Kawamata and Manfredi review proposed mechanisms of how the accumulation of misfolded proteins in neurodegenerative diseases causes mitochondrial dysfunction.
Abstract
Mitochondria participate in essential processes in the nervous system such as energy and intermediate metabolism, calcium homeostasis, and apoptosis. Major neurodegenerative diseases are characterized pathologically by accumulation of misfolded proteins as a result of gene mutations or abnormal protein homeostasis. Misfolded proteins associate with mitochondria, forming oligomeric and fibrillary aggregates. As mitochondrial dysfunction, particularly of the oxidative phosphorylation system (OXPHOS), occurs in neurodegeneration, it is postulated that such defects are caused by the accumulation of misfolded proteins. However, this hypothesis and the pathological role of proteinopathies in mitochondria remain elusive. In this study, we critically review the proposed mechanisms whereby exemplary misfolded proteins associate with mitochondria and their consequences on OXPHOS.
Mitochondrial OXPHOS and neurodegeneration
In the nervous system, mitochondria provide a large proportion of ATP for high energetic expenditure required for neuronal function. The mitochondrial oxidative phosphorylation system (OXPHOS) utilizes substrates derived from glucose, fatty acids, and amino acids to produce reducing equivalents (i.e., NADH) that are delivered to the respiratory chain in the mitochondrial inner membrane (IM). The OXPHOS is comprised of enzymes needed for the oxidation of NADH and for the phosphorylation of ADP. Components of OXPHOS are the respiratory chain and the ATP synthase complex (ATPase and complex V). The respiratory chain consists of four protein complexes (complex I–IV), three of which (I, III, and IV) couple electron transfer to proton pumping across the mitochondrial IM to generate a transmembrane electrochemical potential (Brand and Nicholls, 2011).
Mitochondrial membrane potential is the fundamental energy source for essential mitochondrial processes including ATP synthesis by the ATPase complex as well as calcium uptake from the cytosol into the matrix through the mitochondrial calcium uniporter (MCU) system (De Stefani et al., 2015). Mitochondria are also central to intermediary metabolism, both in the biosynthesis and catabolism of most classes of molecules, from nucleotides to amino acids to lipids. Alterations of intermediary metabolism from impaired respiratory chain function impeding the flow of NADH from the Krebs cycle can contribute to neuronal dysfunction. An example of a key pathway affected by mitochondrial dysfunction is glutamate metabolism, which is essential in neurons and glial cells for catabolic and signaling purposes (McKenna et al., 2016). For these reasons, it can easily be surmised that defective mitochondrial bioenergetics can result in impaired neuronal activity and synaptic transmission.
Mitochondrial biogenesis requires more than 1,500 proteins (Calvo et al., 2016). Although mitochondrial DNA (mtDNA) only encodes for 13 components of OXPHOS, nuclear DNA encodes for a wide array of proteins encompassing structural components, transporters, metabolic enzymes, proteases, kinases, and all the members of the mtDNA replication and transcription systems. Therefore, mitochondria depend on the integration of a few critical mtDNA-encoded proteins synthesized within the matrix along with many nuclear DNA–encoded proteins, which are synthesized by cytosolic ribosomes and imported into mitochondria through specialized import systems (Wiedemann and Pfanner, 2017).
Mitochondria are often involved in the pathogenesis of neurodegeneration, either as primary disease targets or secondary to pathogenic events taking place elsewhere in the cell (DiMauro and Schon, 2008). Numerous examples of mitochondrial alterations have been illustrated in studies of cellular and animal models of neurodegeneration as well as of human biopsies or postmortem tissues from affected individuals. Disease models have been focused mainly on genetic forms of neurodegenerative diseases and have characterized alterations in mitochondrial functions, specifically OXPHOS defects. The value of such models, however, is often questioned because of the limited adherence to the human condition. However, human studies of mitochondrial dysfunction in neurodegeneration have been difficult to pursue, because they have been limited to easily accessible samples such as blood cells and fibroblasts, which are typically unaffected, or to postmortem neural tissue, which is suboptimal for investigating mitochondrial functions. These limitations need to be recognized when reviewing the evidence for and against the involvement of mitochondrial dysfunction in the pathogenesis of neurodegenerative diseases.
Neurodegenerative proteinopathies and mitochondrial dysfunction
Among the multitude of neurodegenerative proteinopathies that have been shown to be associated with mitochondrial dysfunction, the most common and extensively studied are Alzheimer’s disease (AD), Parkinson’s disease (PD), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease. These diseases have very different genetic makeups. In AD, only a small minority of cases is linked to autosomal dominant mutations in amyloid precursor protein (APP) or presenilin-1/2 (PS). In PD, ALS, and FTD, numerous genetic forms with recessive or dominant Mendelian inheritance exist, but sporadic cases are also the majority. Despite genetic differences, one feature that these neurodegenerative diseases have in common is the accumulation of misfolded proteins that can interact with themselves or with other proteins to form aberrant aggregates and inclusions.
Protein misfolding and aggregation are prominent events in the initiation of the pathogenic cascades that occur in neurons and other affected cell types in the degenerating nervous system, and mitochondria are heavily entangled in this process. However, the mechanisms leading to mitochondrial dysfunction are not always well understood, as misfolded proteins can exert a multiplicity of noxious effects on mitochondria. In some cases, they can act from within the boundaries of the mitochondrial membranes. In other cases, they can affect mitochondria from their surface or even exogenously, by interfering with mitochondrial maintenance processes such as mitochondrial dynamics (i.e., mitochondrial fission, fusion, and transport), the interaction with other organelles, or the regulation of mitochondrial biogenesis and turnover (e.g., mitophagy).
Mitochondria possess systems to keep misfolded proteins and damage to mitochondria in check. There are three known pathways: protein degradation, vesicular degradation, and mitophagy. The first involves proteostatic selective elimination of damaged proteins by internal proteases, such as the AAA–protease complex of the IM (Gerdes et al., 2012) and the Lon protease of the matrix (Matsushima and Kaguni, 2012). Mitochondria are also endowed with their own unfolded protein response mechanisms, which are activated when misfolded protein accumulates in the matrix (Pellegrino et al., 2013) or in the intermembrane space (IMS; Papa and Germain, 2011). Mitochondria rely on the cytosolic ubiquitin–proteasome system to eliminate damaged proteins destined to the outer membrane (OM) or, in the case of the IMS, before they engage in the mitochondrial import pathway (Radke et al., 2008; Karbowski and Youle, 2011). Ub ligases such as Parkin ubiquitinate oxidized or misfolded OM proteins (Heo and Rutter, 2011). Parkin recruitment has been ascribed to the kinase PINK1 after its incomplete processing and import across the OM of depolarized mitochondria (Narendra et al., 2008, 2010). OM protein ubiquitination can recruit p97 (Kim et al., 2013), whose segregase activity extracts ubiquitinated proteins from the OM and targets them for proteasomal degradation (Ye et al., 2005). Ubiquitination of OM proteins can also be performed by other ligases such as MUL1 (Li et al., 2008) and MARCH5 (Yonashiro et al., 2006; Karbowski et al., 2007).
Notably, there are pediatric and adult neurodegenerative conditions caused by mutations in mitochondrial genes in the nucleus and in the mtDNA, resulting in primary mitochondrial dysfunction (DiMauro and Schon, 2008). However, in this article, we critically review and discuss the evidence of how misfolded and aggregated proteins accumulating in mitochondria cause bioenergetic defects that lead to neuronal dysfunction and death during disease. In some cases, these proteins have been proposed to normally localize in mitochondria and in other cell compartments. In others, their proposed association with mitochondria has been shown to be pathological. We analyze the literature that addresses the following questions: do proteinopathies associated with neurodegenerative diseases cause OXPHOS dysfunction, and if so, how? We zoom in on OXPHOS defects because they are measurable hallmarks of mitochondrial functional demise. This review is not meant to provide an extensive compendium of countless studies on mitochondrial alterations in neurodegeneration; rather, we wish to convey the intriguing and often controversial nature of the evidence. Although we limit our review to a subset of the most common neurodegenerative disorders and link them to how their related misfolded proteins intersect with mitochondria, we think that events leading to mitochondrial dysfunction in these diseases also occur in other pathologies and apply to different types of proteins.
Mitochondrial Aβ and OXPHOS in AD
Amyloid β (Aβ) derives from APP through processing by β and γ secretases, which produce a 36–44-amino-acid peptide, with Aβ1–42 being the most toxic species. Although oligomerization of Aβ-forming extracellular amyloid plaques is largely regarded as a key event in AD pathogenesis, overwhelming evidence suggests that APP and Aβ play a detrimental role in mitochondria.
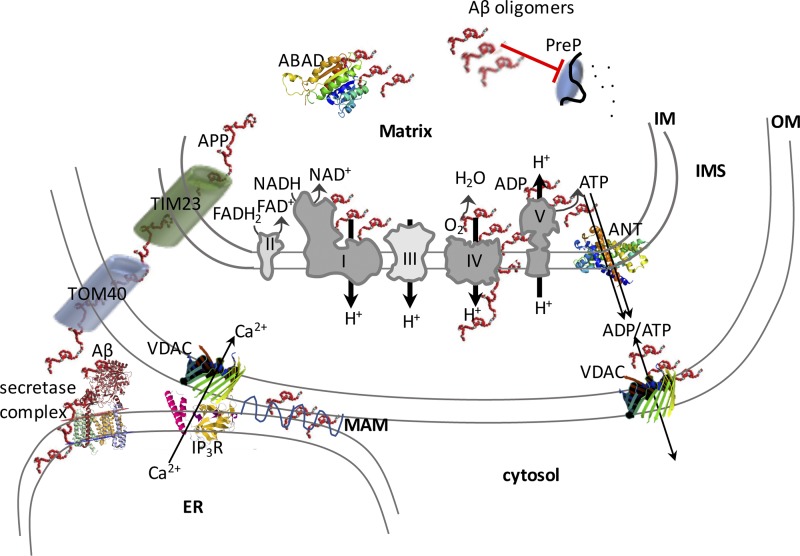
But how does Aβ enter mitochondria? Both APP and Aβ have been shown to localize within mitochondria. Cytosolic APP was shown to engage into the mitochondrial import machinery because of positively charged residues in its N terminus that drive the protein through the translocator complex, whereas the acidic domain in the middle of the protein arrests its import, forcing it to remain in a conformation where the C terminus faces the cytosol (Anandatheerthavarada et al., 2003). In the mitochondrial IMS, APP is cleaved by the HtrA2 serine protease, also known as Omi, generating a C161 fragment (Fig. 1), which is then released into the cytosol (Park et al., 2006). This truncated fragment of APP generated within mitochondria is clearly distinct from the Aβ peptide produced outside mitochondria by the secretase complex. It was shown that both full-length and C-terminal–truncated APP accumulates in the mitochondrial protein import channel of human AD brain, forming protein complexes with the translocase of the outer mitochondrial membrane (TOM) channel (Fig. 1) and with the translocase of the inner mitochondrial membrane channel, supporting the view that APP fragments can span the whole OM, IMS, and IM length. Accumulation of APP in the mitochondrial membrane translocases prevents the normal import of nuclear-encoded OXPHOS proteins, including complex IV components (Devi et al., 2006). Aβ peptide is also imported into mitochondria through TOM and, after import, localizes to the IM, where it accumulates in the proximity of mitochondrial cristae, which are the most OXPHOS-active compartments (Hansson Petersen et al., 2008). However, one study did not identify Aβ associated with the IM, but rather it found that Aβ peptides, particularly Aβ1–42, interact weakly with the surface of the OM (Cenini et al., 2016). The authors proposed that Aβ1–42 impairs the import of mitochondrial precursor proteins by causing extra-mitochondrial protein aggregation, without directly affecting the mitochondrial import machinery. It was also reported that mutations in pitrilysin metallopeptidase 1 (PITRM1), a mitochondrial matrix enzyme that digests oligopeptides, cause a slowly progressive form of neurodegeneration with Aβ deposits, suggesting that failure to degrade Aβ in mitochondria may ultimately lead to amyloid deposition (Brunetti et al., 2016). Together, these observations indicate that Aβ peptides, APP, and APP C-terminal–truncated fragments can participate in impairing mitochondrial protein import, resulting in defects of OXPHOS, and specifically of complex IV activity, in AD mitochondria.
