Abstract
Background
Kidney transplant recipients often receive large volumes of intravenous fluid replacement in the peri-operative period. Administration of 0.9% saline has previously been associated with acidosis, hyperkalaemia and acute kidney injury. The perioperative use of physiologically balanced replacement fluids may reduce the incidence of post-operative renal replacement therapy and hyperkalaemia.
Methods
A retrospective review of consecutive renal transplants before and after a change in perioperative fluid prescription from 0.9% saline to Plasma-Lyte 148.
Results
A total of 97 patients were included in the study, 59 receiving exclusively 0.9% saline and 38 receiving exclusively Plasma-Lyte. Patients in the Plasma-Lyte group were less likely to require emergency postoperative dialysis than those receiving 0.9% saline [odds ratio (OR) 0.15 (95% confidence interval 0.03–0.48), P = 0.004], and these patients had more favourable biochemical parameters with less hyperkalaemia, less acidosis and better diuresis. Patients in the Plasma-Lyte group also had a shorter length of hospital stay (7 days versus 11 days; P < 0.0001) and better graft function at 3 months postoperatively (estimated glomerular filtration rate 51 versus 44 mL/min/1.73 m2; P = 0.03); however, there was no difference in graft function at 1 year.
Conclusions
Plasma-Lyte in the perioperative period is safe in renal transplantation and is associated with a favourable biochemical profile, including a reduced incidence of hyperkalaemia, better diuresis and less frequent use of renal replacement therapy early after surgery. In patients receiving Plasma-Lyte, graft function was better at 3 months, but this difference did not persist up to 1 year after transplantation.
Keywords: acute kidney injury, kidney transplantation, Plasma-Lyte, renal replacement therapy, 0.9%, saline
Introduction
Kidney transplantation is the treatment of choice for patients with end-stage renal disease. A scarcity of organs heightens the need to focus on providing the best possible outcome at every stage of the transplantation process [1].
While there are many determinants of long-term graft outcome after renal transplantation, patient variables in the peri-operative period, such as cold ischaemia time, mean arterial pressure, early urine output and fluid balance, are of importance and they have been shown to have significant influence on long-term as well as short-term graft function [2–6].
Some degree of ischaemic allograft injury is inevitable during renal transplantation; however, severe injury leading to delayed graft function may require early renal replacement therapy (RRT). Delayed graft function has an independent effect on short- and long-term allograft survival, regardless of donor quality [3]. Furthermore, there is growing evidence from the non-transplant acute kidney injury (AKI) literature suggesting that haemodynamic instability associated with intermittent haemodialysis (commonly administered to patients with delayed graft function in kidney transplant centres), as opposed to continuous RRT, may prolong AKI and worsen long-term renal outcomes [4, 5].
As well as maintaining appropriate fluid volume, the choice of fluid may also influence renal outcomes. A 0.9% saline solution remains the most widely used intravenous fluid during the perioperative period, but recent data has emerged questioning its safety [6–8]. A 0.9% saline solution has been shown to reduce renal cortical blood flow in healthy volunteers [9], and animal studies have suggested that sustained renal vasoconstriction is specifically related to hyperchloraemia [10]. The high chloride load in 0.9% saline has also been shown to induce hyperchloraemic acidosis [11], and this in turn has been associated with the development and accentuation of postoperative AKI [12].
Plasma-Lyte is a balanced crystalloid solution that closely resembles the composition of human plasma (Table 1). There is a paucity of data assessing the impact of Plasma-Lyte on renal function in patients who are undergoing renal transplantation, thus the European Renal Best Practice (ERBP) transplantation guidelines [13] and the Kidney Disease: Improving Global Outcomes (KDIGO) transplantation guidelines [14] recommend using 0.9% saline during transplantation.
Table 1.
