Abstract
Atypical haemolytic uraemic syndrome (aHUS) may clinically present as acute renal graft failure resulting from excessive activation of the complement cascade. While mutations of complement-encoding genes predispose for aHUS, it is generally thought to require an additional insult (e.g. drugs) to trigger and manifest the full-blown clinical syndrome. Calcineurin inhibitors (CNIs) used for immunosuppression act as potential triggers, especially in the post-transplantation setting. Therefore, CNI-free immunosuppressive regimens may be beneficial. We report on a 58-year-old woman who developed aHUS with acute graft failure within 20 days after renal transplantation. Genetic investigation revealed a homozygous deletion of the CFH-related 1 (CFHR1) and CFHR3 genes in addition to the presence of autoantibodies against complement factor H (CFH). The patient was treated with plasmapheresis and administration of the complement component 5 (C5) antibody eculizumab, and her immunosuppressive regimen was switched from CNI (tacrolimus) to the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor belatacept. Renal graft function recovered and stabilized over an 18-month follow-up period. We describe the successful management of post-transplant aHUS using a CNI-free immunosuppressive regimen based on eculizumab and belatacept. Ideally, adequate molecular diagnostics, performed prior to transplantation, can identify relevant genetic risk factors for graft failure and help to select patients for individualized immunosuppressive regimens.
Keywords: atypical haemolytic uraemic syndrome, recurrence, renal transplantation
Introduction
Atypical haemolytic uraemic syndrome (aHUS) is a rare severe condition leading to end-stage renal disease (ESRD) and necessity for renal replacement therapy. In addition, aHUS is also known to occur subsequent to renal transplantation (RTX), either de novo or by way of disease recurrence. It therefore takes a well-considered therapeutic regimen to prevent allograft loss due to uncontrolled activation of the complement system leading to the aHUS hallmarks of haemolytic anaemia, thrombocytopenia and thrombotic microangiopathy (TMA). Various mutations in genes encoding the complement system [e.g. complement component 3 (C3)] and its regulators [e.g. complement factor H (CFH), complement factor I (CFI) and membrane cofactor protein (MCP)] are known to predispose for aHUS [1]. The current pathomechanistic understanding, however, suggests a threshold model, where an additional insult (e.g. drugs, infections, pregnancy, graft damage by ischaemia–reperfusion, brain death-related injury) on top of genetic susceptibility is needed for clinical manifestation [2, 3]. Calcineurin inhibitors (CNIs) are one of these potential triggers able to initiate aHUS development. As CNIs are part of the standard immunosuppression after RTX, the question arises whether a CNI-free regimen may be beneficial in selected patients with recurrent or de novo aHUS. In this context, we report the case of a patient with post-transplant aHUS based on a homozygous deletion of complement factor H related 1 (CFHR1) and complement factor H related 3 (CFHR3) and concomitant development of CFH autoantibodies, who was effectively treated with a CNI-free, long-term immunosuppressive regimen, based on eculizumab and belatacept.
Case report
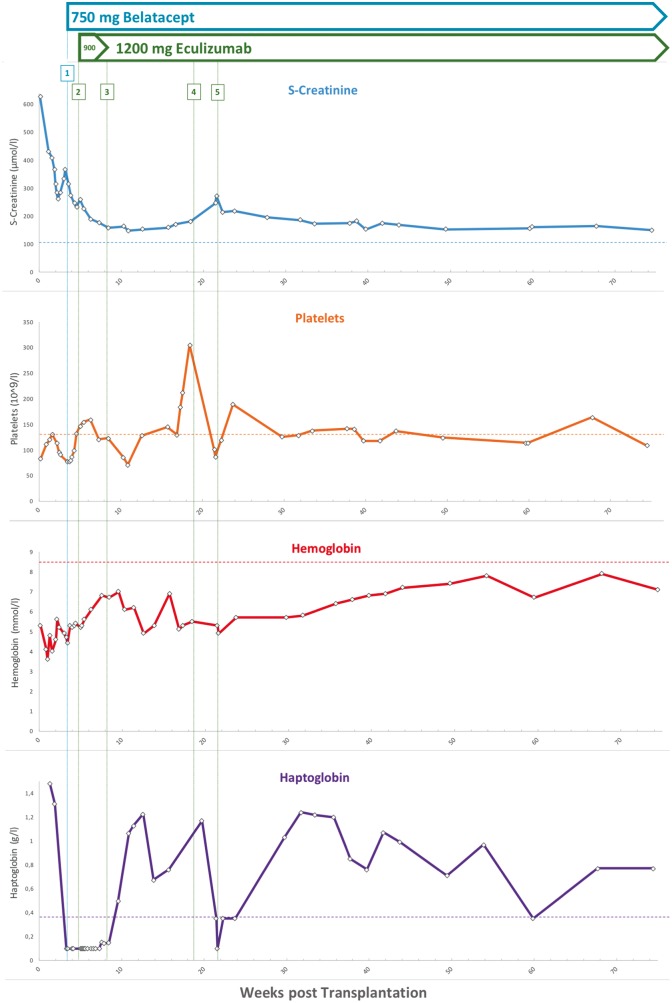
