Abstract
The current study aimed to capture empathy processing in an interpersonal context. Mother–adolescent dyads (N = 22) each completed an empathy task during fMRI, in which they imagined the target person in distressing scenes as either themselves or their family (i.e. child for the mother, mother for the child). Using multi-voxel pattern approach, we compared neural pattern similarity for the self and family conditions and found that mothers showed greater perceptual similarity between self and child in the fusiform face area (FFA), representing high self–child overlap, whereas adolescents showed significantly less self–mother overlap. Adolescents’ pattern similarity was dependent upon family relationship quality, such that they showed greater self–mother overlap with higher relationship quality, whereas mothers’ pattern similarity was independent of relationship quality. Furthermore, adolescents’ perceptual similarity in the FFA was associated with increased social brain activation (e.g. temporal parietal junction). Mediation analyses indicated that high relationship quality was associated with greater social brain activation, which was mediated by greater self–mother overlap in the FFA. Our findings suggest that adolescents show more distinct neural patterns in perceiving their own vs their mother’s distress, and such distinction is sensitive to mother–child relationship quality. In contrast, mothers’ perception for their own and child’s distress is highly similar and unconditional.
Keywords: representational similarity analysis (RSA), mother–child dyads, empathy, maternal empathy, adolescent, self–other overlap
Introduction
Emotional experiences often occur in an interpersonal context. For example, parents may experience more negative feelings themselves when their child is upset. Experiencing empathy with regard to others’ pain or distress is found across species including rodents, non-human primates and humans, in infants as young as a few months of age, as well as across many forms of social relationships (e.g. for strangers, loved ones, etc.; Preston and De Waal, 2002; Decety, 2011 b; Batson, 2009). At its most basic core, empathy facilitates parental care of their offspring and paves the way for successful social and emotional development in youth (Mikulincer et al., 2001; De Waal, 2008; Decety, 2011 b). A parents’ ability to perceive and respond to emotional cues of their offspring, such as hunger, pain or distress, is essential for survival and has been hardwired at the neural level to promote parental care in mammalian species as an innate protective system for their offspring (Decety, 2011 b). Indeed, studies have shown that parental empathic experiences and neural engagement of the empathy network are more intensified when parents see their own child’s pain compared with an unknown child’s pain (Leibenluft et al., 2004; Goubert et al., 2008). Similarly, when mothers make caregiving decisions for their child, neural activation in the empathy network, including the prefrontal cortex (PFC), limbic and sensory regions, increases to meet the needs of their child (Ho et al., 2014). Although emerging evidence supports maternal empathy for their own child’s emotions, it is unclear whether children’s empathy is equivalent to their parents’ emotional perception of their offspring. Thus, the current study aimed to capture empathy processes in an interpersonal context within parent–child dyads.
One important aspect of empathy is the phenomenon by which one blurs the line between self and other (Davis et al., 1996; Hodges and Klein, 2001; Decety and Sommerville, 2003; Galinsky et al., 2005; Decety and Lamm, 2006; Batson, 2009). Theories suggest that understanding others’ emotions and actions begin by perceptually representing or mirroring others’ feelings and actions onto one’s own states (Rizzolatti et al., 2001; Preston and De Waal, 2002; Gallese, 2007; Keysers and Gazzola, 2009). According to Preston and de Waal’s (2002) perception–action model, the initial perception of others’ states can lead perceivers to have high self–other mental representation in feelings and actions, thereby eliciting emotional contagion and empathic responses as behavioral outcomes. Perceiving others’ emotion (i.e. perceptual encoding) enables individuals to simulate others’ states as one’s own states, and thereby forms self–other overlap (Rizzolatti et al., 2001; Gallese, 2007; Keysers and Gazzola, 2009). That is, how we perceive others’ emotions could be an important gateway to how we share mental representations with others for empathic actions. An important consideration for the emergence of empathy is thus whether individuals show a similar representation of the self and other during perceptual encoding and whether such perceptual similarity facilitates further empathic responding.
Perceptual representation for self–other overlap can be characterized as a bottom-up process because it requires one to detect and encode others’ states first (Singer, 2006; Decety, 2011a). Indeed, studies have shown that empathic responses during the observation of others’ pain are associated with increased activation in early perception-sensory regions such as extrastriate body area, fusiform gyrus and sensory-motor area (e.g. Jackson et al., 2005; Lamm et al., 2007; Lamm and Decety, 2008). This bottom-up process may influence social cognitive top-down modulation, in which the perceptual representation for others is evaluated to make subsequent inferences for others’ situation (Decety and Sommerville, 2003; Jackson and Decety, 2004; Singer, 2006; Decety, 2011a). For example, social cognitive regions, such as the temporal parietal junction (TPJ), medial PFC (MPFC) and posterior cingulate cortex (PCC), show increased activity for in-group member’s pain (Amodio and Frith, 2006; Adams et al., 2010; Cheon et al., 2010; Mathur et al., 2010; Morrison et al., 2012), along with increased neural activation of sensory-perception regions (Azevedo et al., 2013; Zuo and Han, 2013). In this vein, researchers highlight the importance of both affective and cognitive components of empathy that influence the extent of empathic concern and potential prosocial behavior (Decety, 2005; Eisenberg and Eggum, 2009; Goubert et al., 2009). In this model, the bottom-up affective component helps perceivers to increase personal distress and attention for understanding other’s affective states, and the top-down cognitive component regulates perceiver’s emotional arousal and leads to prosocial behavior by recruiting cognitive control resources. However, too much personal distress, induced by emotional arousal and contagion, can impede the perceiver’s empathic behaviors as it depletes cognitive resources to regulate their own affective arousal, thereby failing to attend to others’ needs (Eisenberg and Eggum, 2009).
Previous work has focused on the neural involvement of either early sensory-perception processes (e.g. Singer et al., 2004; Jackson et al., 2005) or higher-order social cognitive processes (e.g. Saxe et al., 2004) independently, instead of conceptualizing empathy as a continuum from the initial representation at the perception level to later social cognitive processes. Thus, empirical research which integrates how the self and others are represented during the initial perception and how this perceptual representation is associated with mentalizing processes will shed light on our understanding of how empathy arises in the brain. In the current study, we scanned both mothers and their adolescent child as they engaged in a task that measures empathic responses when perceiving their own and each other’s distress. We sought to examine how empathic experiences are modulated by perceptual representation based on the perception-action perspective (Preston and De Waal, 2002; Preston, 2007). In particular, we examined perceptual encoding of self–other overlap in the fusiform face area (FFA), and whether higher perceptual similarity is associated with empathic responses in social–cognitive brain regions. Our perceptual similarity analysis of self–other overlap focused on neural patterns in the FFA because evidence indicates that the FFA is involved in the initial encoding of faces as an early visual perception process (Ghuman et al., 2014). Importantly, the mental imagination of faces and encoding emotionality and social information from faces significantly increases neural activation in the FFA (O'Craven and Kanwisher, 2000; Adolphs, 2009; Sabatinelli et al., 2011). To quantify the representation similarity, we adopted the representational similarity analysis (RSA; Kriegeskorte et al., 2008) as a form of multi-voxel pattern approach. This pattern-focused approach allows us to test how neural representations when perceiving self and other are similar by focusing on signal variations of neural response across voxels (i.e. multi-voxel pattern), rather than simply changes in the overall fMRI magnitude between parent and adolescent groups (i.e. univariate neural activation).