Composition of intravenous fluids [1, 3]
| Strong ion (mmol/L) | 0.9% saline | Plasma-Lyte |
|---|---|---|
| Sodium (Na+) | 154 | 140 |
| Chloride (Cl−) | 154 | 98 |
| Potassium (K+) | 0 | 5 |
| Calcium (Ca2+) | 0 | 0 |
| Magnesium (Mg2+) | 0 | 3 |
| Acetate | 0 | 27 |
| Gluconate | 0 | 23 |
| Lactate | 0 | 0 |
A change in the intra-operative fluid regimen from 0.9% saline to Plasma-Lyte was implemented in our hospital in response to increasing evidence and national guidance regarding general fluid management of patients undergoing major surgery [15]. This change was also implemented for renal transplant recipients in our centre and extended to include the immediate postoperative period.
We hypothesized that the use of Plasma-Lyte in the perioperative period of renal transplantation would result in a reduction in the need for early RRT and fewer episodes of hyperkalaemia in the early postoperative period. As secondary endpoints, we examined plasma biochemical parameters and urine output, biopsy-proven rejection by 3 months and improved early graft function at 3 months and 1 year after surgery.
Materials and methods
Ethics
Approval for this study was as a retrospective review of data in the form of audit of clinical practice.
Study design
An observational retrospective study was performed in a single large renal transplant centre. A hospital-wide change in intra-operative fluid regimen from 0.9% saline to Plasma-Lyte was implemented in January 2013, which was extended to cover the routine postoperative period in renal transplant patients. We included consecutive renal transplants in the 6 months prior to the policy change, then following a 6-month transition period, consecutive renal transplants for further 6 months. Patients were excluded if their inpatient notes were unavailable or if the fluid administered did not comply with the policy at the time (including the use of sodium bicarbonate solution). This study is thus a per-protocol analysis.
Electrolytes, the need for RRT and the volume and composition of intravenous fluid administered were documented from paper and electronic inpatient medical records. Renal function at 3 months and at 1 year post-surgery was recorded.
Patients in both cohorts were managed postoperatively on either an acute renal ward or a renal high-dependency unit. There were no changes in management policy between the care given to the two cohorts of patients other than the switch from 0.9% saline to Plasma-Lyte. In both cohorts, the rate of fluid administration, decisions regarding RRT, inpatient biopsy and discharge from hospital were recorded by the responsible consultant from a highly experienced pool of clinicians who did not change throughout the study. Any patient who had a pre-operative potassium >6.0 mmol underwent dialysis prior to transplantation. All patients had blood tests preoperatively, postoperatively on recovery and at 7 a.m. each day thereafter, unless additional tests were clinically indicated. All patients underwent protocol transplant biopsy at 3 months unless this was deemed unsuitable or unnecessary based on individual patient characteristics.
Statistical methods
Statistical analysis was done using R (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
We sought to assess the effect of the choice of fluid therapy in the perioperative period on the probability of needing RRT within 48 h of renal transplantation. To exclude the effect of potential confounders we performed a multivariable logistic regression including the following clinically plausible variables: preoperative potassium, modality of RRT prior to transplantation (none, i.e. pre-emptive transplantation; haemodialysis or peritoneal dialysis), donor type (live donor, donation after brain death (DBD) or donation after cardiac death (DCD)), cold ischaemia time >12 h (live donors were imputed a cold ischaemia time <12h), age and sex. Forward and backward selection based on minimization of Akaike’s Information Criterion (AIC) was used to develop the final model.
Time free from RRT, for delayed graft function in the first 5 days after surgery, was plotted as a Kaplan–Meier estimator and compared using the log-rank test for days after transplantation. Patients who needed RRT after early graft loss for surgical reasons necessitating graft nephrectomy were censored from this analysis. Comparisons in biochemical characteristics, such as potassium and chloride, were calculated with a two-way analysis of variance.
