Abstract
Impulsive aggression is common among military personnel after deployment and may arise because of impaired top-down regulation of the amygdala by prefrontal regions. This study sought to further explore this hypothesis via resting-state functional connectivity analyses in impulsively aggressive combat veterans. Male combat veterans with (n = 28) and without (n = 30) impulsive aggression problems underwent resting-state functional magnetic resonance imaging. Functional connectivity analyses were conducted with the following seed-regions: basolateral amygdala (BLA), centromedial amygdala, anterior cingulate cortex (ACC), and anterior insular cortex (AIC). Regions-of-interest analyses focused on the orbitofrontal cortex and periaqueductal gray, and yielded no significant results. In exploratory cluster analyses, we observed reduced functional connectivity between the (bilateral) BLA and left dorsolateral prefrontal cortex in the impulsive aggression group, relative to combat controls. This finding indicates that combat-related impulsive aggression may be marked by weakened functional connectivity between the amygdala and prefrontal regions, already in the absence of explicit emotional stimuli. Group differences in functional connectivity were also observed between the (bilateral) ACC and left cuneus, which may be related to heightened vigilance to potentially threatening visual cues, as well as between the left AIC and right temporal pole, possibly related to negative memory association in impulsive aggression.
Keywords: impulsive aggression, neuroimaging, functional connectivity, amygdala, DLPFC
Introduction
Impulsive aggression is characterized by recurrent incidents of verbal and/or physical aggression, which may be aimed at people, animals, or inanimate objects, and can be differentiated from premeditated aggression by its reactive, affectively driven nature (Coccaro, 2011). Problems with impulsive aggression can occur in many forms of psychiatric dysfunction, such as schizophrenia (Hoptman, 2015), major depressive disorder (Painuly et al., 2005), and post-traumatic stress disorder (Orth & Wieland, 2006), but are neither necessary nor sufficient for a diagnosis of such illnesses. Research has indicated that combat veterans are at an increased risk to develop impulsive aggression problems in the aftermath of military deployment (Heesink et al., 2015; Jakupcak et al., 2007).
The neural basis of impulsive aggression has been investigated extensively both in humans and non-human species (see Nelson & Trainor, 2007 for a review). More recently, the focus of this research area has started to shift towards functional imaging in clinical and non-clinical populations. For instance, Coccaro et al. (2007) observed that the amygdala of patients suffering from impulsive aggression problems was hyperresponsive to the presentation of angry faces in a task-based functional magnetic resonance imaging (fMRI) paradigm; i.e. relative to non-aggressive healthy controls. A decrease in the reactivity of the orbitofrontal cortex (OFC) was also observed in these impulsively aggressive individuals. Moreover, the authors noted a significant reduction in the extent of negative functional connectivity between the amygdala and OFC in the impulsive aggression group (n = 10), relative to the healthy control group (n = 10) (Coccaro et al., 2007). These latter results were replicated in a more recent inquiry by the same group, which included larger sample sizes (n = 20) (McCloskey et al., 2016). In line with the above-mentioned findings, Fulwiler et al. (2012) reported that resting-state functional connectivity between the amygdala and OFC was inversely related to Trait Anger in healthy volunteers (N = 16), as measured by the State-Trait Anger Expression Inventory-2 (STAXI-2; Spielberger, 1999). Taken together, these results indicate that a loss of top-down regulation of the amygdala by the OFC may serve as a neural substrate of impulsive aggression.
Neuroimaging studies on impulsive aggression have typically treated the amygdala as a single uniform structure, which is an oversimplification, given that it consists of multiple and functionally distinct subunits [the basolateral (BLA), centromedial (CeM), and superficial amygdala]. For instance, the BLA is thought to facilitate emotional learning by integrating inputs from sensory cortices (i.e., tones, odors, sounds) and subcortical brain areas (e.g., hippocampus, thalamus) (Davis & Whalen, 2001; Maren & Holmes, 2016; Phelps & LeDoux, 2005). Activity in this subdivision may be modulated by inhibitory projections arising from prefrontal regions, most notably the OFC (Maren & Holmes, 2016). The CeM is believed to serve as the main output structure of the amygdalar complex, and may promote species-specific fight-or-flight responses through descending projections to the midbrain, including the periaqueductal gray (PAG) (Davis & Whalen, 2001; Phelps & LeDoux, 2005; Roy et al., 2009). Consistent with this notion, impulsive aggression has frequently been linked to (hyper)activation of the PAG in the animal literature (Nelson & Trainor, 2007). It currently remains unclear as to what role (if any) subregions of the amygdala may play in human impulsive aggression.
Two additional brain areas which may be relevant to consider in light of the present subject matter are the anterior cingulate cortex (ACC) and anterior insular cortex (AIC): The ACC is believed to be involved in the detection of deviations from expected and/or desired outcomes and has frequently been linked to the emotional appraisal of physical and/or social distress (Carter & van Veen, 2007; Eisenberger & Lieberman, 2004; Etkin et al., 2011). The AIC is believed to play a key role in gaining awareness of one’s own physical and emotional state, as well as that of others (i.e., empathy) (Craig, 2009). Prior neuroimaging inquiries have linked both the ACC and AIC to impulsive aggression, albeit with mixed results (Denson et al., 2009; Dougherty et al., 1999; New et al., 2009): For instance, Denson et al. (2009) reported that the ACC and AIC were activated by a verbal insult in an fMRI study with healthy volunteers (N = 20). The authors also noted a positive relation between the extent of left dorsal ACC activation and scores on the Buss–Perry Aggression Questionnaire (Buss & Perry, 1992; Denson et al., 2009). Dougherty et al. (1999) recorded activation of the right ACC in a sample of healthy volunteers (N = 8), using a script-driven anger-induction protocol with positron emission tomography (PET); contrary to the authors’ hypothesis, no significant activation of the insula was observed. New et al. (2009) conducted PET and compared patients with impulsive aggression (n = 38) to non-aggressive healthy controls (n = 36) on the Point Subtraction Aggression Paradigm (Cherek et al., 1996). No significant group effects were detected in either the cingulate (as region-of-interest) or insular cortex (at whole brain) (New et al., 2009). Thus, although previous work has linked both the ACC and AIC to impulsive aggression, studies have been few and far between, and results have been inconclusive. Hence, additional research is needed to help clarify the role(s) of ACC and/or AIC dysfunction in impulsive aggression.