We tested three key hypotheses. First, mothers’ perceptual representation of distress for self and child would be highly similar, as evidenced by greater neural pattern similarity in the FFA regardless of the quality of their relationship. In contrast, adolescents would show less similar perceptual representation of distress for self and mother, and such representational similarity would depend upon their relationship quality. Previous studies have indicated that parental concerns for their offspring are stable and unconditional regardless of social environmental factors (Acock and Bengtson, 1980; Leibenluft et al., 2004; Goubert et al., 2008; Proulx and Helms, 2008; Driscoll and Pianta, 2011). For instance, parents’ perception of closeness to their child is stable as parents focus on deemphasizing conflicts and increasing support in their roles as parents for their child’s well-being (Acock and Bengtson, 1980; Proulx and Helms, 2008; Driscoll and Pianta, 2011). Indeed, following pregnancy, mothers' socioemotional neural system shifts to support empathy and care of their child (Hoekzema et al., 2016). In contrast, the adolescent period is marked by a social reorientation away from parents and towards peers (Nelson et al., 2005). Adolescents report decreased closeness in parent–child relationship quality (Tsai et al., 2013), combined with exaggeration of conflicts to diminish closeness with parents as a means of establishing autonomy (Fuligni and Eccles, 1993; Larson et al., 1996; Collins and Steinberg, 2006). Thus, adolescents’ perceptual representation of distress for self and mother would be more conditional, such that adolescents will only show high neural pattern similarity in the FFA when they report stronger relationship quality.
Second, we tested whether perceptual encoding (i.e. representational pattern similarity in the FFA) is associated with higher social cognitive processing. Perceptual representation similarity in the FFA, an index of self–family overlap, was used as a regressor in a univariate general linear model (GLM) analysis to link initial perception to subsequent empathic processes in social brain regions, which include the TPJ, MPFC and PCC (Saxe et al., 2004). In other words, the level of neural engagement in the social brain when processing family and self will depend on the perceptual similarity in the FFA. As suggested by theories of empathy (e.g. Preston and de Waal’s, 2002), the initial perceptual representation process for others’ states subsequently elicits emotional contagion and empathic response. Although the sluggish nature of the fMRI signal does not allow us to test direct temporal influences between processes, we hypothesized that higher perceptual similarity in the FFA, a neural index of self–family perceptual overlap, would be associated with greater neural recruitment of mentalizing processes in social brain regions.
Finally, we performed mediation analyses to test an integrated model linking mother–child relationship quality, the perceptual similarity in self––family overlap and neural engagement of social brain regions. We hypothesized that greater relationship quality in adolescents would be associated with the recruitment of social brain regions during empathic responses, and this path would be mediated by greater encoding of perceptual similarity in the FFA. Whereas relationship quality would be a key predictor of perceptional similarity and social brain responding in adolescents, we predicted that relationship quality would not influence empathic responses in mothers because mothers’ socioemotional system is more stable and invariable for their offspring (Acock and Bengtson, 1980; Proulx and Helms, 2008; Driscoll and Pianta, 2011; Hoekzema et al., 2016).
Materials and methods
Participants
Twenty-two healthy mother–adolescent dyads were recruited to participate in this study (adolescents: Mage = 13.90 years, s.d. = 0.11, 21% female; mothers: Mage = 44.26 years, s.d. = 1.57, 100% female). Mothers and their adolescent child each underwent an fMRI scan on the same day. Participants were compensated $50 each for their participation. All participants provided written informed consent and assent approved by the Institutional Review Board.
Task design and procedure
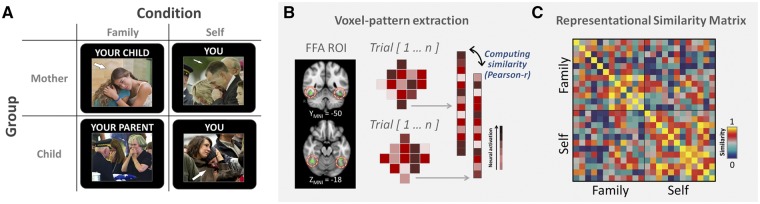
The task was modified from prior work on neural processing of empathy, a task which has reliably recruited neural regions involved in mentalizing (Rameson et al., 2012; Morelli et al., 2014). During the task, adolescents and mothers each saw individuals expressing emotional distress in negative contexts (e.g. at a funeral or being bullied), with instructions to perceive the target face as either their family member (family-condition; mother for the child’s scan or child for the mother’s scan) or themselves (self-condition). Participants were instructed to imagine the person in the picture as themselves and to take the perspective of the person in the picture during the self-condition. For the family-condition, participants were instructed to imagine the person in the picture as their parent/child, and to take the perspective of the person in the picture as if it were their family member. When the target individuals appeared in the social scenes with many other people, a white arrow was inserted to guide participants to the target face (see Figure 1A). The stimulus conditions were balanced by gender of target face. The gender of faces was not matched to the subject, and participants were instructed to imagine the person in each image as themselves (self-condition) or their family member (family-condition) regardless of the gender of the person. The age of the target face for each group (e.g. family and self) was matched. Each trial lasted between 8 and 9.2 s and was followed by 2 s rating phase consisting of a 10-point Likert scale to indicate how they felt from −5 (very negative) to 5 (very positive).1 Two independent blocks for self- and family conditions consisted of 12 event-related trials each (i.e. mixed-design). Each block was separated by a 5-s blank screen, and inter-trial intervals were inserted between trials to separate the BOLD signal jittered between 2.0 and 3.2 s (mean = 2.4 s).
Fig. 1.
(A) Experimental condition for mothers and adolescent children, (B) schematic procedure of computing similarity values within the region of interest between trials and (C) example of the representational similarity matrix.
fMRI data acquisition and analysis
Image acquisition, preprocessing and registration
Imaging data were collected using a 3T-Siemens Trio MRI scanner with a 32-channel matrix coil. High-resolution structural images (T1-MPRAGE) were acquired first (repetition time or TR = 1.9 s; echo time or TE = 2.3 ms; matrix size = 256 × 256; field of view or FOV = 230 mm; flip angle or FA = 90°; 1 mm isotropic voxel). T2*-weighted echoplanar images were acquired during the emotion perception task (38 slices with 0.3 mm inter-slice gap; TR = 2 s; TE = 25 ms; matrix = 92 × 92; FOV = 230 mm; FA = 90°; voxel size 2.5 × 2.5 mm; slice thickness = 3 mm).
Preprocessing was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library; Smith et al., 2004). The following pre-statistics processing was applied: motion correction using MCFLIRT (Jenkinson et al., 2002); non-brain removal using BET (Smith, 2002); grand-mean intensity normalization of the entire 4D data set by a single multiplicative factor; spatial smoothing applied only for univariate whole-brain analysis with Gaussian kernel of full width at half maximum 6 mm. For univariate analysis, registration matrix was estimated additionally between functional images, high-resolution structural images and standard Montreal Neurological Institute (MNI) 2-mm brain using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002).
RSA (multi-voxel pattern analysis)
For the RSA, we first estimated single-trial activation patterns for each trial using least squares single methods (Mumford et al., 2012) where each single-level GLM included regressors for a current trial and all other remaining trials with temporal derivate regressor. We included the rating phase in the model as a regressor of non-interest (i.e. nuisance regressor) as well as motion regressors (six motion parameters and motion-outlier points). For each participant, the standardized voxel-wise pattern activity (i.e. z-transformed parameter estimates) was extracted and vectorized within the FFA region of interest (ROI) (see ROI selection for more details). The extracted patterns were then analyzed by calculating pairwise Pearson coefficient (i.e. similarity value) between each trial with every possible pair of other trials (Figure 1B), and then applied Fisher’s r-to-z transformation, producing a symmetric 24 × 24 similarity matrix for each dyad and the ROI (Figure 1C). Finally, the average of self–family similarity values was used for subsequent analyses (e.g. Visser et al., 2011).