Results
Patient characteristics
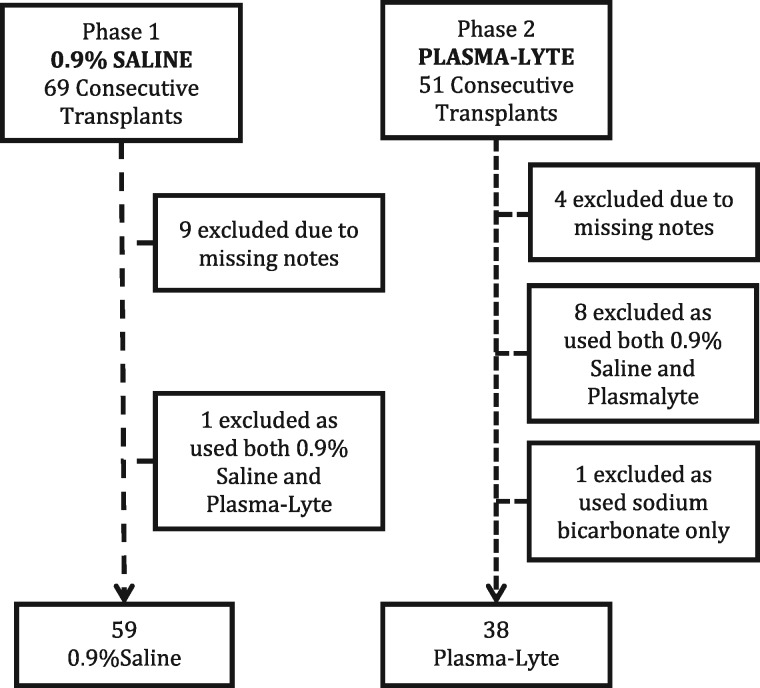
A total of 97 patients were included in the study (Figure 1); 59 received exclusively 0.9% saline and 38 received exclusively Plasma-Lyte. Baseline demographics and details of transplantation are shown in Table 2. There were no significant differences between the two groups: 6/59 (10%) patients in the 0.9% saline group and 3/38 (7.8%) in the Plasma-Lyte group had RRT in the preceding 24 h of transplantation due to either hyperkalaemia or scheduled outpatient dialysis. All patients who received RRT in the pre- or postoperative period underwent intermittent haemodialysis.
Fig. 1.
Study recruitment chart.
Table 2.
Patient demographics and selected donor kidney characteristics
| 0.9% saline | Plasma- Lyte | P-value | |
|---|---|---|---|
| Number | 59 | 38 | |
| Male, n (%) | 45 (76) | 24 (66) | 0.17 |
| Age (years) | 49 (20–70) | 46 (18–73) | 0.43 |
| Ethnicity, n (%) | |||
| White | 29 (49) | 21 (55) | 0.67 |
| Black | 13 (22) | 6 (16) | 0.60 |
| South Asian | 14 (24) | 10 (26) | 0.64 |
| Other | 3 (5) | 1 (3) | 0.36 |
| Time on RRTa (years) | 4 (1–25) | 3 (1–31) | 0.63 |
| RRT mode prior to transplant, n (%) | |||
| Pre-emptive | 6 (10) | 7 (18) | 0.36 |
| Haemodialysis | 44 (75) | 25 (66) | 0.37 |
| Peritoneal dialysis | 9 (15) | 6 (16) | 1 |
| Donor age (years) | 52 (19–70) | 55 (19–71) | 0.41 |
| Donor type, n (%) | |||
| Live related | 15 (25) | 7 (18) | 0.47 |
| Live unrelated | 6 (10) | 3 (8) | 0.4 |
| Live combined, n (%) | 21 (35) | 10 (26) | 0.38 |
| Donation after brain death, n (%) | 21 (36) | 22 (58) | 0.24 |
| Donation after circulatory death, n (%) | 17 (29) | 6 (16) | 0.22 |
| ABO incompatible, n (%) | 6 (10) | 3 (8) | 1 |
| Cold ischaemia timeb (h) | 14 (8–17) | 14 (12–19) | 1 |
Results are presented as median and range unless stated otherwise.
Includes previous renal transplant.
For donors after brain death and donors after cardiac death only.