The above-described functional imaging studies have mostly examined impulsive aggression from a task–response point of view. Although such studies provide valuable insight into the neural architecture of certain cognitive and/or emotional processes, they do not offer much information on the brain’s native or resting state. It is now widely recognized that ongoing spontaneous fluctuations in blood–oxygen-level dependent signal are meaningful in and of themselves, as they are purported to reflect the activity of so-called resting-state networks: constellations of neuron populations that fire in synchrony at rest (Fox & Raichle, 2007). Perturbations in the intrinsic connectivity of such resting-state networks have been recorded in various forms of psychopathology (see Greicius, 2008 for a review), such as major depression (Greicius et al., 2007), attention/deficit hyperactivity disorder (Tian et al., 2006), and schizophrenia (Zhou et al., 2007). It currently is unclear if perturbations in resting-state functional connectivity are similarly present in combat-related impulsive aggression.
In the current study, we conducted functional connectivity analyses of resting-state fMRI in combat veterans with (n = 28) and without (n = 30) impulsive aggression. Based on previous task–response findings in patients who suffer from impulsive aggression (Coccaro et al., 2007; McCloskey et al., 2016), as well as a resting-state analysis of Trait Anger in healthy volunteers (Fulwiler et al., 2012), we hypothesized that combat veterans with impulsive aggression problems would display (i) a decrease in functional connectivity between the OFC and BLA, and (ii) an increase in functional connectivity between the CeM and PAG, relative to non-aggressive combat controls. We also conducted exploratory functional connectivity analyses using the BLA, CeM, as well as the ACC and AIC, as seed-regions-of-interest.
Materials and methods
Participants
Twenty-eight male veterans with impulsive aggression problems participated in this study after giving written and informed consent. We recruited these participants from four outpatient clinics of the Military Mental Healthcare Organization in the Netherlands. An additional 30 male veterans were included as combat controls and were recruited through advertisements or through participation in prior studies. Demographic and clinical information per group are summarized in Table 1. Candidates were considered eligible for participation if the following criteria were met: (i) military deployment for a minimum of 4 months, and (ii) age between 18 and 50 years. Impulsive aggression problems were ascertained via a set of research diagnostic criteria as proposed by Coccaro (2011): (1) acts of verbal and/or physical aggression that occur at least twice weekly over a period of 1 month, or three episodes of physical assault that occur over a period of 1 year; (2) the degree of aggressiveness is grossly out of proportion; (3) the aggressive behavior is impulsive rather than premeditated; (4) the aggressive behavior is distressful or leads to significant impairment in occupational and/or interpersonal function (Coccaro, 2011). Inclusion in the combat control group was contingent on the absence of anger- and aggression-related complaints, as well as the absence of any current DSM-IV Axis-I diagnosis. Exclusion criteria for both groups included a history of neurological disorder, claustrophobia, and the presence of a pacemaker or other metallic implant. This study was in accordance with the Declaration of Helsinki and was approved by the Medical Ethical Review Board of the University Medical Centre Utrecht in the Netherlands.
Table 1.
Demographic and clinical information per group
| Measure | Impulsive aggression group (n = 28) | Combat control group (n = 30) | P-value |
|---|---|---|---|
| Age | 36.54 ± 6.27 | 34.53 ± 7.59 | 0.280 |
| Number of deployments | 2.11 ± 1.17 | 2.37 ± 1.25 | 0.417 |
| Duration of deployment (in months) | 10.02 ± 6.05 | 11.56 ± 7.18 | 0.401 |
| Number of years since last deployment | 8.50 ± 5.25 | 6.23 ± 1.76 | <0.05 |
| Rank | |||
| Enlisted | 0 | 1 (3.3%) | |
| Corporal | 3 (10.7%) | 6 (20%) | |
| NCO | 8 (28.6%) | 9 (30%) | |
| Officer | 0 | 5 (16.7%) | |
| Not currently enlisted | 17 (60.7%) | 9 (30%) | |
| Education | |||
| Lower | 2 (7.1%) | 5 (16.7%) | |
| Middle | 19 (67.9%) | 16 (53.3%) | |
| Higher | 7 (25%) | 9 (30%) | |
| State-Trait Anger Expression Inventory | |||
| Total scores | 46.75 ± 15.97 | 27.33 ± 3.07 | <0.01 |
| State Anger | 23.96 ± 11.49 | 15.20 ± 0.76 | <0.01 |
| Trait Anger | 22.79 ± 7.01 | 12.13 ± 2.47 | <0.01 |
Fig. 1.
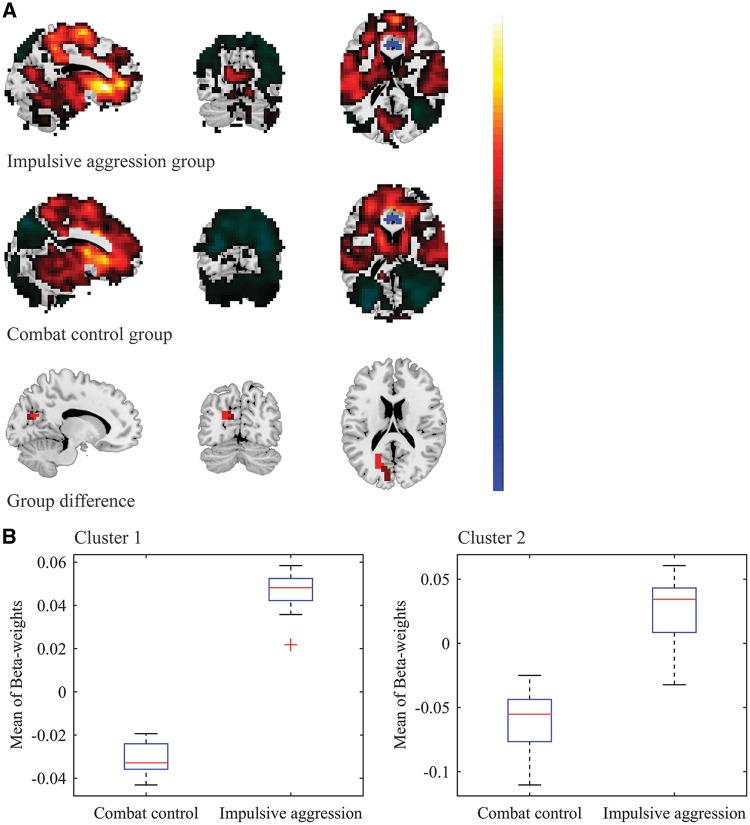
Reduced negative functional connectivity between the right BLA and left DLPFC in veterans suffering from impulsive aggression problems. A. For visualization purposes, the top and middle panel show the whole-brain FWE-significant connectivity patterns with the right BLA seed, for the impulsive aggression (cut-off = 5.774) and combat control groups (cut-off = 5.668), respectively. Note: voxels in the immediate proximity of the seed-region (clear blue) were set to zero for purposes of visualization and scaling. Cluster-based analysis revealed significant group-differences in the left DLPFC, extending into the left premotor cortex (bottom panel; voxel-level p-value < 0.005, k ≥ 20). B. Mean (negative) β-weight values were observed to be diminished in the impulsive aggression group (mean β-weight = -0.009 ± 0.012), relative to the combat control group (mean β-weight = -0.078 ± 0.018).
