Abstract
Anhedonia is considered a core feature of major depressive disorder, and the dopamine system plays a pivotal role in the hedonic deficits described in this disorder. Dopaminergic activity is complex and under the regulation of multiple brain structures, including the ventral subiculum of the hippocampus and the basolateral amygdala. Whereas basic and clinical studies demonstrate deficits of the dopaminergic system in depression, the origin of these deficits likely lies in dysregulation of its regulatory afferent circuits. This review explores the current information regarding the afferent modulation of the dopaminergic system and its relevance to major depressive disorder, as well as some of the system-level effects of novel antidepressants such as agomelatine and ketamine.
Keywords: dopamine, depression, hippocampus, amygdala, ketamine, animal models
Major Depressive Disorders
Major Depressive Disorder (MDD) is one of the most prevalent mental disorders worldwide. Indeed, the lifetime prevalence rates range for most countries between 8% and 12% (Kessler and Bromet, 2013). According to the World Health Organization (WHO, 2008), MDD also carries the heaviest burden of disability among mental and behavioral disorders (Collins et al., 2011). According to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013), a major depressive episode is defined as a period of 2 weeks or longer during which there is either depressed mood or loss of interest or pleasure (i.e., anhedonia) and at least 4 other symptoms that reflect a change in a person’s baseline activity, such as fatigue, suicidality, change in sleep, or change in activity (e.g., psychomotor agitation or retardation). This disorder is complex and likely involves a multitude of unique circuitry, and despite its status as a leading cause of burden of disease as well as decades of research into it, its etiology and pathophysiology remain largely unknown.
At present, the majority of approved antidepressants for MDD act through monoaminergic mechanisms. Thus, the first-generation antidepressants, such as tricyclic antidepressants and monoamine oxidase (MAOs) inhibitors, alter the reuptake, metabolism, or receptor pharmacodynamics of the monoamines serotonin and norepinephrine. MAO inhibitors also inhibit the metabolism of dopamine, enhancing its brain level (Tekes et al., 1988). The next generation of antidepressants, thought to carry less significant side effects than first-generation drugs, targets monoaminergic neurotransmission with molecules such as selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors. Although SSRIs have less severe adverse effects than the first-generation agents, their efficacy is limited. Indeed, when the treatment is effective, it can take weeks to get a therapeutic effect (Katz et al., 2004). This is a particular concern when in some depressed patients, there is an imminent risk of suicide. The last generation of antidepressant includes serotonin antagonists and reuptake inhibitors (SARIs) such as trazodone, noradrenergic and specific serotonergic antidepressant (NaSSA) such as mirtazapine, catecholamine releaser such as bupropion, and triple reuptake inhibitors, such as venlafaxine. This new generation of antidepressant is suggested to have comparable antidepressant efficacy to other drug classes, but is more tolerable (for review Chang and Fava, 2010). Therefore, MDD is very hard to treat, with 2/3 of patients not achieving remission after one course of treatment and 1/3 who fail to remit after 4 treatments (Rush et al., 2006). As a result, there is a pressing need to develop new, fast-acting, and effective drugs and novel pharmacological approaches to treat depression, in particular in patients who are unresponsive to traditional antidepressants.
MDD is a complex disorder, comprised of many symptoms, each symptom likely involving unique neuronal circuits. To study and treat MDD in the most efficient manner, the DSM, based on clusters of clinical symptoms, is unfortunately limited. The National Institute of Mental Health has launched the Research Domain Criteria approach to create a research classification system based on biologically determined variables, such as genetics, imaging, cognitive science, behavior, or neural circuits. Indeed, it is hypothesized that symptoms, more than diagnostic categories, are linked to specific circuit disruptions, and therefore understanding their biological foundations will facilitate the treatment of disorders that include such symptoms. This approach is consistent with fundamental research using animal models of specific disorders, where neurobiological and behavioral disruptions are assessed. Therefore, following the Research Domain Criteria, the present review will focus on anhedonia, a core symptom and a diagnostic criterion for MDD. In particular, we will focus on circuit-based regulation of the dopaminergic system. It should be noted that molecular deficits as well as disruptions in specific neural circuits underlying different reward-related processes have been reviewed in detail in (Der-Avakian and Markou, 2012) and (Nestler and Carlezon, 2006).
Dopamine Deficits in MDD
Anhedonia is a symptom described in various neurodegenerative and psychiatric disorders such as Parkinson’s disease (Isella et al., 2003; Zahodne et al., 2012) and schizophrenia (Strauss and Gold, 2012), respectively. It is also characteristic of withdrawal symptoms described in substance abusers (Gawin and Kleber, 1986) and is suggested to play a central role in the increased risk of relapse (Koob and Le Moal, 2001; Volkow et al., 2002). In MDD, it is 1 of 2 hallmark symptoms of this disorder besides depressed mood, and its presence has been shown to be predictive of poor antidepressant response (Klein, 1974). Indeed, it is suggested that anhedonia contributes to the persistence of MDD treatment resistance (McMakin et al., 2012; Vrieze et al., 2013).The DSM defines anhedonia as diminished interest or pleasure in response to stimuli that were previously perceived as rewarding before the development of the disorder (American Psychiatric Association, 2013). Anhedonia is a particularly difficult symptom to treat, as increasing evidence suggests that second-generation antidepressants, such as SSRIs, are not effective in treating positive affect deficits, such as motivation and reward-related cognitive impairment in depression (Nutt et al., 2007; McCabe et al., 2009). It is therefore critical to determine the possible disrupted regulation of the neuronal circuitry underpinning this symptom. It is important to note that anhedonia is not only defined as a loss of the ability to experience pleasure, but encompasses the complex reward-related deficits observed in MDD or other neuropsychiatric disorders, such as disruption of the anticipation, motivation, and decision-making processes involved in obtaining a reward (Treadway and Zald, 2011). Anhedonia has been linked to dysfunctions in the reward system, and in particular the dopamine (DA) system (Der-Avakian and Markou, 2012). The DA system plays a role in reward prediction (Schultz, 1998b), motivational arousal, and responsiveness to conditioned incentive stimuli (Wise, 1982; Salamone et al., 2003). It is also suggested that DA is necessary for the attribution of incentive salience to motivational stimuli, transforming the perception of liking a reward into a wanted incentive (Berridge and Robinson, 1998), consistent with disruptions of the motivation to seek out pleasurable experiences described in individuals diagnosed with MDD (Sherdell et al., 2012). Although historically depression has been associated with dysfunctions of the serotonin (5-HT)- and norepinephrine-containing circuits (Bunney and Davis, 1965; Schildkraut et al., 1965; Coppen, 1967), research using neuroimaging, pharmacological, and electrophysiological methods in humans and animal models of depression has provided support for the presence of DA dysfunctions (for review, see Yadid and Friedman, 2008). Depression and anhedonia have been shown to be associated with a reduced striatal response to reward (Forbes et al., 2009). Moreover, in depressed patients with anhedonia, PET imaging studies have shown significantly lower DA transporter (DAT) binding compared with healthy subjects (Meyer et al., 2001; Sarchiapone et al., 2006). This suggests a downregulation secondary to lower DA concentrations, as proposed by previous studies showing a decreased DAT density when DA is chronically depleted (Kilbourn et al., 1992; Ikawa et al., 1994; Gordon et al., 1996). Earlier studies have found that MDD patients showed increased striatal D2 receptor binding (D’Haenen and Bossuyt, 1994) as well as an elevated D2/3 receptor binding in the central and basal nuclei of the amygdala of postmortem depressed patients who committed suicide compared with control subjects (Pare et al., 1969), suggesting a decreased DA turnover. These findings are in accordance with the degree of amphetamine-induced rewarding effects described in MDD patients. Indeed, it has been shown that the severity of MDD correlates with the magnitude of euphoria after administration of amphetamine (Tremblay et al., 2002). Thus, the amphetamine-induced DA release would result in an increased DA signal transduction via compensatory mechanisms such as increased postsynaptic DA receptors expression as well as reduced DAT density.
