Abstract
Current recommendations for the use of intravenous iron therapy in the management of anaemia in patients with chronic kidney disease (CKD) are based on limited clinical evidence. Since the publication of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anaemia in Chronic Kidney Disease in 2012, a number of randomized clinical trials [notably, the Ferinject Assessment in Patients with Iron Deficiency Anaemia (FIND-CKD) and Randomized Trial to Evaluate IV and Oral Iron in Chronic Kidney Disease (REVOKE) trials] and observational studies have been completed, and a further large clinical trial—Proactive IV Iron Therapy in Dialysis Patients (PIVOTAL)—is currently underway. In this article, the implications of the findings from these recent studies are discussed and the critical evidence gaps that remain to be addressed are highlighted.
Keywords: CKD, clinical trial, epidemiology, ESRD, iron
Introduction
Iron deficiency anaemia is a common and clinically important concern in patients with chronic kidney disease (CKD). In this patient population, chronic inflammation may lead to increased hepcidin production, in turn inhibiting both the uptake of dietary iron and the mobilization of stored iron from the reticuloendothelial system to circulating transferrin [1]. Insufficient dietary iron uptake may be compounded by poor appetite or dietary restrictions, and intestinal bleeding may result in increased iron losses [2–4]. The problem is exacerbated in patients receiving dialysis who experience significant additional iron losses due to blood remaining in the dialyser circuit after treatment. Patients with CKD receiving treatment with erythropoiesis-stimulating agents (ESAs) are very prone to iron deficiency due to the increased demand for iron to support erythropoiesis, and indeed iron deficiency is the most commonly identified cause of hyporesponsiveness to ESA therapy in dialysis patients [5, 6]. As a result, iron therapy, either alone or in combination with ESA treatment, has been an important component of the management of anaemia in patients with CKD for many years.
The chapter in this supplement authored by Berns provides a detailed discussion of the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline recommendations for the use of iron to treat anaemia in CKD, while the chapter by Roger discusses current opinion regarding the application of the guideline in clinical practice. Both of these chapters highlight the fact that the quality of clinical evidence underpinning the guideline recommendations is relatively poor, with the result that different interpretations are possible. In the 4 years since the publication of the guideline, several new randomized controlled trials (RCTs) have been completed, most notably the Ferinject Assessment in Patients with Iron Deficiency Anaemia (FIND-CKD) [7, 8] and Randomized Trial to Evaluate IV and Oral Iron in Chronic Kidney Disease (REVOKE) [9] studies, while another large RCT, Proactive IV Iron Therapy in Dialysis Patients (PIVOTAL), is currently in progress. In addition, a number of relevant observational studies have been published. The aim of this chapter is to summarize the findings from these more recent studies and evaluate their implications for the management of iron deficiency anaemia in patients with CKD.
Iron therapy in patients with CKD: the evidence base for the 2012 KDIGO guideline
The 2012 KDIGO guideline recommends considering a trial of intravenous (IV) or oral iron in patients with CKD for whom an increase in haemoglobin levels without starting ESA therapy is desired (or when a decrease in the current ESA dose is desired), if transferrin saturation (TSAT) levels are ≤30% and serum ferritin levels are ≤500 µg/L. In patients with non-dialysis-dependent CKD (CKD-ND), it is suggested that the selection of iron administration route (oral versus IV) should be made based on the severity of anaemia, availability of venous access, response or lack of response to prior oral iron therapy, previously observed adverse events (AEs), patient adherence and cost; in patients with end-stage CKD (CKD-5D) receiving regular dialysis, oral iron is not recommended.
The recommendation to use IV rather than oral iron in CKD-5D patients was supported by a number of clinical studies in both haemodialysis and peritoneal dialysis patients (although the number of studies in peritoneal dialysis patients is very low), demonstrating a greater haemoglobin response with IV iron compared with oral iron [10–15]. Moreover, iron therapy, and in particular IV iron therapy, was found to improve the response to ESA treatment and reduce ESA requirements in this patient population [10, 11, 13, 15–21]. At the time of guideline publication, data showing greater efficacy of IV iron compared with oral iron with respect to erythropoietic response in patients with CKD-ND were more limited [22–28]. In both patient populations, the studies on which guideline recommendations were based were generally very short in duration and involved only small numbers of patients such that no conclusions could be drawn with respect to long-term efficacy or safety of IV versus oral iron.
At the time the guidelines were written, very little clinical evidence existed to support the suggested upper limits for iron status targets [29]. The recommendation to discontinue IV iron therapy in patients with a serum ferritin >500 µg/L was based mainly on the reported potential for hepatic deposition of iron at higher ferritin levels, as well as the potential for exacerbation of oxidative stress or infections [30–32]. While serum ferritin and TSAT are the favoured markers for assessing iron status, serum ferritin is an acute-phase reactant and levels may be elevated if inflammation is present. Furthermore, hepcidin-mediated blockade of iron mobilization from the reticuloendothelial system may also contribute to circumstances where patients have elevated serum ferritin levels but low TSAT. Results from the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) study suggested that iron therapy may lead to increases in haemoglobin levels and reduced ESA requirements even in patients with serum ferritin levels in excess of 500 µg/L [20, 33]. However, the study was conducted in only 134 dialysis patients, and the initial period of follow-up was only 6 weeks, with a further 6-week observational extension (DRIVE-II). Clearly, the long-term safety of IV iron administration in patients with elevated serum ferritin could not be ascertained from such a short follow-up period.
Observational data on the safety of iron therapy that were available at the time of guideline publication (but not considered by the panel when making recommendations) were also conflicting: one large US study showed no increase in dialysis patient mortality with iron doses of ≤1000 mg over 6 months [34], while a second study showed an increase in mortality in dialysis patients receiving doses of >400 mg/month [35].