Univariate group-level analysis with RSA as a covariate
To assess how perceptual encoding (i.e. representational pattern similarity in FFA) is associated with higher-level social cognitive processing, an additional standard two-stage mixed-effects analysis was performed. The GLM of the BOLD signal for each perception type (family and self) was estimated with temporal derivate regressors at the first level using a double-gamma hemodynamic response function. We added the rating phase in the model as a regressor of non-interest (i.e. nuisance regressor) to the design matrix as well as motion regressors (six motion parameters and motion-outlier points). The individuals’ data were then inputted into a random-effects model using cluster detection statistics with outlier-deweighting at a threshold of Z > 2.3 and a cluster probability of P < 0.05 (one-tailed), corrected for whole-brain multiple comparisons using Gaussian Random Field Theory. In particular, we entered each individual’s FFA similarity values (demeaned for each group) as covariates in the group-level analysis to examine how perceptual similarity in the FFA to self and other is associated with neural encoding of self and other in social–cognitive regions.
ROI selection
In this study, we indexed neural representation at the encoding stage by examining neural pattern similarity within the FFA, a key region in visual face encoding (Kanwisher et al., 1997). We constructed the ROI mask of a priori voxels associated with ‘FFA’, ‘emotional face’, ‘faces’ and ‘face perception’ from the reversed inference map (q < 0.1 FDR-corrected) in the NeuroSynth database (http://www.neurosynth.org; downloaded on 24 November 2015). The final FFA voxels were then selected along with the ventral temporal cortex at Z = 3.71 (Figure 1B; a total 321 voxels).
Family relationship quality
To capture a comprehensive measure of relationship quality between mothers and adolescent children, three measures were assessed from both mothers and adolescent children: family cohesion (Family Adaptation and Cohesion Evaluation Scales II inventory, FACE II; Olson et al., 1979), family conflict (Ruiz et al., 1998; Telzer et al., 2014) and family identity (Tyler and Degoey, 1995). We created a composite score of positive family relationship quality by taking the score of family cohesion, reverse-scored family conflict and family identity, with higher scores indicating more positive parent–child relationships. This composite score provides us with a comprehensive measure of relationship quality using these three dimensions (Qu et al., 2015). Details for each scale are as follows:
Family cohesion
Participants completed the Cohesion subscale of the FACE II (Olson et al., 1979). They responded to 10 items that assessed how close they feel and how much time they spend with their mother [child] on a 5-point Likert scale from 1 (almost never) to 5 (almost always). Sample items included ‘My mother [child] and I do things together’, ‘My mother [child] and I are supportive of each other during difficult times’, and ‘My mother [child] and I feel close to one another’ (internal consistency α = 0.81 for mother; α = 0.88 for child). The 10 items were averaged, and higher scores indicate greater relationship closeness.
Family conflict
Parent–child conflict was assessed by 10 items (Ruiz et al., 1998; Telzer et al., 2014) (e.g. ‘You and your parents [child] had a serious argument or fight’ and ‘You and your parents [child] yelled or raised your voices at each other’). Participants reported how true each item was for them in the past month on a 5-point scale ranging from ‘almost never’ to ‘almost always’ (α = 0.88 and 0.92 for mother and child, respectively). The 10 items were averaged, such that higher scores indicate greater conflicts with family members.
Family identity
Participants completed an eight-item scale that assessed the extent to which their family is an important aspect of their identity (Tyler and Degoey, 1995). Using a 5-point scale from 1 (strongly disagree) to 5 (strongly agree), participants indicated how much their sense of self was internalized to their family value (e.g. ‘I feel like a valued member of my family’ and ‘My family is important to the way I think of myself as a person’; α = 0.83 and 0.87 for mother and child, respectively). The eight items were averaged, and higher scores indicate greater internalized family values.
Results
RSA: perceptual similarity for self and family
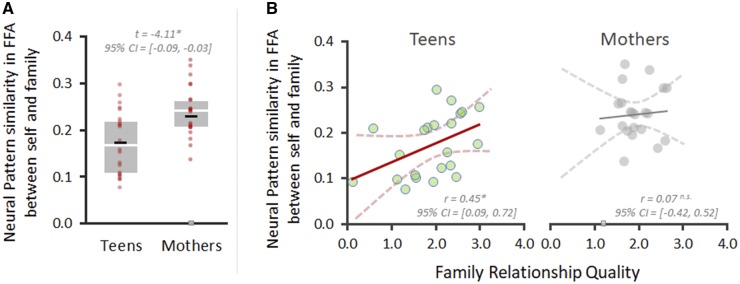
To examine how mothers and adolescent children perceive the emotional distress of their family and self at the perceptual level, we compared the similarity value in FFA of mothers when perceiving their own and their child’s emotional distress and of adolescents when perceiving their own and their mother’s emotional distress. As shown in Figure 2A, mothers showed greater neural similarity in perceptual representation in the FFA when perceiving emotional distress in self vs child (M = 0.24, s.d. = 0.06), whereas their adolescent children showed significantly less neural pattern similarity between the conditions (M = 0.17, s.d. = 0.07), paired-t(20) = −4.11, P < 0.05, 95% confidence interval (CI) 50000 bootstrap resampling = −0.09 to −0.03. That is, mothers’ perception of their child’s emotional distress within each dyad is more similar to their own emotional distress compared with their own adolescent child’s perception of self and mother.
Fig. 2.
(A) Whisker plot for the neural pattern similarity between self and family condition calculated within FFA ROI. Black and white bar within indicate mean and median, respectively. Red-colored dots indicate individual’s similarity point; one mother had extremely low similarity value (0.002; gray-colored square) that was far less than 2.5 s.d. under the mean. We excluded this mother for the final analyses. (B) Scatter plots of the similarity values and relationship quality for adolescents and mothers. Dotted lines indicate 95% CI of the regression line. Note that all statistical results remained same when the outlier mother was included; 95% CI = −0.09 to − 0.03 for panel A and 95% CI = −0.29 to 0.68 for panel B.
As hypothesized, children’s perceptual similarity was dependent upon their family relationship quality, such that they showed greater perceptual similarity when they reported higher relationship quality, r(22) = 0.45, P < 0.05, 95% CI 50000 bootstrap resampling = 0.09–0.72, whereas mothers’ neural pattern similarity was high and independent of relationship quality, r(21) = 0.07, P > 0.05, 95% CI 50000 bootstrap resampling = −0.42 to 0.52 (Figure 2B). To rule out the possibility of ceiling effects relating to the finding that there was no association between mother’s perceptual similarity and relationship quality compared to adolescents, we compared the relationship quality in dyads and found no significant difference between mother and child, paired-t(21) = −0.14, P > 0.05, 95% CI 50000 bootstrap resampling = −0.38 to 0.32. This result indicates that the null finding for mothers was not due to ceiling effects for relationship quality. In sum, mothers show high self–child overlap in perceptual similarity in the FFA regardless of their relationship quality with their child. In contrast, adolescent children only showed high perceptual similarity in the FFA when they also reported high relationship quality with their family, suggesting that adolescent children’s self–family overlap is dependent upon their relationship quality.