Figure 1.
Schematic illustration of the proposed interactions of Aβ with mitochondrial components and their effects on OXPHOS function. Amyloid precursor protein (APP) and its secretase complex processing product, Aβ, can enter mitochondria through the mitochondrial import pore complexes, represented in this figure in a simplified form by TOM40 and TIM23. Proposed interactors of Aβ and Aβ oligomers are depicted on the electron transport chain (Complexes I–V) and on the outer mitochondrial surface. PreP and ABAD are Aβ oligomer targets that have been described in the matrix.
Where does mitochondrial Aβ come from? Recent research has demonstrated that mitochondria do not possess the enzymatic apparatus to produce Aβ peptides from APP (Mamada et al., 2017). Instead, Aβ is abundantly produced at mitochondria–ER contact sites (Schreiner et al., 2015) comprised of outer mitochondrial membranes and mitochondria-associated ER membranes (MAMs). Aβ is found in MAMs in cellular and animal models of AD, and Aβ producing β and γ secretase activities are found in MAMs (Fig. 1; Del Prete et al., 2017). In all cells, including neurons, mitochondria and ER establish extensive and dynamic interactions, which are involved in various cellular functions, from lipid biosynthesis to mitochondrial fission to intracellular calcium homeostasis. Although the normal function of APP and of Aβ production at the mitochondria–ER contact points is completely unknown, it is likely to play a role in the accumulation and aggregation of mitochondrial Aβ in AD by providing abundant local production of the peptide. Importantly, Aβ can also alter the normal physiology of mitochondria–ER contacts, for example by disrupting the regulation of calcium signaling between the two organelles (Fonseca et al., 2015), which may lead to excessive ER calcium release and mitochondrial calcium overload. The interaction between ER and mitochondria is altered in AD, as fibroblasts from AD patients and PS1 knockout (KO) mouse models of AD have significantly increased ER–mitochondrial contacts and MAM function, as shown by elevated cholesteryl ester and phospholipid synthesis (Area-Gomez et al., 2012). These observations suggest that increased mitochondria–ER contacts enhance Aβ production in proximity to the mitochondrial import system, thereby increasing the amount of Aβ available for mitochondrial import. However, increasing ER–mitochondria contacts using a genetic approach to knock down Mitofusin 2 was shown to impair γ secretase complex activity and lower intracellular Aβ production (Leal et al., 2016). Therefore, the link between the extent of ER–mitochondrial contacts, Aβ delivery to mitochondria, and mitochondrial damage is not straightforward, and it cannot be excluded that specific molecular players including Mitofusin 2 could be involved in regulating Aβ production.
Aβ causes OXPHOS dysfunction, characterized by enzymatic defects in the cytochrome oxidase complex (complex IV; Fig. 1). Complex IV activity defects have been consistently observed in brain mitochondria from transgenic mouse models expressing mutant human APP (Manczak et al., 2006; Rönnbäck et al., 2016) and in human AD brain mitochondria (Devi et al., 2006). The reasons for the severe involvement of complex IV have not been not fully elucidated, but several different mechanisms have been proposed. First, biochemical experiments conducted on the isolated enzyme treated with Aβ1–42 suggested that a direct inhibition of complex IV is dependent on dimeric Aβ binding to and reducing the active Cu2+ in the enzyme via the methionine 35 residue of the peptide (Crouch et al., 2005, 2006). In addition, heme-a, an essential prosthetic group needed for electron transfer for complex IV, has been shown to be deficient in AD because of excessive Aβ aberrantly binding to regulatory heme (Atamna and Boyle, 2006). Furthermore, a direct physical interaction between Aβ1–42 and the mtDNA-encoded subunit 1 of complex IV was shown to be responsible for the enzymatic defect (Hernandez-Zimbron et al., 2012). Defective complex IV has been found to associate with increased reactive oxygen species (ROS) production in the mitochondria of transgenic AD mice (Manczak et al., 2006; Rönnbäck et al., 2016). Interestingly, mitochondrial ROS was shown to modulate β secretase activity and potentially increase Aβ production (Mao et al., 2012). This is an intriguing hypothesis because it could result in a feed-forward mechanism that potentiates Aβ-induced mitochondrial damage as well as amyloid deposition and plaque formation. However, it is difficult to establish whether the complex IV deficiency and ROS increase are functionally linked, as complex IV is not particularly susceptible to inactivation by ROS, and its direct role as a significant source of ROS in mitochondria is controversial (Srinivasan and Avadhani, 2012). Furthermore, genetic inactivation of complex IV in neurons of a transgenic mouse model of AD resulted in decreased deposition of amyloid plaques but not in increased ROS production (Fukui et al., 2007). Likewise, although the induction of mtDNA damage in neurons of an AD transgenic mouse led to a partial OXPHOS defect, it did not affect Aβ accumulation and instead decreased plaque burden (Pinto et al., 2013), suggesting that amyloid pathology may not be a direct consequence of mitochondrial OXPHOS impairment. Nevertheless, mitochondrial ROS are likely to play roles as disease modifiers in AD. A recombinant catalase localized ectopically in the mitochondrial matrix of AD mice resulted in a delay of disease onset and progression (Mao et al., 2012). It is possible that mitochondrially localized catalase, an enzyme normally not present in mitochondria, acts as a sink for hydrogen peroxidase, thereby limiting ROS-mediated activation of abnormal APP processing and enhancing Aβ-degrading enzymes.
The mitochondrial ATPase, the molecular engine using the proton motive force across the IM to condense ADP and Pi into ATP, is also defective in AD (Fig. 1). A physical interaction between one of the ATPase protein subunits, the oligomycin sensitivity conferring protein (OSCP), and Aβ was identified in human AD brains and transgenic AD mice. This interaction was accompanied by a decrease of OSCP and loss of ATPase function, leading to reduced ATP production, increased ROS production, and increased probability of mitochondria to undergo a calcium-induced IM permeabilization event defined as a mitochondrial permeability transition (MPT; Beck et al., 2016). The latter observation is particularly intriguing, because dimeric ATPase has been proposed as one of the critical molecular components of MPT, and OSCP was proposed to be the subunit of ATPase to which the MPT activator cyclophilin D binds (Giorgio et al., 2013). Therefore, it is tempting to speculate that the interaction of Aβ with the ATPase may alter the normal regulation of MPT, exposing mitochondria to calcium-induced toxicity. The hypothesis that the c ring of the ATPase complex represents the physical pore (Alavian et al., 2014) has recently been questioned by work showing that the complete loss of the c ring does not hinder MPT (He et al., 2017). Nevertheless, that Aβ could play a role in MPT is supported by in vitro experiments showing that neuronal death caused by Aβ can be attenuated by preventing mitochondrial calcium overload (Sanz-Blasco et al., 2008). Interestingly, the genetic ablation of cyclophilin D ameliorates the pathology, memory, synaptic function, and long-term potentiation in AD mice, further suggesting that calcium-induced MPT is linked to the synaptic dysfunction in AD (Du et al., 2008). In addition to direct binding of Aβ, another mechanism of ATPase inactivation may derive from the observation that the α subunit (ATP5A) of the ATPase is glycosylated with O-linked β-N-acetylglucosamine (O-GlcNAc), and that O-GlcNAcylated ATP5A is decreased in the brains of AD patients, transgenic mice, and Aβ-treated cells (Cha et al., 2015). The alteration in ATPase posttranslational modifications in AD could justify a decline in activity without a decrease in protein levels.
In addition to complex IV and ATPase, other molecular targets of Aβ have been suggested to alter mitochondrial function. A remarkable effect of mitochondrial Aβ is its inhibitory action on preprotein degrading enzymes in the matrix (Fig. 1). Upon import, mitochondrial targeting peptides are cleaved by processing peptidases and then degraded by PreP, a peptidase that is also thought to degrade Aβ. Mitochondrial accumulation of Aβ decreases PreP activity, which triggers peptidase inactivation and the buildup of unprocessed proteins. This in turn exerts a feedback inhibition of the normal targeting peptide processing, mitochondrial protein degradation, and an imbalance in the mitochondrial proteome (Mossmann et al., 2014). Interestingly, an analysis of the mitochondrial proteome in synaptic and nonsynaptic mitochondria of AD mice detected significant changes in the content of OXPHOS proteins, suggesting that altered proteome homeostasis could play a role in impairing OXPHOS function (Völgyi et al., 2017). It is noteworthy that synaptic mitochondria, which are subjected to the high-energy requirements associated with synaptic activity, appear to be particularly affected by OXPHOS impairment in the AD mouse brain (Wang et al., 2016a).
Another mitochondrial protein linked to Aβ through direct binding is amyloid-binding alcohol dehydrogenase (ABAD), also known as 17β-hydroxysteroid dehydrogenase type 10 (17β-HSD10), a NAD+-dependent dehydrogenase with multiple substrates, including steroids and fatty acids (Fig. 1). Although ABAD is not part of the OXPHOS, altered ABAD function caused by conformational modifications induced by Aβ–ABAD interaction was shown to result in mitochondrial dysfunction (Lustbader et al., 2004). In support to the role of ABAD in mediating Aβ mitochondrial toxicity, it was shown that inhibition of ABAD–Aβ interaction by a decoy peptide partially prevented Aβ-induced mitochondrial dysfunction (Yao et al., 2011). Most relevant to OXPHOS, transgenic mice expressing both mutant APP and ABAD have increased ROS production and decreased complex IV activity (Takuma et al., 2005). As mentioned above, complex IV activity is not directly linked to ROS production, but it is possible that a partial decrease in enzymatic activity could slow electron transfer to a degree sufficient to favor superoxide production from electrons escaping the respiratory chain upstream of complex IV. Although the mechanisms whereby the ABAD–Aβ interaction affects OXPHOS function remains to be fully elucidated, ROS-mediated inhibition of PreP peptidase has been proposed as a potential cause because decreased PreP activity was detected in AD brains compared with controls (Alikhani et al., 2011), which could be responsible for enhanced Aβ accumulation and imbalance of OXPHOS components.
