Abstract
Background
B-cell survival is regulated through interactions of B-cell-activating factor and a proliferation-inducing ligand with their receptors transmembrane activator and CAML interactor (TACI) and B-cell maturation antigen (BCMA). We evaluated the diagnostic potential of soluble TACI (sTACI) and soluble BCMA (sBCMA) in CSF and serum as biomarkers in primary CNS lymphoma (PCNSL).
Methods
CSF (n = 176) and serum samples (n = 105) from patients with clinically or radiologically suspected PCNSL as well as from control patients were collected prospectively. Levels of sTACI and sBCMA were analyzed by enzyme-linked immunosorbent assay. Additionally, in patients with PCNSL, CSF was analyzed during disease course (time of diagnosis, n = 26; relapse, n = 10; remission, n = 14), and in 2 patients long-term longitudinal analysis was performed.
Results
Soluble TACI and sBCMA are significantly increased in patients with PCNSL (sTACI, median: 445 pg/mL; sBCMA, median: 760 pg/mL) compared with control patients (sTACI, median: 0 pg/mL; sBCMA, median: 290 pg/mL). At a cutoff value of 68.4 pg/mL, sTACI shows high sensitivity (87.9%) and specificity (88.3%) for the diagnosis of active PCNSL. Soluble BCMA is less sensitive (72.7%) and specific (71.8%) (cutoff: 460 pg/mL). When both markers are combined, specificity increases, however, at the cost of a lower sensitivity. In serum, both sTACI and sBCMA are not increased in PCNSL patients. Both soluble receptors correlate with clinical course and therapy response.
Conclusions
Our results suggest that sTACI and sBCMA in the CSF are promising new biomarkers for diagnosis and therapy monitoring in PCNSL. However, our findings need to be validated in an independent cohort.
Keywords: biomarker, cerebrospinal fluid, primary central nervous system lymphoma, sBCMA, sTACI
Importance of the study
PCNSLs are highly aggressive B-cell tumors. Effective treatment should be initiated as soon as possible. However, MRI characteristics are not specific and in the majority of cases a neuropathological examination is required for diagnosis, which can be technically challenging and potentially harmful and can protract time to diagnosis. We aimed to identify CSF biomarkers that facilitate diagnosis and therapy monitoring in PCNSL. We found the B-cell derived soluble receptors, sTACI and sBCMA, to be elevated in the CSF of PCNSL patients. They represent biomarkers with high sensitivity and specificity for the diagnosis of PCNSL and correlate with clinical course and therapy response. Our findings in combination with other CSF biomarkers and imaging approaches might enable diagnosis of PCNSL in the future without the need for brain biopsy. Furthermore, sTACI and sBCMA could also be promising biomarkers for peripheral B-cell derived lymphomas.
Primary central nervous system lymphoma (PCNSL) represents a form of non-Hodgkin lymphoma which is confined to the brain, leptomeninges, eyes, and spinal cord in the absence of systemic lymphoma at the time of diagnosis.1 PCNSL accounts for 3%–5% of primary brain tumors and are highly aggressive. Therefore, prompt diagnosis is essential to rapidly initiate appropriate treatment. MRI features, suspicious for lymphoma, involve unique or multiple periventricular, homogeneously enhancing lesions. However, the spectrum of radiological presentation is very broad, including non-enhancing infiltrating lesions or diffuse leukencephalopathy2 that simulates inflammatory diseases (multiple sclerosis, neurosarcoidosis), neuroinfectious diseases, or other brain tumors.3 In the majority of cases, a cerebral biopsy is required for diagnosis, with potential complications. Therefore, biomarkers supporting the diagnosis of PCNSL and allowing stratification of patients for biopsy or replacing biopsy would be very helpful.
Regulation of B-cell homeostasis involves a system that comprises 2 ligands, B-cell activating factor of the tumor necrosis factor family (BAFF) and a proliferation inducing ligand (APRIL), and 3 receptors, B-cell maturation antigen (BCMA), transmembrane activator and CAML interactor (TACI), and BAFF-receptor.4 The essential role of this pathway in the pathogenesis of B-cell malignancies such as systemic non-Hodgkin lymphoma has already been described; in this context lymphoma B cells can evade apoptosis through BAFF and APRIL, probably in an autocrine manner.5
Recently, we demonstrated that the receptors TACI and BCMA also exist as soluble forms that can be detected in body fluids and reflect systemic and compartmentalized B-cell accumulation and activation.6,7 We therefore evaluated the role of soluble TACI (sTACI) and soluble BCMA (sBCMA) as biomarkers in PCNSL.
Materials and Methods
Patients
The study was conducted in the Department of Neurology, the Institute of Clinical Chemistry, and the Institute of Clinical Neuroimmunology, Klinikum Grosshadern from February 2012 to May 2015. All patient samples were collected following written informed consent according to local ethics guidelines in Munich and the Declaration of Helsinki. Included in the study were patients (age >18 y) with at least one MRI-proven suspicious brain lesion of unknown origin (tumorous/autoimmune-inflammatory/infectious), in whom diagnostic lumbar puncture was performed during clinical routine. Additionally, patients with other neurological diseases (ONDs) without a focal brain lesion were included as controls. In detail 33 patients with PCNSL (all diffuse large B-cell lymphoma [DLBCL]), 6 patients with secondary CNS lymphoma (SCNSL), 20 patients with primary brain tumors (PBT), 22 patients with secondary brain tumors (SBT), 13 patients with neuroinfectious diseases, 30 patients with neuroinflammatory diseases, and 52 patients with ONDs were included (Table 1). Diagnosis of PCNSL was made by stereotactic brain biopsy and neuropathological examination in 28 patients, by immunophenotyping and flow cytomorphometry of CSF cells in 3 patients, and by immunophenotyping and flow cytomorphometry of vitreous fluid cells in 2 patients. All PCNSLs were of the DLBCL subtype. Diagnosis of SCNSL was made by immunophenotyping and flow cytomorphometry of CSF in 2 patients who had prior histologic confirmation of their systemic tumor manifestation and by stereotactic brain biopsy and neuropathological examination in 4 patients. All SCNSLs were of the DLBCL subtype.
Table 1.