Animal models of depression also demonstrated altered mesolimbic DA system function. Altered DA receptor expression within limbic structures was observed in different models of depression such as the learned helplessness model (Kram et al., 2002) and the chronic mild stress (CMS) model (Dziedzicka-Wasylewska et al., 1997). These changes reflect a decrease in DA release into the synapse, such as, for example, significantly lower homovanillic acid, a DA metabolite (Reddy et al., 1992), as well as reduced striatal dopaminergic activity (Pruessner et al., 2004) also described in depressed patients compared with controls. This downregulation of the DA system is consistent with studies showing altered dopamine release in the nucleus accumbens in animals exposed to the CMS procedure (Di Chiara and Tanda, 1997). Moreover, lesions of the VTA with 6-OHDA in rats induce depressive-like behavior as measured by the learned helpless paradigm (Winter et al., 2007). It has also been reported that the Flinders sensitive rat line, a genetic animal model of depression (Overstreet, 1993), show reduced “burst” firing of VTA neurons (Friedman et al., 2008). Recently, more evidence implicating the activity of the DA system has emerged using animal models of depression (Belujon and Grace, 2014; Tye and Deisseroth, 2012; Chaudhury et al., 2013; Savitz et al., 2013; Chang and Grace, 2014; Moreines et al., 2016). In particular, Tye and collaborators (Tye et al., 2013) showed in 2013 that selective inhibition of DA neurons recorded in the VTA induces specific depression-like behaviors and that CMS induces several depression-like phenotypes that are reversed by selective activation of the mesolimbic DA system.
Therefore, the majority of studies highlighting the role of the DA system in depression converge on a downregulation of this system. However, little is known about which afferent circuits may mediate dysfunctions in the regulation of the DA system. Indeed, although disruption of the DA system may form the basis of anhedonia in several psychiatric disorders, including MDD, the symptom and/or the pathology is more likely to originate in regions involved in their afferent control. To understand different pathology states, it is therefore critical to characterize the mechanisms by which these afferents control DA population activity.
Reward-Related Circuity
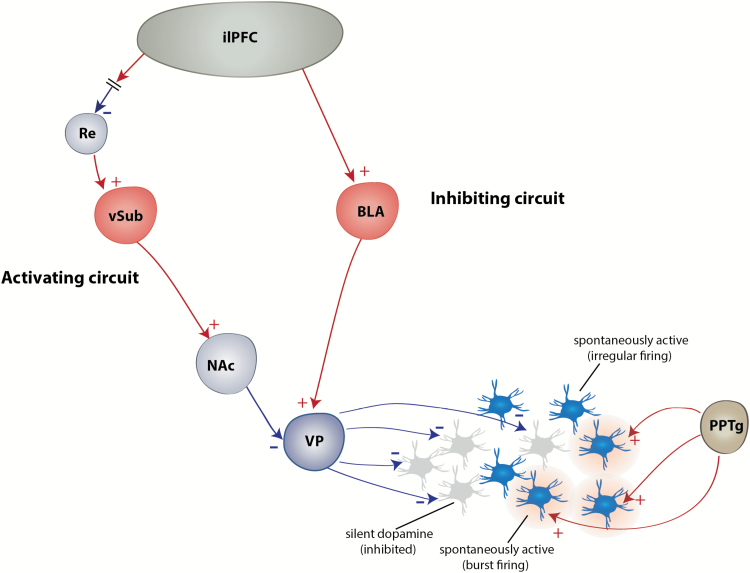
As previously described in numerous reviews (Grace, 1991, 2016; Grace et al., 2007; Belujon and Grace, 2015), the mesolimbic DA neurons can display multiple activity states. It should be noted that in this review, we will refer to neurons that fit the electrophysiological criteria described in Grace and Bunney in 1983 (Grace and Bunney, 1983) as DA neurons (see also Ungless and Grace, 2012). Dopamine neurons can either be spontaneously firing or non-firing; the proportion of neurons spontaneously active is termed population activity (Floresco et al., 2001b). In anesthetized animals, spontaneously active DA neurons can fire either in a slow irregular pattern (tonic activity) or in bursts of action potentials (phasic activity). These bursts are driven by afferents from regions such as the pedunculopontine tegmental nucleus (PPTg), a glutamatergic/cholinergic structure (Floresco et al., 2003; Lodge and Grace, 2006b), and the laterodorsal tegmentum (Lodge and Grace, 2006a). The PPTg, the most potent activator of DA neuron burst firing, is involved in conditioned stimulus responses in reward tasks (Bortolanza et al., 2010). In awake behaving animals, these bursts of action potentials are also present in response to behaviorally salient stimuli (Schultz, 1998a; Zweifel et al., 2009), since DA neurons display phasic burst when an individual is presented with an unpredictable reward or a stimulus previously associated with a rewarding (Schultz, 1998a) or aversive (Lammel et al., 2011; Zweifel et al., 2011) event. In anesthetized and freely moving animals, one-half of DA neurons are not spontaneously active (Grace and Bunney, 1984) due to a constant hyperpolarized, silent state as a result of activation of GABAergic inputs from the ventral pallidum (VP) (Floresco et al., 2003). Activation of the ventral subiculum of the hippocampus (vSub) induces an increase in the number of spontaneously active DA neurons, without affecting the burst firing (Lodge and Grace, 2006b), via a polysynaptic pathway through the nucleus accumbens (NAc) and the VP (Floresco et al., 2001a). Therefore, inactivation of the VP releases inactive DA neurons from inhibition and enables them to fire spontaneously. This supplies a stable baseline level, that is, a tonic state, of extra-synaptic DA in target structures, such as the prefrontal cortex or the NAc. Phasic activation of burst firing can only occur in spontaneously firing neurons; therefore, the PPTg provides the “signal” and the vSub is the gain of this signal. The vSub is involved in the control of contextual processing (Jarrard, 1995; Maren and Quirk, 2004). Therefore, the change in population activity enables the system to be adjusted depending on the stimulus itself but also the physical context in which the stimuli are presented. This is particularly important in several situations, including survival ones, which involves a stress response. For example, in a benign context, where the