A 58-year-old woman from Greece with a history of >20 years of chronic kidney disease of unknown origin since her mid-30s received her first deceased kidney transplantation at 48 years of age. Concomitant diseases included hypothyroidism, repeated thrombotic occlusions of her dialysis shunt and a transient ischaemic attack at the age of 41 years. Family history regarding inherited kidney diseases, thromboembolic events and/or malignancy was unremarkable. The patient developed a rapid and irreversible graft failure 18 months after her first kidney transplantation. Diagnostic kidney biopsy revealed TMA in the transplanted kidney, interpreted as potentially CNI-associated as tacrolimus was part of her immunosuppressive regimen. After another 10 years on haemodialysis, a second deceased RTX was performed under continuous control of haemolytic parameters and complement factors. Despite absence of haemolysis and complement consumption over the first 2 weeks after RTX, onset of graft function was delayed. Subsequently, the patient presented with reduction of urinary volume and rising serum creatinine levels of >300 µmol/L estimated glomerular filtration rate according to chronic kidney disease epidemiology collaboration (eGFR CKD-EPI)-CKD-EPI 12 mL/min/1.73 m2] (Figure 1). On Day +21, laboratory findings finally revealed both a Coombs-negative haemolytic anaemia (haemoglobin 4.9 mmol/L, platelets 93 × 109/L, haptoglobin <0.1 g/L, fragmentocytes 1%, lactate dehydrogenase 10.5 mmol/L) and consumption of complement factors [C3 0.83 g/L, ref. range 0.9–1.7; complement component 4 (C4) 0.07 g/L, ref. range 0.18–0.49] representing clinical recurrence of aHUS. Accordingly, a subsequent kidney biopsy showed pre-glomerulary and intra-glomerulary TMA. Triggering infections, especially Shiga toxin-producing bacteria, streptococcus and CMV were not detected. Complement analysis revealed increased levels of soluble complex of complement component 5b and component 9 (C5b-9) (sC5b-9 655 ng/mL; ref. range <320 ng/mL), also known as the soluble form of the membrane attack complex (MAC), and CFH autoantibodies (IgG anti-CFH 74 U/mL; ref. range <60 U/mL; method ELISA). Specific mutation analysis, using a targeted complement gene panel (including ADAMTS13, C3, CFB, CFD, CFH, CFHR1, complement factor H related 2 (CFHR2), CFHR3, CFHR5, CFI, DGKE, MCP, MMACHC and THBD), yielded a homozygous deletion of the CFHR1 and CFHR3 genes, which was confirmed by multiplex ligation-probe amplification (Figure 2). Additional risk polymorphisms or rare variants were not detected. Based on the clinical and genetic diagnosis of post-transplantation aHUS, plasmapheresis (n = 6) was initiated. Furthermore, the patient received the complement component 5 (C5) convertase inhibitor eculizumab, starting with a loading dose of 900 mg weekly for 4 weeks, followed by 1200 mg every 14 days. To eliminate additional triggers, we switched the initial immunosuppressive regimen from tacrolimus/prednisolone/mycophenolate mofetil (MMF) to a CNI-free protocol consisting of belatacept/prednisolone/MMF and eculizumab. Eight weeks after switch of immunosuppression, haematologic remission with normalization of haptoglobin and platelets as well as normalization of C3 and C4 could be obtained (Figure 1). Simultaneously, renal graft function recovered and remained stable at a moderate level of kidney function with an eGFR (CKD-EPI) of 30–32 mL/min/1.73 m2 during an 18-month follow-up under continued belatacept and eculizumab administration. Re-biopsy of the transplanted kidney on Day +52 showed only slight residual activity of TMA. Interestingly, an attempt to discontinue eculizumab therapy after 19 weeks resulted in a new rise of serum creatinine levels and decreasing platelet counts, whereas immediate re-initiation of eculizumab administration led to a prompt haematological remission (Figure 1). Retrospective measurements of CFH autoantibody titres from samples taken 6 months after failure of the first allograft and immediately before second kidney transplantation were negative.