In summary, there is ample evidence that APP and its product Aβ are abundantly localized in mitochondria, where they establish extensive interactions with crucial components of the mitochondrial protein import and processing machinery as well as components of the OXPHOS apparatus with detrimental consequences on mitochondrial function. Despite this evidence, in our opinion, two important questions remain unanswered. The first regards the existence and the nature of the physiological role of Aβ in mitochondria. One interesting possibility is that monomeric Aβ could be an antioxidant, as suggested by an earlier study (Kontush et al., 2001), which could act in mitochondria to prevent lipid peroxidation. However, this hypothesis contrasts with the large body of evidence on the pro-oxidant role of Aβ in mitochondria. The second one regards the importance of Aβ-induced mitochondrial dysfunction in the context of AD pathogenesis relative to other intervening pathological processes that affect different cellular compartments. AD models where Aβ could be specifically removed from or targeted to mitochondria and not elsewhere in the cell could help start answering these questions.
Mitochondrial α-synuclein and OXPHOS in PD
α-Synuclein (α-syn) is a 140-amino-acid protein normally involved in the regulation of synaptic vesicle release and membrane fusion because of the ability of α-syn to bind lipids. In PD, protein inclusions (Lewy bodies) containing α-syn are typical pathological features of the disease. Moreover, α-syn gene triplications and mutations cause rare forms of familial PD. Together, pathological and genetic evidence clearly indicate that α-syn is involved in PD pathogenesis. However, transgenic animal models in which α-syn is overexpressed often present neurological disease phenotypes that are not related to PD. The involvement of mitochondria in the pathological processes leading to PD is probably the most deeply investigated among the major neurodegenerative disease associated with aging. A strong rationale supporting the hypothesis that mitochondria participate in PD pathogenesis arise from the fact that dopamine metabolism depends on mitochondrial enzymes and from the observations of PD-like syndromes associated with mitochondrial toxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, which impair OXPHOS function and increase ROS production. However, whether direct effects of α-syn on mitochondrial function play a role in PD pathogenesis is still debated.
There is broad consensus that a portion of α-syn is localized in mitochondria. Different groups independently described the physical interaction of α-syn with mitochondria. Using a yeast two-hybrid screen, both WT and disease-associated mutant α-syn were found to interact with complex IV subunits (Elkon et al., 2002), which suggests that α-syn is localized either in the IM or in very close proximity to it in the IMS or the matrix (Fig. 2). Association of α-syn with the mitochondrial membrane was also suggested by early work that used immunoelectron microscopy (Li et al., 2007). Furthermore, it was proposed that the N-terminal portion of α-syn contains a cryptic mitochondria target signal because deletion of the first 32 amino acids of the proteins abrogated its mitochondrial localization (Devi et al., 2008). The same work also showed that α-syn physically interacts with complex I of the respiratory chain, which is an intriguing finding as complex I deficiency has been convincingly linked to PD (Greenamyre et al., 2001; Nakamura, 2013). However, whether complex I deficiency by itself causes dopaminergic neuron loss is unclear. The conditional ablation of the gene encoding for the complex I subunit NDUFS4 in dopaminergic neurons results in complex I deficiency and a decline in striatal dopamine levels but not in dopaminergic neuron death, at least based on this mouse model (Kim et al., 2015). Moreover, in cells with preexisting mtDNA-encoded complex I deficiency, aggregated α-syn did not worsen the complex I defect (Reeve et al., 2013), further suggesting that a complex I defect emerges as a consequence of α-syn but not vice versa.
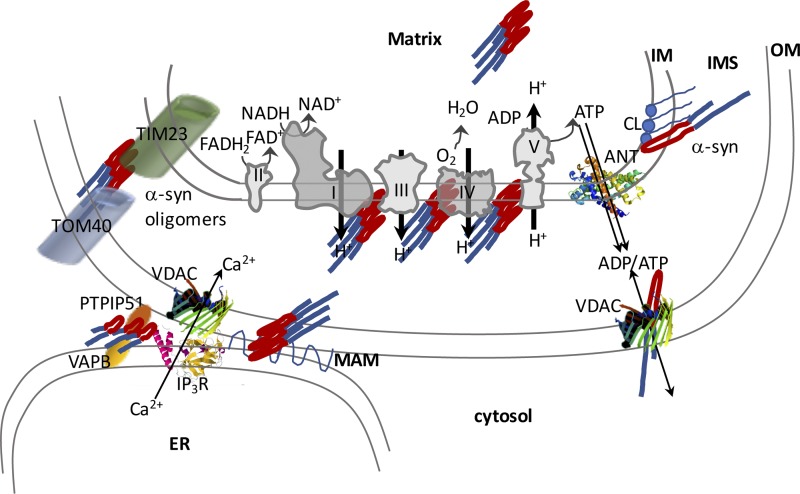
Figure 2.
Schematic illustration of the proposed interactions of α-syn with mitochondrial components and their effects on OXPHOS function. α-Synuclein monomers and oligomers can affect mitochondrial import complexes and various components of the electron transport chain complexes. It is proposed that α-syn affects IM integrity through its interaction with cardiolipin (CL). α-Syn also affects mitochondrial and ER contact regions by interfering with the VAPB-PTPIP51 tethering complex. Mutant α-syn can disrupt MAMs. PTPIP51, protein tyrosine phosphate–interacting protein 51; VAPB, VAMP-associated protein B, an ER resident protein.
The N terminus of α-syn was also proposed to participate in the binding of the protein to the mitochondrial IM because of its positive charges on lysine residues that interact with the phospholipid cardiolipin (Shen et al., 2014), which is a crucial mitochondrial phospholipid of the IM. Biophysical methods have demonstrated the role of cardiolipin in the interactions of α-syn synthetic membranes and isolated mitochondrial membranes (Robotta et al., 2014). One of the pathogenic α-syn variants (A30P) presents altered affinity for the IM (Robotta et al., 2014), suggesting that mutant α-syn could play a pathogenic role in mitochondria by failing to establish physiological interactions with membranes. Translocation from the cytosol to mitochondria and binding of α-syn to cardiolipin is facilitated by low pH (Cole et al., 2008), indicating that this interaction may change based on intracellular environmental conditions. Using FRET-based reporters, it was shown that cytosolic α-syn has a predominantly closed conformation, which is most abundant in neuronal synaptic boutons, whereas a membrane-bound open conformation is more abundant in the cell bodies, where mitochondria are more concentrated (Nakamura et al., 2008).
This evidence suggests that mitochondrial localization of α-syn and its interaction with the IM through cardiolipin may have a physiological role (Fig. 2). Furthermore, the interaction, either direct or through a lipid moiety, with the respiratory chain may regulate OXPHOS. If this hypothesis were correct, loss of α-syn would be expected to have negative consequences on mitochondrial functions. To address this hypothesis, mitochondrial respiration was investigated in α-syn KO neurons, which did not display apparent OXPHOS defects. Furthermore, all three synuclein isoforms (α, β, and γ) were ablated in neurons, without detrimental effects on synaptic mitochondria ATP production (Pathak et al., 2017). In addition, it was shown that α-syn KO mice are more resistant to mitochondrial toxins that affect OXPHOS and increase ROS production, such as MPTP and the complex II inhibitors malonate and 3-nitropropionic acid (Klivenyi et al., 2006). Collectively, these results do not support the hypothesis that α-syn has a significant physiological role in mitochondrial OXPHOS. However, another study identified a partial loss of cardiolipin in brain mitochondria of α-syn KO mice together with a reduction in phosphatidylglycerol, the precursor of cardiolipin. They found that α-syn–deficient mitochondria display a specific but mild defect in complex I + III activity without impairment of individual complex I and III activities (Ellis et al., 2005). This may be explained by the alterations in membrane lipid composition as cardiolipin is essential for the maintenance of respiratory chain supercomplex integrity (Pfeiffer et al., 2003). Furthermore, another study showed that monomeric α-syn localized to mitochondria interacts with subunit α of ATPase and regulates ATP synthesis. This work also reported that brain mitochondria of mice KO for all three forms of synuclein have increased respiration but decreased membrane potential and ATP synthesis, indicative of loss of OXPHOS coupling (Ludtmann et al., 2016).
Although the role of monomeric α-syn in the normal physiology of OXPHOS remains far from settled, the detrimental effects of α-syn oligomers and fibrils on mitochondrial function are not disputed. Addition of soluble preformed α-syn oligomers inhibits complex I activity and promotes calcium-dependent MPT in brain mitochondria (Luth et al., 2014). Moreover, administration of preformed α-syn oligomers to cultured dopaminergic neurons and to mouse brain in vivo results in enhanced mitochondrial reactive oxidation species (ROS) production (Tapias et al., 2017). These effects are likely related to α-syn oligomers and aggregates destabilizing the lipids of the IM, disrupting OXPHOS integrity, and increasing solute permeability (Camilleri et al., 2013). This hypothesis is also supported by the finding that lipid binding–defective α-syn variants do not cause mitochondrial uncoupling and complex I impairment (Zhang et al., 2016). Transgenic mice overexpressing A53T mutant α-syn have clear symptoms of neurodegeneration, albeit not of typical PD, and respiratory defects have been identified in primary neurons (Li et al., 2013). In the transgenic mice, A53T mutant α-syn localizes to the mitochondrial membranes both as monomers and oligomers and is associated with complex I deficiency (Chinta et al., 2010). Interestingly, in neurons, the mitochondrial accumulation of mutant α-syn is potentiated by proteasomal inhibition, suggesting that failure to degrade cytosolic mutant α-syn enhances the probability of its mitochondrial import (Chinta et al., 2010). Both overexpressed WT α-syn (Subramaniam et al., 2014) and disease-associated mutants can oligomerize and cause mitochondrial OXPHOS defects, which strongly suggests that imbalance of mitochondrial α-syn levels are sufficient to alter the putative physiological interactions of the protein with membrane components and result in mitochondrial damage. Furthermore, the ability of overexpressed WT or mutant α-syn to impair OXPHOS, and complex I specifically, do not appear to correlate with the presence of overt α-syn pathology with large inclusions (Loeb et al., 2010). The lack of difference between the effects of WT and those of aggregation-prone mutant α-syn on complex I inhibition suggests that regulation of complex I activity could be one of the physiological functions of α-syn in mitochondria and that the extent of the inhibitory effect of complex I could be mostly correlated with α-syn expression levels (Loeb et al., 2010). Nevertheless, it is logical to hypothesize that mutant α-syn is more prone to forming intramitochondrial aggregates and may impair OXPHOS at lower concentrations and a faster rate compared with the WT protein.