Fig. 2.
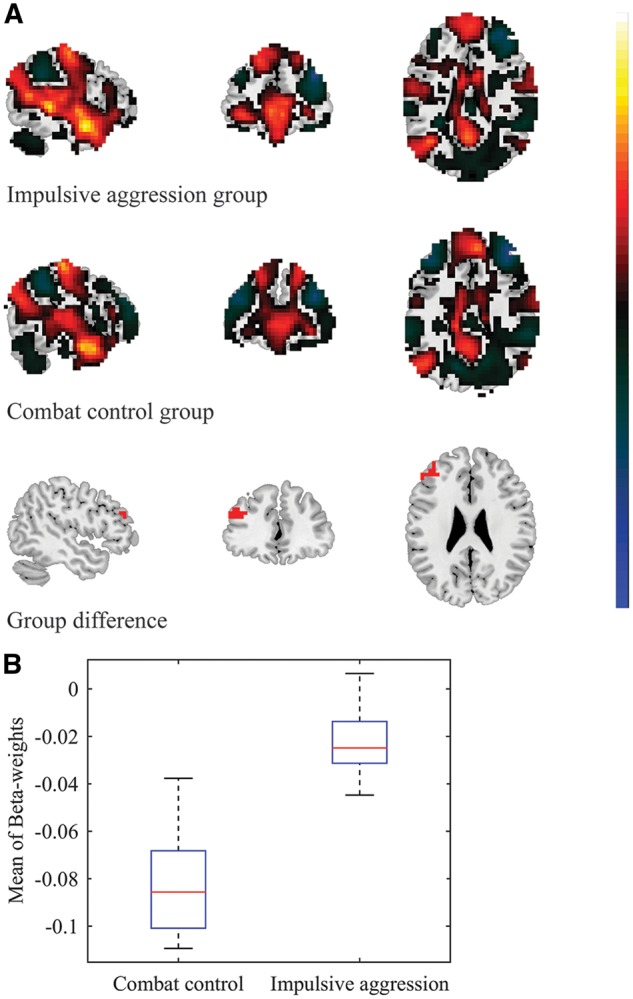
Reduced negative functional connectivity between the left BLA and left DLPFC in veterans suffering from impulsive aggression problems. A. For visualization purposes, the top and middle panel show the whole-brain FWE-significant connectivity patterns with the left BLA seed, for the impulsive aggression (cut-off = 5.774) and combat control groups (cut-off = 5.668), respectively. Note: voxels in the immediate proximity of the seed-region were set to zero for purposes of visualization and scaling. Cluster-based analysis revealed significant group-differences in the left DLPFC (bottom panel; voxel-level p-value < 0.005, k ≥ 20). B. Mean (negative) β-weight values were observed to be diminished in the impulsive aggression group (mean β-weight = -0.021 ± 0.014), relative to the combat control group (mean β-weight = -0.082 ± 0.022)
Fig. 3.
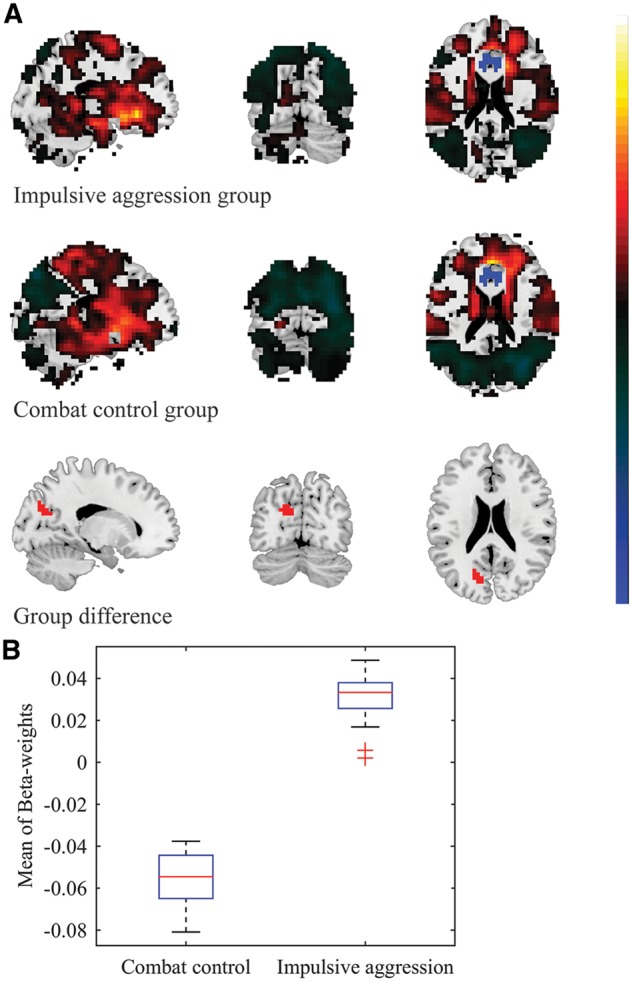
Group differences in functional connectivity between the left ACC and two distinct clusters at/near the left cuneus. A. For visualization purposes, the top and middle panel show the whole-brain FWE-significant connectivity patterns with the left ACC seed, for the impulsive aggression (cut-off = 5.774) and combat control groups (cut-off = 5.668), respectively. Note: voxels in the immediate proximity of the seed-region (clear blue) were set to zero for purposes of visualization and scaling. Cluster-based analysis revealed significant group-differences in a region spanning the left cuneus, calcarine cortex, and superior occipital cortex (cluster 1: dark red), as well as a second region again spanning the left cuneus, calcarine cortex, and superior occipital cortex (cluster 2: light red) (bottom panel; voxel-level p-value < 0.005, k ≥ 20). B. Mean β-weight values were observed to be (relatively) positive in the impulsive aggression group for both the first (cluster 1: mean β-weight = 0.046 ± 0.008) and second (cluster 2: mean β-weight = 0.027 ± 0.023) of these clusters, whereas negative values were observed in the combat control group (cluster 1: mean β-weight = -0.031 ± 0.007; cluster 2: mean β-weight = -0.060 ± 0.022, respectively).