Fig. 1.
Course of disease: diagram showing laboratory values (serum creatinine, platelets, haemoglobin, haptoglobin) and immunosuppressive therapy over 18 months. Initiation of belatacept is indicated by vertical blue line (‘1’) and was given as follows: 750 mg belatacept weekly for 3 weeks, followed by a 4-weekly administration. (‘2’) Initiation of eculizumab is indicated by vertical dark green line and was administered as follows: 900 mg eculizumab weekly for 4 weeks, followed by 1200 mg eculizumab maintenance 2-weekly (‘3’). (‘4’) Discontinuation of eculizumab therapy after 19 weeks post-RTX resulted in rising serum creatinine and recurring haemolytic anaemia; however, re-initiation of eculizumab therapy (‘5’) led to prompt response and subsequent haematological remission. Horizontal lines denote upper (creatinine)/lower range (platelets, haemoglobin, haptoglobin) of respective reference levels.
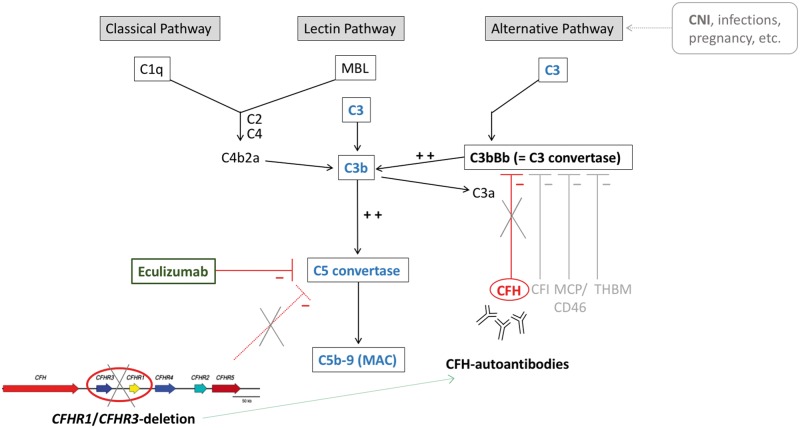
Fig. 2.
Model of excessive activation of the complement cascade by lack of regulatory inhibition: CFH deficiency and insufficient C3b and C5 blockade, due to a homozygous deletion of CFHR1/CFHR3 within the ‘regulator of complement activation locus’ (‘RCA’ on chromosome 1, comprising CFH, CFHR1-5) and development of CFH autoantibodies. Pharmaceutical intervention with eculizumab blocks uncontrolled complement activation by inhibition of the C5 convertase. MBL: mannose-binding lectin; CFI: complement factor I; CD46/MCP: membrane cofactor protein; THBM: thrombomodulin.