Patient data, CSF
| Lymphoma Patients | Controls | ||||||
|---|---|---|---|---|---|---|---|
| PCNSL/ Activea (n = 33) |
SCNSL (n = 6) |
PBT (n = 20) |
SBTb (n = 22) |
Neuroinf. (n = 13) |
Neuroinflamm.c (n = 30) |
ONDsd (n = 52) |
|
| Sex F/M | 14/19 | 1/5 | 6/14 | 12/10 | 3/10 | 18/12 | 30/22 |
| Mean age ± SD (range) | 65 ± 15 (21–89) |
62 ± 19 (33–90) |
54 ± 19 (21–83) |
64 ± 14 (31–84) |
56 ± 15 (30–81) |
43 ± 14 (19–73) |
49 ± 22 (18–85) |
| Mean CSF cell count, cells/µL ± SD (range) | 38 ± 83 (1–408) |
95 ± 148 (2–369) |
12 ± 45 (0–204) |
19 ± 33 (0–132) |
882 ± 2349 (1–8846) |
21 ± 38 (0–144) |
2 ± 1 (0–7) |
| Mean CSF total protein, mg/dL ± SD (range) | 106 ± 124 (9–604) |
101 ± 60 (56–211) |
64 ± 32 (25–160) |
90 ± 81 (15–336) |
137 ± 93 (44–376) |
59 ± 25 (24–116) |
44 ± 16 (22–83) |
| Mean CSF glucose, mg/ dL ± SD (range) | 64 ± 16 (10–93) |
58 ± 18 (34–88) |
62 ± 8 (53–89) |
60 ± 20 (10–98) |
59 ± 18 (32–99) |
63 ± 10 (51–101) |
64 ± 11 (45–104) |
| Mean albumin quotient ± SD (range) | 19 ± 23 (3–96) |
26 ± 23 (12–53) |
9 ± 6 (2–22) |
17 ± 18 (4–55) |
17 ± 18 (4–65) |
7 ± 4 (2–19) |
6 ± 3 (3–12) |
Abbreviations: neuroinf. = neuroinfectious disease; neuroinflamm. = neuroinflammatory disease.
aTwenty-six patients with sampling at the time of diagnosis, 7 patients with sampling during disease relapse.
bSix patients with brain metastases without meningeosis, 10 patients with brain metastases and meningeosis, 6 patients with meningeosis without solid metastases.
cEleven patients with multiple sclerosis, 7 patients with other inflammatory neurological diseases, 5 patients with clinically isolated syndrome, 2 patients with neuromyelitis spectrum disorder, 1 patient with acute demyelinating encephalomyelitis, 1 patient with cerebral vasculitis, 1 patient with neurosarcoidosis, 1 patient with Sjogren’s syndrome, 1 patient with Miller–Fisher syndrome.
dTwenty patients with headache, 4 patients with neurodegenerative diseases, 3 patients with epilepsia, 2 patients with normal pressure hydrocephalus, 4 patients with Bell’s palsy, 8 patients with peripheral neuropathies, 4 patients with ischemia, 1 patient with hypersomnia, 1 patient with narcolepsia, 1 patient with chronic pain, 1 patient with arthritis, 1 patient with metabolic encephalopathy, 2 patients with myasthenia gravis.
Patients were not currently under chemotherapeutic treatment at the time of CSF analysis. Two patients were treated with corticosteroids in the 14 days prior to sTACI and sBCMA analysis and for 2 patients data on corticosteroid treatment prior to CSF analysis was not available. The rest of the patients had not received corticosteroids. According to patient data, clinical performance, MRI characteristics, and respective laboratory parameters, 2 clinically relevant prognostic scores were determined: the International Extranodal Lymphoma Study Group (IELSG) prognostic score 8 and the Memorial Sloan-Kettering Cancer Center prognostic score.9
Magnetic Resonance Imaging
MRI was performed on either a 1.5-Tesla scanner (Magnetom Symphony, Siemens Medical Solutions) or a 3-Tesla scanner (Signa, HDxT, GE Healthcare). Imaging included a T1-weighted, a T1-weighted contrast enhanced, a T2-weighted, a diffusion weighted, and a fluid-attenuated inversion recovery weighted sequence. Soluble TACI and sBCMA levels were compared for patients with different PCNSL MRI characteristics: (i) homogeneous versus heterogeneous contrast enhancement, (ii) contact versus no contact to ventricular system, (iii) involvement versus no involvement of deep brain structures, and (iv) monolocular versus multilocular occurrence. MRI evaluation was performed as central review by an experienced neuro-oncologist (M.H.).
Processing of CSF and Serum Samples
CSF and serum samples were collected, immediately centrifuged, and stored at −80°C. Routine CSF analysis (ie, cell count, microscopy, protein quantification, and glucose levels) as well as immunophenotyping were performed at the Institute of Clinical Chemistry. Additional serum samples were collected in 60% of the patients (64% PCNSL, 67% SCNSL, 55% PBT, 45% SBT, 69% neuroinfectious diseases, 70% neuroinflammatory diseases, 56% ONDs) (Supplementary Table S1).
Enzyme-Linked Immunosorbent Assay
Levels of sTACI and sBCMA in CSF and serum were determined by enzyme-linked immunosorbent assay (ELISA) (Human TACI/TNFRSF13B DuoSet ELISA, R&D Systems and Human BCMA/TNFRSF17 DuoSet ELISA, R&D Systems) following the manufacturer’s instructions. Briefly, protein binding plates were coated with the respective capture antibodies and incubated at 4°C overnight. After blocking of the plates, the diluted samples (dilution for sTACI: CSF and serum 1:2, for sBCMA: CSF 1:3 and serum 1:50) and standards were added and incubated at 4°C overnight. Then, biotinylated detection antibodies and streptavidin‒horseradish peroxidase were added. Absorbance at 450 nm and 540 nm was determined on a plate reader (Victor Multilabel, Perkin Elmer). The detection limits were 93.80 pg/mL for sTACI and 31.20 pg/mL for sBCMA. Day-to-day control sera samples and negative controls were included to control for variability of the assay.
Statistics
Statistical tests were performed using GraphPad Prism software. P-values of *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were considered significant and designated accordingly. All patient subgroups were tested for normality using the D’Agostino–Pearson omnibus test. Differences between subgroups were compared by Kruskal–Wallis test followed by Dunn’s multiple comparisons test if more than 2 subgroups were compared. For comparison of 2 groups, the Mann–Whitney test was applied. To determine correlation between parameters, Spearman correlation was performed, followed by Bonferroni correction to control for multiple testing.
