after complex surgical procedures. Similar findings could be observed in other PET studies using 11C-MET.85,86
Future Directions and Prospects
Future attempts to understand meningioma may combine imaging, histological, and molecular features. Similar to parenchymal brain tumors, the concept of an “integrated diagnosis” will be adopted also for meningiomas.87 Integrated diagnoses consider the histological appearance and genetic and epigenetic aberrations and provide a more precise prediction of risk of recurrence in order to well advise patients, guide management decisions, and stratify for clinical trials. A further step in this context, complementing the evolving landscape of genetic analysis (“genomics”), can be “radiomics”—the extraction of a large number of quantitative imaging features and recognition of imaging phenotypes/patterns by automated data characterization.88 Linkage of these radiomic features to genomic data (“imaging-genomics” or “radiogenomics”) is currently an evolving research field that hopefully will also address meningioma challenges such as tumor growth kinetics and differential diagnoses as outlined above. Ultimately, it will be the goal not only to characterize meningioma tissue and give prognostic information but to offer potential therapeutic strategies—in the form of targeted therapies (eg, NCT02523014) or recently also in the form of immunologic approaches (eg, NCT02648997).
Summary
In summary, neuroimaging with standard MRI is the imaging modality of choice for the initial diagnosis and follow-up of meningioma patients. Functional imaging approaches such as perfusion MRI may help in differential diagnostic problems, and PET imaging especially with SSTR2 ligands may overcome diagnostic challenges with respect to tumor delineation and the evaluation of tumor growth rate. Research on molecular genetic alterations in meningiomas is currently ongoing with the goal to incorporate molecular markers into WHO classification and allow for a more precise assessment of prognosis, risk of recurrence, and guidance of therapy.
Conflict of interest statement. M.B. has received research funding from Siemens, Novartis, Bayer, Stryker, Medtronic, Codman, and Guerbet. He has received speaker honoraria from Codman, Roche, Bayer, Novartis, Teva, and Guerbet. He has a consultant relationship with B. Braun, Boehringer Ingelheim, Codman, Vascular Dynamics, and Guerbet. W.W. has participated in a speaker’s bureau for and has received research funding from MSD. He has received research funding from Apogenix, Boehringer Ingelheim, Genentech Roche, and Pfizer. F.S. has received research support and/or travel bursaries from Roche, Agilent, and Illumina. No other authors report a conflict of interest.
Acknowledgments
The authors confirm the originality of this manuscript. Parts of this review have been presented at the EANO meeting 2016 in Mannheim at the educational day.
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldbrunner R, Minniti G, Preusser M et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 3. Sahm F, Schrimpf D, Olar A et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5):pii: djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 5. Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT. Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg. 2010;113(5):1036–1042. [DOI] [PubMed] [Google Scholar]
- 6. Saloner D, Uzelac A, Hetts S, Martin A, Dillon W. Modern meningioma imaging techniques. J Neurooncol. 2010;99(3):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reubi JC, Schaer JC, Waser B, Mengod G. Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res. 1994;54(13):3455–3459. [PubMed] [Google Scholar]
- 8. Gehler B, Paulsen F, Oksüz MO et al. [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S et al. Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J Nucl Med. 2005;46(5):763–769. [PubMed] [Google Scholar]