The import of cytosolic α-syn into mitochondria to reach the IM and interact with cardiolipin and respiratory chain components remains elusive. One study shows that levels of the major protein translocator of the OM, TOM40, are reduced in the brains of PD patients and in α-syn transgenic mice (Bender et al., 2013), suggesting that the protein may enter mitochondria through the TOM40 channel and establish aberrant interactions with TOM40, triggering its degradation (Fig. 2). Another intriguing observation is that α-syn interacts with the voltage-dependent anion channel (VDAC), the channel allowing metabolite fluxes across the OM. It was shown that α-syn can block VDAC functionally, and it was also proposed that α-syn enters mitochondria through this channel (Rostovtseva et al., 2015). Furthermore, the activation of MPT by α-syn was prevented by a blocker of the adenine nucleotide translocator (ANT), the IM nucleotide exchanger that is in close contact with VDAC (Shen et al., 2014). Combined, these findings suggest that the interaction of α-syn with VDAC and ANT could participate in the translocation process and in the OXPHOS dysfunction.
Accumulation of OXPHOS-defective mitochondria resulting from respiratory defects and depolarization induced by α-syn oligomers and aggregates triggers a quality control program that culminates in mitophagy, a selective form of autophagy. Increased mitophagy associated with overexpression of α-syn in neurons has been extensively reported (Chinta et al., 2010; Choubey et al., 2011; Calì et al., 2012; Chen et al., 2015). Although in the short term, mitophagy represents an effective protective mechanism to clear damaged mitochondria, it was suggested that chronically hyperactive mitophagy can deplete the mitochondrial pool and accelerate bioenergetic crisis in neurons (Choubey et al., 2011). The need for a balanced mitochondrial quality control process in maintaining neuronal viability in the context of PD is highlighted by the fact that two proteins involved in familial autosomal dominant PD, PINK1 and Parkin, are in fact major players of mitochondrial proteostasis and mitophagy (Pickrell and Youle, 2015). Therefore, an imbalance of the interplay between mitochondrial dysfunction and clearance mechanisms may significantly contribute to PD pathogenesis (Norris et al., 2015).
In addition to the intramitochondrial effects and similarly to those described in the previous section for Aβ, α-syn can affect mitochondrial function by altering the physical and functional interactions with the ER. It was shown that α-syn expression in cultured cells results in an increase in the number of ER–mitochondria contacts and increases mitochondrial calcium uptake upon hormonal stimulation of ER calcium release (Fig. 2). Conversely, α-syn silencing decreases mitochondrial calcium uptake (Calì et al., 2012). Alteration of ER–mitochondria calcium signaling was not caused by changes in mitochondrial calcium uptake capacity but exclusively by the abnormal interactions between the two organelles. Abnormal calcium signaling can alter mitochondrial OXPHOS and contribute to mitochondrial dysfunction. Another study proposed that α-syn is not inside mitochondria but rather is localized within the MAMs. It also showed that mutant forms of α-syn linked to PD cause a decrease of α-syn localization in the MAMs and decreased ER–mitochondria contacts (Guardia-Laguarta et al., 2014). The reason behind the discrepancy in the effects on ER–mitochondria contacts by α-syn expression in these two studies is unclear, but it could be a result of different expression levels as well as WT versus mutant α-syn differences. A proposed mechanistic explanation of decreased ER–mitochondrial tethering induced by α-syn is the interference with the binding of ER protein vesicle–associated membrane protein–associated protein B (VAPB) to the outer mitochondrial membrane protein, protein tyrosine phosphatase–interacting protein 51 (PTPIP51). It was shown that both overexpressed WT and mutant α-syn bind to VAPB and disrupt the VAPB-PTPIP51 tethers, thereby impairing ER–mitochondria contacts (Paillusson et al., 2017). Alterations of VAPB-PTPIP51 tether was shown to affect not only ER–mitochondria calcium signaling but also the autophagy process, whereby increasing the tether decreases autophagic vacuoles formation and vice versa (Gomez-Suaga et al., 2017). Another similarity between α-syn in PD and APP in AD is their effect on the mitochondrial protein import machinery as it was shown that posttranslationally modified α-syn binds to TOM20, a key regulatory component of the TOM complex, thereby preventing the interaction of TOM20 with its coreceptor TOM22 and impairing mitochondrial protein import (Di Maio et al., 2016).
To summarize, the majority of the studies discussed in this review describe a direct interaction of α-syn with mitochondrial IM molecules, notably the phospholipid cardiolipin, and a direct or indirect interaction with several OXPHOS components. Fig. 2 summarizes the proposed interactions of α-syn with mitochondria and the functional consequences. Although most studies agree that aggregated/oligomeric α-syn in mitochondria alter OXPHOS function, there is disagreement on whether α-syn plays a physiological role in OXPHOS function and whether loss of α-syn from mitochondria results in OXPHOS dysfunction. This is a very interesting question, as modulation of α-syn expression, conformation, and turnover are being increasingly considered as potential therapeutic targets in PD.
Mitochondrial SOD1 and OXPHOS in familial ALS
Cu,Zn-superoxide dismutase (SOD1) is the first protein to be associated with familial autosomal dominant forms of ALS. SOD1-linked ALS is pathologically characterized by misfolding and aggregation of the mutant protein in the affected motor neurons. In cellular and animal models, mutant SOD1 aggregates localize in multiple cell compartments including mitochondria, where it causes OXPHOS alterations. However, although mutant SOD1 triggers mitochondrial abnormalities, it is well established that WT SOD1 plays a physiological role in mitochondria. SOD1 is an abundant cytosolic enzyme, but studies in yeast (Sturtz et al., 2001) and mammalian cells (Okado-Matsumoto and Fridovich, 2002) have demonstrated that a portion of SOD1 is localized in mitochondria. In mitochondria, SOD1 is concentrated in the IMS, where it serves an antioxidant role (Sturtz et al., 2001) as the intramitochondrial Mn-SOD is localized exclusively in the matrix and cannot metabolize the superoxide that is formed by electrons escaping the respiratory chain on the outer side of the IM.
The physiological import of SOD1 in the IMS can occur only when the protein is in the monomeric state, unfolded, and devoid of metals (Okado-Matsumoto and Fridovich, 2002). Its retention in the IMS depends on the formation of an intramolecular disulfide bond, which is facilitated by the copper chaperone for SOD1 (Ccs1), which in turn is imported and retained in mitochondria by a redox relay system regulated by the IMS proteins Mia40 and Erv1 (Fig. 3; Reddehase et al., 2009; Gross et al., 2011; Klöppel et al., 2011). In addition, it was suggested that, at least in yeast, a portion of SOD1 can be imported in the IMS in a reduced form independently of Ccs1 (Varabyova et al., 2013). The retention of SOD1 is dependent on the redox state of the IMS and the oxidation of its cysteine residues, which are highly sensitive to oxygen concentration in the cell (Kawamata and Manfredi, 2008). Unlike WT SOD1, which traffics in and out of the IMS based on redox state, pathogenic mutant SOD1 gets entrapped in the IMS because of misfolding and aggregation, thereby losing the regulatory properties of normal import and retention (Kawamata and Manfredi, 2008).
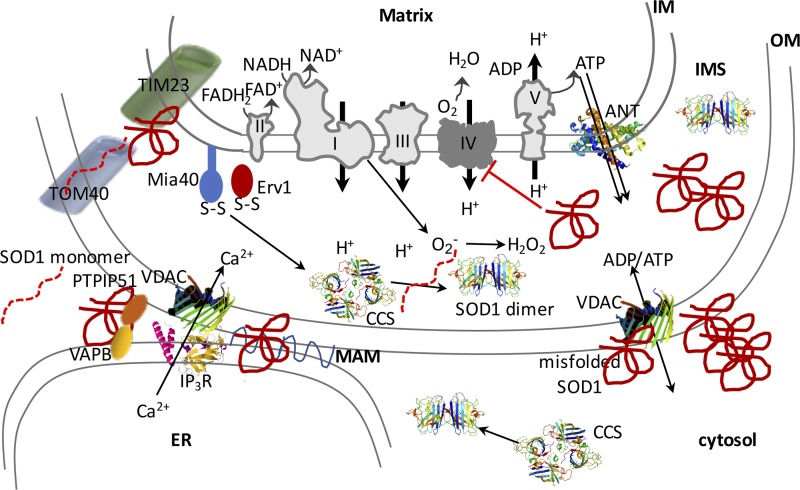
Figure 3.
Schematic illustration of the proposed interactions of SOD1 with mitochondrial components and their effects on OXPHOS function. SOD1 localizes to mitochondria, where it provides dismutase activity in the IMS. SOD1 is imported to the IMS via the Mia40-Erv1 disulfide relay pathway, and its maturation is facilitated by the copper chaperone for SOD1 (CCS). It is proposed that mutant misfolded SOD1 associates with various mitochondrial proteins both within the IMS and at the OM. Misfolded SOD1 interferes with the mitochondrial protein import machinery, ER–mitochondrial contacts, and VDAC. Among the electron transfer chain (ETC) complexes, mutant SOD1 mostly inhibits complex IV activity.
Misfolded SOD1 accumulates in spinal cord mitochondria (Higgins et al., 2002), affecting multiple compartments, including the IMS (Kawamata and Manfredi, 2008), where it can form cross-linked oligomers (Ferri et al., 2006) with itself or with other proteins (Fig. 3). Misfolded SOD1 is also found in the mitochondrial IM and in the matrix (Vijayvergiya et al., 2005) as well as on the OM, where it establishes aberrant interactions with several proteins, including proteins of the OM import machinery (Liu et al., 2004), the antiapoptotic protein Bcl-2 (Pasinelli et al., 2004), and VDAC (Israelson et al., 2010). The interaction of mutant SOD1 with TOM proteins leads to a net decrease in mitochondrial protein import and depletion of OXPHOS components (Li et al., 2010). Interactions with VDAC were proposed to alter the properties of the channel and impede ATP and ADP flow (Israelson et al., 2010). In addition, SOD1 competes with hexokinase 1 for binding to VDAC, thereby displacing the enzyme from the OM (Magrì et al., 2016). The SOD1–Bcl-2 interactions were also proposed to inhibit the ADP entry into mitochondria and to cause an increase in mitochondrial membrane potential because of loss of ADP phosphorylation by the ATPase (Tan et al., 2013).
It was proposed that the accumulation of misfolded SOD1 on the OM is specific to spinal cord mitochondria (Liu et al., 2004; Vande Velde et al., 2008). Furthermore, research with conformation-specific antibodies showed that specific forms of misfolded SOD1 accumulate selectively in the mitochondria of spinal cord motor neurons (Fig. 3; Pickles et al., 2016). These findings suggest that the complement of chaperones to keep proteins unfolded and the mechanisms to eliminate misfolded SOD1 are ineffective in the spinal cord. This is an intriguing hypothesis because it would help to explain the susceptibility of spinal motor neuron mitochondria to mutant SOD1. Recently, using a battery of 19 different disease-related mutants of SOD1 expressed in neuronal cell lines, it was shown that the severity of the disease correlated with the ability of the mutants to associate with mitochondria (Abu-Hamad et al., 2017). Interestingly, accumulation of misfolded SOD1 in mitochondria correlated with disease duration but not with disease onset, suggesting that mitochondrial SOD1 contributes to accelerating rather than initiating the disease process. Although the precise mechanisms to keep mitochondrial accumulation of mutant SOD1 in check are not fully understood, it was suggested that the mitochondrial ubiquitin ligase March5 (MITOL) plays a predominant role in initiating proteasomal degradation of mitochondrial mutant SOD1 (Yonashiro et al., 2009). However, whether March5 is weakly expressed in motor neurons relative to other cell types has not yet been investigated. Furthermore, the expression of the macrophage migration inhibitory factor (MIF), a cytosolic chaperone that prevents SOD1 misfolding, is particularly low in spinal motor neurons, possibly contributing to the tendency to selectively accumulate mutant SOD1 in mitochondria (Israelson et al., 2015).