Results
In our study we collected CSF and serum samples from patients with suspected brain lesions of unknown origin (tumorous/autoimmune-inflammatory/infectious), in whom diagnostic lumbar puncture was performed during clinical routine, prospectively. Additionally, we collected samples from control patients. In total, CSF samples from 176 patients were included. In detail, CSF samples from 33 patients with active PCNSL, 6 patients with SCNSL, 20 patients with PBT, 22 patients with SBT, 13 patients with infectious diseases, 30 with neuroinflammatory diseases, and 52 patients with ONDs were included in the study. A patient with active lymphoma was defined as a patient not currently under treatment who presented at the time of diagnosis (n = 26) or with a relapse (n = 7). Serum samples came from 21 patients with active PCNSL, 4 patients with SCNSL, 11 patients with PBT, 10 patients with SBT, 9 patients with neuroinfectious diseases, 21 patients with neuroinflammatory diseases, and 29 patients with ONDs. A full description of the patient cohort is provided in Table 1 (CSF) and Supplementary Table S1 (serum).
Levels of sTACI and sBCMA Are Elevated in the CSF of PCNSL Patients
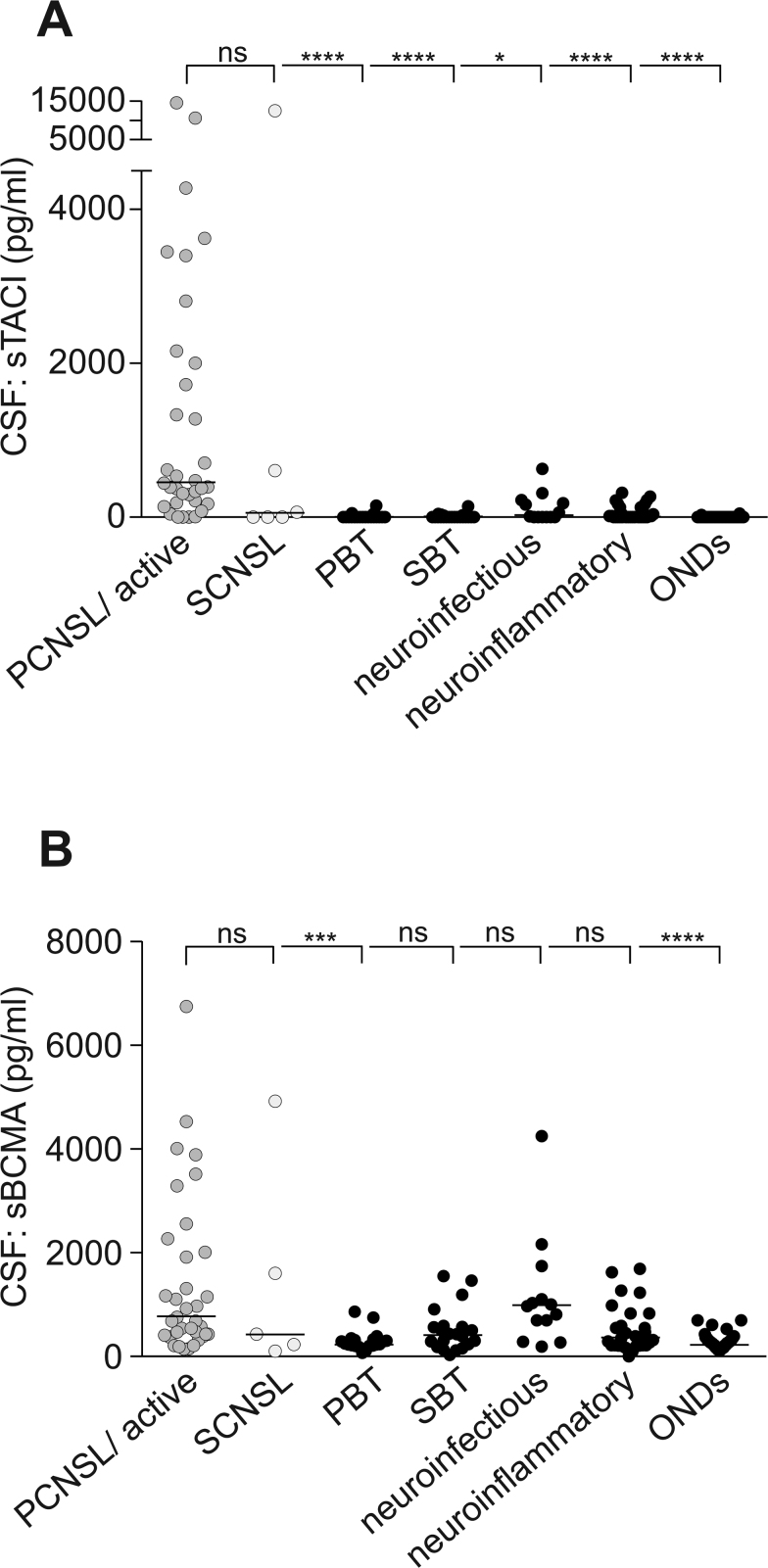
We found highly elevated sTACI levels in the CSF of patients with PCNSL (median: 445 pg/mL, interquartile range [IQR]: 1883 pg/mL) and SCNSL (median: 32 pg/mL, IQR: 3583 pg/mL) compared with all other patients analyzed (median: 0 pg/mL, IQR: 7 pg/mL) (Fig. 1A). Detailed analyses revealed that sTACI levels were significantly elevated in PCNSL patients compared with patients with ONDs (median: 0 pg/mL, IQR: 0 pg/mL) and, even more important, compared with patients with cerebral lesions, including PBT (median: 0 pg/mL, IQR: 0 pg/mL), SBT (median: 0 pg/mL, IQR: 20 pg/mL), neuroinfectious diseases (median: 11 pg/mL, IQR: 205 pg/mL), and neuroinflammatory diseases (median: 8 pg/mL, IQR: 129 pg/mL) (Fig. 1A).
Fig. 1.
Soluble TACI and sBCMA levels are elevated in the CSF of PCNSL patients. (A, B) Soluble TACI and sBCMA levels were determined by ELISA in CSF (active PCNSL: n = 33; SCNSL: n = 6; PBT: n = 20; SBT: n = 22; patients with neuroinfectious diseases: n = 13; patients with neuroinflammatory diseases: n = 30; ONDs: n = 52). Horizontal bars indicate the median; normality testing was performed using the D’Agostino–Pearson omnibus test, as the normality test was not passed in all subgroups; sTACI or sBCMA levels in the subgroups were compared by Kruskal–Wallis test followed by Dunn’s multiple comparison test. (A) Soluble TACI is elevated in the CSF of patients with PCNSL and SCNSL, with sTACI levels being significantly increased compared with all other patient subgroups. (B) Soluble BCMA levels in CSF are significantly increased in PCNSL compared with patients with PBT and ONDs; however, not when compared with patients with SBT, patients with neuroinfectious diseases, and patients with neuroinflammatory diseases.