Primary objective: early postoperative RRT
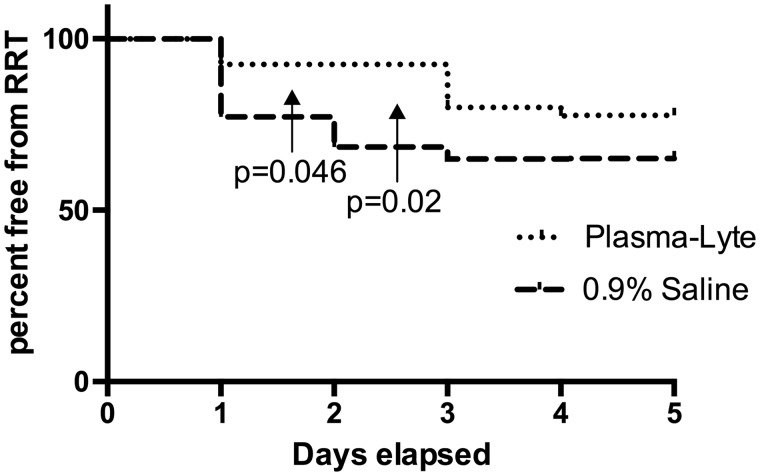
We excluded two patients from this analysis who suffered immediate surgical complications requiring graft nephrectomy (one renal vein and one renal artery thrombosis; both patients were in the 0.9% saline group). At least one episode of RRT in the first 5 days postoperatively was required in 20/57 (35%) patients in the 0.9% saline group compared with 9/38 (24%) patients in the Plasma-Lyte group. The absolute risk reduction was 0.11 [95% confidence interval (CI) −0.07 to −0.28; P = 0.36], which although not statistically significant, does suggest a trend in favour of the Plasma-Lyte group. All patients who required RRT had hyperkalemia: three patients in the 0.9% saline group and one patient in the Plasma-Lyte group, who also had respiratory compromising pulmonary oedema. However, there was significantly more RRT within the first and second 24 h postoperatively in the 0.9% saline group (Figure 2).
Fig. 2.
Kaplan–Meier plot showing the chance of remaining free from renal replacement therapy in the first 5 days post-transplantation, censored for operative complications causing immediate graft failure.
A simplistic comparison between those that did and did not receive RRT within the first 48 h suggests RRT is associated with patients who received 0.9% saline, were older and had a donor following circulatory death (Table 3). A logistic regression analysis was then performed to explore whether these between-group differences in donor and recipient characteristics were confounding our assessment of the effect of perioperative fluid type on the need for RRT in the first 48 h following renal transplant (Table 4).
Table 3.
A comparison of the binary variables used in logistic regression for patients who received RRT in the first 48 h compared with those who did not
| Variable | Needed RRT in first 48 h | Did not need RRT in first 48 h | P-value |
|---|---|---|---|
| Number of patients | 21 | 74 | |
| Plasma-Lyte versus 0.9% saline | 3/38 versus 18/57 | 0.002 | |
| Cold ischaemia time (h), median (range) | 13 (0–18) | 10 (0–21) | 0.09 |
| Recipient age (years), median (range) | 56 (20–64) | 46 (18–70) | 0.005 |
| Pre-transplant mode of RRT, n (%) | |||
| Haemodialysis | 16 (76) | 51 (68) | 0.59 |
| Peritoneal dialysis | 4 (19) | 11 (16) | 0.73 |
| Pre-emptive | 1 (5) | 13 (17) | 0.29 |
| Donor type, n (%) | |||
| Live related | 2 (10) | 20 (26) | 0.14 |
| Live unrelated | 2 (10) | 7 (9) | 1 |
| Donation after brain death | 8 (38) | 34 (45) | 0.6 |
| Donation after circulatory death | 9 (42) | 14 (19) | 0.04 |
Table 4.
Multivariable logistic regression analysis of the need for renal replacement therapy in the first 48 h after transplantation
| Variable | OR | 2.5% | 97.5% | P-value |
|---|---|---|---|---|
| Plasma-Lyte versus 0.9% saline | 0.14 | 0.03 | 0.48 | 0.004 |
| Cold ischaemia time (h) | 3.12 | 1.06 | 9.78 | 0.04 |
| Recipient age (years) | 1.05 | 1.00 | 1.11 | 0.053 |
| Preoperative potassium | 1.41 | 0.84 | 2.44 | 0.2 |
After accounting for these important clinical variables, the use of Plasma-Lyte was strongly associated with a decreased need for dialysis in the first 48 h after surgery, with an OR of 0.15 (95% CI 0.03–0.48; P = 0.004). The only other variable significantly associated with the risk of early RRT once accounting for the type of fluid administered was cold ischaemia time.