- 10. Rachinger W, Stoecklein VM, Terpolilli NA et al. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–353. [DOI] [PubMed] [Google Scholar]
- 11. Zimny A, Sasiadek M. Contribution of perfusion-weighted magnetic resonance imaging in the differentiation of meningiomas and other extra-axial tumors: case reports and literature review. J Neurooncol. 2011;103(3):777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pieper DR, Al-Mefty O, Hanada Y, Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44(4):742–746; discussion 746. [DOI] [PubMed] [Google Scholar]
- 13. Guermazi A, Lafitte F, Miaux Y, Adem C, Bonneville JF, Chiras J. The dural tail sign—beyond meningioma. Clin Radiol. 2005;60(2):171–188. [DOI] [PubMed] [Google Scholar]
- 14. Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223(1):11–29. [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M. Perfusion MR imaging for differentiation of benign and malignant meningiomas. Neuroradiology. 2008;50(6):525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majós C, Cucurella G, Aguilera C, Coll S, Pons LC. Intraventricular meningiomas: MR imaging and MR spectroscopic findings in two cases. AJNR Am J Neuroradiol. 1999;20(5):882–885. [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M. Preoperative subtyping of meningiomas by perfusion MR imaging. Neuroradiology. 2008;50(10):835–840. [DOI] [PubMed] [Google Scholar]
- 18. Menke JR, Raleigh DR, Gown AM, Thomas S, Perry A, Tihan T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015;130(3):441–443. [DOI] [PubMed] [Google Scholar]
- 19. Tagle P, Villanueva P, Torrealba G, Huete I. Intracranial metastasis or meningioma? An uncommon clinical diagnostic dilemma. Surg Neurol. 2002;58(3-4):241–245. [DOI] [PubMed] [Google Scholar]
- 20. Bendszus M, Warmuth-Metz M, Burger R, Klein R, Tonn JC, Solymosi L. Diagnosing dural metastases: the value of 1H magnetic resonance spectroscopy. Neuroradiology. 2001;43(4):285–289. [DOI] [PubMed] [Google Scholar]
- 21. Wilms G, Lammens M, Marchal G et al. Prominent dural enhancement adjacent to nonmeningiomatous malignant lesions on contrast-enhanced MR images. AJNR Am J Neuroradiol. 1991;12(4):761–764. [PMC free article] [PubMed] [Google Scholar]
- 22. Bourekas EC, Wildenhain P, Lewin JS et al. The dural tail sign revisited. AJNR Am J Neuroradiol. 1995;16(7):1514–1516. [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002;33(12):1211–1226. [DOI] [PubMed] [Google Scholar]
- 24. Sandhu FA, Schellinger D, Martuza RL. A vascular sarcoid mass mimicking a convexity meningioma. Neuroradiology. 2000;42(3):195–198. [DOI] [PubMed] [Google Scholar]
- 25. Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996;17(7):1365–1371. [PMC free article] [PubMed] [Google Scholar]
- 26. Kremer S, Grand S, Rémy C et al. Contribution of dynamic contrast MR imaging to the differentiation between dural metastasis and meningioma. Neuroradiology. 2004;46(8):642–648. [DOI] [PubMed] [Google Scholar]
- 27. Kremer S, Grand S, Berger F et al. Dynamic contrast-enhanced MRI: differentiating melanoma and renal carcinoma metastases from high-grade astrocytomas and other metastases. Neuroradiology. 2003;45(1):44–49. [DOI] [PubMed] [Google Scholar]
- 28. Aronen HJ, Gazit IE, Louis DN et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191(1):41–51. [DOI] [PubMed] [Google Scholar]
- 29. Hakyemez B, Erdogan C, Bolca N, Yildirim N, Gokalp G, Parlak M. Evaluation of different cerebral mass lesions by perfusion-weighted MR imaging. J Magn Reson Imaging. 2006;24(4):817–824. [DOI] [PubMed] [Google Scholar]
- 30. Kousi E, Tsougos I, Fountas K et al. Distinct peak at 3.8 ppm observed by 3T MR spectroscopy in meningiomas, while nearly absent in high-grade gliomas and cerebral metastases. Mol Med Rep. 2012;5(4):1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chmielecki J, Crago AM, Rosenberg M et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45(2):131–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koelsche C, Schweizer L, Renner M et al. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology. 2014;65(5):613–622. [DOI] [PubMed] [Google Scholar]