Similarly, sBCMA levels were significantly increased in PCNSL patients (median: 760 pg/mL, IQR: 1740 pg/mL) and SCNSL patients (median: 430 pg/mL, IQR: 3095 pg/mL) compared with all other patients analyzed (median: 290 pg/mL, IQR: 320 pg/mL). Subgroup analysis shows that the elevation is significant compared with patients with ONDs (median: 260 pg/mL, IQR: 170 pg/mL) and patients with PBT (median: 245 pg/mL, IQR: 125 pg/mL) (Fig. 1B). However, sBCMA levels do not allow a discrimination between PCNSL and SBT (median: 435 pg/mL, IQR: 350 pg/mL), neuroinfectious diseases (median: 970 pg/mL, IQR: 930 pg/mL), and neuroinflammatory diseases (median: 350 pg/mL, IQR: 495 pg/mL) (Fig. 1B).
We could previously show that sTACI and sBCMA levels are increased in the CSF of patients with active multiple sclerosis and clinically isolated syndrome.6 The present study partly recapitulates this finding, as sTACI levels were found to be higher in the CSF of patients with neuroinflammatory diseases compared with patients with ONDs (P < 0.05). However, as already highlighted, CSF levels did not reach the values obtained for patients with PCNSL.
For sBCMA we could not find a significant difference between patients with neuroinflammatory diseases and with ONDs. This presumably relates to the fact that the cohort of patients with neuroinflammatory diseases in the present study was more heterogeneous (see Table 1) compared with our previous studies.
Next, we correlated sTACI and sBCMA levels with each other and with results from routine CSF analysis and patient age. Soluble TACI levels in the CSF correlated with sBCMA levels (Supplementary Figure S1A). We detected no correlations of sTACI with CSF cell count, CSF glucose, CSF protein, albumin quotient, or age (Supplementary Figure S1A). Soluble BCMA levels correlated with sTACI levels, but also with CSF glucose and albumin quotient (Supplementary Figure S1A). Soluble BCMA levels did not correlate with CSF cell count, CSF protein, and age (Supplementary Figure S1A). Furthermore, we compared the levels of sTACI and sBCMA in patients with different MRI characteristics. Apparently, neither PCNSL contact to the ventricular system nor homogeneous contrast-enhancement involvement of deep brain structures or multilocular occurrence was associated with increased sTACI or sBCMA levels (Supplementary Figure S1B–I). Also we detected no association with the prognostic scores of IELSG (Supplementary Figures S1J, S2K) and Memorial Sloan-Kettering Cancer Center (data not shown). For sTACI we detected an association with meningeosis (Supplementary Figure S1L); however, meningeosis had no effect on sBCMA levels (Supplementary Figure S1M). Also, in patients with SBT no association of meningeosis and sTACI and sBCMA levels was detected (data not shown).
Levels of sTACI and sBCMA Are Not Elevated in the Serum of PCNSL Patients
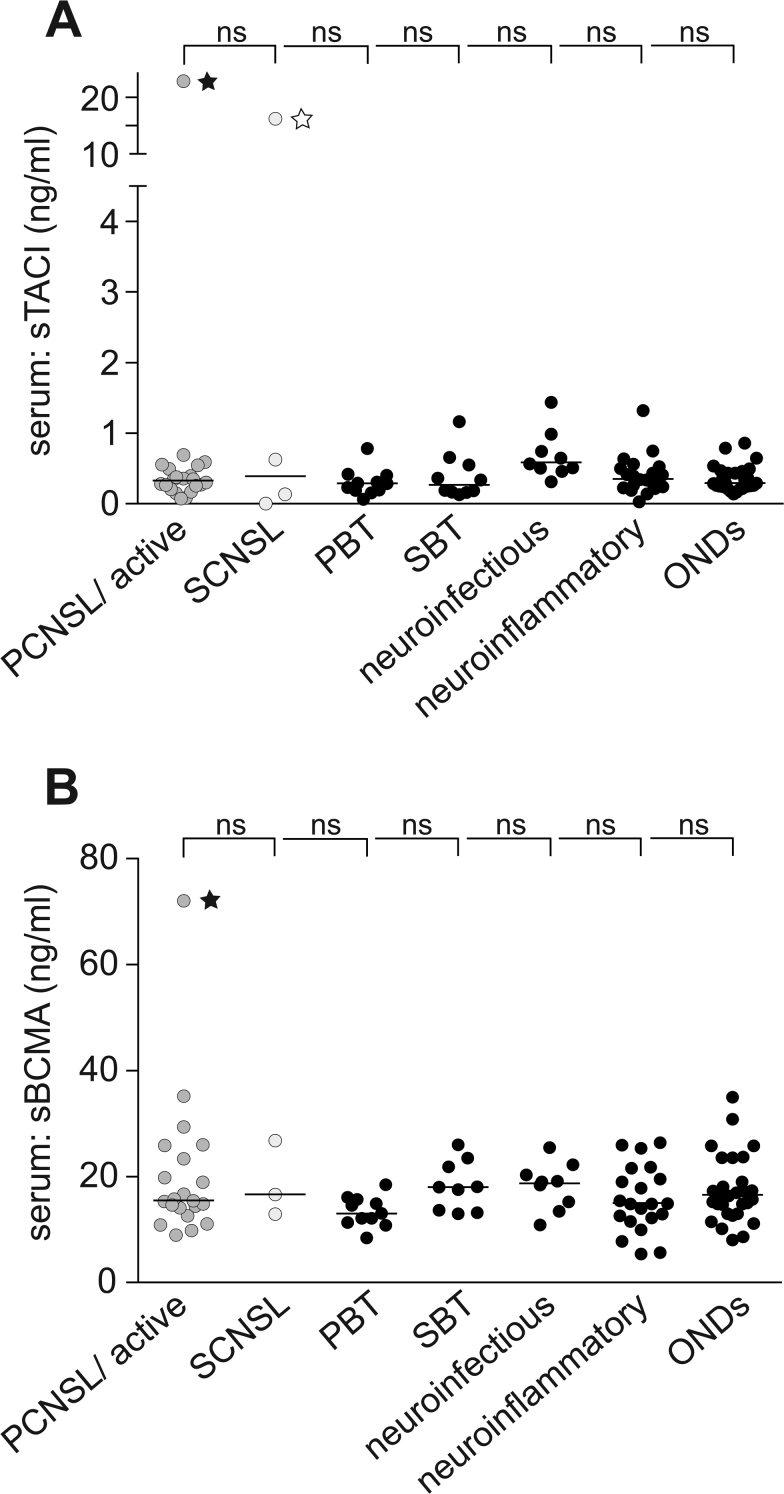
Additionally to CSF levels, we determined sTACI and sBCMA levels in the serum. No significant difference was detected between sTACI levels in PCNSL patients (median: 348 pg/mL, IQR: 289 pg/mL) and all other patients analyzed (median: 338 pg/mL, IQR: 291 pg/mL). Also, sBCMA levels did not differ in PCNSL patients (median: 15.4 ng/mL, 11.2 ng/mL) and all other patients analyzed (median: 15.5 ng/mL, IQR: 6.4 ng/mL), indicating that the elevated levels of sTACI and sBCMA in PCNSL are indeed CNS specific. This is in line with the diagnostic criteria of PCNSL requiring no evidence of systemic disease manifestation at the time of diagnosis (Fig. 2A, B). Soluble TACI and sBCMA levels in the CSF of patients without PCNSL or SCNSL were generally more than 10-fold lower—or for sTACI in many cases even nondetectable—compared with the respective serum levels. However, in PCNSL patients, sTACI CSF levels reached or even exceeded respective serum levels. For sBCMA, however, the CSF levels always remained lower compared with the serum levels. There were 2 patients with very high systemic sTACI levels, one in the PCNSL group who presented with a systemic relapse of disease, and the other in the SCNSL group with a diagnosis of DLBCL presenting with systemic and CNS manifestation.
