Abstract
Meningiomas are the most frequent nonglial primary brain tumors and represent about 30% of brain tumors. Usually, diagnosis and treatment planning are based on neuroimaging using mainly MRI or, rarely, CT. Most common treatment options are neurosurgical resection and radiotherapy (eg, radiosurgery, external fractionated radiotherapy). For follow-up after treatment, a structural imaging technique such as MRI or CT is used. However, these structural imaging modalities have limitations, particularly in terms of tumor delineation as well as diagnosis of posttherapeutic reactive changes. Molecular imaging techniques such as PET can characterize specific metabolic and cellular features which may provide clinically relevant information beyond that obtained from structural MR or CT imaging alone. Currently, the use of PET in meningioma patients is steadily increasing. In the present article, we provide recommendations for the use of PET imaging in the clinical management of meningiomas based on evidence generated from studies being validated by histology or clinical course.
Keywords: MRI, positron emission tomography, ligand, somatostatin, meningiomas
Importance of the study.
This paper seeks to summarize all data published thus far on PET imaging in meningiomas, which account for levels 1–3 evidence according to the Oxford Centre for Evidence-Based Medicine in order to provide recommendations for its use as a guideline for clinicians.
Meningiomas are the most common primary brain tumors and represent approximately 30% of intracranial tumors. According to the classification of the World Health Organization (WHO), the majority of meningiomas are benign (WHO grade I), exhibit slow growth, and have a low recurrence rate (5-y overall recurrence rate of ~5% following complete resection).1 In contrast, WHO grade II (atypical) and WHO grade III (malignant) meningiomas may show a more aggressive clinical behavior.2 Atypical and malignant meningiomas have 5-y overall recurrence rates of 40% and 80%, respectively.3 Molecular factors with strong prognostic information4,5 and potential value as predictive markers for targeted therapies have recently emerged.6–10 Most common treatment options are neurosurgical resection and various radiotherapy options such as radiosurgery and external fractionated radiotherapy.6
Contrast-enhanced structural imaging techniques such as MRI and CT (to delineate bony structures) are routinely used for defining the extent of the meningioma, treatment planning, and monitoring, as well as for follow-up after treatment, especially diagnosis of tumor recurrence. However, these structural imaging techniques have limitations in delineating meningiomas, especially at the skull base and in the case of bony involvement as well as in tumors with complex geometry.6 Furthermore, in the case of suspected residual or recurrent tumor, it can be challenging to distinguish viable tumor from scar tissue or posttherapeutic changes by CT or MRI alone, particularly after radiotherapy.
Molecular imaging modalities, which are not routinely used yet, may provide further diagnostic information. PET has meanwhile gained considerable importance for diagnostic purposes in general oncology. In neuro-oncology, particularly cerebral gliomas have been extensively studied using initially 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) and more recently amino acid PET tracers.11,12 In meningiomas, several tracers have been used, including specific somatostatin receptor (SSTR) ligands such as gallium-68 (68Ga)-DOTA-Tyr3-octreotide (68Ga-DOTATOC), 68Ga -DOTA-D-Phe1-Tyr3-octreotate (68Ga-DOTATATE), and 68Ga-DOTA-1-Nal3-octreotide (68Ga-DOTANOC). Currently, the number of PET examinations in meningioma patients is steadily increasing. The Response Assessment in Neuro-Oncology (RANO) Working Group and the European Association for Neuro-Oncology recently published guidelines for the use of PET in gliomas11; here we have prepared evidence-based recommendations for the use of PET imaging in the diagnosis and follow-up of patients with meningiomas to guide clinicians from all disciplines involved in the management of patients with these tumors.
Search Strategy, Selection Criteria, and Levels of Validation
A PubMed search was performed of the published literature with the combination of the search terms “meningioma,” “PET,” “FDG,” “amino acid,” “somatostatin,” “DOTATOC,” “DOTATATE,” “DOTANOC,” “grading,” “delineation,” “radiotherapy,” and “extent” until September 2016. Additionally, articles identified through searches of the authors’ own files were included in the search. Results of the search were evaluated by the working group with respect to the level of evidence and the grade of validation of the PET studies examined. As described previously,11 any study that correlated the PET findings with histopathology was considered to represent the highest degree of validation. Next, correlation with MRI and with the patient’s clinical course was used for the second level of validation. Only papers constituting levels 1–3 evidence according to the Oxford Centre for Evidence-Based Medicine (“The Oxford 2011 Levels of Evidence”) were included.
Tracers for PET Imaging in Meningioma Patients
Several tracers addressing different molecular structures or pathophysiological pathways in meningioma cells are available for PET imaging and will be summarized in the following paragraphs.
Glucose PET
18F-FDG represents the most widely used tracer in oncological PET imaging.13 With a half-life of the 18F isotope of 110 minutes, the tracer does not need in-house production, which facilitates supply. Therefore, 18F-FDG is available at all PET centers independently of the presence of a cyclotron. Due to an increased glycolysis in neoplastic tissue, uptake of 18F-FDG is generally higher than in nonneoplastic tissue.14,15 However, there are several limitations for the use of 18F-FDG in meningioma. Meningiomas are mostly slow-growing tumors and their glucose metabolism might be only moderately elevated15–17 (Fig. 1). Furthermore, high physiological glucose uptake of the normal cerebral cortex leads to a low tumor-to-background ratio and therefore limits the sensitivity for the detection of meningioma tissue and its delineation from adjacent brain parenchyma.18 Moreover, 18F-FDG uptake is not tumor specific but may be increased in inflammatory tissue.18
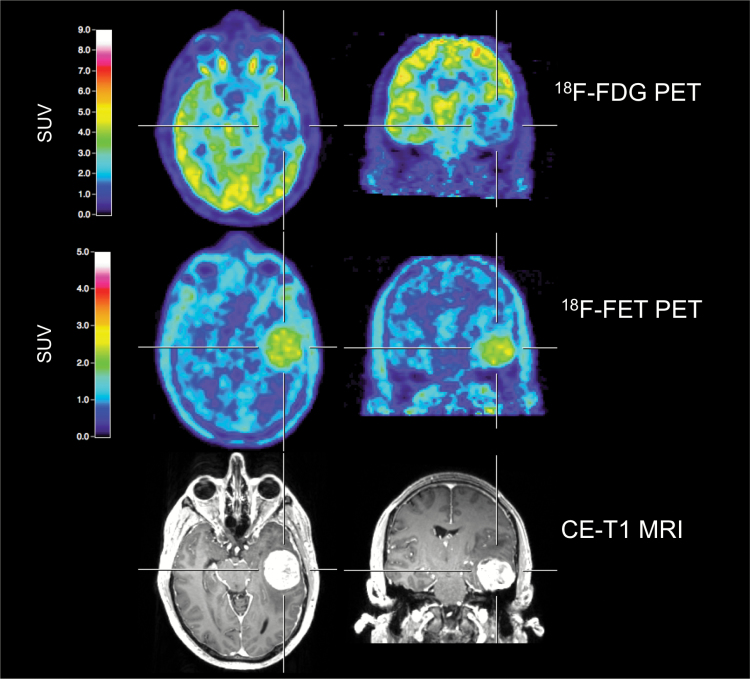
Fig. 1.