New evidence: the FIND-CKD and REVOKE trials
Since the publication of the KDIGO guideline, several new studies have added to the clinical evidence base in support of the efficacy and short-term safety of IV iron therapy in patients with CKD-ND and CKD-5D [36–39]. A recent meta-analysis by Shepshelovich et al. [40], updating their original 2008 analysis [41], assessed data from 24 RCTs—13 of patients with CKD-ND and 11 of patients with CKD-5D—and concluded that patients treated with IV iron were more likely to achieve a haemoglobin increase of >1 g/dL than patients treated with oral iron (risk ratios 1.61 and 2.14 for CKD-ND and CKD-5D, respectively). Rates of mortality and serious AEs (SAEs)/AEs were found to be similar for the IV and oral iron groups, although IV iron administration was associated with a higher risk of hypotension but fewer gastrointestinal AEs. Similarly, in a meta-analysis of RCTs that evaluated IV iron for any clinical indication, treatment with IV iron was not found to be associated with increased risk of SAEs or infections versus comparator (placebo, no iron, oral iron or intramuscular iron), although the median follow-up time for the 103 studies assessed was only 8 weeks [42] (Table 1).
Table 1.
Summary of RCTs of IV iron in patients with non-dialysis-dependent and dialysis-dependent CKD
| Reference | Treatment | n | Follow-up | Study conclusions |
|---|---|---|---|---|
| Studies in patients with dialysis-dependent CKD | ||||
| Fishbane et al. 1995 [10] | IV iron dextran versus oral iron | 52 (HD) | 4 months | Hb response to IV iron superior to oral iron, reduced ESA requirements with IV iron |
| Macdougall et al. 1996 [13] | IV iron dextran versus oral ferrous sulphate versus no iron | 37 (HD) | 4 months | Enhanced Hb response to ESA and lower ESA requirements with IV iron compared with oral iron or no iron |
| Singh et al. 2006 [15] | IV iron sucrose versus no iron | 96 (PD) | 8 weeks | Peak Hb higher and other anaemia interventions occurred later/less often in patients receiving IV iron |
| Coyne et al. 2007 [33] | IV sodium ferric gluconate versus no iron | 134 (HD) | 6 weeks | In patients receiving adequate ESA, Hb response greater at 6 weeks in IV iron group than control, irrespective of serum ferritin levels (≤800 µg/L versus >800 µg/L) |
| Kapoian et al. 2008 [20] | IV sodium ferric gluconate versus no iron | 118 (HD) | 6 weeks | Reduced ESA use with IV iron compared with no iron, fewer AEs with IV iron |
| Li and Wang 2008 [11] | IV iron sucrose versus oral ferrous succinate | 136 (HD) | 8 weeks | Hb response to IV iron superior to oral iron, reduced ESA requirements with IV iron, fewer AEs with IV iron |
| Li and Wang 2008 [12] | IV iron sucrose versus oral ferrous succinate | 46 (PD) | 8 weeks | Hb response to IV iron superior to oral iron, no difference in AEs |
| Provenzano et al. 2009 [14] | IV ferumoxytol versus oral ferrous fumarate | 230 (HD) | 5 weeks | Hb response to IV iron superior to oral iron, no difference in AEs |
| Charytan et al. 2013 [37] | IV ferric carboxymaltose versus standard medical care (oral/IV/no iron) | 97 (CKD-HD subgroup) | 30 days | No significant difference in primary safety outcome (number of AEs), although more SAEs in standard medical care group; no significant difference in secondary efficacy outcomes |
| Bhandari et al. 2015 [36] | IV iron isomaltoside 1000 versus IV iron sucrose | 351 (HD) | 6 weeks | Similar efficacy with respect to Hb in range; significantly greater increase in ferritin from baseline to weeks 1, 2 and 4 and in reticulocyte count at week 4 for iron isomaltoside group; frequency and severity of AEs similar |
| Studies in patients with non-dialysis-dependent CKD | ||||
| Silverberg et al. 2001 [4] | IV iron sucrose with versus without ESA | 90 | 1 year | Target Hb maintained with low dose ESA in two-thirds of patients, and with no ESA in one-third |
| Stoves et al. 2001 [27] | IV iron sucrose versus oral ferrous sulphate | 45 | 6 months | No difference between IV iron and oral iron in patients |
| Aggarwal et al. 2003 [23] | IV iron dextran versus oral ferrous sulphate | 40 | 3 months | IV iron superior to oral iron, no difference in AEs |
| Charytan et al. 2005 [24] | IV iron sucrose versus oral ferrous sulphate | 96 | 6 weeks | IV iron superior to oral iron, no difference in AEs |
| Van Wyck et al. 2005 [28] | IV iron sucrose versus oral ferrous sulphate | 188 | 8 weeks | IV iron superior to oral iron, no difference in AEs, proportion of patients achieving Hb targets unaffected by ESA use |
| Agarwal et al. 2006 [22] | IV sodium ferric gluconate versus oral ferrous sulphate | 75 | 6 weeks | More rapid repletion of iron stores and improved QOL with IV iron compared with oral iron |
| Spinowitz et al. 2008 [26] | IV ferumoxytol versus oral iron | 304 | 5 weeks | IV iron superior to oral iron, no difference in AEs, increased Hb response when ESA given concurrently |
| Bailie et al. 2010 [43] | IV ferric carboxymaltose versus placebo | 598 subjects with IDA; 70 with CKD | 2 weeks | Minimal risk of hypersensitivity or adverse drug reaction with IV iron |
| McMahon et al. 2010 [44] | IV iron sucrose versus oral ferrous sulphate | 100 | 52 weeks | Maintaining iron stores above physiological level does not confer greater Hb response in ESA-naïve, iron-replete patients with Hb >11 g/dL |
| Qunibi et al. 2011 [25] | IV ferric carboxymaltose versus oral ferrous sulphate | 255 | 8 weeks | IV iron more effective than oral iron, fewer AEs with IV iron, increased Hb response when ESA given concurrently |
| Charytan et al. 2013 [37] | IV ferric carboxymaltose versus standard medical care (oral/IV/no iron) | 416 (CKD-ND subgroup) | 30 days | No significant difference in primary safety outcome (number of AEs), although more SAEs in standard medical care group; significant difference in secondary efficacy outcomes |
| Macdougall et al. 2014 [8] | IV ferric carboxymaltose versus oral ferrous sulphate | 626 | 56 weeks | Ferric carboxymaltose targeting ferritin 400–600 µg/L more effective than oral iron at reducing/delaying onset of other anaemia management or consecutive Hb values <10 g/dL; no difference in AEs |