organism is not aroused, that is, not in an alert state, the vSub maintains DA neurons in a low tonic activity state. However, in a threatening situation (i.e., strong context), the activation of the vSub results in an inhibition of the VP, thereby increasing DA neuron population activity. This will cause a behaviorally salient stimulus to induce phasic burst firing of a large proportion of DA neurons leading to an increase in DA release in afferent structures. This will ultimately cause the organism to react appropriately to the arousing situation. An increase in DA population activity has been shown to be accompanied by DA release in the NAc (Floresco et al., 2003) and an increase in amphetamine-induced hyperlocomotion (White et al., 2006), a behavioral consequence of augmented DA neuronal activity (Lodge and Grace, 2007; Gill and Grace, 2011; Chang and Grace, 2013). Moreover, the context-dependent subiculum control of DA population activity is modulated by the medial prefrontal cortex (mPFC), in particular the infralimbic subregion (ilPFC). Indeed, our group has shown that inactivation of the ilPFC increases DA population activity, an effect that is dependent on the vSub (Patton et al., 2013). The mPFC does not project directly to the vSub (Vertes et al., 2006), and we have shown that this effect was mediated by, at least in part, the nucleus reuniens of the thalamus (Zimmerman and Grace, 2016). Indeed, the nucleus reuniens sends dense projections to as well as drives activity in the vSub (Wouterlood et al., 1990; Bertram and Zhang, 1999) (Figure 1). There are direct projections from the mPFC to the entorhinal cortex as well, which also provides powerful excitatory influence over the vSub (van Groen et al., 2003). This structure could also be a relay between the mPFC and the vSub that could potentially affect DA activity.
Figure 1.
Afferent regulation of the dopamine (DA) system. The DA system is under regulation by an inhibitory circuit including the basolateral amygdala (BLA)-ventral pallidum (VP) pathway that is activated by the infralimbic subregion (ilPFC), as well as an activating circuit. including the Re-ventral subiculum of the hippocampus (vSub)-nucleus accumbens (NAc)-VP pathway that is inhibited by the ilPFC. The result is that at baseline, about one-half of the VTA DA neurons are firing spontaneously. Only DA neurons that fire spontaneously can fire in response to rapid, phasic activation with bursts of action potentials, due to activation of pedunculopontine tegmental nucleus (PPTg) afferents.
On the other hand, the amygdala, in particular the basolateral amygdala (BLA), is responsible for the decrease in tonic dopamine neuron firing, that is, population activity (Chang and Grace, 2013), in response to repeated stressors. This decrease is thought to occur via a polysynaptic pathway, since the BLA does not send direct projection to the VTA (Geisler et al., 2007). The VTA receives GABAergic projections from the rostromedial tegmentum as well as the VP and glutamatergic projections from the lateral habenula, among others (Russo and Nestler, 2013). A subset of neurons from the rostromedial tegmentum receives excitatory projections from the lateral habenula (Stamatakis and Stuber, 2012). The BLA sends dense direct projections to these structures (Kaufling et al., 2009; Lee and Kim, 2011) that could act as relay in negatively modulating DA population activity. We have recently shown that the BLA-induced attenuation of DA population activity is reversed by the blockade of glutamate afferents in the VP (Chang and Grace, 2014), which makes the VP a critical relay in the modulation of VTA DA population activity by the BLA. Indeed, BLA sends potent direct excitatory projections onto VP neurons (Maslowski-Cobuzzi and Napier, 1994). The BLA is a limbic structure that attributes emotional significance to external (contextual) stimuli (Aggleton, 1993), which is of significant importance in the stress response (Roozendaal et al., 2009). As previously shown with the vSub, the BLA-dependent attenuation of DA population activity is under control of the ilPFC, since the decrease in DA population activity by activation of the ilPFC is prevented by removal of the BLA influence (Patton et al., 2013). It should be noted that the VTA is not homogenous, the most medial part of the VTA projecting to the NAc shell, which plays an important role in incentive learning, whereas the most lateral part of the VTA, which projects to the NAc core, is involved in the selection of adaptive responding (Ikemoto, 2007). Interestingly, when activated, the ilPFC inhibits preferentially the medial, reward-related part of the VTA (Moreines et al., 2016).
Hence, the tonic DA activity (population) is under 2 distinct and opposing circuits, one activating circuit involving the vSub of the hippocampus (releasing DA neurons from VP GABAergic inhibition) and one inhibitory circuit involving the BLA (activating VP GABAergic inhibition of DA neurons) (Figure 1). Therefore, in a situation where an individual must respond to intense salient stimuli, large proportions of DA neurons will be activated by the vSub, causing a strong burst firing-dependent DA release allowing the appropriate response to the stimulus. This is downregulated by the amygdala, which will decrease the responsivity of the reward-related DA system.