Fig. 2.
Soluble TACI and sBCMA levels are not elevated in the serum of PCNSL patients. (A, B) Soluble TACI and sBCMA levels were determined by ELISA in serum (active PCNSL: n = 21; SCNSL: n = 4; PBT: n = 11; SBT: n = 10; patients with neuroinfectious diseases: n = 9; patients with neuroinflammatory diseases: n = 21; ONDs: n = 29). Horizontal bars indicate the median; normality testing was performed using the D’Agostino–Pearson omnibus test, as the normality test was not passed in all subgroups, sTACI levels in the subgroups were compared by Kruskal–Wallis test followed by Dunn’s multiple comparison test. Filled star: patient initially with a diagnosis of PCNSL who experienced a systemic relapse; empty star: patient with the diagnosis of DLBCL and systemic and CNS manifestation. (A) Soluble TACI is not elevated in the serum of patients with PCNSL compared with all other patient subgroups. (B) Soluble BCMA is not elevated in the serum of patients with PCNSL compared with all other patient subgroups.
Evaluation of sTACI and sBCMA as Biomarkers in PCNSL
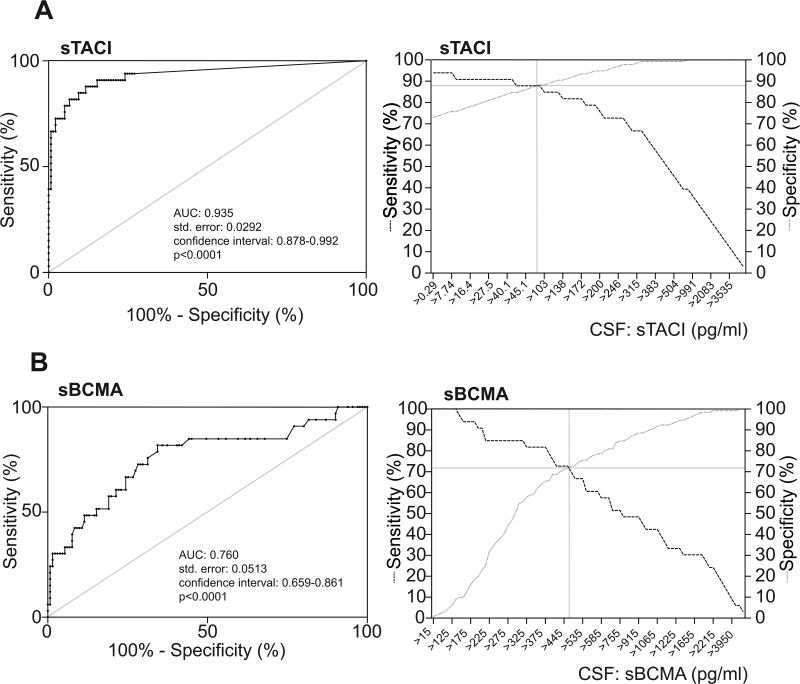
To assess the sensitivity and specificity of sTACI and sBCMA in the CSF as biomarkers for PCNSL, we performed receiver operating characteristic curve analysis (Fig. 3A, B, left panels). Hereby, we confirmed our finding of sTACI (area under the curve [AUC]: 0.935) being superior to sBCMA (AUC: 0.760) in discriminating PCNSL from all other patients analyzed. We calculated sensitivity and specificity of the 2 markers at different cutoff levels and found sensitivity and specificity to be highest at a cutoff at 68.4 pg/mL for sTACI (sensitivity: 87.9%, specificity: 88.3%) and 460 pg/mL for sBCMA (sensitivity: 72.7%, specificity: 71.8%) (Fig. 3A, B, right panels). For sTACI with a cutoff of 68.4 pg/mL, the positive predictive value was 63.6% and the negative predictive value was 96.0%. For sBCMA with a cutoff of 460 pg/mL, the positive predictive value was 50.0% and the negative predictive value 89.9%. Next, we determined whether a combination of both tests added diagnostic specificity. To this aim, we calculated combined sensitivity (sTACI)sens × (sBCMA)sens and combined specificity (sTACI)spec + (sBCMA)spec − [(sTACI)spec × (sBCMA))spec.10 The combined sensitivity was 63.9% and the combined specificity was 96.7%.
Fig. 3.
Evaluation of sTACI and sBCMA as potential biomarkers in PCNSL. Results of receiver operating characteristic analysis are depicted in the left panels for sTACI (A) and sBCMA (B). Patients with PCNSL were compared with all control patients. Sensitivity and specificity were calculated for different cutoff values in the right panels for sTACI (A) and sBCMA (B).