| Onken et al. 2014 [39] | IV ferric carboxymaltose versus IV iron sucrose | 2585 | 56 days efficacy; 120 days safety | More subjects receiving ferric carboxymaltose versus iron sucrose achieved increase in Hb ≥1g/dL; no significant difference in composite safety endpoint |
| Agarwal et al. 2015 [9] | IV iron sucrose versus oral ferrous sulphate | 136 | 2 years | No difference between groups in slope of mGFR change; IV iron associated with increased risk of CV events and infection |
| Kalra et al. 2015 [38] | IV iron isomaltoside 1000 versus oral ferrous sulphate | 351 | 8 weeks | Greater increase in Hb with IV iron from week 3 to week 8; serum ferritin and TSAT also significantly increased with IV iron; ADRs similar; more patients in oral iron group withdrew from study due to ADRs |
ADR, adverse drug reaction; Hb, haemoglobin; HD, haemodialysis; PD, peritoneal dialysis; IDA, iron deficiency anaemia.
Two recent studies that were designed to inform with respect to the long-term efficacy and safety of IV iron in patients with CKD-ND were the FIND-CKD and REVOKE trials. FIND-CKD, published in 2014 [8], was designed to evaluate the efficacy of oral or IV iron (ferric carboxymaltose) to delay the onset of, or reduce the use of, ESA or other anaemia management in patients with CKD-ND and iron-deficiency anaemia when using targeted ferritin levels to determine iron dosing. A total of 626 patients across 193 sites in 20 countries were randomized to receive either high-dose IV ferric carboxymaltose (1000 mg every 4 weeks) targeting ferritin 400–600 µg/L, low-dose IV ferric carboxymaltose (200 mg every 4 weeks) targeting ferritin 100–200 µg/L, or oral iron (200 mg daily), and were followed over 56 weeks. The primary endpoint of the study was initiation of other anaemia management (ESA, other iron therapy, transfusion) or two consecutive haemoglobin measurements <10 g/dL during weeks 8–52. This endpoint occurred in 23.5, 32.2 and 31.8% of patients in the high-ferritin ferric carboxymaltose, low-ferritin ferric carboxymaltose and oral iron groups, respectively. The difference between the high-ferritin ferric carboxymaltose and oral iron groups was statistically significant [hazard ratio (HR), 0.65; 95% confidence interval (CI) 0.44–0.95; P = 0.025]. The increase in mean haemoglobin level from baseline to month 12 was significantly greater for the high-ferritin ferric carboxymaltose group versus the oral iron group, and the haematological response was also faster. Quality of life (QOL), as measured by the SF-36 instrument, did not show significant differences across groups, and AE and SAE rates were similar in all groups.
The primary objective of the REVOKE trial published 2015 [9] was to evaluate the effects of oral or IV iron on kidney function in patients with CKD-ND (stage 3/4) and iron-deficiency anaemia. A total of 136 subjects at a single centre in the USA were randomized to receive open label oral ferrous sulphate (325 mg tablets containing 65 mg elemental iron, three times daily for 8 weeks; n = 69) or IV iron sucrose (200 mg every 2 weeks for total of 1 g; n = 67); patients were followed for 2 years. No difference in the rate of measured glomerular filtration rate (mGFR) decline between groups was observed—i.e. IV iron was not found to accelerate (or delay) a decline in kidney function; statistically significant improvements in haemoglobin that were sustained for 24 months were observed for both the IV and oral iron groups with no significant between-group differences. However, a higher risk of SAEs [adjusted incidence rate ratio (aIRR), 1.60; 95% CI 1.28–2.00; P < 0.0001], cardiovascular (CV) SAEs (aIRR, 2.51; 95% CI 1.56–4.04; P < 0.001) and infection resulting in hospitalization (aIRR, 2.12; 95% CI 1.24–3.64; P < 0.006) in the IV iron group resulted in early termination of the study.
While both of these studies represent a significant advance over previous RCTs in that they involved larger numbers of patients and much longer follow-up periods, the findings were starkly contrasting, with the authors drawing essentially opposing conclusions. The investigators of the REVOKE trial concluded that use of IV iron resulted in elevated risk of infection and CV complications and that in patients with CKD-ND, oral iron may be the preferred first-line treatment for iron deficiency anaemia. In contrast, the FIND-CKD investigators concluded that the use of IV iron to target higher ferritin levels may contribute to improved anaemia management in this patient population, with no safety concerns in terms of CV events or infectious episodes [45].
Potential explanations for the differences in findings from these studies have been discussed in several commentaries [46–48]. One key difference between the two trials is that the dose of oral iron used in the FIND-CKD study was significantly lower than that used in the REVOKE study; this could potentially explain the conflicting conclusions drawn with respect to the efficacy of IV versus oral iron, but would not explain the discrepancy in the safety data. Another point of contention that may have bearing on the interpretation of findings is the appropriateness of adjustments for baseline patient differences that were applied in the analysis of data from the REVOKE study, particularly as some of the key safety findings only achieved statistical significance following such adjustment. In addition, the reporting and methods of adjudication of AEs differ between the two studies, further complicating their comparison. However, a recent post-hoc analysis of data from FIND-CKD examining the incidence of AEs using the same approach as the REVOKE analysis—counting individual AEs as separate events when a patient experienced multiple events—also showed no difference between treatment groups [45]. Other, more fundamental differences between the two studies include the fact that they used different iron formulations (iron sucrose versus ferric carboxymaltose) and were undertaken in different countries (USA versus multiple European countries/Australia).