- 33. Robinson DR, Wu YM, Kalyana-Sundaram S et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45(2):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schweizer L, Koelsche C, Sahm F et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125(5):651–658. [DOI] [PubMed] [Google Scholar]
- 35. Bailey P, Cushing H, Eisenhardt L. Angioblastic meningiomas. Arch Pathol Lab Med. 1928;6:453–490. [Google Scholar]
- 36. Bailey P, Bucy PC. The origin and nature of meningeal tumors. Am J Cancer. 1931;15:15–54. [Google Scholar]
- 37. Couce ME, Aker FV, Scheithauer BW. Chordoid meningioma: a clinicopathologic study of 42 cases. Am J Surg Pathol. 2000;24(7):899–905. [DOI] [PubMed] [Google Scholar]
- 38. Kepes JJ, Moral LA, Wilkinson SB, Abdullah A, Llena JF. Rhabdoid transformation of tumor cells in meningiomas: a histologic indication of increased proliferative activity: report of four cases. Am J Surg Pathol. 1998;22(2):231–238. [DOI] [PubMed] [Google Scholar]
- 39. Perry A, Scheithauer BW, Stafford SL, Abell-Aleff PC, Meyer FB. “Rhabdoid” meningioma: an aggressive variant. Am J Surg Pathol. 1998;22(12):1482–1490. [DOI] [PubMed] [Google Scholar]
- 40. Zorludemir S, Scheithauer BW, Hirose T, Van Houten C, Miller G, Meyer FB. Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol. 1995;19(5):493–505. [PubMed] [Google Scholar]
- 41. Lin BJ, Chou KN, Kao HW et al. Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg. 2014;121(5):1201–1208. [DOI] [PubMed] [Google Scholar]
- 42. Sanverdi SE, Ozgen B, Oguz KK et al. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol. 2012;81(9):2389–2395. [DOI] [PubMed] [Google Scholar]
- 43. Azizyan A, Eboli P, Drazin D, Mirocha J, Maya MM, Bannykh S. Differentiation of benign angiomatous and microcystic meningiomas with extensive peritumoral edema from high grade meningiomas with aid of diffusion weighted MRI. Biomed Res Int. 2014;2014:650939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cornelius JF, Stoffels G, Filß C et al. Uptake and tracer kinetics of O-(2-(18)F-fluoroethyl)-L-tyrosine in meningiomas: preliminary results. Eur J Nucl Med Mol Imaging. 2015;42(3):459–467. [DOI] [PubMed] [Google Scholar]
- 45. Rogers CL, Perry A, Pugh S et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro Oncol. 2016;18(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kros JM, Cella F, Bakker SL, Paz Y Geuze D, Egeler RM. Papillary meningioma with pleural metastasis: case report and literature review. Acta Neurol Scand. 2000;102(3):200–202. [DOI] [PubMed] [Google Scholar]
- 47. Pasquier B, Gasnier F, Pasquier D, Keddari E, Morens A, Couderc P. Papillary meningioma. Clinicopathologic study of seven cases and review of the literature. Cancer. 1986;58(2):299–305. [DOI] [PubMed] [Google Scholar]
- 48. Baumgarten P, Gessler F, Schittenhelm J et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016;132(3):479–481. [DOI] [PubMed] [Google Scholar]
- 49. Pizem J, Velnar T, Prestor B, Mlakar J, Popovic M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin Neuropathol. 2014;33(5):354–363. [DOI] [PubMed] [Google Scholar]
- 50. Spille DC, Heß K, Sauerland C et al. Brain invasion in meningiomas: incidence and correlations with clinical variables and prognosis. World Neurosurg. 2016;93:346–354. [DOI] [PubMed] [Google Scholar]
- 51. Vaubel RA, Chen SG, Raleigh DR et al. Meningiomas with rhabdoid features lacking other histologic features of malignancy: a study of 44 cases and review of the literature. J Neuropathol Exp Neurol. 2016;75(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dumanski JP, Carlbom E, Collins VP, Nordenskjold M. Deletion mapping of a locus on human chromosome 22 involved in the oncogenesis of meningioma. Proc Natl Acad Sci U S A. 1987;84(24):9275–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meese E, Blin N, Zang KD. Loss of heterozygosity and the origin of meningioma. Hum Genet. 1987;77(4):349–351. [DOI] [PubMed] [Google Scholar]