Mutant SOD1 causes OXPHOS impairments characterized by defective mitochondrial respiration and ATP production, which clearly manifest in the spinal cord tissues of mutant SOD1 transgenic mouse models (Jung et al., 2002; Mattiazzi et al., 2002; Kirkinezos et al., 2005) and in cultured neurons expressing recombinant mutant SOD1 (Fig. 3; Menzies et al., 2002). In these models, several respiratory chain enzymes are defective, including complex I (Cacabelos et al., 2016), but complex IV activity appears to be particularly susceptible, suggesting that this enzyme is targeted by mutant SOD1 (Fig. 3). Complex IV impairment was also identified in a subset of muscle biopsies from sporadic ALS patients (Echaniz-Laguna et al., 2006; Crugnola et al., 2010), indicating that complex IV deficiency is not restricted to the spinal cord nor to SOD1 mutants. However, similar defects were also observed in the muscle of patients affected by other forms of neurogenic atrophy (Krasnianski et al., 2005), suggesting that complex IV defects are not only specific to ALS.
Two main lines of evidence indicate that complex IV defects are associated with the accumulation of mutant SOD1 in the IMS. First, when Ccs1 was overexpressed together with mutant SOD1 in transgenic mice, there was an increase of mutant SOD1 in the IMS accompanied by profound complex IV deficiency and vastly accelerated disease course (Son et al., 2007). Second, when mutant SOD1 was selectively targeted to the IMS, mice developed a significant decline of complex IV activity (Igoudjil et al., 2011). Collectively, this evidence suggests that inhibition of complex IV plays a key role in the mitochondrial dysfunction that arises from mutant SOD1 accumulation in mitochondria, but the mechanism is still debated. An attractive hypothesis is that SOD1 in the IMS could interfere with copper delivery to complex IV, thereby impairing the normal assembly process of the active complex. This hypothesis is supported indirectly by the observation that only G93A mutants that can gain copper result in a worsening of the phenotype when Ccs1 is overexpressed, whereas mutants that fail to bind metals do not (Son et al., 2009). Assembly factors in the IMS, such as the metallochaperone cmc2, may also be involved, as modulation of these factors in yeast result in opposing effects on complex IV and SOD1 activities (Horn et al., 2010).
In summary, overwhelming evidence indicates that OXPHOS is altered in transgenic models of SOD1-linked familial ALS. Fig. 3 schematically illustrates the localization of SOD1 in mitochondria and its consequences on OXPHOS. It is important to note that these alterations are accompanied by other mitochondrial deficits, especially related to axonal transport, fusion and fission, calcium uptake, physical and functional interactions with the ER, and mitochondrial quality control processes. Studies investigating these alterations are numerous and their review is beyond of the scopes of this article that focuses on OXPHOS. Nevertheless, many of these mitochondrial alterations can be intimately linked to OXPHOS defects, especially those concerning the integrity of mitochondrial membranes and the ability to generate membrane potential, as OXPHOS-defective mitochondria are prone to undergo MPT and generate excessive ROS. Furthermore, the inability to maintain a proper pool of functional mitochondria in critical energy-requiring neuronal regions because of impaired mitochondrial dynamics and intracellular calcium signaling dysregulation can be equally or more detrimental to neuronal health than the direct effects of mutant SOD1 on OXPHOS function. Of note, in our opinion, whether mutant SOD1 expressed at physiological levels results in mitochondrial abnormalities as seen in the transgenic models overexpressing the mutant proteins remains to be resolved, despite numerous studies in patient-derived induced neurons.
Less is known about the role of other mutant proteins associated with familial FTD/ALS in mitochondria. Nevertheless, recent studies have indicated that alterations in transactivation response element DNA–binding protein 43 (TDP-43) can cause OXPHOS defects. It was proposed that both WT and mutant TDP-43 gain access to the mitochondrial matrix, where they bind the mRNA encoding for two complex I subunits, ND3 and ND6, leading to decreased protein expression and complex I defects. The mitochondrial localization was attributed to specific regions of TDP-43, and peptides directed against these regions prevented import and binding to the mRNAs (Wang et al., 2016b). In the cortical neurons of transgenic mice, the mitochondrial association of mutant TDP-43 was stronger than the endogenous protein in nontransgenic littermates, suggesting that mutant TDP-43 has a higher tropism for mitochondria. A recent study has also proposed that WT TDP43 may serve a physiological function by stabilizing a subset of tRNAs and mRNA intermediates in mitochondria (Izumikawa et al., 2017). However, unlike the studies mentioned above, in human fibroblasts harboring mutant TDP-43, a decrease in mitochondria membrane potential was detected in the absence of alterations in oxygen consumption and mitochondrial localization of TDP-43 (Onesto et al., 2016). This result suggests that in human cells, alterations of energy metabolism could emerge without the direct interference of mutant protein with mitochondria. To complicate things further, in mutant TDP-43 transgenic mouse brain, we detected association but no internalization of the protein in mitochondria, which did not present abnormalities of respiration, membrane potential, or ATP synthesis (Kawamata et al., 2017). These are early studies, and differences in findings may arise from several variables in models, mutants, and reagents used to identify the mitochondrial localization of the proteins. Additional studies are needed to further elucidate the physiological and pathological roles of TDP-43 in mitochondria.
Interestingly, it was shown that mutant TDP-43 dysregulates ER–mitochondria contacts through interference with the VAPB-PTPIP51 tethers (Stoica et al., 2014), similar to the effects of α-syn described in the previous section (Paillusson et al., 2017). Alterations of these important tethers have been reported also in other genetic forms of FTD/ALS, such as those associated with fused in sarcoma (FUS; Stoica et al., 2016) and VAPB (De Vos et al., 2012) mutations. Therefore, it appears that impaired ER–mitochondria physical and functional communication is a common pathogenic pathway in neurodegenerative diseases caused by protein misfolding.
Conclusions
Numerous lines of evidence support the hypothesis that the proteins associated with neurodegenerative diseases such as Aβ, α-syn, and mutant SOD1 localize to mitochondria. For SOD1, the normal function in mitochondria, the mechanisms that regulate import, and the topology within mitochondria are relatively well understood. However, the normal physiological functions of Aβ and α-syn in mitochondria are not yet well characterized, as the results in KO cells and mice are controversial. There is further debate on whether their localization in mitochondria is physiological or if it occurs only when they are overexpressed or aberrantly accumulated in the cytosol. Detailed understanding of their import mechanisms and topology remain to be analyzed.
Regarding the impact on OXPHOS function, there is little doubt that accumulation and aggregation of Aβ, α-syn, and mutant SOD1 in mitochondria cause mitochondrial bioenergetic dysfunction. OXPHOS appears to be almost invariably involved in many models. Table 1 summarizes the OXPHOS defects described in this review. However, in our opinion, it remains to be conclusively determined whether the OXPHOS is a primary target of the proteinopathies or if it is affected downstream of defects in mitochondrial dynamics, structure, and quality control. Both scenarios are possible as all these mitochondrial processes are intimately related.
Table 1. OXPHOS defects associated with the neurodegenerative proteinopathies discussed in this review.
| Protein | CIVa defects | CIb defects | Mito ATP synthesis defects | ΔΨmc defects | Mito dynamics defects | ER–mito contact alterations | Mito Ca2+ handling defects |
|---|---|---|---|---|---|---|---|
| APP/Aβ | + | + | + | + | + | + | |
| α-Syn | + | + | + | + | + | + | + |
| SOD1 | + | + | + | + | + | + |
Mito dynamics refers to the processes of fusion and fission and to mitochondrial axonal transport. ER–mito contacts refers to MAMs and functional interactions between organelles. Mito Ca2+ handling refers to mitochondrial calcium uptake and permeability transition.
Complex IV.
Complex I.
Mitochondrial membrane potential.
Last, and perhaps most important, is the question of whether OXPHOS defects can be prevented or improved and whether this would have a therapeutic impact on the diseases. So far, with very few exceptions, mitochondrial drugs have not provided much relief in AD, PD, or ALS, and in the very few cases where they had an effect, such as Edaravone in ALS (Writing Group on behalf of the Edaravone (MCI-186) ALS 19 Study Group, 2017), the benefit is limited. However, it is also true that in many clinical trials, compounds with unclear targets and mechanisms of action were used. Reliable biomarker strategies and stratification of patient populations may also raise challenges. Furthermore, as the field of primary mitochondrial diseases is painfully aware, there are very few options to improve OXPHOS defects. Therefore, it is quite possible that the perspective of targeting OXPHOS dysfunction in neurodegeneration becomes more optimistic as better targeted and more effective strategies are developed.
Acknowledgments
We thank the ALS Association, the Muscular Dystrophy Association, and National Institutes of Health grants R01NS062055 and R01NS093872 for supporting our research.
The authors declare no competing financial interests.