New evidence: observational studies
In addition to the data from RCTs, a number of epidemiologic analyses pertaining to IV iron safety and efficacy have been published in the years since the release of the KDIGO guideline. An important consideration when interpreting findings from observational studies is that, by nature, such studies may be subject to confounding for various reasons, the most obvious in this case being that it is often the sickest patients who are given the greatest amounts of IV iron. Nonetheless, such studies can still provide insights into anaemia management trends as well as the efficacy and safety of IV iron therapy as applied in real-world clinical practice (Table 2).
Table 2.
Summary of observational studies of IV iron in patients with non-dialysis-dependent and dialysis-dependent CKD (2013−present)
| Reference | Study population | n | Study design | Study conclusions |
|---|---|---|---|---|
| Brookhart et al. 2016 [49] | Medicare ICHD patients (2004–05) | 66 207 | Comparison of short-term safety of IV sodium ferric gluconate versus iron sucrose; 1-month exposure period; outcomes (all-cause mortality, infection-related and CV hospitalization and mortality) assessed over 3-month follow-up period | No difference in mortality outcomes |
| Among CVC patients, slightly reduced risk of infection-related events in ferric gluconate patients | ||||
| Bolus dosing associated with increased infection-related events in both groups | ||||
| Freburger et al. 2016 [50] | ICHD patients of large US dialysis organization (2008–10) | 13 039 | Iron and ESA dosing assessed during 1-month and 2-week exposure periods; HRQOL measured over 3-month outcome period | In patients with low-baseline Hb, higher ESA dosing and bolus iron dosing associated with higher HRQOL scores |
| Airy et al. 2015 [51] | USRDS, incident HD patients (2009–11) | 14 206 | Comparison of HD facilities switching from iron sucrose or ferric gluconate to ferumoxytol with facilities that did not switch; incident patients at these facilities were followed until censoring, facility switch to different iron formulation or end of study (31 Dec 2011); outcomes assessed were all-cause mortality, CV hospitalization/mortality, infectious hospitalization/mortality | No difference in outcomes between facilities that switched to ferumoxytol and those that did not |
| Bailie et al. 2015 [52] | DOPPS facility HD patients (2009–11) | 32 435 | Assessed association between total prescribed IV iron dose over first 4 months in study with clinical outcomes (mortality, cause-specific mortality) | Increased risk of mortality for patients receiving 300–399 (13%) or 400+ mg/month (18%) compared with 100–199 mg/month; associations with cause-specific mortality and hospitalization similar |
| Ishida et al. 2015 [53] | USRDS ICHD patients (2010) | 22 820 | Comparison of outcomes for patients receiving versus not receiving IV iron while in hospital for bacterial infection | Receipt of IV iron not associated with higher 30-day mortality or readmission for infection |
| Karaboyas et al. 2015 [54] | DOPPS facility patients (2009–13) | 9735 | Trends in mean ferritin, haemoglobin, IV iron dose and ESA dose from 2009 to 2013 assessed among patients at 91 DOPPS facilities | IV iron increased from 220 mg/month in 2009/10 to 280 mg/month in 2011 then declined back to 200 mg/month in 2012–13; mean ferritin increased from 601 ng/mL in Q3 2009 to 887 ng/mL in Q1 2012; increase in ferritin not solely due to iron dosing practices |
| Kuo et al. 2015 [55] | Taiwan National Health Insurance Research Database, CKD-ND patients (2000–09) | 31 971 | Prospective cohort study of patients with creatinine >6 mg/dL, haematocrit <28%, treated with ESA; patients receiving versus not receiving IV iron within 90 days of starting ESA compared; outcomes assessed: death before dialysis initiation, hospitalization | Iron supplementation associated with 15% reduction in mortality and reduction in risk of hospitalization but higher risk of faster progression to ESRD |
| Tangri et al. 2015 [56] | HD patients of Dialysis Clinic Inc. (2003-08) | 9544 | Iron exposure assessed over 1-, 3- and 6-month time windows; incident hospitalizations assessed during 30-day outcome window | Higher cumulative dose of IV iron not associated with increased risk of hospitalization |
| Freburger et al. 2014 [57] | Medicare HD patients of small US dialysis provider | 6505 | Iron dosing patterns (bolus, maintenance, no iron) assessed during 1-month exposure windows; outcomes assessed over 3-month follow-up period | Bolus iron dosing associated with increased risk of infection-related hospitalization and use of IV antibiotics; no association between dosing practice and CV outcomes |
| Miskulin et al. 2014 [58] | Incident HD patients of Dialysis Clinic Inc. (2003–08) | 14 078 | Iron exposure assessed over 1-, 3- and 6-month time windows; all-cause, CV and infection-related mortality assessed during 30-day outcome window | Receipt of ≤1050 mg iron in 3 months or ≤ 2100 mg in 6 months not associated with all-cause, CV or infection-related mortality |
| Receipt of >1050 mg iron in 3 months or >2100 mg in 6 months possibly associated with infection-related mortality (non-statistically significant) | ||||
| Schiller et al. 2014 [59] | Patients of three US dialysis chains | 8666 | Patients treated with ferumoxytol at any time in 12-month period assessed; efficacy and safety outcomes considered | Ferumoxytol effective in increasing and maintaining Hb with AE profile similar to that reported in clinical trials |
| Bailie et al. 2013 [60] | DOPPS facility patients (1999–2011) | 32 192 | Trends in iron use and associations of IV iron dose with ferritin and TSAT assessed | IV iron use varied by country and increased over 2009–11 in most countries; increases in ferritin but not TSAT also observed |