- 54. Schneider G, Lutz S, Henn W, Zang KD, Blin N. Search for putative suppressor genes in meningioma: significance of chromosome 22. Hum Genet. 1992;88(5):579–582. [DOI] [PubMed] [Google Scholar]
- 55. Wellenreuther R, Kraus JA, Lenartz D et al. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995;146(4):827–832. [PMC free article] [PubMed] [Google Scholar]
- 56. Rouleau GA, Merel P, Lutchman M et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363(6429):515–521. [DOI] [PubMed] [Google Scholar]
- 57. Trofatter JA, MacCollin MM, Rutter JL et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75(4):826. [DOI] [PubMed] [Google Scholar]
- 58. Tabernero MD, Espinosa AB, Maíllo A et al. Characterization of chromosome 14 abnormalities by interphase in situ hybridization and comparative genomic hybridization in 124 meningiomas: correlation with clinical, histopathologic, and prognostic features. Am J Clin Pathol. 2005;123(5):744–751. [PubMed] [Google Scholar]
- 59. Sayagués JM, Tabernero MD, Maíllo A et al. Intratumoral patterns of clonal evolution in meningiomas as defined by multicolor interphase fluorescence in situ hybridization (FISH): is there a relationship between histopathologically benign and atypical/anaplastic lesions? J Mol Diagn. 2004;6(4):316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maillo A, Orfao A, Espinosa AB et al. Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro Oncol. 2007;9(4):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Domingues PH, Sousa P, Otero Á et al. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro Oncol. 2014;16(5):735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strickland MR, Gill CM, Nayyar N et al. Targeted sequencing of SMO and AKT1 in anterior skull base meningiomas. J Neurosurg. 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 63. Clark VE, Erson-Omay EZ, Serin A et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clark VE, Harmancı AS, Bai H et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. 2016;48(10):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brastianos PK, Horowitz PM, Santagata S et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sahm F, Bissel J, Koelsche C et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol. 2013;126(5):757–762. [DOI] [PubMed] [Google Scholar]
- 67. Reuss DE, Piro RM, Jones DT et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol. 2013;125(3):351–358. [DOI] [PubMed] [Google Scholar]
- 68. Abedalthagafi MS, Bi WL, Merrill PH et al. ARID1A and TERT promoter mutations in dedifferentiated meningioma. Cancer Genet. 2015;208(6):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sahm F, Schrimpf D, Stichel D et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 71. SIMPSON D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gousias K, Schramm J, Simon M. The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg. 2016;125(3):551–560. [DOI] [PubMed] [Google Scholar]
- 73. Oya S, Kim SH, Sade B, Lee JH. The natural history of intracranial meningiomas. J Neurosurg. 2011;114(5):1250–1256. [DOI] [PubMed] [Google Scholar]
- 74. Hwang WL, Marciscano AE, Niemierko A et al. Imaging and extent of surgical resection predict risk of meningioma recurrence better than WHO histopathological grade. Neuro Oncol. 2016;18(6):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sommerauer M, Burkhardt JK, Frontzek K et al. 68Gallium-DOTATATE PET in meningioma: a reliable predictor of tumor growth rate? Neuro Oncol. 2016;18(7):1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ikeda H, Tsuyuguchi N, Kunihiro N, Ishibashi K, Goto T, Ohata K. Analysis of progression and recurrence of meningioma using (11)C-methionine PET. Ann Nucl Med. 2013;27(8):772–780. [DOI] [PubMed] [Google Scholar]