A 43-year-old male patient with a newly diagnosed left temporal meningioma (WHO grade I), preoperatively examined by multimodal imaging. Both contrast-enhanced MRI and 18F-FET PET allow a precise tumor delineation. Conversely, 18F-FDG PET shows decreased metabolic activity, indicating its limitation for the evaluation of meningioma extent.
PET Ligands for Somatostatin Receptors
Because of the overexpression of SSTRs in meningiomas,19–21 radiolabeled SSTR ligands can be used for the visualization of meningioma tissue. Somatostatin receptor subtype 2 has been found to be the most abundant isoform, with almost 100% expression in meningiomas.19 The most commonly applied SSTR ligands for PET imaging are 68Ga-DOTATOC, 68Ga-DOTATATE, and 68Ga-DOTANOC. These tracers are also frequently used for imaging of neuroendocrine tumors, which likewise express high levels of SSTR.2268Ga has a physical half-life of 68 minutes and can be produced with a 68Ge/68Ga generator system, which enables in-house production without need of an on-site cyclotron. PET ligands to SSTR provide high sensitivity with excellent target-to-background contrast due to low uptake in bone and healthy brain tissue.23,24 However, the pituitary gland shows high physiological uptake which serves as a positive control but limits the exact delineation of meningioma extent in its close proximity.25 Up to now, a comparative study of 68Ga-DOTATOC, 68Ga-DOTATATE, and 68Ga-DOTANOC in meningioma patients is not available. An animal study with nude mice bearing xenografts of a human meningioma cell line (CH-157MN) revealed similar uptake kinetics of the 3 tracers, but tumor uptake ratios were higher with 68Ga-DOTATATE, suggesting a higher diagnostic value of 68Ga-DOTATATE for detecting meningiomas.26 However, the uptake of all these tracers is relatively high compared with normal brain; thus, these differences are of less importance. Procedure guidelines for PET imaging with 68Ga-DOTA–conjugated peptides have been published previously.27
Amino Acid PET Tracers
Uptake of radiolabeled amino acids or their analogues, such as [11C-methyl]-methionine (11C-MET) and O-(2-[18F]-fluoroethyl)-L-tyrosine (18F-FET), is mediated by the L-amino acid transporter system, and increased uptake is already seen in slow-growing tumors such as low-grade gliomas28–30 and meningiomas.31,32
Amino acid PET tracers are widely used for glioma imaging as well as for the assessment of brain metastases after radiotherapy and have been integrated in many centers in clinical routine.11,12 Even though amino acid PET exhibits a better tumor-to-background contrast than 18F-FDG PET,33 the availability of specific SSTR ligands with even higher tumor-to-background contrast led to a limited use of amino acid PET in meningioma imaging.34,35 For the use of 11C-MET, an on-site cyclotron is needed due to the short half-life (20 min) of 11C. In contrast, 18F-FET is (like 18F-FDG) labeled with 18F (half-life, 110 min) and can therefore be purchased and delivered independently of a local radiopharmaceutical setting. Interestingly, 18F-FET does not accumulate in the pituitary gland in comparison with 11C-MET and SSTR ligands; it may be superior in detecting intrasellar invasion of meningioma.36 For therapeutic procedures such as boron neutron capture therapy, boronated amino acid PET probes have been used in meningiomas.37,38
Other PET Tracers
11C-choline can be used as a marker of increased phospholipid synthesis in tumor cells.39 Over the last years, it has been mostly used in prostate cancer and is currently being replaced by prostate-specific membrane antigen (PSMA) ligands.39,40 As choline exhibits low uptake in the healthy brain tissue, the target-to-background contrast is good as well, but the experience in meningioma patients is limited to case reports so far, and only one small study on 7 patients compared the value of 11C-choline with 18F-FDG PET in meningiomas, indicating a higher target-to-background contrast for 11C-choline than for 18F-FDG.41
11C-acetate is another possible PET tracer used in extracranial tumors which are difficult to detect by 18F-FDG PET, such as renal carcinoma, prostate cancer, and hepatocellular carcinoma.42–44 Uptake of 11C-acetate in tumor cells depends on the activation of anabolic pathways of fatty acids and sterol synthesis.18,45 The experience with this tracer for meningioma imaging is also very limited. So far, only one study on 22 patients has been published, stating that the tracer is superior to 18F-FDG for the detection of meningioma and delineation of tumor extent for radiosurgery planning and the evaluation of treatment response.18