| Brookhart et al. 2013 [61] | HD patients of large dialysis provider (2004–08) | 117 050 | Iron dosing patterns (bolus versus maintenance) assessed over 1-month exposure periods; mortality and infection-related hospitalization assessed n subsequent 3 months | Bolus iron dosing associated with increased risk of infection-related hospitalization and mortality; maintenance iron dosing not associated with increased risk for adverse outcomes compared with no iron |
| Kshirsagar et al. 2013 [62] | HD patients of large dialysis provider (2004–08) | 117 050 | Compared bolus versus maintenance and high versus low iron dose during 1-month exposure period and 3-month follow-up period; outcomes assessed: MI, stroke and CV mortality | Large doses of IV iron were not associated with increased risk of short-term CV morbidity and mortality |
| Kshirsagar et al. 2013 [63] | HD patients of large dialysis provider (2004–08) | 117 050 | Compared bolus versus maintenance and high versus low iron dose during 1-month exposure period and 6-week follow-up period; outcomes assessed: Hb, ESA dose, TSAT, serum ferritin | Large doses of IV iron associated with improved measures of anaemia management |
| Miskulin et al. 2013 [64] | HD patients from medium-sized US dialysis provider (2004–10) | Indicators of anaemia management assessed in HD patients over 2004–07, 2007–09 and 2010 | Median proportion of patients with Hb >12 g/dL and median weekly ESA doses declined sharply in 2010; iron doses, serum ferritin and TSAT increased over time |
DOPPS, Dialysis Practice Patterns and Outcomes Study; Hb, haemoglobin; HD, haemodialysis; HRQOL, health-related quality of life; ICHD, in-centre haemodialysis.
An analysis of data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) collected between 2002 and 2011, and considering 32 435 patients in 12 countries, showed an association (albeit fairly weak) between high IV iron dose and mortality [52]. Compared with patients receiving 100–199 mg/month, mortality was higher for those receiving 300–399 mg/month (HR, 1.13; 95% CI 1.00–1.27) and 400+ mg/month (HR, 1.18; 95% CI 1.07–1.30). Associations with cause-specific mortality (CV, infection, other) followed a similar pattern, and receipt of 300 mg/month or more was also associated with increased risk of hospitalization (HR, 1.12; 95% CI 1.07–1.18) versus 100–199 mg/month. In contrast, results of an analysis of data from the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE)-ESRD study considering 14 078 patients initiating dialysis between 2003 and 2008 showed no association between receipt of cumulative doses <1050 mg in 3 months or <2100 mg in 6 months with all-cause, CV or infection-related mortality [58]. A second analysis of data from the DEcIDE-ESRD study also showed no association between iron dose level during 1-, 3- or 6-month exposure windows and risk of all-cause hospitalization or death among a cohort of incident haemodialysis patients (n = 9544) [56].
Several studies have examined outcomes in patients receiving bolus versus maintenance IV iron dosing. Notably, Brookhart et al. showed that among 117 050 Medicare patients dialysing at a large dialysis organization, bolus dosing (≥100 mg iron in at least two consecutive dialysis sessions and total dose with potential to exceed 600 mg within 30 days) was associated with a small increase in relative risk of infection-related hospitalizations compared with maintenance dosing, resulting in 25 additional events per 1000 patient-years [61]; this association was found to be most pronounced in patients dialysing with a central venous catheter. Similar results were obtained in a study of 6605 patients of a small dialysis organization in which bolus dosing during a 1-month exposure period was found to be associated with a significant increase in relative risk of hospitalization for infection and receipt of IV antibiotics in the following 3 months; again associations were more pronounced in patients dialysing with catheters [57]. Infection risk associated with IV iron therapy was also assessed in a recent study of United States Renal Data System (USRDS) data: among a cohort of Medicare beneficiaries on in-centre haemodialysis who received IV iron in the 14 days prior to a hospitalization for bacterial infection, no association was observed between receipt of IV iron at any point during the hospitalization and 30-day mortality, mean length of hospital stay or a composite measure of readmission for infection or death within 30 days of discharge [53]. The authors of the study concluded that their findings did not support the withholding of IV iron upon hospitalization for bacterial infection, although they noted that the majority of IV iron administered was received on the day of admission, limiting the ability to draw conclusions about the longer-term use of IV iron during the course of infection.
Observational analyses of outcomes in CKD-ND patients have suggested potential benefits of initiating IV iron therapy prior to dialysis onset. One prospective study found that CKD-ND patients who were treated with ESA and also initiated IV iron supplementation had a lower risk of all-cause death than those who did not receive iron [55]. In this study, the risk of hospitalizations was also lower, although the risk of faster progression to end-stage renal disease (ESRD) was higher. Similarly, a small retrospective study of 102 patients initiating dialysis showed that those who received IV iron and ESA prior to onset of dialysis had higher haemoglobin levels and greater left ventricular ejection fraction at the time of dialysis initiation than patients who received oral iron and ESA therapy [65].
In summary, findings from observational studies have also been equivocal with respect to the long-term safety of IV iron (in particular, mortality and infection risk). While the differing findings may well be explained by differences in study design and patient populations assessed, the lack of a clear consensus again emphasizes the need for additional clinical data.