- 77. Arita H, Kinoshita M, Okita Y et al. Clinical characteristics of meningiomas assessed by ¹¹C-methionine and 18F-fluorodeoxyglucose positron-emission tomography. J Neurooncol. 2012;107(2):379–386. [DOI] [PubMed] [Google Scholar]
- 78. Ryttlefors M, Danfors T, Latini F, Montelius A, Blomquist E, Gudjonsson O. Long-term evaluation of the effect of hypofractionated high-energy proton treatment of benign meningiomas by means of (11)C-L-methionine positron emission tomography. Eur J Nucl Med Mol Imaging. 2016;43(8):1432–1443. [DOI] [PubMed] [Google Scholar]
- 79. Afshar-Oromieh A, Giesel FL, Linhart HG et al. Detection of cranial meningiomas: comparison of 68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39(9):1409–1415. [DOI] [PubMed] [Google Scholar]
- 80. Pieper DR, Al-Mefty O. Management of intracranial meningiomas secondarily involving the infratemporal fossa: radiographic characteristics, pattern of tumor invasion, and surgical implications. Neurosurgery. 1999;45(2):231–237; discussion 237. [DOI] [PubMed] [Google Scholar]
- 81. Song SW, Son YD, Cho ZH, Paek SH. Experience with 7.0 T MRI in patients with supratentorial meningiomas. J Korean Neurosurg Soc. 2016;59(4):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Milker-Zabel S, Zabel-du Bois A, Henze M et al. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int J Radiat Oncol Biol Phys. 2006;65(1):222–227. [DOI] [PubMed] [Google Scholar]
- 83. Graf R, Nyuyki F, Steffen IG et al. Contribution of 68Ga-DOTATOC PET/CT to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):68–73. [DOI] [PubMed] [Google Scholar]
- 84. Nyuyki F, Plotkin M, Graf R et al. Potential impact of (68)Ga-DOTATOC PET/CT on stereotactic radiotherapy planning of meningiomas. Eur J Nucl Med Mol Imaging. 2010;37(2):310–318. [DOI] [PubMed] [Google Scholar]
- 85. Grosu AL, Weber WA, Astner ST et al. 11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(2):339–344. [DOI] [PubMed] [Google Scholar]
- 86. Astner ST, Dobrei-Ciuchendea M, Essler M et al. Effect of 11C-methionine-positron emission tomography on gross tumor volume delineation in stereotactic radiotherapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2008;72(4):1161–1167. [DOI] [PubMed] [Google Scholar]
- 87. Louis DN, Perry A, Burger P et al. ; International Society Of Neuropathology–Haarlem. International Society of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guermazi A, De Kerviler E, Zagdanski AM, Frija J. Diagnostic imaging of choroid plexus disease. Clin Radiol. 2000;55(7):503–516. [DOI] [PubMed] [Google Scholar]
- 90. Fukui MB, Meltzer CC, Kanal E, Smirniotopoulos JG. MR imaging of the meninges. Part II. Neoplastic disease. Radiology. 1996;201(3):605–612. [DOI] [PubMed] [Google Scholar]
- 91. Stevens KL, Carlson ML, Pelosi S, Haynes DS. Middle ear meningiomas: a case series reviewing the clinical presentation, radiologic features, and contemporary management of a rare temporal bone pathology. Am J Otolaryngol. 2014;35(3):384–389. [DOI] [PubMed] [Google Scholar]
- 92. Tan ZG, Zhou Q, Cui Y, Yi L, Ouyang Y, Jiang Y. Extra-axial isolated cerebral varix misdiagnosed as convexity meningioma: A case report and review of literatures. Medicine (Baltimore). 2016;95(26):e4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lavinsky J, Aaron KA, Christian E et al. Solitary plasmacytoma in the internal auditory canal and cerebellopontine angle mimicking meningioma. Otol Neurotol. 2016;37(10):e400–e401. [DOI] [PubMed] [Google Scholar]
- 94. Deep NL, Graffeo CS, Copeland WR 3rd et al. Teflon granulomas mimicking cerebellopontine angle tumors following microvascular decompression. Laryngoscope. 2017;127(3):715–719. [DOI] [PubMed] [Google Scholar]
- 95. Lee JH, Lee HK, Choi CT, Huh J. Mucosa-associated lymphoid tissue lymphoma of the pituitary gland: MR imaging features. AJNR Am J Neuroradiol. 2002;23(5):838–840. [PMC free article] [PubMed] [Google Scholar]