Dysregulation of the Reward-Related Circuit in MDD
Functional imaging studies have highlighted the critical role of the fronto-limbic circuit in modulating mood states. Dysfunction of activity in one region in particular, the subgenual cingulate (Cg25), has been consistently observed in depressed patients. For example, an increased metabolism is observed in MDD patients (Mayberg et al., 2000; Kumano et al., 2007), remitted MDD patients during depressive relapse (Neumeister et al., 2004), or MDD patients in response to sad face stimuli (Keedwell et al., 2009). Anatomically, MDD is associated with gray matter abnormalities and decreased volume in the mPFC (Price and Drevets, 2010; Kempton et al., 2011) as well as a reduction in the size of neurons and a loss of glia (Drevets et al., 2008). Furthermore, treatments for MDD such as antidepressants (Mayberg et al., 2000), electroconvulsive therapy (Nobler et al., 2001), transcranial magnetic stimulation (George et al., 2010), and vagus nerve stimulation (Nahas et al., 2007) cause a return to normal levels of activity of the PFC that correlate with an improved mood in MDD patients. Stimulation of this region using deep brain stimulation is also used for the treatment of depression (Mayberg et al., 2005). It is, however, well known that major depression is not the result of selective regional dysfunction but rather involves dysfunction of neural networks that normally modulates mood and emotions (Mayberg, 2003). Indeed, disruption in PFC-amygdala as well as PFC-hippocampus functional connectivity, among others, have been described (Kong et al., 2013; Genzel et al., 2015), suggesting disruption of reciprocal connection between these structures.
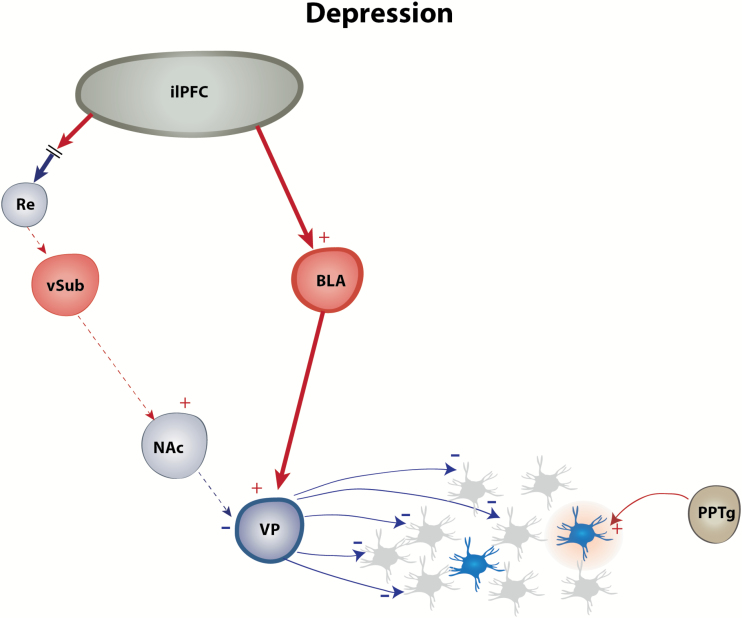
The Cg25 in patients is homologous with the infralimbic part of the mPFC in rodents (Heilbronner et al., 2016), and animal models of depression have been important tools for investigating the etiology of depression, the neurocircuitry involved in some symptoms, as well as developing new, effective, and innovative treatment. Most animal models of depression are based on the induction of a depression-like phenotype by stress, which plays a critical role in the onset of depression (Kendler et al., 1999). Among these, CMS, first reported by Willner and colleagues (Willner et al., 1987), is the most extensively validated and widely used model of major depression (Hill et al., 2012). This model is based on clinical and preclinical research suggesting that uncontrollable and prolonged exposure to life stressors is a factor in the development of the disease (Katz, 1982; Kessler et al., 1985; Kendler et al., 1999). This model has high face, construct, and predictive validity. Thus, rodents exposed to this procedure develop a decreased response to reward (i.e., anhedonia), a core symptom of MDD, as well as decreased motivated behavior, weight loss, and sleep disruption (Willner, 1997). There has been extensive research on the anatomical and physiological modifications observed in rodents exposed to the CMS procedure that are similar to the changes observed in MDD patients (Hill et al., 2012) and described in detail in Willner’s review in 2017 (Willner, 2017). Comparable with MDD in patients, rats exposed to CMS show elevated hypothalamo-pituitary-adrenal axis activity (Goshen et al., 2008), decreased dendritic tree of hippocampal (Sousa et al., 2000) and cortical (Liu and Aghajanian, 2008; Li et al., 2011) pyramidal neurons, and increase in the length of dendrites and density of dendritic spines in amygdala neurons (Sharma and Thakur, 2015). CMS in rats causes a prolonged decrease of DA population activity in the VTA (Chang and Grace, 2014; Moreines et al., 2016). This decrease is reversed by inactivation of the ilPFC (Moreines et al., 2016) or the BLA-VP pathway (Chang and Grace, 2014), suggesting that abnormal hyperactivity in the ilPFC leads to hyperactivation of the BLA-VP pathway, responsible for a downregulation of the DA system in depression (Figure 2). This inhibition of the DA system is accompanied by an increased immobility time in the forced swim test, which relates to “behavioral despair” or resignation, and with a decrease in sucrose preference, which is suggested as a hedonic deficit (Katz, 1982), often described in animals exposed to CMS (Willner et al., 1987; Tye et al., 2013). This downregulation of the DA system has also been described in another well-validated model of depression, the learned helplessness model (Belujon and Grace, 2014). In this model, the stress-sensitive Wistar-Kyoto rats are exposed to uncontrollable, unpredictable, and inescapable stress (e.g., shocks). When reexposed to the same shocks while provided with a means to easily escape the shocks, the animals will lose the ability to show escape behavior (failure to escape) or an increased latency to escape (Seligman and Beagley, 1975). Interestingly, approximately one-half of animals exposed to uncontrollable stress developed learned helplessness (helpless animals), that is, a decreased ability to learn how to escape subsequent stressor (Petty et al., 1997). The other one-half, which do not demonstrate learned helplessness (nonhelpless animals), may have undergone alternate adaptations that protected them from the detrimental effects of inescapable stress. This model has high construct, face, and predictive validity. Indeed, animals exposed to this procedure show weight loss, alterations in sleep patterns, modification of the hypothalamo-pituitary-adrenal axis activity, and a decrease in spine density in the hippocampus and the mPFC (Nestler and Hyman, 2010; Yang et al., 2015). Animals also have symptoms that parallel those of major depression, which are reduced by antidepressant treatment (Takamori et al., 2001). Using this model, altered synaptic plasticity has been described in the activating circuit of the DA system, the vSub-NAc pathway, in helpless rats but not in identically treated rats that are nonhelpless (Belujon and Grace, 2014). In particular, tetanic stimulation of the vSub-NAc pathway induced a long-term depression in NAc neurons in helpless rats, whereas a long-term potentiation is induced in control and non-helpless rats. This suggests a downregulation of the vSub-NAc pathway in helpless rats that could contribute to the decrease in DA neuron activity (Belujon and Grace, 2014). The ilPFC could play a role in the disrupted vSub-NAc plasticity, as described in other psychiatric disorders (Belujon et al., 2014); however, this still needs to be examined in detail.