Group-level univariate analysis with FFA similarity predicting social brain activation for self and family
To test the prediction that higher neural similarity for self and family at the perceptual level is associated with neural processing in higher-level, social–cognitive regions, we examined which brain regions covaried with perceptual similarity in the FFA. To this end, we used FFA similarity from the self and family conditions and regressed this similarity value onto neural activation when perceiving self vs family’s emotional distress (self > family). Note that greater activation for this contrast represents greater differences in neural responses to self and family. We found that greater perceptual similarity between self and family in adolescents was negatively associated with neural activation in several social brain regions including bilateral TPJ, PCC and mPFC (Figure 3A and Table 1), suggesting that greater similarly in the FFA at the encoding stage was associated with lower differentiation of self and family in the social brain processing for empathy.
Fig. 3.
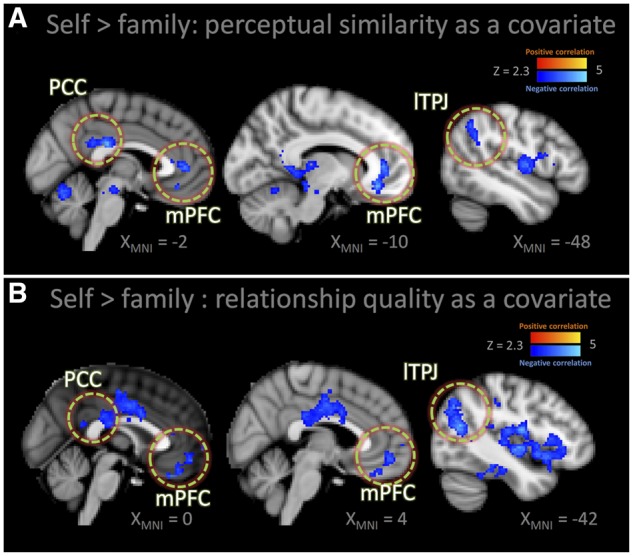
Negatively covaried social brain regions (circled area) with (A) the increased family–self perceptual similarity of FFA (B) the degree of family relationship quality in family–self contrast of adolescent children. See also Table 1 and 2 for other clusters.
Table 1.
Brain regions which correlated with perceptual similarity on family-self condition in adolescents
| Adolescent | |||||||
|---|---|---|---|---|---|---|---|
| MNI |
|||||||
| Self > Family | H | BA | Z-score | k | x | y | z |
| Perceptual similarity: negative covariation | |||||||
| Temporal parietal junction (TPJ) | L | 39 | 4.21 | 279 | −34 | −68 | 44 |
| R | 39 | 4.17 | 468 | 42 | −64 | 48 | |
| Medial PFC (mPFC) | L | 11 | 3.64 | 28 | −4 | 36 | −12 |
| Posterior cingulate gyrus (PCC) | – | – | 4.12 | 209 | −2 | −28 | 28 |
| Angular gyrus/inferior parietal lobe (IPL) | R | 40 | 3.55 | 63 | 46 | −52 | 54 |
| Anterior cingulate gyrus (ACC) | – | 32 | 3.78 | 194 | −6 | 42 | 4 |
| Brain stem | – | – | 2.98 | 43 | −10 | −30 | −10 |
| Insula | L | 4 | 4.02 | 186 | −42 | −8 | 16 |
| L | 13 | 3.41 | 85 | −40 | −8 | 0 | |
| R | 1 | 2.75 | 28 | 44 | −18 | 16 | |
| Dorsal lateral PFC (DLPFC) | L | 46 | 3.17 | 53 | −40 | 44 | 6 |
| R | 9 | 2.86 | 22 | 42 | 40 | 34 | |
| Inferior temporal gyrus (ITG) | R | 37 | 3.59 | 47 | 56 | −52 | −16 |
| DLPFC | R | 8 | 4.07 | 137 | 42 | 16 | 52 |
| Auditory cortex | R | 41 | 2.93 | 20 | 62 | −24 | 12 |
| Primary somatosensory cortex | R | 1 | 3.13 | 60 | 62 | −16 | 36 |
| Secondary somatosensory cortex | R | 40 | 3.11 | 20 | 42 | −26 | 18 |
| Primary motor cortex | R | 6 | 3.37 | 151 | 42 | −10 | 64 |
| Superior parietal lobule (SPL) | R | 7 | 3.11 | 22 | 30 | −54 | 62 |
| Superior temporal gyrus (STG) | R | 41 | 3.27 | 49 | 68 | −26 | 10 |
| TPJ | R | 40 | 3.95 | 181 | 64 | −24 | 42 |
| R | 40 | 3.38 | 45 | 56 | −36 | 52 | |
| Fusiform gyrus | R | 37 | 3.65 | 67 | 40 | −56 | −16 |
| Thalamus | L | 50 | 3.33 | 151 | −14 | −30 | 8 |
| Perceptual similarity: positive covariation | No significant activations | ||||||
| Mothers | |||||||
| Perceptual similarity: negative covariation | |||||||
| Auditory Cortex | L | 41 | 3.32 | 58 | −40 | −22 | 10 |
| L | 41 | 3.08 | 29 | −42 | −34 | 10 | |
| Insula | L | 6 | 3.35 | 88 | −52 | −2 | 2 |
| L | 13 | 2.9 | 20 | −42 | −10 | 0 | |
| R | 6 | 2.74 | 20 | 54 | −4 | 8 | |
| Primary somatosensory cortex | L | 4 | 4.2 | 88 | −64 | −12 | 18 |
| Secondary somatosensory cortex | L | 40 | 3.01 | 29 | −54 | −26 | 16 |
| Primary motor cortex | R | 6 | 3.41 | 39 | 64 | 4 | 16 |
| STG | R | 22 | 4.44 | 30 | 68 | −12 | 0 |
| L | 6 | 3.74 | 31 | −54 | −2 | 0 | |
| Perceptual similarity: positive covariation | No significant activations | ||||||
Notes: Reported regions and their ‘local maxima’ were at 50% probability locations on the Harvard–Oxford atlas with more than 20 voxels. H, hemisphere; BA, Brodmann area; k, the number of voxels.
Next, we regressed family relationship quality onto neural activation when perceiving self vs family’s emotional distress and found negative correlations in several social brain regions as a function of adolescents’ family relationship quality (Figure 3B and Table 2). That is, adolescents who reported greater relationship quality showed lower differentiation of self and family in the social brain during empathic responding. Social brain regions of mothers were not modulated by the perceptual similarity for self and child (see Table 1 for local maxima regions in the clusters).
Table 2.