References
- Abu-Hamad S., Kahn J., Leyton-Jaimes M.F., Rosenblatt J., and Israelson A.. 2017. Misfolded SOD1 Accumulation and Mitochondrial Association Contribute to the Selective Vulnerability of Motor Neurons in Familial ALS: Correlation to Human Disease. ACS Chem. Neurosci. 18:2225–2234. 10.1021/acschemneuro.7b00140 [DOI] [PubMed] [Google Scholar]
- Alavian K.N., Beutner G., Lazrove E., Sacchetti S., Park H.A., Licznerski P., Li H., Nabili P., Hockensmith K., Graham M., et al. 2014. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA. 111:10580–10585. 10.1073/pnas.1401591111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhani N., Guo L., Yan S., Du H., Pinho C.M., Chen J.X., Glaser E., and Yan S.S.. 2011. Decreased proteolytic activity of the mitochondrial amyloid-β degrading enzyme, PreP peptidasome, in Alzheimer’s disease brain mitochondria. J. Alzheimers Dis. 27:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandatheerthavarada H.K., Biswas G., Robin M.A., and Avadhani N.G.. 2003. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell Biol. 161:41–54. 10.1083/jcb.200207030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area-Gomez E., Del Carmen Lara Castillo M., Tambini M.D., Guardia-Laguarta C., de Groof A.J., Madra M., Ikenouchi J., Umeda M., Bird T.D., Sturley S.L., and Schon E.A.. 2012. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 31:4106–4123. 10.1038/emboj.2012.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H., and Boyle K.. 2006. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 103:3381–3386. 10.1073/pnas.0600134103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S.J., Guo L., Phensy A., Tian J., Wang L., Tandon N., Gauba E., Lu L., Pascual J.M., Kroener S., and Du H.. 2016. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer’s disease. Nat. Commun. 7:11483 10.1038/ncomms11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A., Desplats P., Spencer B., Rockenstein E., Adame A., Elstner M., Laub C., Mueller S., Koob A.O., Mante M., et al. 2013. TOM40 mediates mitochondrial dysfunction induced by α-synuclein accumulation in Parkinson’s disease. PLoS One. 8:e62277 10.1371/journal.pone.0062277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M.D., and Nicholls D.G.. 2011. Assessing mitochondrial dysfunction in cells. Biochem. J. 435:297–312. 10.1042/BJ20110162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti D., Torsvik J., Dallabona C., Teixeira P., Sztromwasser P., Fernandez-Vizarra E., Cerutti R., Reyes A., Preziuso C., D’Amati G., et al. 2016. Defective PITRM1 mitochondrial peptidase is associated with Aβ amyloidotic neurodegeneration. EMBO Mol. Med. 8:176–190. 10.15252/emmm.201505894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos D., Ramírez-Núñez O., Granado-Serrano A.B., Torres P., Ayala V., Moiseeva V., Povedano M., Ferrer I., Pamplona R., Portero-Otin M., and Boada J.. 2016. Early and gender-specific differences in spinal cord mitochondrial function and oxidative stress markers in a mouse model of ALS. Acta Neuropathol. Commun. 4:3 10.1186/s40478-015-0271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T., Ottolini D., Negro A., and Brini M.. 2012. α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J. Biol. Chem. 287:17914–17929. 10.1074/jbc.M111.302794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo S.E., Clauser K.R., and Mootha V.K.. 2016. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44(D1):D1251–D1257. 10.1093/nar/gkv1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri A., Zarb C., Caruana M., Ostermeier U., Ghio S., Högen T., Schmidt F., Giese A., and Vassallo N.. 2013. Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim. Biophys. Acta. 1828:2532–2543. 10.1016/j.bbamem.2013.06.026 [DOI] [PubMed] [Google Scholar]
- Cenini G., Rüb C., Bruderek M., and Voos W.. 2016. Amyloid β-peptides interfere with mitochondrial preprotein import competence by a coaggregation process. Mol. Biol. Cell. 27:3257–3272. 10.1091/mbc.E16-05-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha M.Y., Cho H.J., Kim C., Jung Y.O., Kang M.J., Murray M.E., Hong H.S., Choi Y.J., Choi H., Kim D.K., et al. 2015. Mitochondrial ATP synthase activity is impaired by suppressed O-GlcNAcylation in Alzheimer’s disease. Hum. Mol. Genet. 24:6492–6504. 10.1093/hmg/ddv358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xie Z., Turkson S., and Zhuang X.. 2015. A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration. J. Neurosci. 35:890–905. 10.1523/JNEUROSCI.0089-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta S.J., Mallajosyula J.K., Rane A., and Andersen J.K.. 2010. Mitochondrial α-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci. Lett. 486:235–239. 10.1016/j.neulet.2010.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey V., Safiulina D., Vaarmann A., Cagalinec M., Wareski P., Kuum M., Zharkovsky A., and Kaasik A.. 2011. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J. Biol. Chem. 286:10814–10824. 10.1074/jbc.M110.132514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N.B., Dieuliis D., Leo P., Mitchell D.C., and Nussbaum R.L.. 2008. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp. Cell Res. 314:2076–2089. 10.1016/j.yexcr.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch P.J., Blake R., Duce J.A., Ciccotosto G.D., Li Q.X., Barnham K.J., Curtain C.C., Cherny R.A., Cappai R., Dyrks T., et al. 2005. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J. Neurosci. 25:672–679. 10.1523/JNEUROSCI.4276-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch P.J., Barnham K.J., Duce J.A., Blake R.E., Masters C.L., and Trounce I.A.. 2006. Copper-dependent inhibition of cytochrome c oxidase by Aβ1–42) requires reduced methionine at residue 35 of the Aβ peptide. J. Neurochem. 99:226–236. 10.1111/j.1471-4159.2006.04050.x [DOI] [PubMed] [Google Scholar]
- Crugnola V., Lamperti C., Lucchini V., Ronchi D., Peverelli L., Prelle A., Sciacco M., Bordoni A., Fassone E., Fortunato F., et al. 2010. Mitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosis. Arch. Neurol. 67:849–854. 10.1001/archneurol.2010.128 [DOI] [PubMed] [Google Scholar]
- Del Prete D., Suski J.M., Oulès B., Debayle D., Gay A.S., Lacas-Gervais S., Bussiere R., Bauer C., Pinton P., Paterlini-Bréchot P., et al. 2017. Localization and Processing of the Amyloid-β Protein Precursor in Mitochondria-Associated Membranes. J. Alzheimers Dis. 55:1549–1570. 10.3233/JAD-160953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D., Patron M., and Rizzuto R.. 2015. Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta. 1853:2006–2011. 10.1016/j.bbamcr.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L., Prabhu B.M., Galati D.F., Avadhani N.G., and Anandatheerthavarada H.K.. 2006. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J. Neurosci. 26:9057–9068. 10.1523/JNEUROSCI.1469-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L., Raghavendran V., Prabhu B.M., Avadhani N.G., and Anandatheerthavarada H.K.. 2008. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 283:9089–9100. 10.1074/jbc.M710012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos K.J., Mórotz G.M., Stoica R., Tudor E.L., Lau K.F., Ackerley S., Warley A., Shaw C.E., and Miller C.C.. 2012. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet. 21:1299–1311. 10.1093/hmg/ddr559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio R., Barrett P.J., Hoffman E.K., Barrett C.W., Zharikov A., Borah A., Hu X., McCoy J., Chu C.T., Burton E.A., et al. 2016. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 8:342ra78 10.1126/scitranslmed.aaf3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S., and Schon E.A.. 2008. Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 31:91–123. 10.1146/annurev.neuro.30.051606.094302 [DOI] [PubMed] [Google Scholar]
- Du H., Guo L., Fang F., Chen D., Sosunov A.A., McKhann G.M., Yan Y., Wang C., Zhang H., Molkentin J.D., et al. 2008. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 14:1097–1105. 10.1038/nm.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echaniz-Laguna A., Zoll J., Ponsot E., N’guessan B., Tranchant C., Loeffler J.P., and Lampert E.. 2006. Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: a temporal study in man. Exp. Neurol. 198:25–30. 10.1016/j.expneurol.2005.07.020 [DOI] [PubMed] [Google Scholar]
- Elkon H., Don J., Melamed E., Ziv I., Shirvan A., and Offen D.. 2002. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J. Mol. Neurosci. 18:229–238. 10.1385/JMN:18:3:229 [DOI] [PubMed] [Google Scholar]
- Ellis C.E., Murphy E.J., Mitchell D.C., Golovko M.Y., Scaglia F., Barceló-Coblijn G.C., and Nussbaum R.L.. 2005. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol. Cell. Biol. 25:10190–10201. 10.1128/MCB.25.22.10190-10201.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A., Cozzolino M., Crosio C., Nencini M., Casciati A., Gralla E.B., Rotilio G., Valentine J.S., and Carrì M.T.. 2006. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc. Natl. Acad. Sci. USA. 103:13860–13865. 10.1073/pnas.0605814103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca A.C., Moreira P.I., Oliveira C.R., Cardoso S.M., Pinton P., and Pereira C.F.. 2015. Amyloid-beta disrupts calcium and redox homeostasis in brain endothelial cells. Mol. Neurobiol. 51:610–622. 10.1007/s12035-014-8740-7 [DOI] [PubMed] [Google Scholar]
- Fukui H., Diaz F., Garcia S., and Moraes C.T.. 2007. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 104:14163–14168. 10.1073/pnas.0705738104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes F., Tatsuta T., and Langer T.. 2012. Mitochondrial AAA proteases--towards a molecular understanding of membrane-bound proteolytic machines. Biochim. Biophys. Acta. 1823:49–55. 10.1016/j.bbamcr.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G.D., Petronilli V., Zoratti M., Szabó I., et al. 2013. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA. 110:5887–5892. 10.1073/pnas.1217823110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suaga P., Paillusson S., Stoica R., Noble W., Hanger D.P., and Miller C.C.. 2017. The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy. Curr. Biol. 27:371–385. 10.1016/j.cub.2016.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre J.T., Sherer T.B., Betarbet R., and Panov A.V.. 2001. Complex I and Parkinson’s disease. IUBMB Life. 52:135–141. 10.1080/15216540152845939 [DOI] [PubMed] [Google Scholar]