Figure 2.
Afferent dysregulation of the dopamine (DA) system in major depressive disorder (MDD). In animal models of depression, the DA system is downregulated, as measured by a decrease in the number of DA neurons that fire spontaneously. This decrease is due to hyperactivity of the infralimbic subregion (ilPFC), driving activity in the inhibitory basolateral amygdala (BLA)-ventral pallidum (VP) pathway while attenuating excitation via the Re-ventral subiculum of the hippocampus (vSub)-nucleus accumbens (NAc)-VP pathway.
Although decreased DA activity forms the basis of some symptoms described in MDD, such as anhedonia, its downregulation originates via hyperexcitation of the ilPFC-BLA-VP pathway and possibly via disrupted synaptic plasticity in the vSub-NAc pathway. Therefore, new therapeutics aiming at these regions are more likely to be effective than therapeutics targeting the DA system directly.
Antidepressants and the Reward-Related Circuit in MDD
As previously mentioned, there is now a consensus that the majority of depressed patients treated with SSRIs do not obtain remission. The effect of these antidepressants on DA neurons may contribute to their low efficacy. Indeed, it has been shown that the administration of SSRIs such as fluoxetine or escitalopram induced a decrease in DA neuron firing rate in the VTA, whereas citalopram decreased the firing rate and the number of spikes per burst (Prisco and Esposito, 1995; Di Mascio et al., 1998; Dremencov et al., 2009). It is suggested that this class of antidepressant acts through 5-HT2C receptors (Prisco and Esposito, 1995; Dremencov et al., 2009). This is consistent with studies using lesions of the raphe nucleus showing an increase in the firing and bursting of DA neurons in the VTA (Guiard et al., 2008). Considering the critical role of DA in hedonic processes, the decrease in firing as well as the bursting activity by SSRIs might contribute to the resistance to antidepressants in some patients. Therefore, augmentation strategies, involving the addition of a second drug to an existing antidepressant therapy, are often used to optimize treatment, such as the addition of an atypical antipsychotic to an SSRI treatment (Ostroff and Nelson, 1999; Shelton et al., 2001; Thase et al., 2007). The United States Food and Drug Administration has approved several atypical antipsychotics as supplement to ongoing antidepressant treatment for treatment-resistant patients (Thase et al., 2007; Berman et al., 2009; Kato and Chang, 2013). The role of antipsychotics in increasing antidepressant activity has been confirmed by animal models. Thus, aripiprazole, an atypical antipsychotic, has been shown to potentiate the effect of citalopram in the forced swimming test (Bourin et al., 2009) and the effect of fluoxetine in the tail suspension test (Kamei et al., 2008). Interestingly, the combination of aripiprazole with the SSRI escitalopram reversed the inhibitory action of the antidepressant on serotoninergic, dopaminergic, and noradrenergic neuron firing (Chernoloz et al., 2009). The combination of fluoxetine with olanzapine, another atypical antipsychotic, also induces an increase in DA, 5-HT, and norepinephrine extracellular levels in the prefrontal cortex (Zhang et al., 2000). One question would be how would a D2 antagonist improve DA system function in a depressed state? We have found recently that repeated treatment with the second-generation antipsychotic quetiapine to the CMS rat model of depression effectively reversed the decrease in DA neuron population activity (Moreines et al., 2017). We believe this is due to the low-dose quetiapine activating the “silent” DA neurons to restore population activity, whereas the low level D2 blockade can be overcome via homeostatic compensation (e.g., increased D2 receptors, increased tyrosine hydroxylase activity, etc.).
Another augmentation strategy consists of the use of pramipexole, a D2 subfamily receptor agonist in treatment-resistant patients (Cusin et al., 2013). In the CMS model, pramipexole has been shown to increase sucrose intake, a putative indicator of anhedonia in rodents, in stressed animals (Willner et al., 1994). Recently, studies going beyond monoamine transporters and MAOIs highlighted a novel class of promising antidepressant drugs such as agomelatine. Agomelatine is a potent melatonin receptor agonist (Yous et al., 1992) and a selective antagonist of the 5-HT2C receptors (Millan et al., 2003). In the CMS model of depression, agomelatine has been shown to have potent antidepressant activity. Indeed, CMS animals showed decreased sucrose consumption, which is normalized by chronic administration of agomelatine (Papp et al., 2003). In the forced swim test, a predictive model of antidepressant activity, acute and repeated administration of agomelatine, induced an antidepressant-like effect in rats, that is, an increase in swimming, without modification of locomotor activity (Bourin et al., 2004). In patients, both venlafaxine (serotonin and norepinephrine reuptake inhibitor) and agomelatine decreased the score of MDD patients on the Hamilton depression scale as well as the Hamilton anxiety scale (Martinotti et al., 2012). Interestingly, on the Snaith Hamilton pleasure scale for anhedonia, agomelatine induced an improvement in anhedonia scores as early as 1 week after the start of the treatment (Martinotti et al., 2012). The anhedonia score improvement is consistent with animals studies showing that chronic administration of agomelatine increased the number of DA spontaneously active neurons, as well as the bursting activity (Chenu et al., 2013). Although the effect of agomelatine on DA neuron activity in the VTA has yet to be studied in animal models of depression, several studies have highlighted its effect on the PFC, the HPC, and the amygdala (Dagyte et al., 2010; Ladurelle et al., 2012; Grillo et al., 2015), which modulate VTA DA neuron activity. Research on glutamate targets might also hold promise, but it is still at an early stage.