Fig. 4.
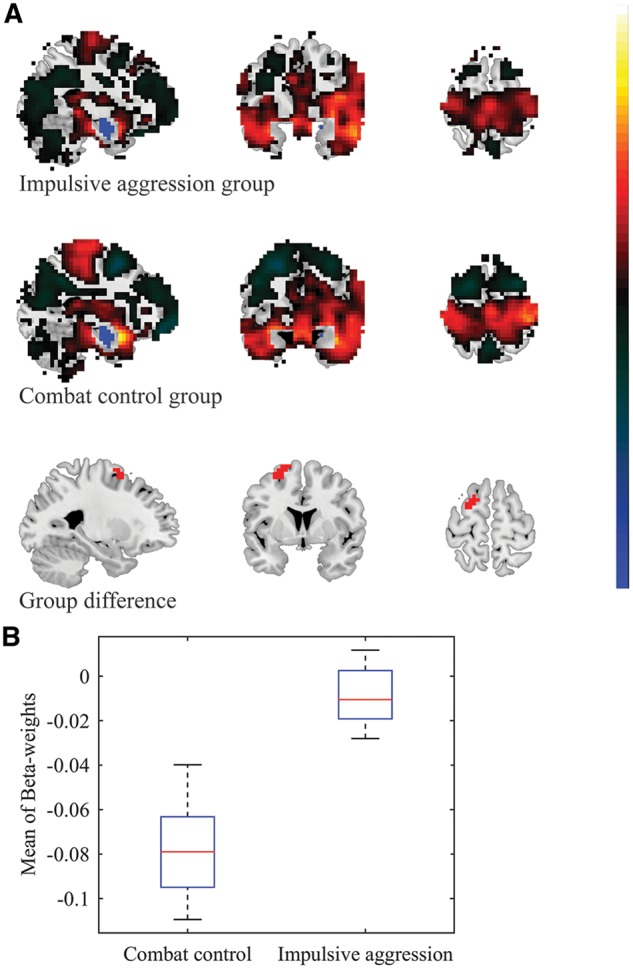
Group differences in functional connectivity between the right ACC and left cuneus. A. For visualization purposes, the top and middle panel show the whole-brain FWE-significant connectivity patterns with the right ACC seed, for the impulsive aggression (cut-off = 5.774) and combat control groups (cut-off = 5.668), respectively. Note: voxels in the immediate proximity of the seed-region (clear blue) were set to zero for purposes of visualization and scaling. Cluster-based analysis revealed significant group-differences in a region spanning the left cuneus, calcarine cortex, superior occipital cortex, and precuneus (bottom panel; voxel-level p-value < 0.005, k ≥ 20). B. Mean β-weight values were observed to be positive in the impulsive aggression group (mean β-weight = 0.030 ± 0.012), whereas negative β-weight values were observed in the combat control group (mean β-weight = -0.057 ± 0.013).
To further quantify the degree of anger- and aggression-related thoughts and behaviors, all participants completed the Dutch version of the STAXI-2 (Hovens et al., 2014; Spielberger, 1999) prior to being scanned.
Data acquisition and image preprocessing
Resting-state data were acquired on a 3.0 Tesla scanner (Philips Medical System, Best, the Netherlands). During the 9-minute scan period, participants were instructed to rest with their eyes open, focus on a fixation cross displayed on a computer screen, and let their minds wander. We collected a total of 320 T2*-weighted echo planar images (TR = 1600 ms; TE = 23 ms; flip angle = 72.5°; 30 transverse slices interleaved, FOV = 256 × 208 × 120; matrix = 64 × 64). A T1-weighted anatomical image was also obtained for spatial normalization and localization purposes (TR = 10 ms; TE = 4.6 ms; flip angle 8°; 200 slices sagittal orientation; FOV = 240 × 240 × 160; matrix = 304 × 299). Image preprocessing was conducted using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) and consisted of (1) realignment (i.e., motion correction), (2) co-registration to the anatomical image, and (3) normalization to standard MNI space (voxel size = 4 × 4 × 4 mm). The data were band-pass filtered at 0.01–0.08 Hz.