Future prospects: the Proactive IV Iron Therapy in Dialysis Patients (PIVOTAL) trial
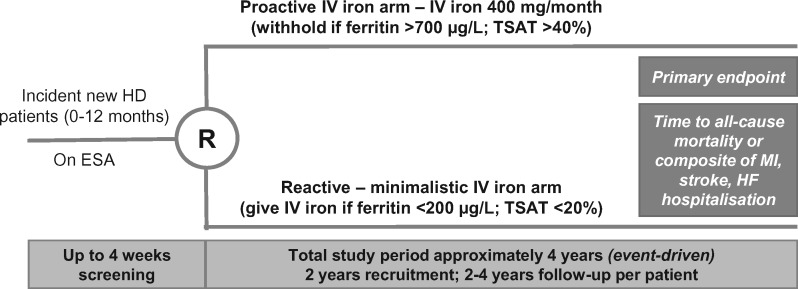
One ongoing study that may prove to be informative is the PIVOTAL trial (EudraCT number 2013-002267-25). This study is the first to assess the long-term safety and efficacy of the currently accepted practices of liberal IV iron use in patients with CKD-5D. The specific objective of the study is to compare proactive high-dose and reactive low-dose IV iron regimens with respect to all-cause mortality and incidence of non-fatal CV endpoints as well as ESA dose requirements, the need for transfusions, incidence of infections and other complications of haemodialysis and indicators of QOL. The trial is a multicentre, prospective, open-label, two-arm RCT, enrolling 2080 haemodialysis patients (new to dialysis, or on dialysis for <12 months) at 50 dialysis units across the UK. Patients included in the study must be at least 18 years of age, receiving ESA therapy, and have serum ferritin levels < 400 µg/L and TSAT <30% at baseline. Key exclusion criteria are life expectancy of <12 months, scheduled transplant within 12 months, C-reactive protein levels > 50 mg/L, and presence of active infection or malignancy.
Study subjects are being randomized into two treatment arms: Those in the proactive IV iron arm will receive 400 mg of iron sucrose monthly provided that ferritin levels are <700 µg/L; iron dose will be withheld if ferritin levels are >700 µg/L and/or TSAT levels are >40%. Subjects in the reactive IV iron arm will receive 200 mg iron sucrose if serum ferritin levels are <100 µg/L, regardless of TSAT levels, and will receive 100 mg IV iron sucrose if ferritin levels are >100 µg/L, with TSAT <20%; iron will be withheld if ferritin levels are >200 µg/L and TSAT levels are >20% (Figure 1).
Fig. 1.
Proactive IV Iron Therapy in Dialysis Patients (PIVOTAL) Trial Design. ESA, erythropoiesis-stimulating agent; HD, haemodialysis; HF, heart failure; IV, intravenous; MI, myocardial infarction; R, randomisation; TSAT, transferrin saturation.
The primary endpoint of the study is time to all-cause death or a composite of non-fatal CV events [myocardial infarction (MI), stroke or hospitalization for heart failure (HF)]. Secondary efficacy endpoints include: incidence of all-cause death and a composite of MI, stroke and hospitalization for HF as recurrent events; time to (and incidence of) all-cause death, composite CV event, MI, stroke, hospitalization for HF; ESA dose requirements; transfusion requirements; and patient functioning and QOL evaluated using the EuroQOL 5 dimensions questionnaire (EQ-5D) and the Kidney Disease Quality of Life (KDQOL) instrument. Secondary safety endpoints to be assessed are incidence of vascular access thrombosis, all-cause hospitalization, hospitalization for infection and infectious episodes, as well as incidence of SAEs, adverse drug reactions and suspected unexpected serious adverse reactions. Tertiary endpoints to be assessed include cumulative iron dose as well as haemoglobin, mean cell volume, red cell distribution width, ferritin, TSAT and platelet levels. The trial is event-driven and evaluation of outcomes will be conducted only once the required number of events are accrued (follow-up time of ∼2–4 years is expected). At the time of writing, all 2080 patients have been recruited and randomized to the trial, and follow-up is ongoing. It is expected that the results of this trial will not be available until 2018, but it is likely that the data from this trial will significantly impact future iron management in CKD patients.
Concluding remarks
Guidelines on iron management in CKD patients are somewhat dated, conflicting and based on sparse evidence. Two new RCTs in non-dialysis CKD patients (FIND-CKD and REVOKE) significantly improve the quality of the evidence base, but unfortunately produced conflicting results, which leaves us as confused as ever. Observational data are hypothesis-generating, but of limited clinical value due to significant confounding; moreover, the results of the new observational studies published since the 2012 KDIGO guideline are also conflicting. The PIVOTAL study seeks to fill the evidence gap, but results are not due until 2018.
Acknowledgements
The author thanks Abigail Hunt, PhD, an employee of DaVita Clinical Research, Minneapolis, MN, USA, for medical writing and editorial support in the preparation of this article.
Funding
Funding for medical writing support was provided by Vifor Pharma.
Conflict of interest statement
I.C.M. has received speaker and consultancy fees, as well as research funding from a number of IV iron manufacturers, including AMAG, Pharmacosmos and Vifor Pharma.