Secondary objectives
Plasma biochemistry
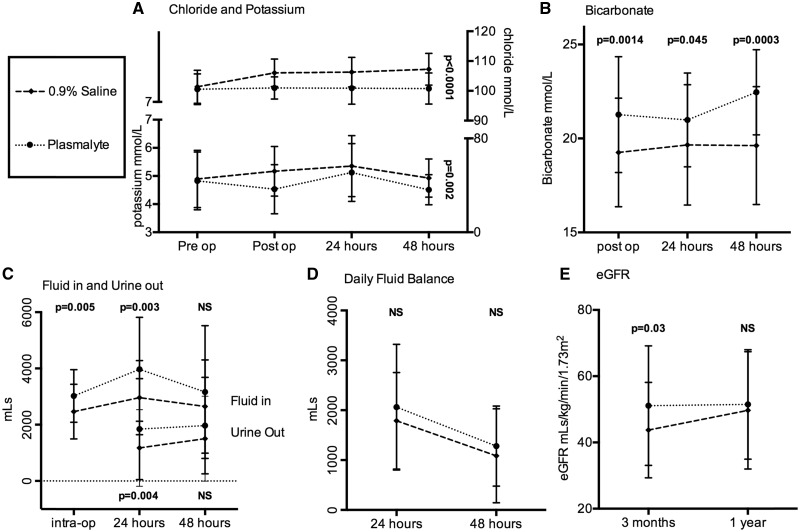
Patients in the Plasma-Lyte group had lower serum potassium and chloride levels postoperatively (despite identical pre-operative values) and had higher levels of serum bicarbonate (our routine chemistry panel omits bicarbonate, hence the lack of preoperative values), as shown in Figure 3A and 3B. We subsequently assessed the incidence of moderate to severe hyperkalaemia (serum potassium >6.0 mmol/L). All patients were included in the analysis up to the point they received RRT.
Fig. 3.
Changes in potassium, chloride, bicarbonate, associated fluid volume administration and urine output with fluid balance censored for the need for dialysis. (A) Kruskal–Wallis analysis of variance for 0.9% saline versus Plasma-Lyte over time; serum chloride P < 0.0001 and serum potassium P = 0.002. (B) Significant differences between 0.9% saline and Plasma-Lyte for serum bicarbonate levels; P = 0.0014, P = 0.045 and P = 0.0003, respectively, at each time point after transplantation. (C) More fluid was administered in the Plasma-Lyte group both during surgery (P = 0.005) and in the first 24 h (P = 0.003) and this was in line with significantly more urine output (P = 0.04) in the first 24 h, but neither parameter was statistically different at 48 h after transplantation. (D) The daily fluid balance was no different between the two groups on each day. (E) The eGFR was significantly higher at 3 months in the Plasma-Lyte group compared with the 0.9% saline group (P = 0.03), but by 1 year there was no difference.
Perioperative use of Plasma-Lyte was significantly associated with a reduced incidence of postoperative hyperkalaemia in the immediate postoperative period (10/57 versus 1/38 in the 0.9% saline and Plasma-Lyte groups, respectively; P = 0.045).
Fluid balance
Patients in the Plasma-Lyte group had a higher urine output than those in the 0.9% saline group (Figure 3C). The improved diuresis was associated with an increase in fluid volume administration, but there was no difference in daily fluid balance (Figure 3D).