Brain regions which correlated with mother-child relationship quality on family-self condition in adolescents
| Adolescent | |||||||
|---|---|---|---|---|---|---|---|
| MNI |
|||||||
| Self > Family | H | BA | Z-score | k | x | y | z |
| Relationship quality: negative covariation\ | |||||||
| Medial PFC (mPFC) | L | 10 | 3.97 | 125 | −2 | 50 | −12 |
| R | 10 | 3.55 | 79 | 2 | 50 | −10 | |
| L | 10 | 3.45 | 74 | −6 | 54 | 4 | |
| R | 10 | 3.14 | 23 | 4 | 48 | −8 | |
| Posterior cingulate cortex (PCC) | 31 | 3.22 | 212 | −4 | −30 | 46 | |
| Temporal parietal junction (TPJ) | L | 19 | 3.82 | 394 | −44 | −66 | 20 |
| Anterior cingulate gyrus (ACC) | 32 | 3.5 | 371 | 6 | 6 | 36 | |
| Amygdala | L | 34 | 3.95 | 230 | −26 | 2 | −20 |
| R | 49 | 3.52 | 123 | 26 | 2 | −10 | |
| Angular gyrus/inferior parietal lobe (IPL) | L | 39 | 3.5 | 21 | −56 | −54 | 24 |
| R | 39 | 2.76 | 48 | 60 | −50 | 36 | |
| Insula | L | 13 | 4.4 | 255 | −40 | −14 | −2 |
| R | 44 | 4.26 | 266 | 42 | 6 | 6 | |
| R | 13 | 4.21 | 250 | 38 | −8 | 6 | |
| L | 4 | 4.17 | 216 | −42 | −8 | 16 | |
| Orbitofrontal cortex (OFC) | L | 47 | 4.3 | 197 | −28 | 34 | −14 |
| R | 3.37 | 176 | 22 | 12 | −16 | ||
| mPFC | L | 10 | 3.84 | 240 | −2 | 62 | 2 |
| R | 2.86 | 22 | 2 | 62 | 2 | ||
| Auditory cortex | R | 41 | 3.85 | 59 | 46 | −28 | 12 |
| R | 41 | 3.37 | 31 | 44 | −22 | 12 | |
| L | 41 | 3.14 | 30 | −40 | −22 | 10 | |
| Hippocampus | L | 54 | 3.76 | 219 | −28 | −16 | −18 |
| R | 54 | 3.09 | 121 | 28 | −32 | −6 | |
| Inferior frontal gyrus (IFG) | L | 45 | 3.62 | 29 | −52 | 16 | 0 |
| L | 45 | 3.35 | 54 | −40 | 22 | 6 | |
| R | 9 | 2.65 | 20 | 56 | 28 | 16 | |
| Inferior temporal gyrus (ITG) | L | 37 | 3.49 | 108 | −36 | −38 | −28 |
| R | 20 | 3.06 | 24 | 58 | −30 | −26 | |
| Lateral occipital cortex—inferior division | R | 37 | 3.45 | 67 | 54 | −64 | −12 |
| L | 19 | 3.09 | 32 | −50 | −70 | 12 | |
| Lingual gyrus | L | 19 | 3.18 | 70 | −8 | −60 | −2 |
| Middle temporal gyrus (extended to posterior superior temporal sulcus pSTS) | R | 21 | 3.98 | 38 | 62 | −2 | −18 |
| R | 37 | 3.63 | 106 | 60 | −54 | 10 | |
| L | 39 | 3.24 | 32 | −56 | −58 | 6 | |
| R | 21 | 3.06 | 115 | 66 | −24 | −20 | |
| L | 21 | 2.99 | 54 | −56 | −26 | −8 | |
| Basal ganglia: pallidum | R | 51 | 3.65 | 87 | 22 | −6 | 2 |
| L | 3.41 | 34 | −26 | −16 | 0 | ||
| Putamen | R | 49 | 3.88 | 258 | 24 | 8 | −10 |
| L | 49 | 3.75 | 273 | −30 | −14 | 0 | |
| Parahippocampal gyrus | L | 37 | 3.46 | 31 | −28 | −30 | −22 |
| Primary somatosensory cortex | L | 1 | 3.72 | 138 | −56 | −18 | 30 |
| R | 40 | 3.69 | 112 | 66 | −16 | 20 | |
| Secondary somatosensory cortex | R | 40 | 4.59 | 85 | 58 | −24 | 18 |
| L | 40 | 3.24 | 119 | −48 | −34 | 20 | |
| Superior temporal gyrus (STG) | R | 22 | 3.92 | 27 | 56 | 2 | −2 |
| R | 22 | 3.72 | 58 | 68 | −14 | −2 | |
| L | 22 | 3.53 | 23 | −42 | −16 | −4 | |
| R | 22 | 3.02 | 26 | 56 | 2 | −14 | |
| Primary motor cortex | R | 6 | 4.17 | 101 | 42 | −10 | 56 |
| Supplementary motor area | R | 6 | 2.86 | 21 | 6 | −4 | 52 |
| Precuneous | L | 18 | 2.96 | 31 | −14 | −58 | 16 |
| Supramarginal gyrus | L | 21 | 4.05 | 99 | −62 | −26 | 38 |
| R | 40 | 3.9 | 164 | 62 | −22 | 36 | |
| R | 40 | 3.15 | 80 | 56 | −36 | 52 | |
| Fusiform gyrus | R | 37 | 4.06 | 66 | 38 | −32 | −20 |
| R | 37 | 3.49 | 101 | 50 | −44 | −22 | |
| R | 37 | 2.99 | 25 | 32 | −68 | −14 | |
| Temporal pole | R | 38 | 3.94 | 281 | 56 | 8 | −16 |
| L | 38 | 3.83 | 105 | −54 | 14 | −8 | |
| Thalamus | L | 50 | 3.24 | 128 | −10 | −16 | 4 |
| R | 3.09 | 71 | 22 | −24 | 12 | ||
| Relationship quality: positive covariation | No significant activations | ||||||
| Mothers | |||||||
| Relationship quality: negative covariation | No significant activations | ||||||
| Relationship quality: positive covariation | No significant activations | ||||||
| ACC | 24 | 3.53 | 50 | 0 | 28 | 16 | |
Notes: Reported regions and their ‘local maxima’ were at 50% probability locations on the Harvard–Oxford atlas with more than 20 voxels. H, hemisphere; BA, Brodmann area; k, the number of voxels.
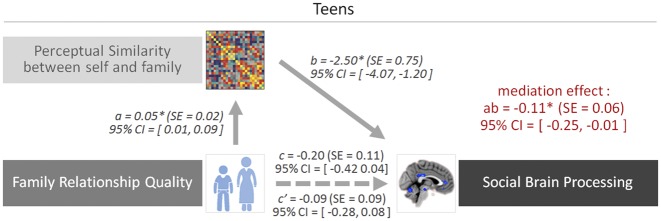
Mediation analysis
To integrate the results of perceptual similarity in the FFA, neural engagement in the social brain and family relationship quality, we performed a mediation analysis. We tested whether relationship quality (i.e. independent variable) is associated with less differentiation of self and family in social–cognitive regions (i.e. outcome) through increased perceptual similarity in the FFA (i.e. mediator). To this end, we extracted and averaged activation from the social brain regions which were identified in the analyses above, and performed mediation analyses using the mediation toolbox in Matlab (Wager et al., 2008). As shown in Figure 4, the path between family relationship quality and perceptual similarity in the FFA for self and mother (path a; a = 0.05, P < 0.05, 95% CI 50 000 bootstrap resampling = 0.01–0.09) and the path between perceptual similarity and social brain regions identified in the univariate analysis (path b; b = −2.50, P < 0.05, 95% CI 50 000 bootstrap resampling = −4.07 to −1.20) were statistically significant. Importantly, the mediation effect was significant (ab = −0.11, P < 0.05, 95% CI 50 000 bootstrap resampling = −0.25 to −0.01), indicating that greater perceptual similarity for self and family explains the link between strong family relationship quality and less differentiation in the social brain for self vs family. Only adolescents showed this significant mediating effect, whereas mothers did not (ab = 0.01, P > 0.05, 95% CI 50000 bootstrap resampling = −0.07 to 0.09). In sum, these results suggest that not only is the quality of the relationship important for high perceptual similarity for both the self and their family but also that high self–other overlap at the perceptual level explains the link between family relationship quality and empathic responding in higher-level social–cognitive regions.
Fig. 4.