18F-fluoride, which is used in imaging of bone metastasis of neoplastic tissue, might facilitate detection of bone invasion of meningiomas. There are 2 studies reporting superior detection of bone involvement with 18F-fluoride compared with CT and MRI, which might assist in planning of surgery.46,47
Aside from PET imaging with the specific purpose of meningioma imaging, meningiomas might be detected incidentally on PET scans using 11C-PIB (Pittsburgh compound B) in patients with Alzheimer’s disease,4868Ga-labeled PSMA ligand PET,49 or dopamine transporter imaging in patients with Parkinson syndromes.50
Clinical Applications for PET Imaging
Diagnosis/Differential Diagnosis
Mostly, meningiomas are well-defined, extra-axial masses, which may displace the adjacent brain. Furthermore, the cerebrospinal fluid (CSF) cleft sign can be present, representing a thin rim of CSF between tumor and brain parenchyma. Sometimes, however, meningiomas may become very large before causing clinical symptoms, and furthermore the distinction between an intra-axial and extra-axial origin may be difficult.51 Several other disease processes have a propensity for primary involvement of the dura mater or subdural space, giving a meningioma-like appearance, including lymphomas, brain metastases, other benign tumors (eg, schwannomas), inflammatory lesions (eg, neurosarcoidosis, Wegener’s granulomatosis), and infections of the central nervous system (eg, tuberculosis).52
Although PET plays no major role in the primary diagnosis of meningiomas, SSTR imaging may be helpful in terms of definition of gross target volume (GTV) and clinical target volume (CTV). A study comparing contrast-enhanced MRI and 68Ga-DOTATOC PET/CT prior to radiotherapy reported that all meningiomas (n = 190) were detected by PET/CT. In contrast, only 171 meningiomas were detected by contrast-enhanced MRI (90%), indicating an improved sensitivity for 68Ga-DOTATOC PET in meningioma detection compared with contrast-enhanced MRI.24 Particularly difficult to detect by standard MRI alone were tumors adjacent to the falx cerebri, tumors located at the skull base, tumors infiltrating bony structures, and tumors obscured by imaging artefacts or calcification (Fig. 2). The authors concluded that 68Ga-DOTATOC PET/CT may provide additional information in patients with uncertain or equivocal results on MRI or could help to confirm a diagnosis of meningioma based on MRI. Moreover, 68Ga-DOTATATE PET/CT helps to discriminate optic nerve sheath meningiomas in the differential diagnosis of other lesions being associated with the optic nerve53 (Fig. 3). In a comparative study between MRI and 68Ga-DOTATATE PET/CT, additional meningiomas were detected by PET, some of them even in retrospect not being visible yet in MRI.23
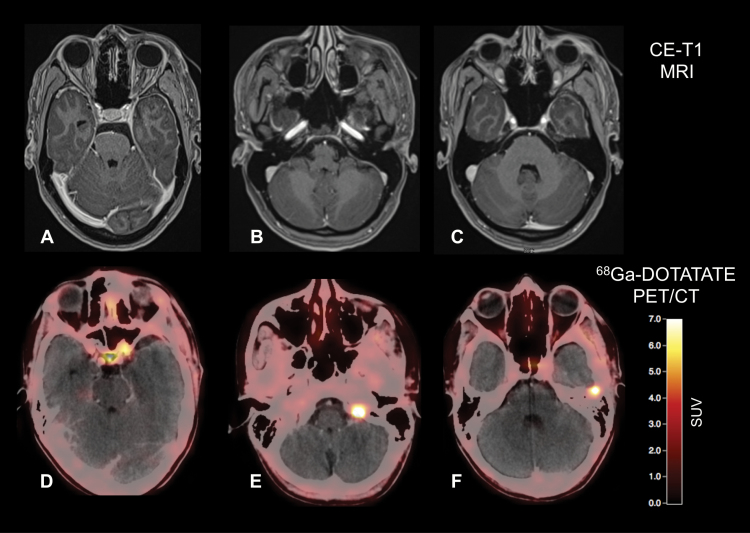
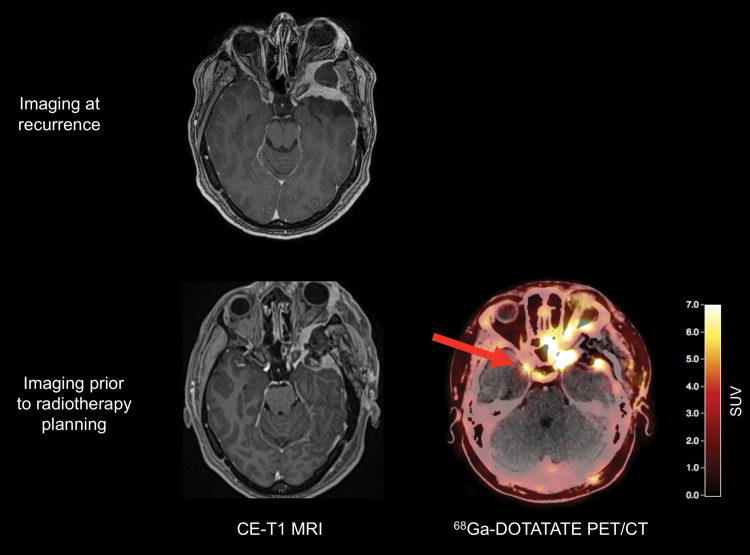
Fig. 2.
Postoperative contrast-enhanced MRI and 68Ga-DOTATATE PET/CT of a 32-year-old patient after resection of a WHO grade I meningioma show residual tumor located at the left internal carotid artery and at tumor at the tip of the left orbit (A, D). Surprisingly, 2 additional meningiomas were also visible on the 68Ga-DOTATATE PET/CT (E, F), without corresponding contrast enhancement on MRI (B, C).
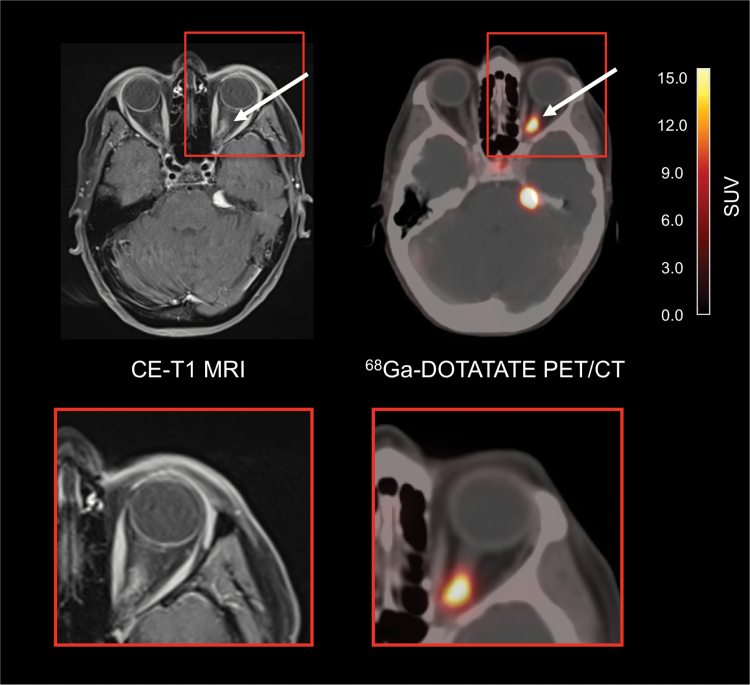
Fig. 3.
A 43-year-old female patient with a history of a meningioma of the left optical sheath treated with surgery and radiotherapy 8 years ago. At follow-up, 68Ga-DOTATATE PET/CT reveals multiple meningioma lesions, including meningioma recurrence at the optical sheath (arrows).
However, expression of SSTRs may also be observed in esthesioneuroblastomas, leukocytes accumulating in chronic inflammatory tissue, pituitary tumors, gliomas, fibrous dysplasia of the bone, Paget’s disease, and brain metastases originating from various extracranial tumors (eg, breast cancer).24,27 Such lesions, however, usually present with a lower uptake and with a distinct morphology and location that differ from meningiomas.