- Gross D.P., Burgard C.A., Reddehase S., Leitch J.M., Culotta V.C., and Hell K.. 2011. Mitochondrial Ccs1 contains a structural disulfide bond crucial for the import of this unconventional substrate by the disulfide relay system. Mol. Biol. Cell. 22:3758–3767. 10.1091/mbc.E11-04-0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C., Area-Gomez E., Rüb C., Liu Y., Magrané J., Becker D., Voos W., Schon E.A., and Przedborski S.. 2014. α-Synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 34:249–259. 10.1523/JNEUROSCI.2507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson Petersen C.A., Alikhani N., Behbahani H., Wiehager B., Pavlov P.F., Alafuzoff I., Leinonen V., Ito A., Winblad B., Glaser E., and Ankarcrona M.. 2008. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA. 105:13145–13150. 10.1073/pnas.0806192105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Ford H.C., Carroll J., Ding S., Fearnley I.M., and Walker J.E.. 2017. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc. Natl. Acad. Sci. USA. 114:3409–3414. 10.1073/pnas.1702357114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.M., and Rutter J.. 2011. Ubiquitin-dependent mitochondrial protein degradation. Int. J. Biochem. Cell Biol. 43:1422–1426. 10.1016/j.biocel.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Zimbron L.F., Luna-Muñoz J., Mena R., Vazquez-Ramirez R., Kubli-Garfias C., Cribbs D.H., Manoutcharian K., and Gevorkian G.. 2012. Amyloid-β peptide binds to cytochrome C oxidase subunit 1. PLoS One. 7:e42344 10.1371/journal.pone.0042344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C.M., Jung C., Ding H., and Xu Z.. 2002. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J. Neurosci. 22:RC215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D., Zhou W., Trevisson E., Al-Ali H., Harris T.K., Salviati L., and Barrientos A.. 2010. The conserved mitochondrial twin Cx9C protein Cmc2 Is a Cmc1 homologue essential for cytochrome c oxidase biogenesis. J. Biol. Chem. 285:15088–15099. 10.1074/jbc.M110.104786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igoudjil A., Magrané J., Fischer L.R., Kim H.J., Hervias I., Dumont M., Cortez C., Glass J.D., Starkov A.A., and Manfredi G.. 2011. In vivo pathogenic role of mutant SOD1 localized in the mitochondrial intermembrane space. J. Neurosci. 31:15826–15837. 10.1523/JNEUROSCI.1965-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelson A., Arbel N., Da Cruz S., Ilieva H., Yamanaka K., Shoshan-Barmatz V., and Cleveland D.W.. 2010. Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron. 67:575–587. 10.1016/j.neuron.2010.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelson A., Ditsworth D., Sun S., Song S., Liang J., Hruska-Plochan M., McAlonis-Downes M., Abu-Hamad S., Zoltsman G., Shani T., et al. 2015. Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1. Neuron. 86:218–232. 10.1016/j.neuron.2015.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa K., Nobe Y., Yoshikawa H., Ishikawa H., Miura Y., Nakayama H., Nonaka T., Hasegawa M., Egawa N., Inoue H., et al. 2017. TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci. Rep. 7:7709 10.1038/s41598-017-06953-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C., Higgins C.M., and Xu Z.. 2002. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J. Neurochem. 83:535–545. 10.1046/j.1471-4159.2002.01112.x [DOI] [PubMed] [Google Scholar]
- Karbowski M., and Youle R.J.. 2011. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr. Opin. Cell Biol. 23:476–482. 10.1016/j.ceb.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M., Neutzner A., and Youle R.J.. 2007. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J. Cell Biol. 178:71–84. 10.1083/jcb.200611064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata H., and Manfredi G.. 2008. Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum. Mol. Genet. 17:3303–3317. 10.1093/hmg/ddn226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata H., Peixoto P., Konrad C., Palomo G., Bredvik K., Gerges M., Valsecchi F., Petrucelli L., Ravits J.M., Starkov A., and Manfredi G.. 2017. Mutant TDP-43 does not impair mitochondrial bioenergetics in vitro and in vivo. Mol. Neurodegener. 12:37 10.1186/s13024-017-0180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.W., Choi W.S., Sorscher N., Park H.J., Tronche F., Palmiter R.D., and Xia Z.. 2015. Genetic reduction of mitochondrial complex I function does not lead to loss of dopamine neurons in vivo. Neurobiol. Aging. 36:2617–2627. 10.1016/j.neurobiolaging.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.C., Tresse E., Kolaitis R.M., Molliex A., Thomas R.E., Alami N.H., Wang B., Joshi A., Smith R.B., Ritson G.P., et al. 2013. VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron. 78:65–80. 10.1016/j.neuron.2013.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkinezos I.G., Bacman S.R., Hernandez D., Oca-Cossio J., Arias L.J., Perez-Pinzon M.A., Bradley W.G., and Moraes C.T.. 2005. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J. Neurosci. 25:164–172. 10.1523/JNEUROSCI.3829-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P., Siwek D., Gardian G., Yang L., Starkov A., Cleren C., Ferrante R.J., Kowall N.W., Abeliovich A., and Beal M.F.. 2006. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 21:541–548. 10.1016/j.nbd.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Klöppel C., Suzuki Y., Kojer K., Petrungaro C., Longen S., Fiedler S., Keller S., and Riemer J.. 2011. Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol. Mol. Biol. Cell. 22:3749–3757. 10.1091/mbc.E11-04-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontush A., Berndt C., Weber W., Akopyan V., Arlt S., Schippling S., and Beisiegel U.. 2001. Amyloid-beta is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radic. Biol. Med. 30:119–128. 10.1016/S0891-5849(00)00458-5 [DOI] [PubMed] [Google Scholar]
- Krasnianski A., Deschauer M., Neudecker S., Gellerich F.N., Müller T., Schoser B.G., Krasnianski M., and Zierz S.. 2005. Mitochondrial changes in skeletal muscle in amyotrophic lateral sclerosis and other neurogenic atrophies. Brain. 128:1870–1876. 10.1093/brain/awh540 [DOI] [PubMed] [Google Scholar]
- Leal N.S., Schreiner B., Pinho C.M., Filadi R., Wiehager B., Karlström H., Pizzo P., and Ankarcrona M.. 2016. Mitofusin-2 knockdown increases ER-mitochondria contact and decreases amyloid β-peptide production. J. Cell. Mol. Med. 20:1686–1695. 10.1111/jcmm.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Nadanaciva S., Berger Z., Shen W., Paumier K., Schwartz J., Mou K., Loos P., Milici A.J., Dunlop J., and Hirst W.D.. 2013. Human A53T α-synuclein causes reversible deficits in mitochondrial function and dynamics in primary mouse cortical neurons. PLoS One. 8:e85815 10.1371/journal.pone.0085815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Vande Velde C., Israelson A., Xie J., Bailey A.O., Dong M.Q., Chun S.J., Roy T., Winer L., Yates J.R., et al. 2010. ALS-linked mutant superoxide dismutase 1 (SOD1) alters mitochondrial protein composition and decreases protein import. Proc. Natl. Acad. Sci. USA. 107:21146–21151. 10.1073/pnas.1014862107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.W., Yang R., Guo J.C., Ren H.M., Zha X.L., Cheng J.S., and Cai D.F.. 2007. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 18:1543–1546. 10.1097/WNR.0b013e3282f03db4 [DOI] [PubMed] [Google Scholar]
- Li W., Bengtson M.H., Ulbrich A., Matsuda A., Reddy V.A., Orth A., Chanda S.K., Batalov S., and Joazeiro C.A.. 2008. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS One. 3:e1487 10.1371/journal.pone.0001487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lillo C., Jonsson P.A., Vande Velde C., Ward C.M., Miller T.M., Subramaniam J.R., Rothstein J.D., Marklund S., Andersen P.M., et al. 2004. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 43:5–17. 10.1016/j.neuron.2004.06.016 [DOI] [PubMed] [Google Scholar]
- Loeb V., Yakunin E., Saada A., and Sharon R.. 2010. The transgenic overexpression of alpha-synuclein and not its related pathology associates with complex I inhibition. J. Biol. Chem. 285:7334–7343. 10.1074/jbc.M109.061051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtmann M.H., Angelova P.R., Ninkina N.N., Gandhi S., Buchman V.L., and Abramov A.Y.. 2016. Monomeric Alpha-Synuclein Exerts a Physiological Role on Brain ATP Synthase. J. Neurosci. 36:10510–10521. 10.1523/JNEUROSCI.1659-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader J.W., Cirilli M., Lin C., Xu H.W., Takuma K., Wang N., Caspersen C., Chen X., Pollak S., Chaney M., et al. 2004. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 304:448–452. 10.1126/science.1091230 [DOI] [PubMed] [Google Scholar]
- Luth E.S., Stavrovskaya I.G., Bartels T., Kristal B.S., and Selkoe D.J.. 2014. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J. Biol. Chem. 289:21490–21507. 10.1074/jbc.M113.545749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrì A., Belfiore R., Reina S., Tomasello M.F., Di Rosa M.C., Guarino F., Leggio L., De Pinto V., and Messina A.. 2016. Hexokinase I N-terminal based peptide prevents the VDAC1-SOD1 G93A interaction and re-establishes ALS cell viability. Sci. Rep. 6:34802 10.1038/srep34802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamada N., Tanokashira D., Ishii K., Tamaoka A., and Araki W.. 2017. Mitochondria are devoid of amyloid β-protein (Aβ)-producing secretases: Evidence for unlikely occurrence within mitochondria of Aβ generation from amyloid precursor protein. Biochem. Biophys. Res. Commun. 486:321–328. 10.1016/j.bbrc.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Manczak M., Anekonda T.S., Henson E., Park B.S., Quinn J., and Reddy P.H.. 2006. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 15:1437–1449. 10.1093/hmg/ddl066 [DOI] [PubMed] [Google Scholar]
- Mao P., Manczak M., Calkins M.J., Truong Q., Reddy T.P., Reddy A.P., Shirendeb U., Lo H.H., Rabinovitch P.S., and Reddy P.H.. 2012. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer’s disease: implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 21:2973–2990. 10.1093/hmg/dds128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y., and Kaguni L.S.. 2012. Matrix proteases in mitochondrial DNA function. Biochim. Biophys. Acta. 1819:1080–1087. 10.1016/j.bbagrm.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M., D’Aurelio M., Gajewski C.D., Martushova K., Kiaei M., Beal M.F., and Manfredi G.. 2002. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 277:29626–29633. 10.1074/jbc.M203065200 [DOI] [PubMed] [Google Scholar]
- McKenna M.C., Stridh M.H., McNair L.F., Sonnewald U., Waagepetersen H.S., and Schousboe A.. 2016. Glutamate oxidation in astrocytes: Roles of glutamate dehydrogenase and aminotransferases. J. Neurosci. Res. 94:1561–1571. 10.1002/jnr.23908 [DOI] [PubMed] [Google Scholar]
- Menzies F.M., Cookson M.R., Taylor R.W., Turnbull D.M., Chrzanowska-Lightowlers Z.M., Dong L., Figlewicz D.A., and Shaw P.J.. 2002. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain. 125:1522–1533. 10.1093/brain/awf167 [DOI] [PubMed] [Google Scholar]
- Mossmann D., Vögtle F.N., Taskin A.A., Teixeira P.F., Ring J., Burkhart J.M., Burger N., Pinho C.M., Tadic J., Loreth D., et al. 2014. Amyloid-β peptide induces mitochondrial dysfunction by inhibition of preprotein maturation. Cell Metab. 20:662–669. 10.1016/j.cmet.2014.07.024 [DOI] [PubMed] [Google Scholar]