References
- 1. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 2006; 1 Suppl 1: S4–S8 [DOI] [PubMed] [Google Scholar]
- 2. Fishbane S, Pollack S, Feldman HI. et al. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol 2009; 4: 57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gotloib L, Silverberg D, Fudin R. et al. Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol 2006; 19: 161–167 [PubMed] [Google Scholar]
- 4. Silverberg DS, Blum M, Agbaria Z. et al. The effect of IV iron alone or in combination with low-dose erythropoietin in the rapid correction of anaemia of chronic renal failure in the predialysis period. Clin Nephrol 2001; 55: 212–219 [PubMed] [Google Scholar]
- 5. Horl WH. Non-erythropoietin-based anaemia management in chronic kidney disease. Nephrol Dial Transplant 2002; 17 Suppl 11: 35–38 [DOI] [PubMed] [Google Scholar]
- 6. Li S, Foley RN, Gilbertson DT. et al. Clinical factors associated with achieving K/DOQI haemoglobin targets in hemodialysis patients. Int Urol Nephrol 2003; 35: 399–405 [DOI] [PubMed] [Google Scholar]
- 7. Macdougall IC, Bock A, Carrera F. et al. The FIND-CKD study–a randomized controlled trial of intravenous iron versus oral iron in non-dialysis chronic kidney disease patients: background and rationale. Nephrol Dial Transplant 2014; 29: 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macdougall IC, Bock AH, Carrera F. et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014; 29: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agarwal R, Kusek JW, Pappas MK.. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 2015; 88: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fishbane S, Frei GL, Maesaka J.. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis 1995; 26: 41–46 [DOI] [PubMed] [Google Scholar]
- 11. Li H, Wang SX.. Intravenous iron sucrose in Chinese hemodialysis patients with renal anaemia. Blood Purif 2008; 26: 151–156 [DOI] [PubMed] [Google Scholar]
- 12. Li H, Wang SX.. Intravenous iron sucrose in peritoneal dialysis patients with renal anaemia. Perit Dial Int 2008; 28: 149–154 [PubMed] [Google Scholar]
- 13. Macdougall IC, Tucker B, Thompson J. et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 1996; 50: 1694–1699 [DOI] [PubMed] [Google Scholar]
- 14. Provenzano R, Schiller B, Rao M. et al. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol 2009; 4: 386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh H, Reed J, Noble S. et al. Effect of intravenous iron sucrose in peritoneal dialysis patients who receive erythropoiesis-stimulating agents for anaemia: a randomized, controlled trial. Clin J Am Soc Nephrol 2006; 1: 475–482 [DOI] [PubMed] [Google Scholar]
- 16. Besarab A, Kaiser JW, Frinak S.. A study of parenteral iron regimens in hemodialysis patients. Am J Kidney Dis 1999; 34: 21–28 [DOI] [PubMed] [Google Scholar]
- 17. Chang CH, Chang CC, Chiang SS.. Reduction in erythropoietin doses by the use of chronic intravenous iron supplementation in iron-replete hemodialysis patients. Clin Nephrol 2002; 57: 136–141 [DOI] [PubMed] [Google Scholar]
- 18. Covic A, Mircescu G.. The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: a multi-centre, open-label, clinical study. Nephrol Dial Transplant 2010; 25: 2722–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeVita MV, Frumkin D, Mittal S. et al. Targeting higher ferritin concentrations with intravenous iron dextran lowers erythropoietin requirement in hemodialysis patients. Clin Nephrol 2003; 60: 335–340 [DOI] [PubMed] [Google Scholar]
- 20. Kapoian T, O'Mara NB, Singh AK. et al. Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol 2008; 19: 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Senger JM, Weiss RJ.. Hematologic and erythropoietin responses to iron dextran in the hemodialysis environment. ANNA J 1996; 23: 319–323; discussion 324–315 [PubMed] [Google Scholar]
- 22. Agarwal R, Rizkala AR, Bastani B. et al. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol 2006; 26: 445–454 [DOI] [PubMed] [Google Scholar]
- 23. Aggarwal HK, Nand N, Singh S. et al. Comparison of oral versus intravenous iron therapy in predialysis patients of chronic renal failure receiving recombinant human erythropoietin. J Assoc Physicians India 2003; 51: 170–174 [PubMed] [Google Scholar]
- 24. Charytan C, Qunibi W, Bailie GR.. Comparison of intravenous iron sucrose to oral iron in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron Clin Pract 2005; 100: c55–c62 [DOI] [PubMed] [Google Scholar]
- 25. Qunibi WY, Martinez C, Smith M. et al. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant 2011; 26: 1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spinowitz BS, Kausz AT, Baptista J. et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol 2008; 19: 1599–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoves J, Inglis H, Newstead CG.. A randomized study of oral vs intravenous iron supplementation in patients with progressive renal insufficiency treated with erythropoietin. Nephrol Dial Transplant 2001; 16: 967–974 [DOI] [PubMed] [Google Scholar]
- 28. Van Wyck DB, Roppolo M, Martinez CO. et al. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int 2005; 68: 2846–2856 [DOI] [PubMed] [Google Scholar]
- 29. Fishbane S, Kalantar-Zadeh K, Nissenson AR.. Serum ferritin in chronic kidney disease: reconsidering the upper limit for iron treatment. Semin Dial 2004; 17: 336–341 [DOI] [PubMed] [Google Scholar]
- 30. Canavese C, Bergamo D, Ciccone G. et al. Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int 2004; 65: 1091–1098 [DOI] [PubMed] [Google Scholar]
- 31. Ferrari P, Kulkarni H, Dheda S. et al. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macdougall IC, Bircher AJ, Eckardt KU. et al. Iron management in chronic kidney disease: conclusions from a ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) Controversies Conference. Kidney Int 2016; 89: 28–39 [DOI] [PubMed] [Google Scholar]
- 33. Coyne DW, Kapoian T, Suki W. et al. Ferric gluconate is highly efficacious in anaemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients' Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 2007; 18: 975–984 [DOI] [PubMed] [Google Scholar]
- 34. Feldman HI, Joffe M, Robinson B. et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 2004; 15: 1623–1632 [DOI] [PubMed] [Google Scholar]
- 35. Kalantar-Zadeh K, Regidor DL, McAllister CJ. et al. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 2005; 16: 3070–3080 [DOI] [PubMed] [Google Scholar]