• PET ligands for SSTRs may add valuable diagnostic information to standard MRI in newly diagnosed brain lesions suspicious for meningiomas, especially concerning differential diagnosis and sensitivity to detect lesions (evidence level 2).
Tumor Grading
The uptake of 18F-FDG correlates significantly with the WHO grade in meningiomas,14,15 but as a major limitation its uptake is not tumor specific and may be increased in inflammatory tissue.1811C-choline may overcome this limitation and may be helpful for meningioma grading as well,41 but the present results are preliminary. Regarding PET ligands to SSTR, 68Ga-DOTATATE binding correlates with tumor growth rate in WHO grades I and II meningiomas but is abolished in anaplastic (WHO grade III) meningiomas.35 Data on the amino acid tracer 11C-MET suggest a correlation with proliferative activity in meningiomas54 but are controversial for noninvasive meningioma grading.34,55 Furthermore, its use is strictly limited to centers with an on-site cyclotron unit. Preliminary findings revealed that static and dynamic 18F-FET parameters may provide additional information for noninvasive grading of meningiomas.32 The tracer 11C-acetate seems not to be helpful for meningioma grading.18
• Up to now, only preliminary evidence for a potential benefit of PET for noninvasive meningioma grading is present (evidence level 3).
Delineation of Tumor Extent
A prerequisite for an improved delineation of tumor extent is a high tumor-to-background ratio derived from the administered PET tracer, preferably higher than the contrast that can be achieved by contrast-enhanced MRI (Fig. 4). Furthermore, regarding meningioma delineation, several different tissues are to be respected as background (eg, brain, bone, blood, fibrotic tissue, inflammatory lesions). Due to usually high levels of glucose in healthy brain parenchyma causing a poor tumor-to-background contrast, the tracer 18F-FDG is not suitable for precise tumor delineation.17 On the other hand, PET ligands to SSTR and radiolabeled amino acids generally elicit high tumor-to-background ratios. In a comparative study using neuronavigated tissue sampling with histological confirmation, in various tumor locations 68Ga-DOTATATE revealed a more precise delineation of tumor extent than contrast-enhanced MRI.23 Furthermore, in meningiomas with osseous infiltration as well as in regions such as the skull base, orbita, and cavernous sinus, PET using 68Ga-DOTATATE and 68Ga-DOTATOC was also reported to provide a better tumor delineation than MRI.25,56,57 In the latter studies, however, histological confirmation of imaging findings was not performed. Similarly, studies using 11C-MET or 2-[18F]-fluoro-L-tyrosine reported an improved tumor delineation compared with MRI, but again without histological confirmation.31,58,59 In one retrospective study with histological evaluation, 18F-fluoride improved preoperative detection of bone infiltration.46
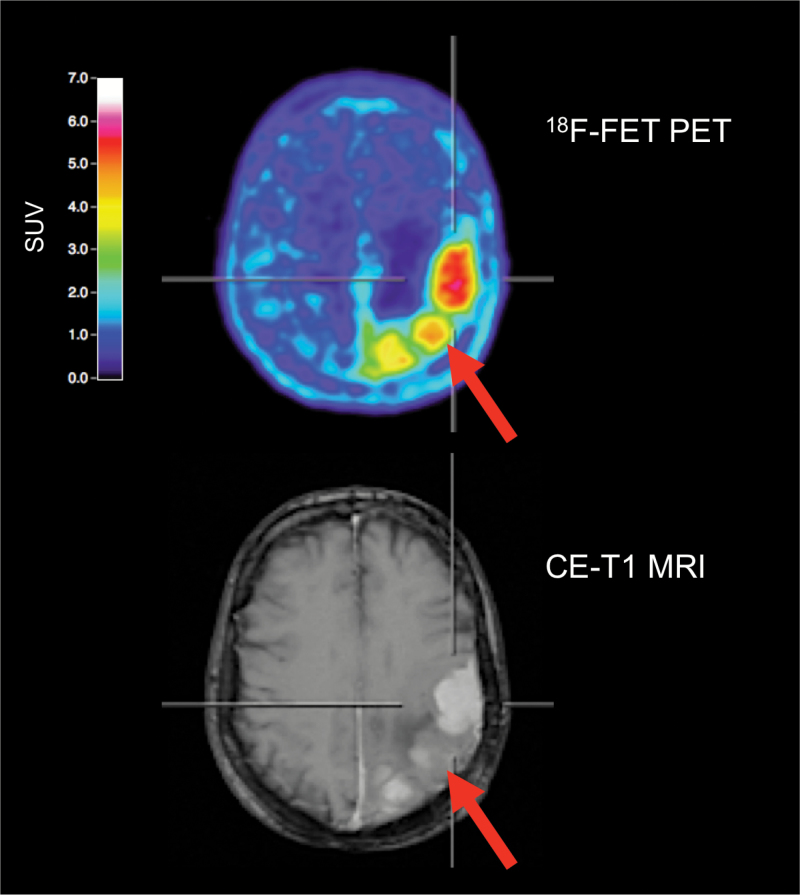
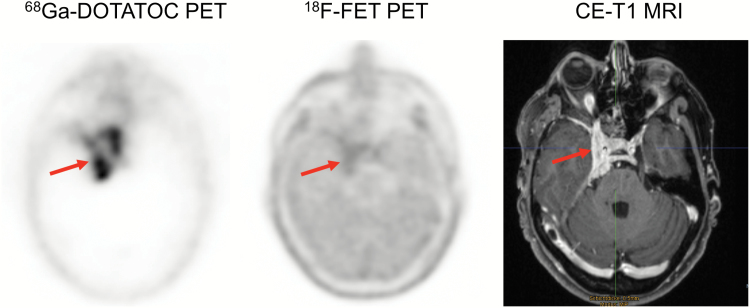
Fig. 4.
Amino acid PET with 18F-FET and contrast-enhanced MR images of a 68-year-old female meningioma patient (WHO grade I) with suspected recurrence 9 years after tumor resection at initial diagnosis. 18F-FET PET identifies 3 hypermetabolic lesions, consistent with meningioma recurrence. In contrast, MRI shows prominent contrast enhancement in only 2 of 3 lesions. In that lesion (arrow, bottom), contrast enhancement is subtle and not well defined. 18F-FET PET allows an improved delineation of this lesion (arrow, top).
• Different PET tracers might facilitate tumor delineation in meningiomas, especially in regions with low MR and CT contrast such as the skull base, orbita, parafalcine area with involvement of the sagittal sinus, cavernous sinus, and any transosseous growth; best evidence exists currently for 68Ga-DOTATATE (evidence level 2).