Subject-level analysis
Analyses were performed using in-house software in Matlab (MATLAB 8.4; The MathWorks Inc., Natick, MA, 2014), and the hiro3 toolbox (Gladwin et al., 2016). Selected regions for seed-based functional connectivity analysis were defined by using probabilistic maps included in the Anatomy toolbox of SPM12 (Amunts et al., 2005; Eickhoff et al., 2005), and when probabilistic maps were not available, by using the Atlas of Intrinsic Connectivity of Homotopic Areas (AICHA) by Joliot et al. (2015). The probabilistic maps of the BLA (Amunts et al., 2005), CeM (Amunts et al., 2005), and ACC (Palomero-Gallagher et al., 2015) were first resliced (nearest-neighbor) into a resolution equivalent to that of the preprocessed functional data (2 × 2 × 2 mm in MNI space). Probability weights were then extracted from these resliced probabilistic maps and assigned to the functional data on a voxel-by-voxel basis. A substantial degree of volumetric overlap was noted between the probabilistic maps of the BLA and CeM. Hence, in an effort to overcome the potential problem of partial volume contamination, we assigned each voxel to the seed-region with the highest probability weight value at the corresponding locus in standard MNI space; all other voxels were set to zero (see also Roy et al., 2009). The time-courses of the BLA, CeM, and ACC seeds were then calculated by taking, for each volume, the sum over all voxels weighted by probability. The time-course of the AIC—which was defined by using the AICHA connectivity atlas (Joliot et al., 2015)—was obtained by calculating the mean over all voxels, separately for each scan volume. Next, the time-series of each seed was entered as a predictor variable in voxel-wise regression analysis [General Linear Model (GLM)], alongside the global mean signal, white matter signal, cerebrospinal fluid signal, and the six motion parameters as nuisance variables (Weissenbacher et al., 2009). The predictor variables of the BLA and CeM seed-regions were entered into regression analysis simultaneously so as to further control for partial volume contamination (Brown et al., 2014); all other seed-regions were entered into regression analysis separately. Extraction of the global mean signal was accomplished by averaging across all brain voxels, separately for each scan volume. The binary masks for extraction of white-matter and cerebrospinal fluid signal were created via segmentation of the anatomical data in SPM12 and were eroded by one voxel along each axis to prevent for partial volume contamination with gray matter signal (Chai et al., 2012). These binary masks were subsequently used to extract white matter and cerebrospinal fluid signal from the preprocessed functional data. The subject-level β-weight maps were smoothed using a Gaussian filter at a Full Width Half Maximum of [2, 2, 2] mm. All regression analyses were conducted separately for the left and right hemispheres.
Regions-of-interest
Region-of-interest analyses were conducted to evaluate the extent of functional connectivity between the OFC and BLA (Hypothesis 1), and between the CeM and PAG (Hypothesis 2). The OFC region-of-interest was defined by using a set of (resliced) probabilistic maps included in the Anatomy toolbox of SPM12 (Eickhoff et al., 2005; Henssen et al., 2016). (The approach to creating this region-of-interest was similar to that of the BLA, CeM, and ACC seed-regions.) The time-course of the OFC was calculated by taking, for each volume, the sum over all voxels weighted by probability. Since probabilistic maps were not available for the PAG, we defined this region-of-interest as a 216 mm3 cube, centered around the standard MNI coordinates of x = 1, y = −29, and z = −12. These coordinates were obtained from Linnman et al. (2012), who reported this locus to be most frequently cited as PAG in a meta-analysis of the neuroimaging literature. The time-course of the PAG was calculated by taking the mean over all voxels within this cubic region, separately for each scan volume. Next, the time-series of the OFC and PAG were entered as outcome variables in regression analyses (as described above), alongside both the BLA and CeM time-series as predictor variables.
Statistical analyses
Group-level statistical analyses for the a priori regions-of-interest were conducted via independent-samples t-tests. The significance threshold of these analyses was set at P < 0.05, corrected for the number of tests (i.e., left BLA vs left OFC; right BLA vs left OFC; left BLA vs right OFC; right BLA vs right OFC; left CeM vs PAG; and right CeM vs PAG). We also conducted exploratory cluster analyses to evaluate the significance of group-level effects outside of our a priori regions-of-interest. These analyses were conducted using permutation tests with a nominal voxel-level significance threshold of P < 0.001 (number of permutations = 1000) (Bullmore et al., 1999). Each participant was assigned randomly to one of the groups on every iteration of these tests. Between-group t-tests were performed for each voxel, and the maximum size over all super-threshold clusters was stored on every iteration. This provided a distribution of maximum cluster sizes under the null hypothesis. The critical value for whole-brain significance of cluster size was defined as the largest value in the null hypothesis distribution, such that fewer than 5% of the iterations would exceed this value. To reduce the probability of false negatives due to a lack of sensitivity, we also opted to report clusters at a more lenient voxel-level significance threshold of P < 0.005 (two-sided), and at an extent of k ≥ 20 voxels (Lieberman & Cunningham, 2009).
Results
Group differences in functional connectivity: confirmatory regions-of-interest analyses
Statistical analyses did not reveal any significant group differences in resting-state functional connectivity between the selected seed-regions and the a priori regions-of-interest (left BLA vs left OFC, P = 0.931; left BLA vs right OFC, P = 0.918; right BLA vs left OFC, P = 0.168; right BLA vs right OFC, P = 0.159; left CeM vs PAG, P = 0.893; and right CeM vs PAG, P = 0.080). Note that the functional interplay between the right CeM and PAG was nominally significant at trend level. Nevertheless, extraction of the mean β-weight values indicated similar connectivity values for the impulsive aggression group (mean β-weight = −0.016 ± 0.188), relative to the combat control group (mean β-weight = 0.068 ± 0.169).
Group differences in functional connectivity: exploratory cluster analyses
The results of the exploratory cluster-based analyses are summarized in Table 2. All reported clusters were significant at a voxel-level P-value of < 0.005 (two-sided), and a cluster-extent of k ≥ 20. Group effects were quantified by calculating the mean of β-weight values per cluster, separately for each group.
Table 2.