Long-term outcomes
In the first year after transplantation there were three deaths in the 0.9% saline group (two from sepsis, one complication following kidney biopsy), compared with one death in the Plasma-Lyte group (sepsis). Length of hospital stay was significantly shorter in the Plasma-Lyte group, at a median of 7 days (range 5–25), compared with 11 days in the 0.9% saline group (range 5–55; P < 0.0001; Mann–Whitney U test). After removing three outliers from the 0.9% saline group with lengths of stay of 55, 35 and 33 days, the length of stay remained significantly longer in the 0.9% saline group [median length of stay 11 days (range 5–30), P = 0.0007]. Of those discharged from the hospital with functioning grafts, there were two graft failures at 3 months post-transplant and another by 12 months in the 0.9% saline group, compared with one graft failure by 3 months and another by 12 months in the Plasma-Lyte group.
There were fewer biopsies carried out in the Plasma-Lyte group during the inpatient period following transplantation (13.1% versus 30.1%; P = 0.055) and they demonstrated less rejection (2.63% versus 18.6%; P = 0.02). A similar proportion in the Plasma-Lyte and 0.9% saline groups had a protocol biopsy carried out at 3 months (59.3% versus 57.8%) and the rates of rejection were 13.1% versus 28.8% (P = 0.08), respectively.
Three months following transplantation there was a significant difference in estimated glomerular filtration rate (eGFR) between the two groups (44 versus 51 mL/min in the 0.9% saline and Plasma-Lyte groups, respectively; P = 0.03). However, there was no difference in eGFR at 1 year (Figure 3E).
Discussion
The use of Plasma-Lyte during the perioperative period of renal transplantation is safe and is associated with a reduction in the need for dialysis and a reduced incidence of hyperkalaemia in the immediate postoperative period when compared with 0.9% saline. The Plasma-Lyte group demonstrates more physiological biochemistry and greater postoperative diuresis; hence we observed a higher fluid volume administration compared with the 0.9% saline group while the total fluid balance of each group remained essentially the same. Although there is no difference in renal function at 1-year post-transplant, the Plasma-Lyte group reached a significantly better eGFR at 3 months, with biopsy results suggesting there may be less early rejection compared with the 0.9% saline group.
This is the first study in renal transplant recipients comparing Plasma-Lyte to 0.9% saline use throughout the perioperative period of renal transplantation and to include information regarding outcomes at 3 months and 1 year. The significant reduction in the need for dialysis in the first 48 h postoperatively is related to the use of Plasma-Lyte, as is the reduction in incidence of hyperkalaemia. There is an ongoing trend for less dialysis after 48 h, and this is important as haemodynamic changes and risks associated with anticoagulation decrease as the time from surgery increases.
A 2016 Cochrane summary of studies that compared 0.9% saline with balanced crystalloid solutions during renal transplant surgery supports our findings [16]. O’Malley et al. [17] gave either lactated Ringer’s solution or 0.9% saline during anaesthesia to patients undergoing renal transplantation followed by dextrose and bicarbonate postoperatively. This study was stopped early, as there was a significant reduction in hyperkalaemia immediately post-transplantation in the Ringer’s lactate group. A subsequent study compared the use of Ringer’s lactate, Plasma-Lyte and 0.9% saline during surgery [18]. After 90 min there was a statistically significant increase in chloride with a decrease in pH and base excess in the 0.9% saline group. These changes did not persist in the postoperative period, when fluid therapy was the same in all patients (dextrose saline).
A key feature of our study compared with others is the extension of Plasma-Lyte use into the postoperative period. The likely beneficial effects seen in the Plasma-Lyte group (better diuresis, improved potassium and acid base homeostasis, reduced incidence of early RRT) may be most pronounced as volumes of fluid administration are at their highest (frequently 8–10 L in 24 h in our study). The evidence that high-volume chloride administration effects renal blood flow [13], which in turn has been suggested as the cause for an increased prevalence of AKI and the need for RRT in critically ill patients [22, 23], may be an important factor in our study. However, the association between the development and accentuation of AKI and 0.9% saline administration in critically ill patients remains controversial, with data both supporting and rejecting the hypothesis [19, 20]. A recent study failed to demonstrate a benefit of Plasma-Lyte on the incidence of AKI, but the mean fluid volume administered was only 2 L in 24 h, considerably less than in our study [10].