Levels of sTACI and sBCMA Correlate with Clinical Activity
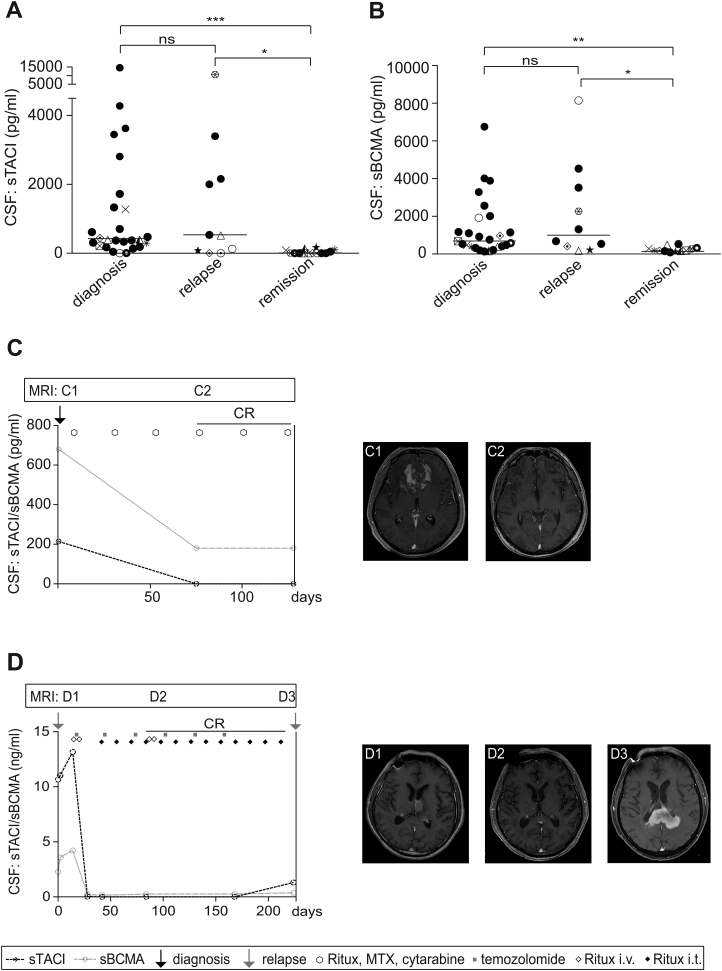
As a next step, we aimed to determine whether disease activity correlates with levels of sTACI and sBCMA. Therefore, we compared the CSF levels of 26 patients at the time of diagnosis with those of 10 patients experiencing a relapse and 14 patients in complete remission (Supplementary Table S2). We found that levels of both sTACI and sBCMA were elevated at the time of diagnosis (sTACI, median: 392 pg/mL, IQR: 1222 pg/mL; sBCMA, median: 720 pg/mL, IQR: 1555 pg/mL) and during relapses (sTACI, median: 524 pg/mL, IQR: 2084 pg/mL; sBCMA, median: 995 pg/mL, IQR: 3420 pg/mL) (Fig. 4A, B). No significant difference between these groups was observed. At remission, however, sTACI and sBCMA levels were both significantly lower (sTACI, median: 0 pg/mL, IQR: 98 pg/mL; sBCMA, median: 185 pg/mL, IQR: 160 pg/mL) compared with patients with newly diagnosed or recurrent PCNSL, suggesting a correlation with disease course (Fig. 4A, B). To further elucidate this correlation, we analyzed sTACI and sBCMA levels longitudinally in 2 patients. A 50-year-old patient was diagnosed with PCNSL and was treated with 6 cycles of rituximab, methotrexate, and cytarabine according to the IELSG protocol,11 which resulted in a complete remission. Levels of the soluble receptors dropped continuously (Fig. 4C). The second patient was 62 years old when the first analysis was made. At that time he experienced the second relapse of PCNSL. As during prior systemic chemotherapy, severe pancytopenia, hepatotoxicity, and renal failure were observed and whole brain radiation had already been applied; for the second relapse he was treated with intermittent intrathecal or intravenous rituximab as well as temozolomide, which resulted in complete remission. Soluble TACI and sBCMA reflected the clinical course with high levels during relapse and very low levels or even undetectable levels for sTACI at complete remission. However, at the time of the last CSF sampling, the patient experienced a third relapse. A rise in sTACI levels could already be detected, while sBCMA levels remained normal (Fig. 4D).
Fig. 4.
Correlation of sTACI and sBCMA levels with disease activity. (A, B) Soluble TACI and sBCMA levels were determined by ELISA in CSF (PCNSL diagnosis: n = 26; PCNSL relapse: n = 10; PCNSL remission: n = 14). Horizontal bars indicate the median. Some patients were sampled at different time points of disease course. Corresponding patients are depicted with corresponding symbols. For statistical analysis every patient was just considered once with the following priority: relapse > remission > diagnosis. Both sTACI and sBCMA levels are significantly increased during diagnosis and relapse compared with remission; normality testing was performed using the D’Agostino‒Pearson omnibus test, as the normality test was not passed in all subgroups, sTACI or sBCMA levels in the subgroups were compared by Kruskal‒Wallis test followed by Dunn’s multiple comparison test. (C, D) Soluble TACI and sBCMA levels were determined by ELISA in CSF in 2 patients with PCNSL during disease course. Soluble TACI and sBCMA levels are depicted together with treatment regimens given to the patient during the observational time. On the right panels MRI findings are depicted. (C) The patient was treated with 6 cycles of rituximab, methotrexate, and cytarabine according to the IELSG protocol. (C1) Cerebral (c)MRI (fast spoiled gradient echo plus gadolinium [FSPGR + Gd]) at the time of diagnosis shows a contrast-enhancing tumor emanating from the corpus callosum and the cingulum and extending bilaterally to the basal parts of both frontal lobes; (C2) cMRI (FSPGR + Gd) after 3 cycles of chemotherapy shows no tumor mass and no contrast enhancement. (D) The patient was experiencing the second relapse of a PCNSL at the time of the first sTACI and sBCMA analysis. As severe pancytopenia, renal failure, and hepatotoxicity had been observed during previous chemotherapies for the second relapse, a treatment regimen with intermittent intrathecal rituximab, intravenous rituximab, and temozolomide was applied. (D1) Cerebral MRI (T1-weighted + Gd) shows a contrast enhancing tumor affecting the left thalamus with exophytic growth into the left lateral ventricle; (D2) cMRI (FSPGR + Gd) shows complete remission of the tumor; (D3) cMRI (T1-weighted + Gd) shows a tumor emanating from the splenium with homogeneous contrast enhancement.
Discussion
This prospective monocentric study demonstrates for the first time that sTACI and sBCMA levels in the CSF are significantly increased in patients with active PCNSL compared with patients with brain lesions of other malignant, inflammatory, or infectious origin. In line with the concept of PCNSL exhibiting no peripheral disease activity, elevated sTACI and sBCMA levels were indeed CSF specific and were not altered in the serum of the analyzed samples. In patients with SCNSL and systemic disease involvement, however, an increase in sTACI and sBCMA serum levels could be detected. Soluble TACI and sBCMA proved to be valuable biomarkers allowing diagnosis of PCNSL with high sensitivity and specificity. Furthermore, CSF level reflects disease activity and might be a promising therapeutic marker.