Brain areas demonstrating significant group-differences in resting-state functional connectivity with the selected seed-regions-of-interest
| Seed-region | Cluster location | BA | Center of gravity |
Peak t-score | Mean β-weight (±standard deviation) |
|||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Aggression group | Control group | ||||
| BLA (left) | Dorsolateral prefrontal (left) | 45/46 | −42 | 44 | 26 | 3.721 | −0.021 (±0.014) | −0.082 (±0.022) |
| BLA (right) | Dorsolateral prefrontal/supplementary motor area (left) | 8/6 | −18 | 8 | 70 | 4.273 | −0.009 (±0.012) | −0.078 (±0.018) |
| CMA (left) | Fusiform gyrus/Lingual gyrus (left) | 37/19/18 | −30 | −52 | −10 | 3.721 | 0.041 (±0.010) | −0.007 (±0.007) |
| ACC (left) | Calcarine cortex/Occipital superior/ Cuneus/Precuneus (left) | 18/17/23/19 | −18 | −68 | 22 | 4.909 | 0.027 (±0.023) | −0.060 (±0.022) |
| ACC (left) | Cuneus/Calcarine cortex (left) | 18 | −6 | −84 | 22 | 4.297 | 0.046 (±0.008) | −0.031 (±0.007) |
| ACC (right) | Cuneus/Occipital superior/Calcarine cortex/ Occipital mid (left) | 18 | −18 | −72 | 26 | 3.583 | 0.030 (±0.012) | −0.057 (±0.013) |
| AIC (left) | Inferior temporal/Middle temporal/ Temporal pole (right) | 20/21/38 | 46 | 16 | −34 | 4.984 | 0.036 (±0.015) | −0.041 (±0.012) |
Spontaneous activity of the left BLA anti-correlated with activation at a region corresponding to the left dorsolateral prefrontal cortex (DLPFC) in the impulsive aggression group (mean β-weight = −0.021 ± 0.014), as well as the combat control group (mean β-weight = −0.082 ± 0.022) (see Supplementary Tables S1 and S2). The exploratory cluster-based analyses revealed that these anti-correlations were significantly diminished in the veterans who suffered from impulsive aggression, relative to the non-aggressive combat controls (see Table 2 and Figure 1). Spontaneous activity of the right BLA also anti-correlated with activation at the left DLPFC in the combat control group (mean β-weight = −0.078 ± 0.018), but not the impulsive aggression group (mean β-weight = −0.009 ± 0.012). Again, these anti-correlations were found to be significantly diminished in the group of veterans who suffered from impulsive aggression, relative to the non-aggressive combat controls (see Table 2 and Figure 2). Significant group differences in functional connectivity were observed between the left CeM and a region spanning the left fusiform gyrus and lingual gyrus: Positive correlations were observed in the impulsive aggression group (mean β-weight = 0.041 ± 0.010), whereas anti-correlations were observed in the combat control group (mean β-weight = −0.007 ± 0.007).
Significant group-differences in functional connectivity were observed between the left ACC and a region spanning the left cuneus, calcarine cortex, and superior occipital cortex: Positive correlations were observed in the impulsive aggression group (mean β-weight = 0.046 ± 0.008), whereas anti-correlations were observed in the combat control group (mean β-weight = −0.031 ± 0.007). A similar pattern of opposite β-weight values was observed between the left ACC and an adjacent region covering the same brain areas, i.e. the left cuneus, calcarine cortex, and superior occipital cortex: Again, relatively positive correlations were observed in the impulsive aggression group (mean β-weight = 0.027 ± 0.023), whereas anti-correlations were observed in the combat control group (mean β-weight = −0.060 ± 0.022) (see Table 2 and Figure 3). Significant group differences in functional connectivity were observed between the right ACC and a region spanning the left cuneus, calcarine cortex, superior occipital cortex, and precuneus: Positive correlations were observed in the impulsive aggression group (mean β-weight = 0.030 ± 0.012), whereas anti-correlations were observed in the combat control group (mean β-weight = −0.057 ± 0.013) (see Figure 4).
Significant group differences in functional connectivity were observed between the left AIC and the right temporal pole: Positive correlations were observed in the impulsive aggression group (mean β-weight = 0.036 ± 0.015), whereas anti-correlations were observed in the combat control group (mean β-weight = −0.041 ± 0.012).
Discussion
The current study sought to examine the neural substrates of impulsive aggression in combat veterans during rest. Based on the available task–response literature (Coccaro et al., 2007; McCloskey et al., 2016), and a resting-state study on Trait Anger in healthy volunteers (Fulwiler et al., 2012), we hypothesized that impulsive aggression would be marked by (i) a decrease in functional connectivity between the OFC and BLA, and (ii) an increase in connectivity between the CeM and PAG. To test these hypotheses, we conducted functional connectivity analyses of resting-state fMRI in combat veterans with and without impulsive aggression problems. Contrary to our expectations, we noted no significant group differences for any of the a priori regions-of-interest (OFC, PAG). Instead, we observed significant group differences in functional connectivity between the bilateral BLA and left DLPFC, between the bilateral ACC and a set of regions clustered around the left cuneus, as well as between the left AIC and a cluster centered around the right temporal pole, in the exploratory cluster analyses (see Table 2).
Consistent with the findings from the animal literature (see Nelson & Trainor, 2007), functional imaging studies have associated impulsive aggression with a loss of top-down regulation of the amygdala by the OFC (Coccaro et al., 2007; Fulwiler et al., 2012; McCloskey et al., 2016). In the current inquiry, no loss of orbitofrontal–amygdalar connectivity was observed. Rather, exploratory cluster analyses revealed a significant reduction in (bilateral) BLA-with-left DLPFC connectivity in the impulsive aggression group, relative to combat controls. This finding is in line with previous results by Yang & Raine (2009), who reported abnormalities in both the structure and function of the left DLPFC, in a meta-analysis of the neuroimaging literature on antisocial and violent behavior. Although the OFC has traditionally been viewed as an impulse control area, more recent insights indicate that its overall function may better be described in terms of sensory integration and stimulus evaluation (Kringelbach, 2005; Rothkirch et al., 2012; Rudebeck & Murray, 2014). This notion fits well with the above-cited previous results by Coccaro and colleagues, who reported stimulus-driven decreases in OFC reactivity, and reduced OFC-with-amygdala connectivity, in patients who suffer from impulsive aggression (Coccaro et al., 2007; McCloskey et al., 2016). The present work recorded no significant group differences in functional connectivity between the OFC and (basolateral) amygdala, which again fits well with the stimulus integration/evaluation perspective on OFC function, given that no particular stimulus was presented to the participants. Possibly then, the processes required to regulate one’s anger when provoked by situational factors, such as seeing an angry face, differ from the processes required to regulate one’s anger as an internally driven state: The former may require regulation of amygdala (re)activity through inhibitory processes governed by the OFC, whereas the latter may involve more an interplay between the (basolateral) amygdala and (left) DLPFC. Indeed, functional imaging studies on mindfulness have indicated that the left DLPFC may play an integral part in emotion regulation already during the anticipation of negative emotional faces, but not during the actual perception thereof (Lutz et al., 2014; Opialla et al., 2015). Taken together, we propose that impulsive aggression may be marked by functional aberrations in both types of anger regulation, but that the precise pattern of amygdala-related (dis)connectivity may depend on situational factors. In particular, the (left) DLPFC may monitor one’s internal state of anger in the absence of environmental cues (e.g., during resting-state), whereas the OFC may regulate one’s anger when it is provoked by external factors.