Perceptual similarity in the FFA mediates the link between perceived family connectedness and social brain activation in adolescents. Note that asterisk indicates statistical significance at P =0.05 (95% CI) based on 50 000 bootstrapping resampling.
Discussion
In this study, we examined mothers and their child's empathic neural representations for the self and family. Using representation similarity analysis, we found that mothers’ perceptual similarity in the FFA for self and their child’s emotional distress was significantly higher compared to their child's self–mother perceptual similarity, indicating that mothers show high self–child overlap compared to their adolescent child. In contrast, adolescents’ perceptual similarity was dependent upon their relationship quality, such that they showed high self–other overlap for their mother’s emotional distress and their own only when they reported high relationship quality with their family. It is worth noting that these similarity results were based on within-family comparisons, indicating that each mother shows higher perceptual similarity than her own child. Moreover, this initial perceptual representation in the fusiform in adolescents was associated with neural activation in social brain regions required to put oneself in another’s shoes (Saxe et al., 2006), whereas no such modulatory effects of perceptual similarity on the social brain were found in mothers. Consistent with previous evidence showing that parents’ perspective to their child is more stable and robust, whereas adolescent children begin to reduce closeness with their parents as a means of establishing autonomy (Acock and Bengtson, 1980; Proulx and Helms, 2008; Driscoll and Pianta, 2011), these results suggest that mothers’ empathy to their child is more unconditional compared to that of their adolescent child. Finally, the mediation model confirmed that family relationship quality is associated with adolescent's mental representation for both the self and their family at the perceptual (i.e. fusiform perceptual representation similarity) and higher-level social cognitive processing level.
Mothers’ attention is unconditionally directed toward their offspring to understand, make sense of and respond to their child’s feelings and behaviors across the lifespan. This maternal empathic bias has deep evolutionary underpinnings at the neural level to be selective and protective of her own offspring (De Waal, 2008; Decety, 2011 b) such that mothers’ empathy promotes positive developmental outcomes, such as mood stability and regulated stress reactivity in developing youth (Abraham et al., 2017). Our findings support this perspective for maternal empathy by showing that maternal empathic processes for their child are more robust compared to children. In our mediation model, we found that mothers’ mentalizing process for their child’s emotional distress was modulated by neither the relationship quality nor the degree of self–child overlap in the FFA. It is possible that mothers were at ceiling with less variability in perceptual similarity in the FFA as well as social brain activation. Importantly, a recent neuroimaging study revealed that brain regions associated with empathy are dramatically reconfigured after pregnancy in a mother’s brain (Hoekzema et al., 2016), which enables mothers to be more selective and sensitive to their own offspring. In other words, the neural connections in mothers’ brains become fine-tuned and strengthened to utilize neural resources optimally for their own offspring. This neural reconfiguration is enduring for mothers after pregnancy and into adolescence, highlighting mothers’ strong self–child overlap. In contrast, adolescents are establishing a sense of identity by expanding their social network, spending more time and sharing their affection with others outside of the family (Larson et al., 1996). Unlike mothers, adolescents’ brains are more likely to be sensitive to their social environment (Blakemore and Mills, 2014), particularly their peers (Nelson et al., 2005). Therefore, adolescents’ mental representations are more flexible depending on social contexts (Crone and Dahl, 2012). Thus, this study suggests that adolescents’ empathy is more conditional whereas mothers show empathy for their child that is stable and unconditional.
The current findings elaborate upon previous theories (Rizzolatti et al., 2001; Preston and De Waal, 2002; Gallese, 2007; Keysers and Gazzola, 2009) by showing how empathic experiences arise based on the interaction of two different processes, the bottom-up perceptual encoding process and the top-down social cognition process. Theories of empathy propose that empathy to others includes various mental and behavioral phenomena, such as shared and vicarious emotional experiences associated with prosocial (helping) reactions as a result of perceiving others’ emotional states (Preston and De Waal, 2002; Singer, 2006; Preston, 2007; Decety, 2011a). Therefore, an approach to integrate these two empathy components (‘perception and action’ or ‘bottom-up and top-down processing’) with a continuum perspective is critical for understanding empathic experiences. The current study demonstrates that perceptual representation similarity in the fusiform, representing an initial mental representation of self–other emotional distress, is an important gateway to neural activation involved in higher social cognitive processing. Interestingly, this two-component model was only valid for adolescents.
In our study, the quality of perceptual representation at the encoding level in the FFA was quantified using RSA as a form of multi-voxel pattern approach. Employing neural pattern approach allows us to examine neural representational patterns in the perceptual region (i.e. FFA for facial expression) rather than simply examining whether this region shows differential neural activation to self vs others’ emotional distress (i.e. univariate analysis). To our knowledge, this is the first study to examine how perceptual representation at the encoding level is related to the degree of empathy processing at the social–cognitive level. However, it should be noted that our model implying the temporal aspects of empathic experience from the initial encoding to later social cognition could not be tested directly. Although we built this model based on previous theories and empirical evidence in empathy (Rizzolatti et al., 2001; Preston and De Waal, 2002; Jackson et al., 2005; Singer, 2006; Gallese, 2007; Lamm et al., 2007; Lamm and Decety, 2008; Keysers and Gazzola, 2009; Decety, 2011a), the sluggish nature of the BOLD signal precludes our ability to test the model in a temporal manner, and thus the current results cannot confirm causality explicitly between these two distinct processes. Experimental designs that can disentangle these two processes in a causal manner would help us to probe the temporal influences between the bottom-up and top-down processes in empathic experiences.
Nonetheless, some evidence highlights the importance of early sensory-perception processing for later social–cognitive processing. For example, emotion perception studies have demonstrated that emotional information is pre-evaluated during the early encoding in perception-sensory regions, such as somatosensory and early visual cortex (e.g. Stolarova et al., 2005; Greening et al., 2016;). Importantly, early sensory-perception bottom-up processing shifts the quality of later cognitive top-down processing, such that impairment of somatosensory processing can impair recognition of face emotions at the later top-down stage (Adolphs et al., 2000). Similarly, event-related potential (ERP) research has shown that late top-down processing in face emotion recognition reflected by late the cognitive component (400–800 ms) is significantly modulated depending on how face stimuli are encoded at the early bottom-up stage represented at the early sensory-perception component (200–300 ms; Lee et al., 2010). Thus, it is plausible that social brain region changes observed in the current study are influenced by perceptual representation at the encoding level first. Our regression model using mediation also supports this pathway, such that perceptual similarity in the FFA significantly mediated the association between relationship quality and social brain activation to self and family.
In the current study, we recruited mothers and adolescents using dyadic recruitment (i.e. mothers and adolescents from the same family), which is a strength of the sample. However, the sample size is relatively small, precluding our ability to examine sex differences among adolescents or between mothers and fathers. Furthermore, our adolescent-only sample does not allow us to probe why adolescents show less self–other overlap compared to adults (i.e. mothers). For example, it is possible that there may be linear increases in self–family overlap across development, as social cognitive abilities increase across this age range. Thus, recruitment of pre-adolescent children would further enable us to probe whether the effects we found change linearly with age. Moreover, examining other targets in the task (e.g. peers) would help us tease apart whether the source of low self–mother overlap is due to adolescents beginning to show greater self–other overlap with their peers as they transition into adolescence (Nelson et al., 2005). Longitudinal research and studies recruiting larger and different samples including children, adolescents, peers and other family members (e.g. sibling and father) will help to unpack developmental trajectories in empathic responding for self and other.