There is an association between sodium intake and inflammation in both human and animal models [21–28]. There is also an increasing understanding that inflammation may precipitate or enhance rejection, and this provides a biologically plausible explanation for a reduced incidence of rejection and improved eGFR at 3 months in the Plasma-Lyte group [29]. However, this may be a chance finding and needs considerably more investigation, though delayed graft function and the need for postoperative dialysis is a risk factor for rejection [30].
The strengths of this study are the inclusion of consecutive transplants looked after by the same group of specialty consultants with all decisions regarding RRT made by the senior nephrologist. The rate of missing data is low and deviation from the fluid administration protocol was also low. However, the study was not randomized and is limited to one transplant centre. Our sample size is not large enough to address our hypothesis with certainty. We recognize the difference in sample size between both groups; patients in the Plasma-Lyte group were excluded because they did not exclusively receive Plasma-Lyte intra-operatively, despite leaving a washout period between both periods studied. We also excluded patients who did not have complete notes from both groups, which may have led to some selection bias. Despite the two groups being well matched, it is possible that other factors in patient management between the two periods studied may have accounted for some of the differences in clinical outcomes. While we accept the 0.9% saline group had a greater number of donors after cardiac death compared with the Plasma-Lyte group, there were more donors after brain death and fewer live donors in the Plasma-Lyte group. Multivariable logistic regression analysis does not show donor type affecting the primary outcome. Very close to the time of transition, a renal high dependency unit was opened where most of the Plasma-Lyte group were cared for. The improved nursing skill mix may have had an influence on outcomes in the Plasma-Lyte group compared with the 0.9% saline group, who were managed in a specialized area of the acute renal ward. All patients were looked after by the same clinicians during both periods studied. Recipients in both groups had potassium levels measured immediately postoperatively on recovery, when arriving in the ward and at 7 a.m. each day thereafter. Fluid balance and administration was accurately recorded in all patients included in the study.
In summary, the use of Plasma-Lyte in the perioperative period of kidney transplantation reduces the need for RRT in the immediate postoperative period and improves plasma biochemistry. There is a suggestion that there is better early graft function and a reduction in the number of rejection episodes. The administration of large volumes of 0.9% saline represents a physiologically abnormal load of sodium and chloride, so it is biologically plausible that balanced solutions may lessen this metabolic insult. Switching to Plasma-Lyte offers a relatively cheap and easy intervention that contributes to improvements in the perioperative care of renal transplant patients.
Authors’ contributions
J.P., M.B. and C.K. were involved in the design of the study. A.A. and D.R. collected and summarized the data. Statistical analysis was performed by C.K. and J.P. The initial draft of the manuscript was written by A.A. and D.R. with equal contribution. It was subsequently revised by C.K. and then agreed upon by all authors.
Funding
As a retrospective study of a change in institutional policy, no funding was received for this study.
Conflict of interest statement
None declared.