Value for Treatment Planning
Resection
Whenever treatment is considered in newly diagnosed meningiomas, surgical resection is the mainstay of therapy in the majority of locations. The surgical goal should be total excision of the lesion, including the involved dura.6,60 In order to achieve this goal, the exact delineation of the tumor has to be fully visualized prior to surgery, since bony involvement and extended dural infiltration might not be recognizable, even with the use of an operating microscope. This is especially the case for regions with low MR and CT contrast, such as the skull base, orbita, parafalcine area with involvement of the sagittal sinus, cavernous sinus, and any transosseous growth. Histology-controlled and imaging-guided resection studies using both 68Ga-DOTATATE PET and MRI showed that 68Ga-DOTATATE PET better delineates the extent of meningiomas than does contrast-enhanced MRI alone.23,61 Equally important, 68Ga-DOTATATE PET helps to discriminate between recurrent tumor and scar tissue after previous surgery or radiotherapy with higher sensitivity and equal specificity compared with MRI.23 This is of additional value to tailor the resection, especially in recurrent, pretreated tumors. Thus, 68Ga-DOTATATE PET provides additional valuable information regarding extent and localization of meningioma tissue, especially when this information is being integrated into neuronavigation systems.62
• 68Ga-DOTATATE PET improves the delineation of tumor extent in meningiomas with potential benefits for tumor resection (evidence level 2).
Radiation treatment planning
Target volume delineation plays a crucial role in the planning of high precision radiation therapy such as radiosurgery and stereotactic fractionated radiotherapy. In meningiomas, the GTV and CTV are delineated based on image fusion of contrast-enhanced CT and MRI. Usually, contrast-enhanced MRI visualizes the GTV very well. However, in a considerable number of cases, especially in tumors located at the skull base (meningiomas of the suprasellar region and the sphenoid wings are ~30% of cases), it is difficult to differentiate between normal dura tissue and tumor tissue, because both normal dura as well as bone show a high contrast enhancement. Moreover, in tumors infiltrating the bone it is difficult to define the infiltration depth with high precision, despite using the bone window on CT images. In these cases, PET imaging may add helpful information. Furthermore, in postoperative MRI with inconclusive findings (eg, reactive changes), PET may aid in the identification of active tumor remnants in the planning of adjuvant radiotherapy after subtotal or partial tumor resection. For radiotherapy planning, it is necessary to fuse PET with MRI/CT due to the lower spatial resolution of PET alone.
11C-MET PET can be integrated into radiation treatment planning63 and significantly influence GTV delineation in meningiomas. Astner and colleagues demonstrated that in 32 patients with benign skull base meningiomas treated with stereotactic fractionated radiotherapy, the addition of 11C-MET PET changed the GTV in all but 3 patients.31 In that study, 11C-MET PET detected tumor areas with a mean volume of 1.6 mL which were not visualized on CT or MRI, leading to an enlargement of GTV of approximately 9%. At the same time, areas without tumor infiltration could be excluded from the GTV, and critical structures like optic nerves, the chiasm, and the pituitary gland could be spared more effectively.31 Furthermore, regarding the GTV definition, the addition of 11C-MET PET to CT and MRI helps to significantly lower the interobserver variability in comparison to MRI and CT alone.59 Subsequently, other groups have confirmed these findings using other radiolabeled amino acids.58
Milker-Zabel et al demonstrated an optimized target volume delineation for stereotactic fractionated radiation therapy in grades I–III meningiomas using 68Ga-DOTATOC PET coregistered to CT and MRI.56 In all patients, 68Ga-DOTATOC PET delivered additional information concerning meningioma extent for fractionated stereotactic radiotherapy target definition. These results are supported by data reported subsequently by other groups57,64,65 (Figs. 5, 6).
Fig. 5.
A 42-year-old patient with exophthalmos and a history of a left sphenoid wing meningioma. Preoperative MRI shows tumor recurrence (top). Postoperative MRI (bottom left) shows an incomplete resection of the tumor, necessitating adjuvant radiotherapy. For radiotherapy planning, 68Ga-DOTATATE PET/CT reveals an additional tumor located at the tip of the right sphenoid wing (arrow, bottom right).
Fig. 6.
Patient with a WHO grade I meningioma (arrows). In contrast to structural MRI, particularly 68Ga-DOTATOC PET delineates meningioma extent more precisely. For radiotherapy planning, the contralateral unaffected side can be spared.
• Amino acid PET and 68Ga-DOTATOC PET add valuable diagnostic information for meningioma delineation, particularly helpful for radiotherapy planning by improving both GTV definition and dose sparing of organs at risk (evidence level 2).
Follow-up: Treatment Response, Progression
In a prospective study with 19 meningioma patients, serial 11C-MET PET scans were used to evaluate the effect of stereotactic high-energy proton beam treatment (24 Gy in 4 consecutive daily 6-Gy fractions).66 The authors observed no significant reduction of tumor size but an average tumor/brain ratio reduction of 19% in the total patient group, suggesting that 11C-MET PET may enable an earlier evaluation of treatment effects than CT or MRI. The long-term evaluation over 10 years of the same patient cohort revealed that in the majority of patients, MET uptake ratios showed a further decrease, whereas tumor size was predominantly unchanged throughout the follow-up.67 As 68Ga-DOTATOC PET is superior in both discriminating meningioma tissue from scars related to pretreatment and detecting meningiomas not (yet) seen in MRI, it is useful in cases of unclear differential diagnosis between tumor progression and posttherapeutic reactive changes.23,24,52 For the discrimination of scar tissue from vital tumor, Rachinger and colleagues demonstrated that standard MRI has a lower diagnostic performance than 68Ga-DOTATATE PET: sensitivity, 79% versus 90%; specificity, 65% versus 74%; and positive predictive value, 84% versus 89%.23 In line with this, a more recent 68Ga-DOTATATE PET study with focus on transosseous growing meningiomas showed an even better diagnostic performance for pretreated lesions than standard MRI (sensitivity, 97% versus 54%; specificity, 100% versus 83%; positive predictive value, 100% versus 95%; and negative predictive value, 86% versus 23%).61
Up to now, only preliminary evidence for a potential benefit of amino acid PET for treatment monitoring of radiotherapy is present (evidence level 3).
68Ga-DOTATOC PET can be useful in cases of unclear differential diagnosis between tumor progression and posttherapeutic reactive changes (evidence level 2).