This study shows that relationship quality may set the stage for social–cognitive processing and may positively affect brain development in social–cognitive regions. Such development may have broader implications for empathy development and adolescents’ ability to mentalize and take the perspective of others’ emotions. Our results can inform future interventions for increasing empathy and related prosocial behavior by elucidating the role of relationship quality in promoting social–cognitive processing. Thus, interventions designed to enhance relationship quality with others may promote greater self–other overlap, thereby enhancing empathy and prosocial relationships in youth.
To our knowledge, this is the first study to directly examine empathy processes with a continuum perspective from the initial perceptual representation to the later mentalizing stage at the neural level by employing both multivariate (i.e. representational neural pattern analysis) and univariate approaches (i.e. general linear model). Moreover, by scanning both mothers and their children, we compared how adolescents’ and their mothers’ empathy for their family and the self are similar. Our results suggest that mothers have unconditional empathic responses to their child’s emotional distress based on higher self–child overlap in their mental representation, whereas adolescents’ empathy is more conditional. This study contributes to our knowledge about empathy processes with the critical importance of perceptual representation in mentalizing others’ emotional distress.
Funding
This work was supported by the National Institute of Health (1R01DA039923) and generous funds from the Department of Psychology at the University of Illinois Urbana Champaign.
Conflict of interest. None declared.
Footnotes
A repeated measure of ANOVA (condition: self and family X group: mother and adolescent) found that there was no main effect of condition or interaction (all ps > 0.29), but a significant main effect of group (p = 0.001), indicating mothers rated painful scenes as more negative than adolescents regardless of stimulus types (children: -0.84 vs. mothers: -2.19). Subsequent t-tests indicated that all ratings for both self and family conditions of mothers and adolescents were significantly different from 0 (0 = rating as non-arousing, all ps < 0.001), indicating that both mothers and children perceived painful scenes as negative across all stimulus types.
References
- Abraham E., Raz G., Zagoory-Sharon O., Feldman R. (2017). Empathy networks in the parental brain and their long-term effects on children's stress reactivity and behavior adaptation. Neuropsychologia [DOI] [PubMed] [Google Scholar]
- Acock A.C., Bengtson V.L. (1980). Socialization and attribution processes: actual versus perceived similarity among parents and youth. Journal of Marriage and the Family, 42(3), 501–15.http://dx.doi.org/10.2307/351895 [Google Scholar]
- Adams R.B., Rule N.O., Franklin R.G.,et al. (2010). Cross-cultural reading the mind in the eyes: an fMRI investigation. Journal of Cognitive Neuroscience, 22(1), 97–108.http://dx.doi.org/10.1162/jocn.2009.21187 [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology, 60, 693–716.http://dx.doi.org/10.1146/annurev.psych.60.110707.163514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R., Damasio H., Tranel D., Cooper G., Damasio A.R. (2000). A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neuroscience, 20(7), 2683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–77.http://dx.doi.org/10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Azevedo R.T., Macaluso E., Avenanti A., Santangelo V., Cazzato V., Aglioti S.M. (2013). Their pain is not our pain: brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Human Brain Mapping, 34(12), 3168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson C.D. (2009). These Things Called Empathy: eight Related but Distinct Phenomena. Cambridge, MA: MIT press. [Google Scholar]
- Blakemore S.-J., Mills K.L. (2014). Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol, 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Cheon B.K., Mathur V.A., Chiao J.Y. (2010). Empathy as cultural process: insights from the cultural neuroscience of empathy. World Cultural Psychiatry Research Review, 5(1), 32–42. [Google Scholar]
- Collins W.A., Steinberg L. (2006). Adolescent Development in Interpersonal Context. Hoboken, NJ: Wiley. [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–50.http://dx.doi.org/10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Davis M.H., Conklin L., Smith A., Luce C. (1996). Effect of perspective taking on the cognitive representation of persons: a merging of self and other. Journal of Personality and Social Psychology, 70(4), 713–26.http://dx.doi.org/10.1037/0022-3514.70.4.713 [DOI] [PubMed] [Google Scholar]
- De Waal F.B. (2008). Putting the altruism back into altruism: the evolution of empathy. Annual Review of Psychology, 59(1), 279–300.http://dx.doi.org/10.1146/annurev.psych.59.103006.093625 [DOI] [PubMed] [Google Scholar]
- Decety J. (2005). Perspective taking as the royal avenue to empathy In Malle B., Hodges S. D. (Eds.), Other Minds: How Humans Bridge the Divide Between Self and Others (pp., 135-149). New York, NY: Guilford Press. [Google Scholar]
- Decety J. (2011a). Dissecting the neural mechanisms mediating empathy. Emotion Review, 3(1), 92–108. [Google Scholar]
- Decety J. (2011). The neuroevolution of empathy. Annals of the New York Academy of Sciences, 1231(1), 35–45.http://dx.doi.org/10.1111/j.1749-6632.2011.06027.x [DOI] [PubMed] [Google Scholar]
- Decety J., Lamm C. (2006). Human empathy through the lens of social neuroscience. The Scientific World Journal, 6, 1146–63.http://dx.doi.org/10.1100/tsw.2006.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Sommerville J.A. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences, 7(12), 527–33.http://dx.doi.org/10.1016/j.tics.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Driscoll K., Pianta R.C. (2011). Mothers' and fathers' perceptions of conflict and closeness in parent-child relationships during early childhood. Journal of Early Childhood & Infant Psychology (7), 1–24. [Google Scholar]
- Eisenberg N., Eggum N.D. (2009). Empathic responding: sympathy and personal distress In Decety J., Ickes W. (Eds.), The Social Neuroscience of Empathy (pp., 71-83). Cambridge, MA: MIT Press. [Google Scholar]
- Fuligni A.J., Eccles J.S. (1993). Perceived parent-child relationships and early adolescents' orientation toward peers. Developmental Psychology, 29(4), 622–32.http://dx.doi.org/10.1037/0012-1649.29.4.622 [Google Scholar]
- Galinsky A.D., Ku G., Wang C.S. (2005). Perspective-taking and self-other overlap: fostering social bonds and facilitating social coordination. Group Processes & Intergroup Relations, 8(2), 109–24.http://dx.doi.org/10.1177/1368430205051060 [Google Scholar]
- Gallese V. (2007). Before and below ‘theory of mind’: embodied simulation and the neural correlates of social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1480), 659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuman A.S., Brunet N.M., Li Y., et al. (2014). Dynamic encoding of face information in the human fusiform gyrus. Nature Communications, 5(5672), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubert L., Craig K., Buysse A. (2009). Perceiving others in pain: experimental and clinical evidence on the role of empathy In Decety J., Ickes W. (Eds.), The Social Neuroscience of Empathy (pp., 153–65). Cambridge, MA: MIT Press. [Google Scholar]
- Goubert L., Vervoort T., Sullivan M.J., Verhoeven K., Crombez G. (2008). Parental emotional responses to their child’s pain: the role of dispositional empathy and catastrophizing about their child’s pain. The Journal of Pain, 9(3), 272–9. [DOI] [PubMed] [Google Scholar]
- Greening, S.G., Lee, T.H., Mather, M. (2016). Individual differences in anticipatory somatosensory cortex activity for shock is positively related with trait anxiety and multisensory integration. Brain sciences, 6(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.S., Konrath S., Brown S., Swain J.E. (2014). Empathy and stress related neural responses in maternal decision making. Frontiers in neuroscience, 8, 152–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges S.D., Klein K.J. (2001). Regulating the costs of empathy: the price of being human. The Journal of Socio-Economics, 30(5), 437–52.http://dx.doi.org/10.1016/S1053-5357(01)00112-3 [Google Scholar]