Discussion
Post-transplant aHUS, regardless of whether de novo or recurrent, is a severe complication of RTX, posing an imminent risk of graft loss. The underlying mechanism constitutes an excessive activation of the complement system, which leads to endothelial damage, microthrombosis and ultimate impairment of renal function. Various mutations of complement-encoding genes associated with aHUS have been described. Apart from the established aHUS-susceptibility genes CFH and MCP (CD46 molecule), the so-called ‘regulator of complement activation locus (RCA)’ on chromosome 1 also harbours five related homologues, CFHR1-5 [1]. The physiological role of CFH-related proteins is not fully understood. CFHR1, however, is believed to constitute a competitive antagonist to CFH by binding to complement component 3b (C3b) [4] and was further shown to antagonize C5 convertase activity [5]. Interestingly, while loss of CFHR1 confers protection from age-related macular degeneration [6] and IgA nephropathy [7], it associates with susceptibility to aHUS, especially via formation of CFH autoantibodies [8]. Development of CFH autoantibodies has been reported to in ∼10% of patients with aHUS, >90% of which harbour deletions of CFHR1/CFHR3 [8, 9]. Valid data concerning the recurrence of aHUS in renal grafts in individuals with CFH autoantibodies and concomitant CFHR1/CFHR3 deletion are lacking; however, the solitary presence of CFH autoantibodies is associated with increased risk of recurrence of aHUS post-transplant and frequent graft failure [10, 11]. CFH autoantibodies were found to be directed against the C-terminal binding domain of CFH that is necessary for interaction with glycosaminoglycans and C3b [4]. It was further hypothesized that microbial proteins are able to induce a CFH neoepitope through conformational change, which results in CFH autoantibody production upon deletion of CFHR1 [4]. By interference with C3b binding, the presence of CFH antibodies leads to decreased inhibition of C3b on the alternative pathway [12, 13]. Taken together, the CFHR1/CFHR3 deletion constitutes a risk allele for development of CFH antibodies, which consecutively results in excessive activation of the complement system, via deactivation of CFH, an important inhibitory regulator of the alternative pathway (Figure 2). The combination of both conditions, CFH antibodies and a homozygous CFHR1/CFHR3 deletion, represents a unique subgroup of complement-mediated aHUS, sometimes referred to as DEAP-HUS (deficiency of CFHR plasma proteins and autoantibody-positive form of haemolytic uraemic syndrome) [14]. In a German cohort of children and adults, 11% of aHUS cases presented with CFH autoantibodies [8]. However, there is no epidemiologic data regarding frequency of DEAP-HUS in a Greek population. In our patient, CFH autoantibody levels were only measured after detection of the homozygous CFHR1/CFHR3 deletion. Although eculizumab therapy and plasmaphereses were already initiated, antibody titres were still found to be elevated. In contrast, retrospective measurements from previous time points, such as 6 months after first allograft failure and immediately before second transplantation, did not show elevated CFH antibody titres, supporting the triggering role of CNIs after transplantation and point to the value of CFH antibody monitoring in the course of disease.
CNIs are routinely used as immunosuppressive agents after RTX and their role as triggers for aHUS is discussed controversially [2, 15, 16]. By inducing endothelial damage, however, CNIs are able to activate the complement cascade and may even provoke an uncontrolled activation with extensive endothelial damage through formation of the so-called MAC, leading to microthrombosis, haemolytic anaemia and organ failure [17]. Eculizumab, an inhibitor of C5 cleavage and consecutive MAC formation, is an approved therapeutic option to stop uncontrolled complement activation and can be combined with plasmapheresis [18, 19]. The timely initiation of eculizumab is crucial in order to prevent progressive damage to the graft [20]. Because of missing data, recommendations for the duration of eculizumab therapy beyond clinical and haematological remission are vague; a lifelong treatment, however, may be necessary for patients with a robust genetic susceptibility, especially after RTX. Regarding its endothelial toxicity and potency of complement activation, a CNI-free immunosuppressive regimen might be favourable for long-term management of patients at risk. However, administration of mechanistic target of rapamycin (mTOR) inhibitors can itself induce TMA after RTX and recent registry data demonstrates an increased prevalence of aHUS recurrence under mTOR-based regimens [21, 19]. Therefore, belatacept, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) Ig-fusion protein blocking T-cell co-stimulation, offers an attractive alternative as a sufficient immunosuppression for reducing the risk of graft rejection. Recently, the 7-year follow-up data on belatacept in RTX recipients confirmed the previously reported positive outcome of superior kidney function with similar safety profile when compared with a CNI-based protocol with cyclosporine A [22]. Just recently, successful long-term management of de novo post-transplant aHUS with a tailored eculizumab–belatacept combination, substituting tacrolimus, has been reported for the first time, although merely heterozygous for the CFHR1/CFHR3 gene deletion [23]. The downside of this regimen, however, is the high cost of these two intravenously administered drugs (eculizumab and belatacept), confining it to selected aHUS patients at risk. In case of acute allograft failure, aHUS should be generally considered as a differential diagnosis and has to prompt timely investigations and therapeutic consequences. As knowledge of the causative renal disorder is indispensable for risk assessment and planning of RTX, we recommend the consideration of complement analysis and targeted mutation analysis in patients with ESRD of unknown origin awaiting RTX. The presented case illustrates the consequences of delayed diagnostics, as a comprehensive genetic workup prior to second kidney transplantation would have allowed us to better estimate the individual genetic susceptibility and to consequently adapt the immunosuppressive regimen in order to prevent aHUS recurrence.
Informed consent
The described patient provided written informed consent.