References
- 1. Tonelli M, Wiebe N, Knoll G. et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011; 11: 2093–2109 [DOI] [PubMed] [Google Scholar]
- 2. Warlé MC, Cheung CL, Teerenstra S. et al. Cold ischaemia time and outcome of renal transplantation. Ned Tijdschr Geneeskd 2010; 154: B539. [PubMed] [Google Scholar]
- 3. Gingell-Littlejohn M, Koh H, Aitken E. et al. Below-target postoperative arterial blood pressure but not central venous pressure is associated with delayed graft function. Transplant Proc 2013; 45: 46–50 [DOI] [PubMed] [Google Scholar]
- 4. Schnuelle P, Gottmann U, Köppel H. et al. Comparison of early renal function parameters for the prediction of 5-year graft survival after kidney transplantation. Nephrol Dial Transplant 2007; 22: 235–245 [DOI] [PubMed] [Google Scholar]
- 5. Lai Q, Pretagostini R, Poli L. et al. Early urine output predicts graft survival after kidney transplantation. Transplant Proc 2010; 42: 1090–1092 [DOI] [PubMed] [Google Scholar]
- 6. Yarlagadda SG, Coca SG, Formica RN. et al. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant 2009; 24: 1039–1047 [DOI] [PubMed] [Google Scholar]
- 7. Gill J, Dong J, Rose C. et al. The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function. Kidney Int 2016; 89: 1331–1336 [DOI] [PubMed] [Google Scholar]
- 8. Bellomo R, Cass A, Cole L. et al. Intensity of continuous renal replacement therapy in critically ill patients. N Engl J Med 2009; 361: 1627–1638 [DOI] [PubMed] [Google Scholar]
- 9. Wald R, Shariff SZ, Adhikari NK. et al. The association between renal replacement modality and long term outcomes amongst critically ill adults with acute kidney injury: a retrospective cohort study. Crit Care Med 2014; 42: 868–877. [DOI] [PubMed] [Google Scholar]
- 10. Young PJ, Joannidis M.. Crystalloid fluid therapy: is the balance tipping towards balanced solutions? Intensive Care Med 2014; 40: 1966–1968 [DOI] [PubMed] [Google Scholar]
- 11. Raghunathan K, Shaw A, Nathanson B. et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill patients with sepsis. Crit Care Med 2014; 42: 1585–1591 [DOI] [PubMed] [Google Scholar]
- 12. Story DA, Lees L, Weinberg L. et al. Cognitive changes after saline or plasmalyte infusion in healthy volunteers. Anesthesiology 2013; 119: 569–575 [DOI] [PubMed] [Google Scholar]
- 13. Chowdhury AH, Cox EF, Francis ST. et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012; 256: 18–24 [DOI] [PubMed] [Google Scholar]
- 14. Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest 1983; 71: 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prough D S, Bidani A.. Hyperchloremic metabolic acidosis is a predictable consequence of intraoperative infusion of 0.9% saline. Anesthesiology 1999; 90: 1247–1249 [DOI] [PubMed] [Google Scholar]
- 16. Toyonaga Y, Kikura M.. Hyperchloremic acidosis is associated with acute kidney injury after abdominal surgery. Nephrology (Carlton) 2016. doi: 10.1111/nep.12840 [DOI] [PubMed] [Google Scholar]
- 17. European Renal Best Practice (ERBP) Transplantation Guideline Development Group. Kidney transplantation guideline. Nephrol Dial Transplant 2013; 28(Suppl 2): ii1–ii71 [DOI] [PubMed] [Google Scholar]
- 18. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 19. Powell-Tuck J, Gosling P, Lobo DN. et al. British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP). London: NHS National Library of Health, 2009 [Google Scholar]
- 20. Wan S, Roberts MA, Mount P.. Normal saline versus lower-chloride solutions for kidney transplantation. Cochrane Database Syst Rev 2016; 9: 1–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Malley CM, Frumento RJ, Hardy MA. et al. A randomized, double-blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesth Analg 2005; 100: 1518–1524 [DOI] [PubMed] [Google Scholar]
- 22. Hadimioglu N, Saadawy I, Saglam T. et al. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg 2008; 107: 264–269 [DOI] [PubMed] [Google Scholar]
- 23. Yunos NM, Bellomo R, Hegarty C. et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012; 308: 1566–1572 [DOI] [PubMed] [Google Scholar]
- 24. Young P, Bailey M, Beasley R. et al. Effect of a buffered crytalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the split randomized clinical trial. JAMA 2015; 314: 1701–1710 [DOI] [PubMed] [Google Scholar]
- 25. Farez MF, Fiol MP, Gaitan MI. et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015; 86: 26–31 [DOI] [PubMed] [Google Scholar]
- 26. Wu C, Yosef N, Thalhamer T. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013; 496: 513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kleinewietfeld M, Manzel A, Titze J. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013; 496: 518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yi B, Titze J, Rykova M. et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Translat Res 2014; 166: 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mori NM, Kreisal D, Fullerton JN. et al. Inflammatory triggers of acute rejection of organ allografts. Immunol Rev 2014; 258: 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu WK, Famure O, Li Y. et al. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int 2015; 88: 851–858. [DOI] [PubMed] [Google Scholar]