Exploratory cluster analyses also indicated a pattern of relatively positive connectivity between the (bilateral) ACC and a set of regions centered around the left cuneus in the impulsive aggression group; in contrast, a negative ACC-with-left cuneus relation was observed in the combat control group. The ACC is believed to be involved in the detection of deviations from expected and/or desired outcomes (Botvinick et al., 2004; Carter & van Veen, 2007). Also, the empirical literature has frequently associated ACC activation with the emotional appraisal of physical and/or social distress (Eisenberger & Lieberman, 2004). This dual role has led Eisenberger and Lieberman (2004) to conceptualize the ACC as a ‘neural alarm system’, with its discrepancy-detecting function being likened to a smoke-detector, and its affective-appraisal function to a sounding-mechanism. The present data indicate that spontaneous activity of this neural alarm system may normally be coupled to deactivation of the cuneus, whilst co-activation is observed in individuals suffering from impulsive aggression. Notably, previous fMRI reports have linked both the cuneus and adjacent visual areas to the allocation of attention towards emotionally salient environmental features (Fu et al., 2007; Moreno-Lopez et al., 2016; Nomi et al., 2008). Co-activation of the ACC with such visual attention areas could indicate that combat veterans who suffer from impulsive aggression may keep a constant vigilant eye on their immediate surroundings, and continuously scan their environment for potentially provoking and/or threatening visual cues.
The anterior insula seems to play a key role in gaining awareness of one’s own physical and emotional states (Craig, 2009). In the current inquiry, a pattern of negative functional connectivity was observed between the left AIC and right temporal pole in the non-aggressive combat control group; conversely, a positive left AIC-with-right temporal pole relation was noted in the impulsive aggression group. The functionality of the temporal poles is thought to revolve around the coupling of high-level sensory information to emotional behaviors and social memories (Olson et al., 2007). The current data indicate that individuals suffering from impulsive aggression may be more prone to superimpose their sensory impressions with (negative) emotional content. Such an interpretation would be consistent with the concept of anger rumination in cognitive psychology, which can be defined as the ‘prolonged allocation of attention towards negative information’ (Wilkowski & Robinson, 2008).
The present study was subject to a number of limitations. First, although sample sizes were large in comparison to previous reports (e.g. Fulwiler et al., 2012; McCloskey et al., 2016), the current analyses may still have suffered from a lack of statistical power. Hence, in an effort to minimize the rate of false negatives, we opted to report clusters at a voxel-level significance threshold of P < 0.005, and an extent of k ≥ 20 voxels (Lieberman & Cunningham, 2009). We reasoned that the gain in sensitivity yielded by this approach would outweigh its loss in controlling for the rate of false positives, and therefore, be better suited to accommodate the exploratory aims of these (cluster) analyses. Second, the conclusions drawn from the current findings may be limited by the fact that correlation does not equal causation, and functional connectivity does not inform us as to the direction of any observed effects. Thus, any causal conclusions drawn with regard to the directionality of the observed effects in this study should be treated as speculative. Indeed, for such causal inferences to be drawn, effective connectivity analyses, e.g. Granger causality, or Dynamic Causal Modeling, will be required. Third, since we did not obtain any information on traumatic brain injury (TBI) in this study, we cannot fully exclude the possibility that group differences in lifetime history of TBI influenced the results. We note, however, that none of the participants was physically injured during deployment. Fourth, we recommend that future imaging studies on impulsive aggression include in their design a control group with high scores on Trait Anger. This would allow one to disentangle the influence of anger as a personality trait, from the effects driven purely by impulsive aggression as a clinical phenotype. The current study is limited by the absence of such a (combat) control group. In addition, we recommend that future imaging studies take into account the functional heterogeneity of the ACC, and study the potentially different roles that subdivisions of this brain region may play in the neurobiology of impulsive aggression problems (e.g., see Bush et al., 2000 and Etkin et al., 2011).
In sum, the present study marks the first to have examined functional connectivity in combat veterans who suffer from impulsive aggression. The inclusion of a non-aggressive combat control group, the focus on specific subregions of the amygdalar complex, and the addition of the ACC and AIC as exploratory seed-regions-of-interest, further lend to the strength of our inquiry. Functional alterations were observed in neural circuits that govern the regulation of affect (DLPFC and BLA), as well as the allocation of attention (ACC and cuneus). We believe such circuits may be viable targets for novel prevention and/or treatment strategies, such as real-time fMRI neurofeedback (cf., Zotev et al., 2013), and transcranial direct current stimulation (cf., Feeser et al., 2014).
Supplementary data
Supplementary data are available at SCAN online.
Funding
This research was supported by the Dutch Ministry of Defense.
Conflict of interest. None declared.