While we did not define the cellular source of sTACI and sBCMA in the CSF, it is known that TACI is expressed late during B-cell development with increasing expression, as B cells differentiate toward immunoglobulin-secreting cells.12 It is highest expressed on marginal zone B cells, CD27+ memory B cells, and plasma cells13,14 and is induced upon B-cell activation.4 However, TACI is described to be absent on germinal center (GC) B cells.15 BCMA is expressed mainly on plasmablasts and long-lived plasma cells but can also be detected on GC B cells.4 In previous studies we could show by using immunohistochemistry that in PCNSL specimens, TACI and BCMA are expressed by CD20+ lymphocytes in human PCNSL lesions. TACI hereby showed a higher expression than BCMA.16 Similarly, quantitative PCR revealed high TACI and a moderate BCMA expression in malignant lymphoma cells of human PCNSL.17 In the present study, however, we cannot rule out that sTACI and sBCMA in the CSF might at least in part be linked also to the infiltration of other activated immune cells.
Our previous studies suggest that sTACI and sBCMA levels reflect the expression of TACI and BCMA on the cell surface.6,7 However, in the present study, the levels of soluble receptors did not correlate with CSF cell count. This might be related to the fact that the total CSF count does not necessarily reflect the number of lymphocytes and at least for sTACI we detected an association with meningeosis.
Both soluble receptors could be valuable biomarkers, with sTACI showing higher sensitivity and specificity compared with sBCMA. The combined analysis of sTACI and sBCMA levels in our study showed that while the combination of both receptors increases specificity, this comes at the cost of a decreased sensitivity.
Several other potential biomarkers for PCNSL have already been identified in the past. The proteins neopterin,18 CXCL13,19 interleukin (IL)-10,19–22 IL-6,20–22 β2-microglobulin,21,23 osteopontin,24 and soluble cluster of differentiation (sCD)2725 seem to be promising candidates. Quantitative analysis based on ELISA and chemiluminescent enzyme immunoassay showed sensitivities ranging from 68% (ß2-microglobulin23) to 100% (sCD2725) and specificities ranging from 63% (IL-620) to 100% (IL-1020). Among other factors, these studies varied with regard to the study design (retro- vs prospective, mono- vs multicentric, etc), the number of patients included (4521–22019), and the composition of the control cohort (focal brain lesions of malignant, inflammatory, and infectious origin, control patients without focal lesions). However, varying results on the diagnostic potential of some biomarkers were obtained by different groups. For instance, the diagnostic sensitivity of IL-10 in the detection of PCNSL among other brain lesions ranged from 64%19 to 94%,21 the respective specificity from 94%19 to 100%.21,22 This indicates that validation studies with a broad patient spectrum are crucial to identify relevant cutoff values and to assess the respective diagnostic potentials of reliable biomarkers.
Despite protein biomarkers, microRNAs seem to be promising novel biomarkers in the diagnosis of brain tumors, especially of PCNSL.26–30 MicroRNA expression level can be measured within the CSF using quantitative real-time PCR assays. In a pioneering study, Baraniskin et al could show that the microRNAs miR-21, miR-19b, and miR-92a were significantly higher in the CSF of patients with PCNSL compared with those from control patients. The combination allowed for a diagnostic accuracy of 95.7% sensitivity and 96.7% specificity,26 confirmed in a second, enlarged study, which could also show their potential as biomarkers for treatment monitoring and follow-up.31 Similarly, measurement of U2 small nuclear RNA fragment levels enabled the differentiation of patients with PCNSL from controls with a sensitivity of 68.1% and a specificity of 91.4%.32 Most importantly, microRNA profiles or selected microRNAs in the peripheral blood of PCNSL patients seem to be suited to prognosticate the survival of patients under therapy.33–35 However, these studies warrant further validation in greater patient cohorts.
Overall, we think that in the context of the abovementioned CSF biomarker studies, the present prospective study comprises a clinically relevant patient cohort and includes a broad spectrum of control patients with neoplastic, neuroinflammatory, or neuroinfectious brain lesions that could represent a relevant differential diagnosis in patients with PCNSL. We show that determination of sTACI allows a discrimination of PCNSL from all other subgroups. High sTACI levels nearly exclude any alternative diagnosis to PCNSL or SCNSL. Soluble TACI could therefore serve as a confirming biomarker in patients in which brain biopsy is ambiguous, not feasible, or too dangerous. Aside from their diagnostic value, levels of both soluble receptors closely correlated with clinical course. Therefore, sTACI and sBCMA constitute potential markers allowing early detection of relapses if patients are followed longitudinally or facilitating monitoring of treatment response. Furthermore, the analysis of sTACI and sBCMA by ELISA is stable, easy to perform, and cost-efficient.
However, our study has some limitations. Our results have to be recapitulated in an independent cohort, possibly of a larger sample size. Furthermore, the restriction of long-term follow-up data on few patients is a clear limitation. Apart from this, in clinical routine not all patients are amenable for a CSF analysis due to the risk of herniation in patients with large, space occupying brain tumors.
Determining sTACI and sBCMA levels in our cohort was not sufficient to discriminate between PCNSL and SCNSL. However, during the initial diagnostic evaluation of a patient presenting with CNS lymphoma, potential systemic involvement should always be ruled out by CT of the chest, abdomen, and pelvis, ultrasonography of the testes, and bone marrow biopsy.1 The fact that we also observe elevated sTACI and sBCMA levels in the serum of SCNSL or PCNSL patients with systemic disease involvement indicates that sTACI and sBCMA might represent promising candidates as biomarkers also in patients with systemic lymphomas.
In the future, it would be very interesting to evaluate which combination of different biomarkers would allow diagnosing PCNSL with highest accuracy. As the analysis of neopterin showed a high sensitivity (96%),18 a combination with the analysis of sTACI and sBCMA could be an interesting option. Similarly, a combined analysis of microRNAs and protein markers would be an intriguing approach.
In conclusion, we identified sTACI and sBCMA as promising biomarkers for diagnosis and therapy monitoring of PCNSL. Our findings encourage further studies validating our results, particularly regarding the prognostic value of sTACI and sBCMA in PCNSL patients.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the Else Kröner Fresenius Stiftung (2011_A154), the DFG (SFB TR128), and the Munich Cluster for Systems Neurology (ExC 1010 SyNergy).
Conflict of interest statement. All authors report no disclosures relevant to this manuscript.