Current Limitations
PET data in relation to the clinical management of meningioma patients have predominantly been reported for small, retrospectively assembled patient series, and data were usually obtained in monocentric studies. Recent encouraging findings in this field should therefore be validated in larger clinical prospective multicenter cohorts and trials. Moreover, further studies evaluating the correlation between PET imaging findings and histology are necessary and essential to define more accurately the impact of PET in this group of patients. Importantly, it has still to be demonstrated that a better tumor delineation allows better long-term tumor control. Another methodical concern of using PET for planning radiotherapy of meningioma is the definition of threshold values defining the radiation volumes (eg, GTV). Because meningiomas may have microscopic tumor growth and PET has a limited spatial resolution, empirical margins have to be added.
Outlook Perspective
Radiopeptide Therapy
By exchanging the radionuclide, the same tracer can be used either for diagnostics or for therapy (“theranostics”). The principle of peptide receptor radionuclide therapy (PRRT) is well established in the management of neuroendocrine tumors68 and, more recently, has been introduced into meningioma treatment. An exchange of the short-lived positron emitter gallium-68 used for PET with a longer-lived β-emitter like lutetium-177 or yttrium-90 allows for receptor-targeted therapy. Due to the wide application in neuroendocrine tumors, the safety profile of SSTR-based PRRT is known and therapy is generally well tolerated.
Eight studies and one single-case study on PRRT treatment in meningioma have been published, reporting on 90Y-DOTATOC, 177Lu-DOTATATE, and 111In-pentetreotide therapy in 124 patients.69–77 However, due to retrospective and prospective study designs, mixed patient populations, differences in administered doses, and varying response assessments as well as follow-up interval, pooling of the present data is complex. Nevertheless, the high rate of reported disease stabilization and the possibility of a patient- or lesion-tailored therapy make PRRT a promising tool; however, future studies should include an adequate sample size with clear inclusion criteria, preferably a comparator to PRRT, and rigorous response assessment to determine the role of PRRT in meningioma management. In the future perspective, PRRT may be further optimized by a change to α-emitters and local application of the substance to increase the locally administered dose.78,79
Conclusion
Compared with standard MRI, particularly PET ligands to SSTR (receptor subtype 2) add valuable additional diagnostic information. Based on the current levels of evidence, the most relevant indications for this group of tracers are differential diagnosis of newly diagnosed brain lesions suspicious for meningiomas, the delineation of meningioma extent in regions with low MR and CT contrast (eg, osseous infiltration) and complex anatomy (eg, skull base) for resection or radiotherapy planning, and the differentiation of tumor progression from a posttherapeutic reactive change such as scar tissue or radiation necrosis (Table 1). The evidence in this field justifies therefore a further validation in larger prospective multicenter clinical cohorts and trials for which standardized technical guidelines for imaging and readout procedures will now be developed.
Table 1.
Overview of the most relevant indications for PET imaging in meningioma patients
| Clinical Indication | PET Ligands for Somatostatin Receptors | Amino Acid PET Tracers | Other PET Tracers |
|---|---|---|---|
| Detection of meningioma tissue/ differential diagnosis | 68Ga-DOTATOC and 68Ga-DOTATATE PET may add valuable diagnostic information24 ,53 | na | na |
| Meningioma grading | 68Ga-DOTATATE binding correlates with tumor growth rate in WHO grades I and II meningiomas35 |
11C-MET correlates with proliferative activity,54
but data on grading are controversial.34
,55
Static and dynamic 18F-FET PET may provide additional information for meningioma grading32 |
11C-choline seems to be helpful for meningioma grading.41
11C-acetate seems not to be helpful18 |
| Delineation of tumor extent for resection planning |
68Ga-DOTATATE PET delineates the meningioma extent better than standard MRI23 ,61 | na | na |
| Delineation of tumor extent for radiation treatment planning |
68Ga-DOTATOC PET delivers additional information on tumor extent for radiotherapy target definition56 ,57 ,64 ,65 | 11C-MET PET significantly influences GTV delineation in meningiomas31 ,59 | na |
| Treatment monitoring |
na |
11C-MET PET allows an earlier evaluation of treatment effects than standard imaging.66 ,67 Boronated amino acid PET probes may help to evaluate treatment effects38 |
na |
| Diagnosis of tumor progression/differentiation of tumor progression from posttreatment changes |
68Ga-DOTATOC/68Ga-DOTATATE PET is useful for differentiation between progression and posttreatment changes23 ,24 ,52 | na | na |
na = not available.
Funding
None.
Conflict of interest statement. Related to the present work, all authors report no conflicts of interest.
References
- 1. Louis DN, Perry A, Reifenberger G et al. . The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5(12):1045–1054. [DOI] [PubMed] [Google Scholar]
- 3. Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99(3):393–405. [DOI] [PubMed] [Google Scholar]
- 4. Sahm F, Schrimpf D, Olar A et al. . TERT Promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016; 108(5):djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahm F, Schrimpf D, Stichel D et al. . DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–694. [DOI] [PubMed] [Google Scholar]
- 6. Goldbrunner R, Minniti G, Preusser M et al. . EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 7. Abedalthagafi M, Bi WL, Aizer AA et al. . Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol. 2016;18(5):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brastianos PK, Horowitz PM, Santagata S et al. . Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark VE, Erson-Omay EZ, Serin A et al. . Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reuss DE, Piro RM, Jones DT et al. . Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol. 2013;125(3):351–358. [DOI] [PubMed] [Google Scholar]