- Nakamura K. 2013. α-Synuclein and mitochondria: partners in crime? Neurotherapeutics. 10:391–399. 10.1007/s13311-013-0182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Nemani V.M., Wallender E.K., Kaehlcke K., Ott M., and Edwards R.H.. 2008. Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J. Neurosci. 28:12305–12317. 10.1523/JNEUROSCI.3088-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D., Tanaka A., Suen D.F., and Youle R.J.. 2008. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183:795–803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D.P., Jin S.M., Tanaka A., Suen D.F., Gautier C.A., Shen J., Cookson M.R., and Youle R.J.. 2010. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8:e1000298 10.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K.L., Hao R., Chen L.F., Lai C.H., Kapur M., Shaughnessy P.J., Chou D., Yan J., Taylor J.P., Engelender S., et al. 2015. Convergence of Parkin, PINK1, and α-Synuclein on Stress-induced Mitochondrial Morphological Remodeling. J. Biol. Chem. 290:13862–13874. 10.1074/jbc.M114.634063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado-Matsumoto A., and Fridovich I.. 2002. Amyotrophic lateral sclerosis: a proposed mechanism. Proc. Natl. Acad. Sci. USA. 99:9010–9014. 10.1073/pnas.132260399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onesto E., Colombrita C., Gumina V., Borghi M.O., Dusi S., Doretti A., Fagiolari G., Invernizzi F., Moggio M., Tiranti V., et al. 2016. Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta Neuropathol. Commun. 4:47 10.1186/s40478-016-0316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillusson S., Gomez-Suaga P., Stoica R., Little D., Gissen P., Devine M.J., Noble W., Hanger D.P., and Miller C.C.J.. 2017. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca(2+) homeostasis and mitochondrial ATP production. Acta Neuropathol. 134:129–149. 10.1007/s00401-017-1704-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L., and Germain D.. 2011. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J. Cell Sci. 124:1396–1402. 10.1242/jcs.078220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-J., Kim S.-S., Seong Y.-M., Kim K.-H., Goo H.G., Yoon E.J., Min S., Kang S., and Rhim H.. 2006. Beta-amyloid precursor protein is a direct cleavage target of HtrA2 serine protease. Implications for the physiological function of HtrA2 in the mitochondria. J. Biol. Chem. 281:34277–34287. 10.1074/jbc.M603443200 [DOI] [PubMed] [Google Scholar]
- Pasinelli P., Belford M.E., Lennon N., Bacskai B.J., Hyman B.T., Trotti D., and Brown R.H. Jr. 2004. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 43:19–30. 10.1016/j.neuron.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Pathak D., Berthet A., Bendor J.T., Yu K., Sellnow R.C., Orr A.L., Nguyen M.K., Edwards R.H., Manfredsson F.P., and Nakamura K.. 2017. Loss of α-Synuclein Does Not Affect Mitochondrial Bioenergetics in Rodent Neurons. eNeuro. 4:e2016-16.2017 10.1523/ENEURO.0216-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M.W., Nargund A.M., and Haynes C.M.. 2013. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta. 1833:410–416. 10.1016/j.bbamcr.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer K., Gohil V., Stuart R.A., Hunte C., Brandt U., Greenberg M.L., and Schägger H.. 2003. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278:52873–52880. 10.1074/jbc.M308366200 [DOI] [PubMed] [Google Scholar]
- Pickles S., Semmler S., Broom H.R., Destroismaisons L., Legroux L., Arbour N., Meiering E., Cashman N.R., and Vande Velde C.. 2016. ALS-linked misfolded SOD1 species have divergent impacts on mitochondria. Acta Neuropathol. Commun. 4:43 10.1186/s40478-016-0313-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell A.M., and Youle R.J.. 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 85:257–273. 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M., Pickrell A.M., Fukui H., and Moraes C.T.. 2013. Mitochondrial DNA damage in a mouse model of Alzheimer’s disease decreases amyloid beta plaque formation. Neurobiol. Aging. 34:2399–2407. 10.1016/j.neurobiolaging.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S., Chander H., Schäfer P., Meiss G., Krüger R., Schulz J.B., and Germain D.. 2008. Mitochondrial protein quality control by the proteasome involves ubiquitination and the protease Omi. J. Biol. Chem. 283:12681–12685. 10.1074/jbc.C800036200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase S., Grumbt B., Neupert W., and Hell K.. 2009. The disulfide relay system of mitochondria is required for the biogenesis of mitochondrial Ccs1 and Sod1. J. Mol. Biol. 385:331–338. 10.1016/j.jmb.2008.10.088 [DOI] [PubMed] [Google Scholar]
- Reeve A., Meagher M., Lax N., Simcox E., Hepplewhite P., Jaros E., and Turnbull D.. 2013. The impact of pathogenic mitochondrial DNA mutations on substantia nigra neurons. J. Neurosci. 33:10790–10801. 10.1523/JNEUROSCI.3525-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robotta M., Gerding H.R., Vogel A., Hauser K., Schildknecht S., Karreman C., Leist M., Subramaniam V., and Drescher M.. 2014. Alpha-synuclein binds to the inner membrane of mitochondria in an α-helical conformation. ChemBioChem. 15:2499–2502. 10.1002/cbic.201402281 [DOI] [PubMed] [Google Scholar]
- Rönnbäck A., Pavlov P.F., Mansory M., Gonze P., Marlière N., Winblad B., Graff C., and Behbahani H.. 2016. Mitochondrial dysfunction in a transgenic mouse model expressing human amyloid precursor protein (APP) with the Arctic mutation. J. Neurochem. 136:497–502. 10.1111/jnc.13410 [DOI] [PubMed] [Google Scholar]
- Rostovtseva T.K., Gurnev P.A., Protchenko O., Hoogerheide D.P., Yap T.L., Philpott C.C., Lee J.C., and Bezrukov S.M.. 2015. α-Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease. J. Biol. Chem. 290:18467–18477. 10.1074/jbc.M115.641746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Blasco S., Valero R.A., Rodríguez-Crespo I., Villalobos C., and Núñez L.. 2008. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One. 3:e2718 10.1371/journal.pone.0002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner B., Hedskog L., Wiehager B., and Ankarcrona M.. 2015. Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers Dis. 43:369–374. [DOI] [PubMed] [Google Scholar]
- Shen J., Du T., Wang X., Duan C., Gao G., Zhang J., Lu L., and Yang H.. 2014. α-Synuclein amino terminus regulates mitochondrial membrane permeability. Brain Res. 1591:14–26. 10.1016/j.brainres.2014.09.046 [DOI] [PubMed] [Google Scholar]
- Son M., Puttaparthi K., Kawamata H., Rajendran B., Boyer P.J., Manfredi G., and Elliott J.L.. 2007. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc. Natl. Acad. Sci. USA. 104:6072–6077. 10.1073/pnas.0610923104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M., Fu Q., Puttaparthi K., Matthews C.M., and Elliott J.L.. 2009. Redox susceptibility of SOD1 mutants is associated with the differential response to CCS over-expression in vivo. Neurobiol. Dis. 34:155–162. 10.1016/j.nbd.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., and Avadhani N.G.. 2012. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic. Biol. Med. 53:1252–1263. 10.1016/j.freeradbiomed.2012.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica R., De Vos K.J., Paillusson S., Mueller S., Sancho R.M., Lau K.F., Vizcay-Barrena G., Lin W.L., Xu Y.F., Lewis J., et al. 2014. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 5:3996 10.1038/ncomms4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica R., Paillusson S., Gomez-Suaga P., Mitchell J.C., Lau D.H., Gray E.H., Sancho R.M., Vizcay-Barrena G., De Vos K.J., Shaw C.E., et al. 2016. ALS/FTD-associated FUS activates GSK-3β to disrupt the VAPB-PTPIP51 interaction and ER-mitochondria associations. EMBO Rep. 17:1326–1342. 10.15252/embr.201541726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtz L.A., Diekert K., Jensen L.T., Lill R., and Culotta V.C.. 2001. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 276:38084–38089. [DOI] [PubMed] [Google Scholar]
- Subramaniam S.R., Vergnes L., Franich N.R., Reue K., and Chesselet M.F.. 2014. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol. Dis. 70:204–213. 10.1016/j.nbd.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K., Yao J., Huang J., Xu H., Chen X., Luddy J., Trillat A.C., Stern D.M., Arancio O., and Yan S.S.. 2005. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 19:597–598. [DOI] [PubMed] [Google Scholar]
- Tan W., Naniche N., Bogush A., Pedrini S., Trotti D., and Pasinelli P.. 2013. Small peptides against the mutant SOD1/Bcl-2 toxic mitochondrial complex restore mitochondrial function and cell viability in mutant SOD1-mediated ALS. J. Neurosci. 33:11588–11598. 10.1523/JNEUROSCI.5385-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias V., Hu X., Luk K.C., Sanders L.H., Lee V.M., and Greenamyre J.T.. 2017. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell. Mol. Life Sci. 74:2851–2874. 10.1007/s00018-017-2541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Velde C., Miller T.M., Cashman N.R., and Cleveland D.W.. 2008. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc. Natl. Acad. Sci. USA. 105:4022–4027. 10.1073/pnas.0712209105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varabyova A., Topf U., Kwiatkowska P., Wrobel L., Kaus-Drobek M., and Chacinska A.. 2013. Mia40 and MINOS act in parallel with Ccs1 in the biogenesis of mitochondrial Sod1. FEBS J. 280:4943–4959. 10.1111/febs.12409 [DOI] [PubMed] [Google Scholar]
- Vijayvergiya C., Beal M.F., Buck J., and Manfredi G.. 2005. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J. Neurosci. 25:2463–2470. 10.1523/JNEUROSCI.4385-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi K., Háden K., Kis V., Gulyássy P., Badics K., Györffy B.A., Simor A., Szabó Z., Janáky T., Drahos L., et al. 2017. Mitochondrial Proteome Changes Correlating with β-Amyloid Accumulation. Mol. Neurobiol. 54:2060–2078. 10.1007/s12035-015-9682-4 [DOI] [PubMed] [Google Scholar]
- Wang L., Guo L., Lu L., Sun H., Shao M., Beck S.J., Li L., Ramachandran J., Du Y., and Du H.. 2016a Synaptosomal Mitochondrial Dysfunction in 5xFAD Mouse Model of Alzheimer’s Disease. PLoS One. 11:e0150441 10.1371/journal.pone.0150441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang L., Lu J., Siedlak S.L., Fujioka H., Liang J., Jiang S., Ma X., Jiang Z., da Rocha E.L., et al. 2016b The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat. Med. 22:869–878. 10.1038/nm.4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N., and Pfanner N.. 2017. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 86:685–714. 10.1146/annurev-biochem-060815-014352 [DOI] [PubMed] [Google Scholar]
- Writing Group on behalf of the Edaravone (MCI-186) ALS 19 Study Group 2017. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16:505–512. 10.1016/S1474-4422(17)30115-1 [DOI] [PubMed] [Google Scholar]
- Yao J., Du H., Yan S., Fang F., Wang C., Lue L.F., Guo L., Chen D., Stern D.M., Gunn Moore F.J., et al. 2011. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. J. Neurosci. 31:2313–2320. 10.1523/JNEUROSCI.4717-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Shibata Y., Kikkert M., van Voorden S., Wiertz E., and Rapoport T.A.. 2005. Recruitment of the p97 ATPase and ubiquitin ligases to the site of retrotranslocation at the endoplasmic reticulum membrane. Proc. Natl. Acad. Sci. USA. 102:14132–14138. 10.1073/pnas.0505006102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonashiro R., Ishido S., Kyo S., Fukuda T., Goto E., Matsuki Y., Ohmura-Hoshino M., Sada K., Hotta H., Yamamura H., et al. 2006. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 25:3618–3626. 10.1038/sj.emboj.7601249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonashiro R., Sugiura A., Miyachi M., Fukuda T., Matsushita N., Inatome R., Ogata Y., Suzuki T., Dohmae N., and Yanagi S.. 2009. Mitochondrial ubiquitin ligase MITOL ubiquitinates mutant SOD1 and attenuates mutant SOD1-induced reactive oxygen species generation. Mol. Biol. Cell. 20:4524–4530. 10.1091/mbc.E09-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu J., Wang X., Duan C., Wang X., and Yang H.. 2016. V63 and N65 of overexpressed α-synuclein are involved in mitochondrial dysfunction. Brain Res. 1642:308–318. 10.1016/j.brainres.2016.04.002 [DOI] [PubMed] [Google Scholar]