In depressed patients, ketamine, a non-competitive, glutamatergic N-methyl-d-aspartate receptor antagonist, exerts rapid (within hours) and prolonged (up to 1–2 weeks) antidepressant effects after a single dose (Berman et al., 2000; Zarate et al., 2006; Murrough et al., 2013). It is now well-known that ketamine rapidly increases AMPA-dependent glutamate transmission, particularly in the PFC, either via an increased synthesis of synaptic proteins in pyramidal neurons leading to an increase in excitatory synaptic input or via antagonism of N-methyl-d-aspartate receptor on cortical interneurons, leading to disinhibition of pyramidal neurons (for review, see Miller et al., 2016). The cellular and molecular effects of ketamine have been extensively studied in the past years (Wohleb et al., 2016). At the circuit level, we have shown that ketamine restores DA population activity in helpless rats comparable with control and non-helpless animals. We have also shown that ketamine restores long-term potentiation in the vSub-NAc pathway (Belujon and Grace, 2014). It is important to note that this effect is observed in rodent learned helplessness depression models, but not in normal rats in which the vSub-NAc pathway is not abnormally downregulated (Carreno et al., 2016). The effect of ketamine on DA population activity has also been observed following acute amphetamine withdrawal (Belujon et al., 2016). Indeed, a decreased DA population activity after acute withdrawal, suggested to underlie a negative emotional state after withdrawal from psychostimulants, is restored by prior administration of ketamine as well as by inhibition of the BLA (Belujon et al., 2016). Whereas ketamine is known to have potent, rapid, and prolonged antidepressant properties, it also induces short-term dissociative side effects. However, understanding some of ketamine antidepressant properties at a systems level will help in finding new treatment strategies that could induce remission with few side effects in treatment-resistant patients.
Conclusion
Patients diagnosed with MDD exhibit a multitude of symptom clusters, which differ from one patient to another, making it difficult to diagnose and treat effectively. To find new and effective treatments, it is critical to understand some of the complex and unique circuitry underlying these symptoms. Anhedonia, a core symptom of MDD, involves a downregulation of the DA system. It is now clear that the DA system is under intricate regulation via an activating vSub-NAc-VP pathway and via an inhibiting BLA-VP pathway, both under the influence of the ilPFC. It is critical to understand the detailed circuitry modulating the DA system and in particular its disruption underlying anhedonia. Future investigations will facilitate the development of a new, fast-acting, and efficient treatment that will ultimately reverse depression without inducing severe side effects.
Statement of Interest
Dr Belujon reported no biomedical financial interests or potential conflict of interest. Dr. Grace has received funds from the following organizations: Johnson & Johnson, Lundbeck, Pfizer, GSK, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, and Alkermes.
Acknowledgments
Dr Grace was supported by the United States Public Health Service Grant no. MH191180. Dr Belujon was supported by the Agence Nationale de la Recherche Grant no. ANR-15-CE37-0010.
References
- Aggleton JP. (1993) The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci 16:328–333. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. Fifth Ed American Psychiatric Association Publishing. [Google Scholar]
- Belujon P, Grace AA (2014) Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2015) Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Jakobowski NL, Dollish HK, Grace AA (2016) Withdrawal from acute amphetamine induces an amygdala-driven attenuation of dopamine neuron activity: reversal by ketamine. Neuropsychopharmacology 41:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Patton MH, Grace AA (2014) Role of the prefrontal cortex in altered hippocampal-accumbens synaptic plasticity in a developmental animal model of schizophrenia. Cerebral Cortex 24:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD, Carson WH, Adson D, Taylor L, Hazel J, Marcus RN (2009) Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr 14:197–206. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28:309–369. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX (1999) Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience 92:15–26. [DOI] [PubMed] [Google Scholar]
- Bortolanza M, Wietzikoski EC, Boschen SL, Dombrowski PA, Latimer M, Maclaren DA, Winn P, Da Cunha C (2010) Functional disconnection of the substantia nigra pars compacta from the pedunculopontine nucleus impairs learning of a conditioned avoidance task. Neurobiol Learn Mem 94:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Mocaer E, Porsolt R (2004) Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci 29:126–133. [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Chenu F, Prica C, Hascoet M (2009) Augmentation effect of combination therapy of aripiprazole and antidepressants on forced swimming test in mice. Psychopharmacology 206:97–107. [DOI] [PubMed] [Google Scholar]
- Bunney WE Jr., Davis JM (1965) Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry 13:483–494. [DOI] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ (2016) Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21:1298–1308. [DOI] [PubMed] [Google Scholar]
- Chang T, Fava M (2010) The future of psychopharmacology of depression. J Clin Psychiatry 71:971–975. [DOI] [PubMed] [Google Scholar]
- Chang CH, Grace AA (2013) Amygdala beta-noradrenergic receptors modulate delayed downregulation of dopamine activity following restraint. J Neurosci 33:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Grace AA (2014) Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 76:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, et al. (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu F, El Mansari M, Blier P (2013) Electrophysiological effects of repeated administration of agomelatine on the dopamine, norepinephrine, and serotonin systems in the rat brain. Neuropsychopharmacology 38:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoloz O, El Mansari M, Blier P (2009) Electrophysiological studies in the rat brain on the basis for aripiprazole augmentation of antidepressants in major depressive disorder. Psychopharmacology 206:335–344. [DOI] [PubMed] [Google Scholar]
- Collins PY, et al. (2011) Grand challenges in global mental health. Nature 475:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppen A. (1967) The biochemistry of affective disorders. Br J Psychiatry 113:1237–1264. [DOI] [PubMed] [Google Scholar]
- Cusin C, Iovieno N, Iosifescu DV, Nierenberg AA, Fava M, Rush AJ, Perlis RH (2013) A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Psychiatry 74:e636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagyte G, Trentani A, Postema F, Luiten PG, Den Boer JA, Gabriel C, Mocaer E, Meerlo P, Van der Zee EA (2010) The novel antidepressant agomelatine normalizes hippocampal neuronal activity and promotes neurogenesis in chronically stressed rats. CNS Neurosci Ther 16:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A (2012) The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Haenen HA, Bossuyt A (1994) Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiatry 35:128–132. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G (1997) Blunting of reactivity of dopamine transmission to palatable food: a biochemical marker of anhedonia in the CMS model? Psychopharmacology 134:351–353; discussion 371–357. [DOI] [PubMed] [Google Scholar]