- 11. Albert NL, Weller M, Suchorska B et al. . Response Assessment in Neuro-Oncology Working Group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view—what is the status quo of positron emission tomography in patients with brain tumors?Neuro Oncol. 2015;17(11):1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel CN, Goldstone AR, Chowdhury FU, Scarsbrook AF. FDG PET/CT in oncology: “raising the bar”. Clin Radiol. 2010;65(7):522–535. [DOI] [PubMed] [Google Scholar]
- 14. Lee JW, Kang KW, Park SH et al. . 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36(10):1574–1582. [DOI] [PubMed] [Google Scholar]
- 15. Di Chiro G, Hatazawa J, Katz DA, Rizzoli HV, De Michele DJ. Glucose utilization by intracranial meningiomas as an index of tumor aggressivity and probability of recurrence: a PET study. Radiology. 1987;164(2):521–526. [DOI] [PubMed] [Google Scholar]
- 16. Cremerius U, Bares R, Weis J et al. . Fasting improves discrimination of grade 1 and atypical or malignant meningioma in FDG-PET. J Nucl Med. 1997;38(1):26–30. [PubMed] [Google Scholar]
- 17. Delbeke D, Meyerowitz C, Lapidus RL et al. . Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology. 1995;195(1):47–52. [DOI] [PubMed] [Google Scholar]
- 18. Liu RS, Chang CP, Guo WY et al. . 1-11C-acetate versus 18F-FDG PET in detection of meningioma and monitoring the effect of gamma-knife radiosurgery. J Nucl Med. 2010;51(6):883–891. [DOI] [PubMed] [Google Scholar]
- 19. Dutour A, Kumar U, Panetta R et al. . Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. 1998;76(5):620–627. [DOI] [PubMed] [Google Scholar]
- 20. Reubi JC, Maurer R, Klijn JG et al. . High incidence of somatostatin receptors in human meningiomas: biochemical characterization. J Clin Endocrinol Metab. 1986;63(2):433–438. [DOI] [PubMed] [Google Scholar]
- 21. Menke JR, Raleigh DR, Gown AM, Thomas S, Perry A, Tihan T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015;130(3):441–443. [DOI] [PubMed] [Google Scholar]
- 22. Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10(14):2259–2277. [DOI] [PubMed] [Google Scholar]
- 23. Rachinger W, Stoecklein VM, Terpolilli NA et al. . Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–353. [DOI] [PubMed] [Google Scholar]
- 24. Afshar-Oromieh A, Giesel FL, Linhart HG et al. . Detection of cranial meningiomas: comparison of 68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39(9):1409–1415. [DOI] [PubMed] [Google Scholar]
- 25. Henze M, Schuhmacher J, Hipp P et al. . PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42(7):1053–1056. [PubMed] [Google Scholar]
- 26. Soto-Montenegro ML, Peña-Zalbidea S, Mateos-Pérez JM et al. . Meningiomas: a comparative study of 68Ga-DOTATOC, 68Ga-DOTANOC and 68Ga-DOTATATE for molecular imaging in mice. PLoS One. 2014;9(11):e111624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Virgolini I, Ambrosini V, Bomanji JB et al. . Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37(10):2004–2010. [DOI] [PubMed] [Google Scholar]
- 28. Jansen NL, Graute V, Armbruster L et al. . MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET?Eur J Nucl Med Mol Imaging. 2012;39(6):1021–1029. [DOI] [PubMed] [Google Scholar]
- 29. Smits A, Baumert BG. The clinical value of PET with amino acid tracers for gliomas WHO grade II. Int J Mol Imaging. 2011;2011:372509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galldiks N, Stoffels G, Ruge MI et al. . Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med. 2013;54(12):2046–2054. [DOI] [PubMed] [Google Scholar]
- 31. Astner ST, Dobrei-Ciuchendea M, Essler M et al. . Effect of 11C-methionine-positron emission tomography on gross tumor volume delineation in stereotactic radiotherapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2008;72(4):1161–1167. [DOI] [PubMed] [Google Scholar]
- 32. Cornelius JF, Stoffels G, Filß C et al. . Uptake and tracer kinetics of O-(2-(18)F-fluoroethyl)-L-tyrosine in meningiomas: preliminary results. Eur J Nucl Med Mol Imaging. 2015;42(3):459–467. [DOI] [PubMed] [Google Scholar]
- 33. Chung JK, Kim YK, Kim SK et al. . Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29(2):176–182. [DOI] [PubMed] [Google Scholar]
- 34. Arita H, Kinoshita M, Okita Y et al. . Clinical characteristics of meningiomas assessed by 11C-methionine and 18F-fluorodeoxyglucose positron-emission tomography. J Neurooncol. 2012;107(2):379–386. [DOI] [PubMed] [Google Scholar]
- 35. Sommerauer M, Burkhardt JK, Frontzek K et al. . 68Gallium-DOTATATE PET in meningioma: a reliable predictor of tumor growth rate?Neuro Oncol. 2016;18(7):1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cornelius JF, Langen KJ, Stoffels G, Hänggi D, Sabel M, Jakob Steiger H. Positron emission tomography imaging of meningioma in clinical practice: review of literature and future directions. Neurosurgery. 2012;70(4):1033–1041; discussion 1042. [DOI] [PubMed] [Google Scholar]
- 37. Kawabata S, Hiramatsu R, Kuroiwa T, Ono K, Miyatake S. Boron neutron capture therapy for recurrent high-grade meningiomas. J Neurosurg. 2013;119(4):837–844. [DOI] [PubMed] [Google Scholar]
- 38. Miyatake S, Kawabata S, Nonoguchi N et al. . Pseudoprogression in boron neutron capture therapy for malignant gliomas and meningiomas. Neuro Oncol. 2009;11(4):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krause BJ, Souvatzoglou M, Treiber U. Imaging of prostate cancer with PET/CT and radioactively labeled choline derivates. Urol Oncol. 2013;31(4):427–435. [DOI] [PubMed] [Google Scholar]
- 40. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–235. [DOI] [PubMed] [Google Scholar]
- 41. Giovacchini G, Fallanca F, Landoni C et al. . C-11 choline versus F-18 fluorodeoxyglucose for imaging meningiomas: an initial experience. Clin Nucl Med. 2009;34(1):7–10. [DOI] [PubMed] [Google Scholar]
- 42. Oyama N, Ito H, Takahara N et al. . Diagnosis of complex renal cystic masses and solid renal lesions using PET imaging: comparison of 11C-acetate and 18F-FDG PET imaging. Clin Nucl Med. 2014;39(3):e208–e214. [DOI] [PubMed] [Google Scholar]
- 43. Huo L, Guo J, Dang Y et al. . Kinetic analysis of dynamic (11)C-acetate PET/CT imaging as a potential method for differentiation of hepatocellular carcinoma and benign liver lesions. Theranostics. 2015;5(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mohsen B, Giorgio T, Rasoul ZS et al. . Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU Int. 2013;112(8):1062–1072. [DOI] [PubMed] [Google Scholar]
- 45. Oyama N, Akino H, Kanamaru H et al. . 11C-acetate PET imaging of prostate cancer. J Nucl Med. 2002;43(2):181–186. [PubMed] [Google Scholar]