Conflict of interest statement
The authors declare no conflicts of interest. However, J.H. received one-time travel funding by Alexion.
References
- 1. Vieira-Martins P, El Sissy C, Bordereau P. et al. Defining the genetics of thrombotic microangiopathies. Transfus Apher Sci 2016; 54: 212–219 [DOI] [PubMed] [Google Scholar]
- 2. Zuber J, Le Quintrec M, Morris H. et al. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev 2013; 27: 117–125 [DOI] [PubMed] [Google Scholar]
- 3. Naesens M, Li L, Ying L. et al. Expression of complement components differs between kidney allografts from living and deceased donors. J Am Soc Nephrol 2009; 20: 1839–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattacharjee A, Reuter S, Trojnár E. et al. The major autoantibody epitope on factor H in atypical hemolytic uremic syndrome is structurally different from its homologous site in factor H-related protein 1, supporting a novel model for induction of autoimmunity in this disease. J Biol Chem 2015; 290: 9500–9510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heinen S, Hartmann A, Lauer N. et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 2009; 114:2439–2447 [DOI] [PubMed] [Google Scholar]
- 6. Hughes AE, Orr N, Esfandiary H. et al. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet 2006; 38: 1173–1177 [DOI] [PubMed] [Google Scholar]
- 7. Xie J, Kiryluk K, Li Y. et al. Fine mapping implicates a deletion of CFHR1 and CFHR3 in protection from IgA nephropathy in Han Chinese. J Am Soc Nephrol 2016; 27: 3187–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Józsi M, Licht C, Strobel S. et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111: 1512–1514 [DOI] [PubMed] [Google Scholar]
- 9. Dragon-Durey MA, Blanc C, Marliot F. et al. The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical haemolytic uraemic syndrome. J Med Genet 2009; 46: 447–450 [DOI] [PubMed] [Google Scholar]
- 10. Noris M, Remuzzi G.. Managing and preventing atypical hemolytic uremic syndrome recurrence after kidney transplantation. Curr Opin Nephrol Hypertens 2013; 22: 704–712 [DOI] [PubMed] [Google Scholar]
- 11. Alasfar S, Alachkar N.. Atypical hemolytic uremic syndrome post-kidney transplantation: two case reports and review of the literature. Front Med 2014; 1: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore I, Strain L, Pappworth I. et al. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 2010; 115: 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hofer J, Janecke AR, Zimmerhackl LB. et al. Complement factor H-related protein 1 deficiency and factor H antibodies in pediatric patients with atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2013; 8: 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skerka C, Zipfel PF, Müller D. et al. The autoimmune disease DEAP-hemolytic uremic syndrome. Semin Thromb Hemost 2010; 36: 625–632 [DOI] [PubMed] [Google Scholar]
- 15. Zuber J, Le Quintrec M, Sberro-Soussan R. et al. New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol 2011; 7: 23–35 [DOI] [PubMed] [Google Scholar]
- 16. Le Quintrec M, Lionet A, Kamar N. et al. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant 2008; 8: 1694–1701 [DOI] [PubMed] [Google Scholar]
- 17. Schwimmer J, Nadasdy TA, Spitalnik PF. et al. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis 2003; 41: 471–479 [DOI] [PubMed] [Google Scholar]
- 18. Alachkar N, Bagnasco SM, Montgomery RA.. Eculizumab for the treatment of two recurrences of atypical hemolytic uremic syndrome in a kidney allograft. Transplant Int 2012; 25: e93–e95 [DOI] [PubMed] [Google Scholar]
- 19. Le Quintrec M, Zuber J, Moulin B. et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant 2013; 13: 663–675 [DOI] [PubMed] [Google Scholar]
- 20. Walle JV, Delmas Y, Ardissino G. et al. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J Nephrol 2016; 30: 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sartelet H, Toupance O, Lorenzato M. et al. Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant 2005; 5: 2441–2447 [DOI] [PubMed] [Google Scholar]
- 22. Vincenti F, Rostaing L, Grinyo J. et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 2016; 374: 333–343 [DOI] [PubMed] [Google Scholar]
- 23. Dedhia P, Govil A, Mogilishetty G. et al. Eculizumab and belatacept for de novo atypical hemolytic uremic syndrome associated with CFHR3-CFHR1 deletion in a kidney transplant recipient: a case report. Transplant Proc 2017; 49: 188–192 [DOI] [PubMed] [Google Scholar]