- 36. Bhandari S, Kalra PA, Kothari J. et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer(R)) compared with iron sucrose (Venofer(R)) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant 2015; 30: 1577–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charytan C, Bernardo MV, Koch TA. et al. Intravenous ferric carboxymaltose versus standard medical care in the treatment of iron deficiency anemia in patients with chronic kidney disease: a randomized, active-controlled, multi-center study. Nephrol Dial Transplant 2013; 28: 953–964 [DOI] [PubMed] [Google Scholar]
- 38. Kalra PA, Bhandari S, Saxena S. et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant 2016; 31: 646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Onken JE, Bregman DB, Harrington RA. et al. Ferric carboxymaltose in patients with iron-deficiency anaemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transplant 2014; 29: 833–842 [DOI] [PubMed] [Google Scholar]
- 40. Shepshelovich D, Rozen-Zvi B, Avni T. et al. Intravenous versus oral iron supplementation for the treatment of anaemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis 2016; 68: 677–690 [DOI] [PubMed] [Google Scholar]
- 41. Rozen-Zvi B, Gafter-Gvili A, Paul M. et al. Intravenous versus oral iron supplementation for the treatment of anaemia in CKD: systematic review and meta-analysis. Am J Kidney Dis 2008; 52: 897–906 [DOI] [PubMed] [Google Scholar]
- 42. Avni T, Bieber A, Grossman A. et al. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc 2015; 90: 12–23 [DOI] [PubMed] [Google Scholar]
- 43. Bailie GR, Mason NA, Valaoras TG.. Safety and tolerability of intravenous ferric carboxymaltose in patients with iron deficiency anaemia. Hemodial Int 2010; 14: 47–54 [DOI] [PubMed] [Google Scholar]
- 44. McMahon LP, Kent AB, Kerr PG. et al. Maintenance of elevated versus physiological iron indices in non-anaemic patients with chronic kidney disease: a randomized controlled trial. Nephrol Dial Transplant 2010; 25: 920–926 [DOI] [PubMed] [Google Scholar]
- 45. Roger SD, Gaillard CA, Bock AH. et al. Safety of intravenous ferric carboxymaltose versus oral iron in patients with nondialysis-dependent CKD: and analysis of the 1-year FIND-CKD trial. Nephrol Dial Transplant 2016https://doi.org/10.1093/ndt/gfw264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhandari S, Kalra PA, Coyne DW.. Data confusion. Kidney Int 2015; 88: 1445. [DOI] [PubMed] [Google Scholar]
- 47. Macdougall IC, Roger SD.. New data on the safety of IV iron-but why the discrepancy with FIND-CKD? Kidney Int 2015; 88: 1445–1446 [DOI] [PubMed] [Google Scholar]
- 48. Agarwal R. The author replies. Kidney Int 2015; 88: 1446–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brookhart MA, Freburger JK, Ellis AR. et al. Comparative short-term safety of sodium ferric gluconate versus iron sucrose in hemodialysis patients. Am J Kidney Dis 2016; 67: 119–127 [DOI] [PubMed] [Google Scholar]
- 50. Freburger JK, Ellis AR, Wang L. et al. Comparative effectiveness of iron and erythropoiesis-stimulating agent dosing on health-related quality of life in patients receiving hemodialysis. Am J Kidney Dis 2016; 67: 271–282 [DOI] [PubMed] [Google Scholar]
- 51. Airy M, Mandayam S, Mitani AA. et al. Comparative outcomes of predominant facility-level use of ferumoxytol versus other intravenous iron formulations in incident hemodialysis patients. Nephrol Dial Transplant 2015; 30: 2068–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bailie GR, Larkina M, Goodkin DA. et al. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int 2015; 87: 162–168 [DOI] [PubMed] [Google Scholar]
- 53. Ishida JH, Marafino BJ, McCulloch CE. et al. Receipt of intravenous iron and clinical outcomes among hemodialysis patients hospitalized for infection. Clin J Am Soc Nephrol 2015; 10: 1799–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karaboyas A, Zee J, Morgenstern H. et al. Understanding the recent increase in ferritin levels in united states dialysis patients: potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. Clin J Am Soc Nephrol 2015; 10: 1814–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kuo KL, Hung SC, Liu JS. et al. Iron supplementation associates with low mortality in pre-dialyzed advanced chronic kidney disease patients receiving erythropoiesis-stimulating agents: a nationwide database analysis. Nephrol Dial Transplant 2015; 30: 1518–1525 [DOI] [PubMed] [Google Scholar]
- 56. Tangri N, Miskulin DC, Zhou J. et al. Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: a comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol Dial Transplant 2015; 30: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Freburger JK, Ellis AR, Kshirsagar AV. et al. Comparative short-term safety of bolus versus maintenance iron dosing in hemodialysis patients: a replication study. BMC Nephrol 2014; 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miskulin DC, Tangri N, Bandeen-Roche K. et al. Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 2014; 9: 1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schiller B, Bhat P, Sharma A.. Safety and effectiveness of ferumoxytol in hemodialysis patients at 3 dialysis chains in the United States over a 12-month period. Clin Ther 2014; 36: 70–83 [DOI] [PubMed] [Google Scholar]
- 60. Bailie GR, Larkina M, Goodkin DA. et al. Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2013; 28: 2570–2579 [DOI] [PubMed] [Google Scholar]
- 61. Brookhart MA, Freburger JK, Ellis AR. et al. Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 2013; 24: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kshirsagar AV, Freburger JK, Ellis AR. et al. Intravenous iron supplementation practices and short-term risk of cardiovascular events in hemodialysis patients. PLoS One 2013; 8: e78930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kshirsagar AV, Freburger JK, Ellis AR. et al. The comparative short-term effectiveness of iron dosing and formulations in US hemodialysis patients. Am J Med 2013; 126: 541.e1–541.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miskulin DC, Zhou J, Tangri N. et al. Trends in anaemia management in US hemodialysis patients 2004–2010. BMC Nephrol 2013; 14: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rottembourg J, Sonigo Y, Dansaert A. et al. Intravenous iron during predialysis period improves anemia management and cardiovascular parameters in incident hemodialysis patients. Nephrol Ther 2013; 9: 486–493 [DOI] [PubMed] [Google Scholar]