- 46. Tateishi U, Tateishi K, Hino-Shishikura A, Torii I, Inoue T, Kawahara N. Multimodal approach to detect osseous involvement in meningioma: additional value of (18)F-fluoride PET/CT for conventional imaging. Radiology. 2014;273(2):521–528. [DOI] [PubMed] [Google Scholar]
- 47. Tateishi U, Tateishi K, Shizukuishi K et al. . 18F-fluoride PET/CT allows detection of hyperostosis and osseous involvement in meningioma: initial experience. Clin Nucl Med. 2013;38(3):e125–e131. [DOI] [PubMed] [Google Scholar]
- 48. Chaves H, Bergamo Y, Paz S, Sanchez F, Vazquez S. Sphenoid wing meningioma behavior on 11C-PiB and 18F-FDG PET. Clin Nucl Med. 2015;40(1):e81–e82. [DOI] [PubMed] [Google Scholar]
- 49. Bilgin R, Ergül N, Çermik TF. Incidental meningioma mimicking metastasis of prostate adenocarcinoma in 68Ga-labeled PSMA ligand PET/CT. Clin Nucl Med. 2016;41(12):956–958. [DOI] [PubMed] [Google Scholar]
- 50. Song IU, Lee SH, Chung YA. The incidental suggestive meningioma presenting as high 18F FP-CIT uptake on PET/CT study. Clin Nucl Med. 2014;39(1):e97–e98. [DOI] [PubMed] [Google Scholar]
- 51. Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 52. Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002;33(12):1211–1226. [DOI] [PubMed] [Google Scholar]
- 53. Klingenstein A, Haug AR, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit. 2015;34(1):16–22. [DOI] [PubMed] [Google Scholar]
- 54. Iuchi T, Iwadate Y, Namba H et al. . Glucose and methionine uptake and proliferative activity in meningiomas. Neurol Res. 1999;21(7):640–644. [DOI] [PubMed] [Google Scholar]
- 55. Ikeda H, Tsuyuguchi N, Kunihiro N, Ishibashi K, Goto T, Ohata K. Analysis of progression and recurrence of meningioma using (11)C-methionine PET. Ann Nucl Med. 2013;27(8):772–780. [DOI] [PubMed] [Google Scholar]
- 56. Milker-Zabel S, Zabel-du Bois A, Henze M et al. . Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int J Radiat Oncol Biol Phys. 2006;65(1):222–227. [DOI] [PubMed] [Google Scholar]
- 57. Nyuyki F, Plotkin M, Graf R et al. . Potential impact of (68)Ga-DOTATOC PET/CT on stereotactic radiotherapy planning of meningiomas. Eur J Nucl Med Mol Imaging. 2010;37(2):310–318. [DOI] [PubMed] [Google Scholar]
- 58. Rutten I, Cabay JE, Withofs N et al. . PET/CT of skull base meningiomas using 2-18F-fluoro-L-tyrosine: initial report. J Nucl Med. 2007;48(5):720–725. [DOI] [PubMed] [Google Scholar]
- 59. Grosu AL, Weber WA, Astner ST et al. . 11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(2):339–344. [DOI] [PubMed] [Google Scholar]
- 60. Rogers L, Barani I, Chamberlain M et al. . Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kunz WG, Jungblut LM, Kazmierczak PM et al. . Improved detection of transosseous meningiomas using 68Ga-DOTATATE PET-CT compared to contrast-enhanced MRI. J Nucl Med. 2017; Apr 27 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 62. Terpolilli NA, Rachinger W, Kunz M et al. . Orbit-associated tumors: navigation and control of resection using intraoperative computed tomography. J Neurosurg. 2016;124(5):1319–1327. [DOI] [PubMed] [Google Scholar]
- 63. Grosu AL, Lachner R, Wiedenmann N et al. . Validation of a method for automatic image fusion (BrainLAB System) of CT data and 11C-methionine-PET data for stereotactic radiotherapy using a LINAC: first clinical experience. Int J Radiat Oncol Biol Phys. 2003;56(5):1450–1463. [DOI] [PubMed] [Google Scholar]
- 64. Gehler B, Paulsen F, Oksüz MO et al. . [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Graf R, Nyuyki F, Steffen IG et al. . Contribution of 68Ga-DOTATOC PET/CT to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):68–73. [DOI] [PubMed] [Google Scholar]
- 66. Gudjonsson O, Blomquist E, Lilja A, Ericson H, Bergström M, Nyberg G. Evaluation of the effect of high-energy proton irradiation treatment on meningiomas by means of 11C-L-methionine PET. Eur J Nucl Med. 2000;27(12):1793–1799. [DOI] [PubMed] [Google Scholar]
- 67. Ryttlefors M, Danfors T, Latini F, Montelius A, Blomquist E, Gudjonsson O. Long-term evaluation of the effect of hypofractionated high-energy proton treatment of benign meningiomas by means of (11)C-L-methionine positron emission tomography. Eur J Nucl Med Mol Imaging. 2016;43(8):1432–1443. [DOI] [PubMed] [Google Scholar]
- 68. Chatalic KL, Kwekkeboom DJ, de Jong M. Radiopeptides for imaging and therapy: a radiant future. J Nucl Med. 2015;56(12):1809–1812. [DOI] [PubMed] [Google Scholar]
- 69. Otte A, Herrmann R, Heppeler A et al. . Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26(11):1439–1447. [PubMed] [Google Scholar]
- 70. Bartolomei M, Bodei L, De Cicco C et al. . Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging. 2009;36(9):1407–1416. [DOI] [PubMed] [Google Scholar]
- 71. Minutoli F, Amato E, Sindoni A et al. . Peptide receptor radionuclide therapy in patients with inoperable meningiomas: our experience and review of the literature. Cancer Biother Radiopharm. 2014;29(5):193–199. [DOI] [PubMed] [Google Scholar]
- 72. Marincek N, Radojewski P, Dumont RA et al. . Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med. 2015;56(2):171–176. [DOI] [PubMed] [Google Scholar]
- 73. Gerster-Gilliéron K, Forrer F, Maecke H, Mueller-Brand J, Merlo A, Cordier D. 90Y-DOTATOC as a therapeutic option for complex recurrent or progressive meningiomas. J Nucl Med. 2015;56(11):1748–1751. [DOI] [PubMed] [Google Scholar]
- 74. Kreissl MC, Hänscheid H, Löhr M et al. . Combination of peptide receptor radionuclide therapy with fractionated external beam radiotherapy for treatment of advanced symptomatic meningioma. Radiat Oncol. 2012;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sabet A, Ahmadzadehfar H, Herrlinger U, Wilinek W, Biersack HJ, Ezziddin S. Successful radiopeptide targeting of metastatic anaplastic meningioma: case report. Radiat Oncol. 2011;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van Essen M, Krenning EP, Kooij PP et al. . Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47(10):1599–1606. [PubMed] [Google Scholar]
- 77. Seystahl K, Stoecklein V, Schüller U et al. . Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016;18(11):1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kratochwil C, Giesel FL, Bruchertseifer F et al. . ²¹³Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41(11):2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schumacher T, Hofer S, Eichhorn K et al. . Local injection of the 90Y-labelled peptidic vector DOTATOC to control gliomas of WHO grades II and III: an extended pilot study. Eur J Nucl Med Mol Imaging. 2002;29(4):486–493. [DOI] [PubMed] [Google Scholar]