Supplementary Material
References
- Amunts K., Kedo O., Kindler M., et al. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology 210(5), 343–52. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences 8(12), 539–46. [DOI] [PubMed] [Google Scholar]
- Brown V.M., LaBar K.S., Haswell C.C., et al. (2014). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39(2), 361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Suckling J., Overmeyer S., Rabe-Hesketh S., Taylor E., Brammer M.J. (1999). Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Transactions on Medical Imaging 18(1), 32–42. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences 4(6), 215–22. [DOI] [PubMed] [Google Scholar]
- Buss A.H., Perry M. (1992). The aggression questionnaire. Journal of Personality and Social Psychology 63(3), 452–9. [DOI] [PubMed] [Google Scholar]
- Carter C.S., van Veen V. (2007). Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience 7(4), 367–79. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Castañón A.N., Öngür D., Whitfield-Gabrieli S. (2012). Anticorrelations in resting state networks without global signal regression. NeuroImage 59(2), 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek D.R., Schnapp W., Moeller F.G., Dougherty D.M. (1996). Laboratory measures of aggressive responding in male parolees with violent and nonviolent histories. Aggressive Behavior 22(1), 27–36. [Google Scholar]
- Coccaro E.F. (2011). Intermittent explosive disorder: Development of integrated research criteria for diagnostic and statistical manual of mental disorders, fifth edition. Comprehensive Psychiatry 52(2), 119–25. [DOI] [PubMed] [Google Scholar]
- Coccaro E.F., McCloskey M.S., Fitzgerald D.A., Phan K.L. (2007). Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry 62(2), 168–78. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry 6(1), 13–34. [DOI] [PubMed] [Google Scholar]
- Denson T.F., Pedersen W.C., Ronquillo J., Nandy A.S. (2009). The angry brain: neural correlates of anger, angry rumination, and aggressive personality. Journal of Cognitive Neuroscience 21(4), 734–44. [DOI] [PubMed] [Google Scholar]
- Dougherty D.D., Shin L.M., Alpert N.M., et al. (1999). Anger in healthy men: a PET study using script-driven imagery. Biological Psychiatry 46(4), 466–72. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25(4), 1325–35. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D. (2004). Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences 8(7), 294–300. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeser M., Prehn K., Kazzer P., Mungee A., Bajbouj M. (2014). Transcranial direct current stimulation enhances cognitive control during emotion regulation. Brain Stimulation 7(1), 105–12. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience 8(9), 700–11. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Williams S.C.R., Brammer M.J., et al. (2007). Neural responses to happy facial expressions in major depression following antidepressant treatment. American Journal of Psychiatry 164(4), 599–607. [DOI] [PubMed] [Google Scholar]
- Fulwiler C.E., King J.A., Zhang N. (2012). Amygdala-orbitofrontal resting state functional connectivity is associated with trait anger. NeuroReport 23(10), 606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin T.E., Vink M., Mars R.B. (2016). A landscape-based cluster analysis using recursive search instead of a threshold parameter. MethodsX 3, 477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology, 21(4), 424–30. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry 62(5), 429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesink L., Rademaker A., Vermetten E., Geuze E., Kleber R. (2015). Longitudinal measures of hostility in deployed military personnel. Psychiatry Research 229(1–2), 479–84. [DOI] [PubMed] [Google Scholar]
- Henssen A., Zilles K., Palomero-Gallagher N., et al. (2016). Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex 75, 87–112. [DOI] [PubMed] [Google Scholar]
- Hoptman M.J. (2015). Impulsivity and aggression in schizophrenia: a neural circuitry perspective with implications for treatment. CNS Spectrums 20(3), 280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens J.E., Lievaart M., Rodenburg J.J. (2014). STAXI-2: Vragenlijst Over Boosheid. Amsterdam: Hografe. [Google Scholar]
- Jakupcak M., Conybeare D., Phelps L., et al. (2007). Anger, hostility, and aggression among Iraq and Afghanistan war veterans reporting PTSD and subthreshold PTSD. Journal of Traumatic Stress 20(6), 945–54. [DOI] [PubMed] [Google Scholar]
- Joliot M., Jobard G., Naveau M., et al. (2015). AICHA: an atlas of intrinsic connectivity of homotopic areas. Journal of Neuroscience Methods 254, 46–59. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L. (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience 6(9), 691–702. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience 4(4), 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C., Moulton E.A., Barmettler G., Becerra L., Borsook D. (2012). Neuroimaging of the periaqueductal gray: state of the field. NeuroImage 60(1), 505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J., Herwig U., Opialla S., et al. (2014). Mindfulness and emotion regulation - an fMRI study. Social Cognitive and Affective Neuroscience 9(6), 776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Holmes A. (2016). Stress and fear extinction. Neuropsychopharmacology Reviews 41(1), 58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M.S., Phan K.L., Angstadt M., Fettich K.C., Keedy S., Coccaro E.F. (2016). Amygdala hyperactivation to angry faces in intermittent explosive disorder. Journal of Psychiatric Research 79, 34–41. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez L., Contreras-Rodriguez O., Soriano-Mas C., Stamatakis E.A., Verdejo-Garcia A. (2016). Disrupted functional connectivity in adolescent obesity. NeuroImage: Clinical 12, 262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R.J., Trainor B.C. (2007). Neural mechanisms of aggression. Nature Reviews Neuroscience 8(7), 536–46. [DOI] [PubMed] [Google Scholar]
- New A.S., Hazlett E.A., Newmark R.E., et al. (2009). Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biological Psychiatry 66(12), 1107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi J.S., Scherfeld D., Friederichs S., et al. (2008). On the neural networks of empathy: a principal component analysis of an fMRI study. Behavioral and Brain Functions 4(1), 41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. (2007). The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130(7), 1718–31. [DOI] [PubMed] [Google Scholar]
- Opialla S., Lutz J., Scherpiet S., et al. (2015). Neural circuits of emotion regulation: a comparison of mindfulness-based and cognitive reappraisal strategies. European Archives of Psychiatry and Clinical Neuroscience 265(1), 45–55. [DOI] [PubMed] [Google Scholar]
- Orth U., Wieland E. (2006). Anger, hostility, and posttraumatic stress disorder in trauma-exposed adults: a meta-analysis. Journal of Consulting and Clinical Psychology 74(4), 698–706. [DOI] [PubMed] [Google Scholar]
- Painuly N., Sharan P., Mattoo S.K. (2005). Relationship of anger and anger attacks with depression: a brief review. European Archives of Psychiatry and Clinical Neuroscience 255(4), 215–22. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Eickhoff S.B., Hoffstaedter F., et al. (2015). Functional organization of human subgenual cortical areas: relationship between architectonical segregation and connectional heterogeneity. NeuroImage 115, 177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Rothkirch M., Schmack K., Schlagenhauf F., Sterzer P. (2012). Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. NeuroImage 62(3), 1717–25. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., et al. (2009). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage 45(2), 614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P.H., Murray E.A. (2014). The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron 84(6), 1143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. (1999). Staxi-2: State-Trait Anger Expression Inventory-2: Professional Manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Tian L., Jiang T., Wang Y., et al. (2006). Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neuroscience Letters 400(1-2), 39–43. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. (2009). Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage 47(4), 1408–16. [DOI] [PubMed] [Google Scholar]
- Wilkowski B.M., Robinson M.D. (2008). The cognitive basis of trait anger and reactive aggression: an integrative analysis. Personality and Social Psychology Review 12(1), 3–21. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raine A. (2009). Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Research: Neuroimaging 174(2), 81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liang M., Tian L., et al. (2007). Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophrenia Research 97(1-3), 194–205. [DOI] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Young K.D., Drevets W.C., Bodurka J., Soriano-Mas C. (2013). Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS ONE 8(11), e79184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.