Supplementary Material
Acknowledgments
We thank Naoto Kawakami and Eduardo Beltrán for critical comments on the manuscript and Sigrid Langer and Barbara Angele for excellent technical assistance.
References
- 1. Baraniskin A, Deckert M, Schulte-Altedorneburg G, Schlegel U, Schroers R. Current strategies in the diagnosis of diffuse large B-cell lymphoma of the central nervous system. Br J Haematol. 2012;156(4):421–432. [DOI] [PubMed] [Google Scholar]
- 2. Jimenez de la Pena MD, Vicente LG, Alonso RC, Cabero SF, Suarez AM, de Vega VM. The multiple faces of nervous system lymphoma: atypical magnetic resonance imaging features and contribution of the advanced imaging. Curr Probl Diagn Radiol. 2016;46(2):136–145. [DOI] [PubMed] [Google Scholar]
- 3. Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. [DOI] [PubMed] [Google Scholar]
- 4. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. [DOI] [PubMed] [Google Scholar]
- 5. Novak AJ, Grote DM, Stenson M et al. . Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104(8):2247–2253. [DOI] [PubMed] [Google Scholar]
- 6. Hoffmann FS, Kuhn PH, Laurent SA et al. . The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol. 2015;194(2):542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laurent SA, Hoffmann FS, Kuhn PH et al. . γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015;6:7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferreri AJ, Reni M. Establishing a prognostic score for primary CNS lymphomas. Int J Radiat Oncol Biol Phys. 2005;61(1):303–304; author reply 304–305. [DOI] [PubMed] [Google Scholar]
- 9. Abrey LE, Ben-Porat L, Panageas KS et al. . Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 10. Weinstein S, Obuchowski NA, Lieber ML. Clinical evaluation of diagnostic tests. AJR Am J Roentgenol. 2005;184(1):14–19. [DOI] [PubMed] [Google Scholar]
- 11. Ferreri AJ, Reni M, Foppoli M et al. ; International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. [DOI] [PubMed] [Google Scholar]
- 12. Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179(11):7276–7286. [DOI] [PubMed] [Google Scholar]
- 13. Castigli E, Wilson SA, Garibyan L et al. . TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37(8):829–834. [DOI] [PubMed] [Google Scholar]
- 14. von Bülow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14(5):573–582. [DOI] [PubMed] [Google Scholar]
- 15. Ng LG, Sutherland AP, Newton R et al. . B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173(2):807–817. [DOI] [PubMed] [Google Scholar]
- 16. Birnbaum T, Langer S, Roeber S, von Baumgarten L, Straube A. Expression of B-cell activating factor, a proliferating inducing ligand and its receptors in primary central nervous system lymphoma. Neurol Int. 2013;5(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krumbholz M, Theil D, Derfuss T et al. . BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201(2):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viaccoz A, Ducray F, Tholance Y et al. . CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro Oncol. 2015;17(11):1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubenstein JL, Wong VS, Kadoch C et al. . CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121(23):4740–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasayama T, Nakamizo S, Nishihara M et al. . Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro Oncol. 2012;14(3):368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sasagawa Y, Akai T, Tachibana O, Iizuka H. Diagnostic value of interleukin-10 in cerebrospinal fluid for diffuse large B-cell lymphoma of the central nervous system. J Neurooncol. 2015;121(1):177–183. [DOI] [PubMed] [Google Scholar]
- 22. Song Y, Zhang W, Zhang L et al. . Cerebrospinal fluid IL-10 and IL-10/IL-6 as accurate diagnostic biomarkers for primary central nervous system large B-cell lymphoma. Sci Rep. 2016;6:38671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caudie C, Bancel J, Dupont M, Matanza D, Poitevin F, Honnorat J. CSF levels and diagnostic utility of cerebrospinal fluid beta2-microglobulin. Ann Biol Clin (Paris). 2005;63(6):631–637. [PubMed] [Google Scholar]
- 24. Strehlow F, Bauer S, Martus P et al. . Osteopontin in cerebrospinal fluid as diagnostic biomarker for central nervous system lymphoma. J Neurooncol. 2016;129(1):165–171. [DOI] [PubMed] [Google Scholar]
- 25. Kersten MJ, Evers LM, Dellemijn PL et al. . Elevation of cerebrospinal fluid soluble CD27 levels in patients with meningeal localization of lymphoid malignancies. Blood. 1996;87(5):1985–1989. [PubMed] [Google Scholar]
- 26. Baraniskin A, Kuhnhenn J, Schlegel U et al. . Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117(11):3140–3146. [DOI] [PubMed] [Google Scholar]
- 27. Fischer L, Hummel M, Korfel A, Lenze D, Joehrens K, Thiel E. Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro Oncol. 2011;13(10):1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertus JL, Harms G, Blokzijl T et al. . Specific expression of miR-17-5p and miR-127 in testicular and central nervous system diffuse large B-cell lymphoma. Mod Pathol. 2009;22(4):547–555. [DOI] [PubMed] [Google Scholar]
- 29. Zheng J, Xu J, Ma S, Sun X, Geng M, Wang L. Clinicopathological study of gene rearrangement and microRNA expression of primary central nervous system diffuse large B-cell lymphomas. Int J Clin Exp Pathol. 2013;6(10):2048–2055. [PMC free article] [PubMed] [Google Scholar]
- 30. Yu X, Li Z, Shen J, Chan MT, Wu WK. Role of microRNAs in primary central nervous system lymphomas. Cell Prolif. 2016;49(2):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baraniskin A, Kuhnhenn J, Schlegel U, Schmiegel W, Hahn S, Schroers R. MicroRNAs in cerebrospinal fluid as biomarker for disease course monitoring in primary central nervous system lymphoma. J Neurooncol. 2012;109(2):239–244. [DOI] [PubMed] [Google Scholar]
- 32. Baraniskin A, Zaslavska E, Nöpel-Dünnebacke S et al. . Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for primary central nervous system lymphoma. Neuro Oncol. 2016;18(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roth P, Keller A, Hoheisel JD et al. . Differentially regulated miRNAs as prognostic biomarkers in the blood of primary CNS lymphoma patients. Eur J Cancer. 2015;51(3):382–390. [DOI] [PubMed] [Google Scholar]
- 34. Mao X, Sun Y, Tang J. Serum miR-21 is a diagnostic and prognostic marker of primary central nervous system lymphoma. Neurol Sci. 2014;35(2):233–238. [DOI] [PubMed] [Google Scholar]
- 35. Zhao HT, Chen J, Shi SB, Tian J, Tao RJ. Pemetrexed plus rituximab as second-line treatment for primary central nervous system lymphoma. Med Oncol. 2015;32(1):351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.