- Hoekzema E., Barba-Müller E., Pozzobon C., et al. (2016). Pregnancy leads to long-lasting changes in human brain structure. Nature Neuroscience, 20(2), 287–96. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Decety J. (2004). Motor cognition: a new paradigm to study self–other interactions. Current Opinion in Neurobiology, 14(2), 259–63.http://dx.doi.org/10.1016/j.conb.2004.01.020 [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Meltzoff A.N., Decety J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24(3), 771–9.http://dx.doi.org/10.1016/j.neuroimage.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–56.http://dx.doi.org/10.1016/S1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17(11), 4302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. (2009). Expanding the mirror: vicarious activity for actions, emotions, and sensations. Current Opinion in Neurobiology, 19(6), 666–71.http://dx.doi.org/10.1016/j.conb.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N., Mur M., Bandettini P. (2008). Representational similarity analysis—connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience, 2, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Batson C.D., Decety J. (2007). The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19(1), 42–58.http://dx.doi.org/10.1162/jocn.2007.19.1.42 [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J. (2008). Is the extrastriate body area (EBA) sensitive to the perception of pain in others? Cerebral Cortex, 18(10), 2369–73. [DOI] [PubMed] [Google Scholar]
- Larson R.W., Richards M.H., Moneta G., Holmbeck G., Duckett E. (1996). Changes in adolescents' daily interactions with their families from ages 10 to 18: disengagement and transformation. Developmental Psychology, 32(4), 744. [Google Scholar]
- Lee K.-Y., Lee T.-H., Yoon S.-J., et al. (2010). Neural correlates of top–down processing in emotion perception: an ERP study of emotional faces in white noise versus noise-alone stimuli. Brain Research, 1337, 56–63.http://dx.doi.org/10.1016/j.brainres.2010.03.094 [DOI] [PubMed] [Google Scholar]
- Leibenluft E., Gobbini M.I., Harrison T., Haxby J.V. (2004). Mothers' neural activation in response to pictures of their children and other children. Biological Psychiatry, 56(4), 225–32. [DOI] [PubMed] [Google Scholar]
- Mathur V.A., Harada T., Lipke T., Chiao J.Y. (2010). Neural basis of extraordinary empathy and altruistic motivation. NeuroImage, 51(4), 1468–75.http://dx.doi.org/10.1016/j.neuroimage.2010.03.025 [DOI] [PubMed] [Google Scholar]
- Mikulincer M., Gillath O., Halevy V., Avihou N., Avidan S., Eshkoli N. (2001). Attachment theory and reactions to others' needs: evidence that activation of the sense of attachment security promotes empathic responses. Journal of Personality and Social Psychology, 81(6), 1205. [PubMed] [Google Scholar]
- Morelli S.A., Rameson L.T., Lieberman M.D. (2014). The neural components of empathy: predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience, 9(1), 39–47.http://dx.doi.org/10.1093/scan/nss088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S., Decety J., Molenberghs P. (2012). The neuroscience of group membership. Neuropsychologia, 50(8), 2114–20.http://dx.doi.org/10.1016/j.neuropsychologia.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Mumford J.A., Turner B.O., Ashby F.G., Poldrack R.A. (2012). Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage, 59(3), 2636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E., Leibenluft E., McClure E., Pine D. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(2), 163–74.http://dx.doi.org/10.1017/S0033291704003915 [DOI] [PubMed] [Google Scholar]
- O'Craven K.M., Kanwisher N. (2000). Mental imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience, 12(6), 1013–23. [DOI] [PubMed] [Google Scholar]
- Olson D.H., Sprenkle D.H., Russell C.S. (1979). Circumplex model of marital and family systems: I. Cohesion and adaptability dimensions, family types, and clinical applications. Family Process, 18(1), 3–28. [DOI] [PubMed] [Google Scholar]
- Preston S.D. (2007). A Perception-Action Model for Empathy. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Preston S.D., De Waal F.B. (2002). Empathy: its ultimate and proximate bases. Behavioral and Brain Sciences, 25(01), 1–20. [DOI] [PubMed] [Google Scholar]
- Proulx C.M., Helms H.M. (2008). Mothers' and fathers' perceptions of change and continuity in their relationships with young adult sons and daughters. Journal of Family Issues, 29(2), 234–61.http://dx.doi.org/10.1177/0192513X07307855 [Google Scholar]
- Qu Y., Fuligni A.J., Galvan A., Telzer E.H. (2015). Buffering effect of positive parent–child relationships on adolescent risk taking: a longitudinal neuroimaging investigation. Developmental Cognitive Neuroscience, 15, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson L.T., Morelli S.A., Lieberman M.D. (2012). The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience, 24(1), 235–45.http://dx.doi.org/10.1162/jocn_a_00130 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fogassi L., Gallese V. (2001). Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience, 2(9), 661–70.http://dx.doi.org/10.1038/35090060 [DOI] [PubMed] [Google Scholar]
- Ruiz S., Gonzales N., Formoso D. (1998). Paper Presented at the Multicultural, Multidimensional Assessment of Parent-Adolescent Conflict: The Biennial Meeting 1998 of the Society for Research on Adolescence San Diego, CA.
- Sabatinelli D., Fortune E.E., Li Q., et al. (2011). Emotional perception: meta-analyses of face and natural scene processing. NeuroImage, 54(3), 2524–33.http://dx.doi.org/10.1016/j.neuroimage.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Saxe R., Carey S., Kanwisher N. (2004). Understanding other minds: linking developmental psychology and functional neuroimaging. Annual Review of Psychology, 55(1), 87–124.http://dx.doi.org/10.1146/annurev.psych.55.090902.142044 [DOI] [PubMed] [Google Scholar]
- Saxe R., Moran J.M., Scholz J., Gabrieli J. (2006). Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience, 1(3), 229–34.http://dx.doi.org/10.1093/scan/nsl034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. (2006). The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neuroscience & Biobehavioral Reviews, 30(6), 855–63.http://dx.doi.org/10.1016/j.neubiorev.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O'Doherty J., Kaube H., Dolan R.J., Frith C.D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–62. [DOI] [PubMed] [Google Scholar]
- Smith S. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–55.http://dx.doi.org/10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, 208–19.http://dx.doi.org/10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Stolarova, M., Keil, A., Moratti, S. (2005). Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cerebral cortex, 16(6), 876–87. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Gonzales N., Fuligni A.J. (2014). Family obligation values and family assistance behaviors: protective and risk factors for Mexican–American adolescents’ substance use. Journal of Youth and Adolescence, 43(2), 270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.M., Telzer E.H., Fuligni A.J. (2013). Continuity and discontinuity in perceptions of family relationships from adolescence to young adulthood. Child Development, 84(2), 471–84.http://dx.doi.org/10.1111/j.1467-8624.2012.01858.x [DOI] [PubMed] [Google Scholar]
- Tyler T.R., Degoey P. (1995). Community, family, and the social good: the psychological dynamics of procedural justice and social identification. Nebraska Symposium on Motivation, 42, 53–91. [PubMed] [Google Scholar]
- Visser R.M., Scholte H.S., Kindt M. (2011). Associative learning increases trial-by-trial similarity of BOLD-MRI patterns. The Journal of Neuroscience, 31(33), 12021–8.http://dx.doi.org/10.1523/JNEUROSCI.2178-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X., Han S. (2013). Cultural experiences reduce racial bias in neural responses to others’ suffering. Culture and Brain, 1(1), 34–46. [Google Scholar]