- Di Mascio M, Di Giovanni G, Di Matteo V, Prisco S, Esposito E (1998) Selective serotonin reuptake inhibitors reduce the spontaneous activity of dopaminergic neurons in the ventral tegmental area. Brain Research Bull 46:547–554. [DOI] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P (2009) Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. J Psychiatry Neurosci 34:223–229. [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Func 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Willner P, Papp M (1997) Changes in dopamine receptor mRNA expression following chronic mild stress and chronic antidepressant treatment. Behav Pharmacol 8:607–618. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA (2001a) Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21:4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG (2001b) Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci 21:6370–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE (2009) Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry 166:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Friedman Y, Dremencov E, Yadid G (2008) VTA dopamine neuron bursting is altered in an animal model of depression and corrected by desipramine. J Mol Neurosci 34:201–209. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD (1986) Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 43:107–113. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS (2007) Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci 27:5730–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Cornu M, Jager E, Konrad B, Adamczyk M, Friess E, Steiger A, Czisch M, Goya-Maldonado R (2015) Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry 77:177–186. [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE 3rd, Schwartz T, Sackeim HA (2010) Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 67:507–516. [DOI] [PubMed] [Google Scholar]
- Gill KM, Grace AA (2011) Heterogeneous processing of amygdala and hippocampal inputs in the rostral and caudal subregions of the nucleus accumbens. Int J Neuropsychopharmacol 14:1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Weizman R, Rehavi M (1996) Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol 298:27–30. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R (2008) Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13:717–728. [DOI] [PubMed] [Google Scholar]
- Grace AA. (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24. [DOI] [PubMed] [Google Scholar]
- Grace AA. (2016) Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience 10:301–315. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4:2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30:220–227. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Risher M, Macht VA, Bumgardner AL, Hang A, Gabriel C, Mocaer E, Piroli GG, Fadel JR, Reagan LP (2015) Repeated restraint stress-induced atrophy of glutamatergic pyramidal neurons and decreases in glutamatergic efflux in the rat amygdala are prevented by the antidepressant agomelatine. Neuroscience 284:430–443. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P (2008) Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol 11:625–639. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN (2016) Circuit-Based corticostriatal homologies between rat and primate. Biol Psychiatry 80:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J (2012) Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36:2085–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa K, Watanabe A, Motohashi N, Kaneno S (1994) The effect of repeated administration of methamphetamine on dopamine uptake sites in rat striatum. Neurosci Lett 167:37–40. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isella V, Iurlaro S, Piolti R, Ferrarese C, Frattola L, Appollonio I, Melzi P, Grimaldi M (2003) Physical anhedonia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 74:1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. (1995) What does the hippocampus really do? Behav Brain Res 71:1–10. [DOI] [PubMed] [Google Scholar]
- Kamei J, Miyata S, Sunohara T, Kamei A, Shimada M, Ohsawa M (2008) Potentiation of the antidepressant-like effect of fluoxetine by aripiprazole in the mouse tail suspension test. J Pharmacol Sci 108:381–384. [DOI] [PubMed] [Google Scholar]
- Kato M, Chang CM (2013) Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs 27 Suppl 1:S11–19. [DOI] [PubMed] [Google Scholar]
- Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, Frazer A (2004) Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology 29:566–579. [DOI] [PubMed] [Google Scholar]
- Katz RJ. (1982) Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav 16:965–968. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M (2009) Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol 513:597–621. [DOI] [PubMed] [Google Scholar]
- Keedwell P, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M (2009) Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J Psychopharmacol 23:775–788. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC (2011) Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68:675–690. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ (2013) The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Price RH, Wortman CB (1985) Social factors in psychopathology: stress, social support, and coping processes. Annu Rev Psychol 36:531–572. [DOI] [PubMed] [Google Scholar]
- Kilbourn MR, Sherman PS, Pisani T (1992) Repeated reserpine administration reduces in vivo [18F]GBR 13119 binding to the dopamine uptake site. Eur J Pharmacol 216:109–112. [DOI] [PubMed] [Google Scholar]
- Klein DF. (1974) Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry 31:447–454. [DOI] [PubMed] [Google Scholar]
- Kong L, Chen K, Tang Y, Wu F, Driesen N, Womer F, Fan G, Ren L, Jiang W, Cao Y, Blumberg HP, Xu K, Wang F (2013) Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci 38:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129. [DOI] [PubMed] [Google Scholar]
- Kram ML, Kramer GL, Ronan PJ, Steciuk M, Petty F (2002) Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Prog Neuropsychopharmacol Biol Psychiatry 26:639–645. [DOI] [PubMed] [Google Scholar]
- Kumano H, Ida I, Oshima A, Takahashi K, Yuuki N, Amanuma M, Oriuchi N, Endo K, Matsuda H, Mikuni M (2007) Brain metabolic changes associated with predispotion to onset of major depressive disorder and adjustment disorder in cancer patients--a preliminary PET study. J Psychiatr Res 41:591–599. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Gabriel C, Viggiano A, Mocaer E, Baulieu EE, Bianchi M (2012) Agomelatine (S20098) modulates the expression of cytoskeletal microtubular proteins, synaptic markers and BDNF in the rat hippocampus, amygdala and PFC. Psychopharmacology 221:493–509. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC (2011) Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Kim U (2011) Topography of projections from the amygdala to the lateral habenula of the epithalamus in rats. Program No 20116 2011 Neuroscience Meeting Planner Washington, DC: Society for Neuroscience, 2011 Online. [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK (2008) Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 105:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2006a) The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A 103:5167–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2006b) The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology 31:1356–1361. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2007) Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27:11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ (2004) Neuronal signalling of fear memory. Nat Rev Neurosci 5:844–852. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Sepede G, Gambi F, Di Iorio G, De Berardis D, Di Nicola M, Onofrj M, Janiri L, Di Giannantonio M (2012) Agomelatine versus venlafaxine XR in the treatment of anhedonia in major depressive disorder: a pilot study. J Clin Psychopharmacol 32:487–491. [DOI] [PubMed] [Google Scholar]
- Maslowski-Cobuzzi RJ, Napier TC (1994) Activation of dopaminergic neurons modulates ventral pallidal responses evoked by amygdala stimulation. Neuroscience 62:1103–1119. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. (2003) Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Brit Med Bull 65:193–207. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA (2000) Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48:830–843. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ (2009) Neural representation of reward in recovered depressed patients. Psychopharmacology 205:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Wagner KD, Asarnow JR, Ryan ND, Birmaher B, Shamseddeen W, Mayes T, Kennard B, Spirito A, Keller M, Lynch FL, Dickerson JF, Brent DA (2012) Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry 51:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Minifie C, Houle S, Hussey D, Kennedy SH (2001) Lower dopamine transporter binding potential in striatum during depression. Neuroreport 12:4121–4125. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, Pasteau V, Rivet JM, Cussac D (2003) The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 306:954–964. [DOI] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ (2016) Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 100:17–26. [DOI] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, Grace AA (2016) Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology 42:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, Gagnon K, Grace AA (2017) Divergent effects of acute and repeated quetiapine treatment on dopamine system in normal and chronic mild stress induced hypodopaminergic states. Biol Psychiatry 81:S267–S268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas Z, Teneback C, Chae JH, Mu Q, Molnar C, Kozel FA, Walker J, Anderson B, Koola J, Kose S, Lomarev M, Bohning DE, George MS (2007) Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology 32:1649–1660. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WAJr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC (2004) Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry 61:765–773. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, Mann JJ (2001) Decreased regional brain metabolism after ect. Am J Psychiatry 158:305–308. [DOI] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S (2007) The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol 21:461–471. [DOI] [PubMed] [Google Scholar]
- Ostroff RB, Nelson JC (1999) Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry 60:256–259. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. (1993) The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev 17:51–68. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Boyer PA, Mocaer E (2003) Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology 28:694–703. [DOI] [PubMed] [Google Scholar]
- Pare CM, Yeung DP, Price K, Stacey RS (1969) 5-hydroxytryptamine, noradrenaline, and dopamine in brainstem, hypothalamus, and caudate nucleus of controls and of patients committing suicide by coal-gas poisoning. Lancet 2:133–135. [DOI] [PubMed] [Google Scholar]
- Patton MH, Bizup BT, Grace AA (2013) The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci 33:16865–16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty F, Kramer GL, Wu J (1997) Serotonergic modulation of learned helplessness. Ann N Y Acad Sci 821:538–541. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco S, Esposito E (1995) Differential effects of acute and chronic fluoxetine administration on the spontaneous activity of dopaminergic neurones in the ventral tegmental area. Br J Pharmacol 116:1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A (2004) Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 24:2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS (1992) CSF amine metabolites in depression. Biol Psychiatry 31:112–118. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10:423–433. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM (2003) Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther 305:1–8. [DOI] [PubMed] [Google Scholar]
- Sarchiapone M, Carli V, Camardese G, Cuomo C, Di Giuda D, Calcagni ML, Focacci C, De Risio S (2006) Dopamine transporter binding in depressed patients with anhedonia. Psychiatry Res 147:243–248. [DOI] [PubMed] [Google Scholar]
- Savitz J, Hodgkinson CA, Martin-Soelch C, Shen PH, Szczepanik J, Nugent A, Herscovitch P, Grace AA, Goldman D, Drevets WC (2013) The functional DRD3 Ser9Gly polymorphism (rs6280) is pleiotropic, affecting reward as well as movement. PloS one 8:e54108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ, Gordon EK, Durell J (1965) Catecholamine metabolism in affective disorders. I. Normetanephrine and VMA excretion in depressed patients treated with imipramine. J Psychiatr Res 3:213–228. [DOI] [PubMed] [Google Scholar]
- Schultz W. (1998a) The phasic reward signal of primate dopamine neurons. Adv Pharmacol 42:686–690. [DOI] [PubMed] [Google Scholar]
- Schultz W. (1998b) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Beagley G (1975) Learned helplessness in the rat. J Comp Physiol Psychol 88:534–541. [DOI] [PubMed] [Google Scholar]
- Sharma HR, Thakur MK (2015) Correlation of ERalpha/ERbeta expression with dendritic and behavioural changes in CUMS mice. Physiol Behav 145:71–83. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tollefson GD, Tohen M, Stahl S, Gannon KS, Jacobs TG, Buras WR, Bymaster FP, Zhang W, Spencer KA, Feldman PD, Meltzer HY (2001) A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 158:131–134. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH (2012) Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol 121:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM (2000) Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97:253–266. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD (2012) Optogenetic strategies to dissect the neural circuits that underlie reward and addiction. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Gold JM (2012) A new perspective on anhedonia in schizophrenia. Am J Psychiatry 169:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori K, Yoshida S, Okuyama S (2001) Availability of learned helplessness test as a model of depression compared to a forced swimming test in rats. Pharmacology 63:147–153. [DOI] [PubMed] [Google Scholar]
- Tekes K, Tothfalusi L, Gaal J, Magyar K (1988) Effect of MAO inhibitors on the uptake and metabolism of dopamine in rat and human brain. Pol J Pharmacol Pharm 40:653–658. [PubMed] [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, Case M, Henley DB, Sanger TM, Watson SB, Dube S (2007) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry 68:224–236. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH (2011) Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE (2002) Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry 59:409–416. [DOI] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K (2012) Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 13:251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Miettinen P, Kadish I (2003) The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus 13:133–149. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ (2006) Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol 499:768–796. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13:355–366. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S (2013) Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry 73:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IM, Whitaker C, White W (2006) Amphetamine-induced hyperlocomotion in rats: hippocampal modulation of the nucleus accumbens. Hippocampus 16:596–603. [DOI] [PubMed] [Google Scholar]
- WHO (2008) The Global Burden of Disease 2004 update. [Google Scholar]
- Willner P. (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134:319–329. [DOI] [PubMed] [Google Scholar]
- Willner P. (2017) The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress 6:78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Lappas S, Cheeta S, Muscat R (1994) Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology 115:454–462. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364. [DOI] [PubMed] [Google Scholar]
- Winter C, von Rumohr A, Mundt A, Petrus D, Klein J, Lee T, Morgenstern R, Kupsch A, Juckel G (2007) Lesions of dopaminergic neurons in the substantia nigra pars compacta and in the ventral tegmental area enhance depressive-like behavior in rats. Behav Brain Res 184:133–141. [DOI] [PubMed] [Google Scholar]
- Wise RA. (1982) Neuroleptics and operant behavior: the anhedonia hypothesis. Behavioral and Brain Sciences 5:39–53. [Google Scholar]
- Wohleb ES, Gerhard D, Thomas A, Duman RS (2016) Molecular and Cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP (1990) Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 296:179–203. [DOI] [PubMed] [Google Scholar]
- Yadid G, Friedman A (2008) Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res 172:265–286. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K (2015) Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol 18:pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yous S, Andrieux J, Howell HE, Morgan PJ, Renard P, Pfeiffer B, Lesieur D, Guardiola-Lemaitre B (1992) Novel naphthalenic ligands with high affinity for the melatonin receptor. J Med Chem 35:1484–1486. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Marsiske M, Okun MS, Bowers D (2012) Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol 25:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zhang W, Perry KW, Wong DT, Potts BD, Bao J, Tollefson GD, Bymaster FP (2000) Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology 23:250–262. [DOI] [PubMed] [Google Scholar]
- Zimmerman EC, Grace AA (2016) The nucleus reuniens of the midline thalamus gates prefrontal-hippocampal modulation of ventral tegmental area dopamine neuron activity. J Neurosci 36:8977–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD (2009) Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A 106:7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD (2011) Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci 14:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]